Gamma-Tocopherol: A Comprehensive Review of Its Antioxidant, Anti-Inflammatory, and Anticancer Properties
Abstract
1. Introduction
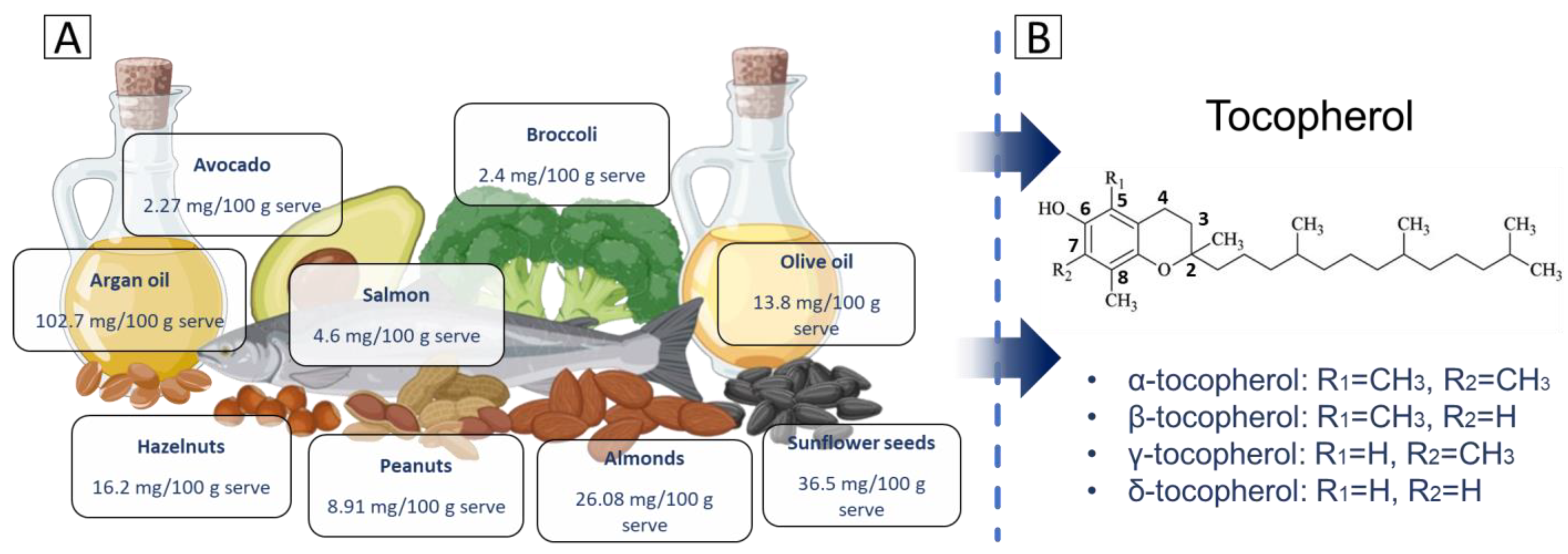
2. Structure and Properties
- α-tocopherol: the chromanol ring has methyl groups at positions R1 = CH₃ and R2 = CH₃. This fully methylated structure allows α-tocopherol to have the highest antioxidant activity among tocopherols and makes it the most bioavailable form.
- β-tocopherol: structurally, β-tocopherol has one methyl group located at R1 = CH₃ and one hydrogen atom in R2 = H. Its activity is intermediate, between that of α-tocopherol and γ-tocopherol, due to this arrangement.
- γ-tocopherol: in this variant, the hydrogen atom is located at R1 = H, while the methyl group is located at R2 = CH₃. The absence of a methyl group at R1 alters its antioxidant properties. While its radical-scavenging activity is slightly lower than α-tocopherol, γ-tocopherol has a unique ability to trap RNS, such as peroxynitrite, which may confer additional protective effects.
- δ-tocopherol: the chromanol ring in δ-tocopherol has hydrogen atoms in both substituents, which are positioned at R1 = H and R2 = H. This structure results in δ-tocopherol having the highest ability to neutralize ROS among the tocopherol isomers.
3. Antioxidant Effects
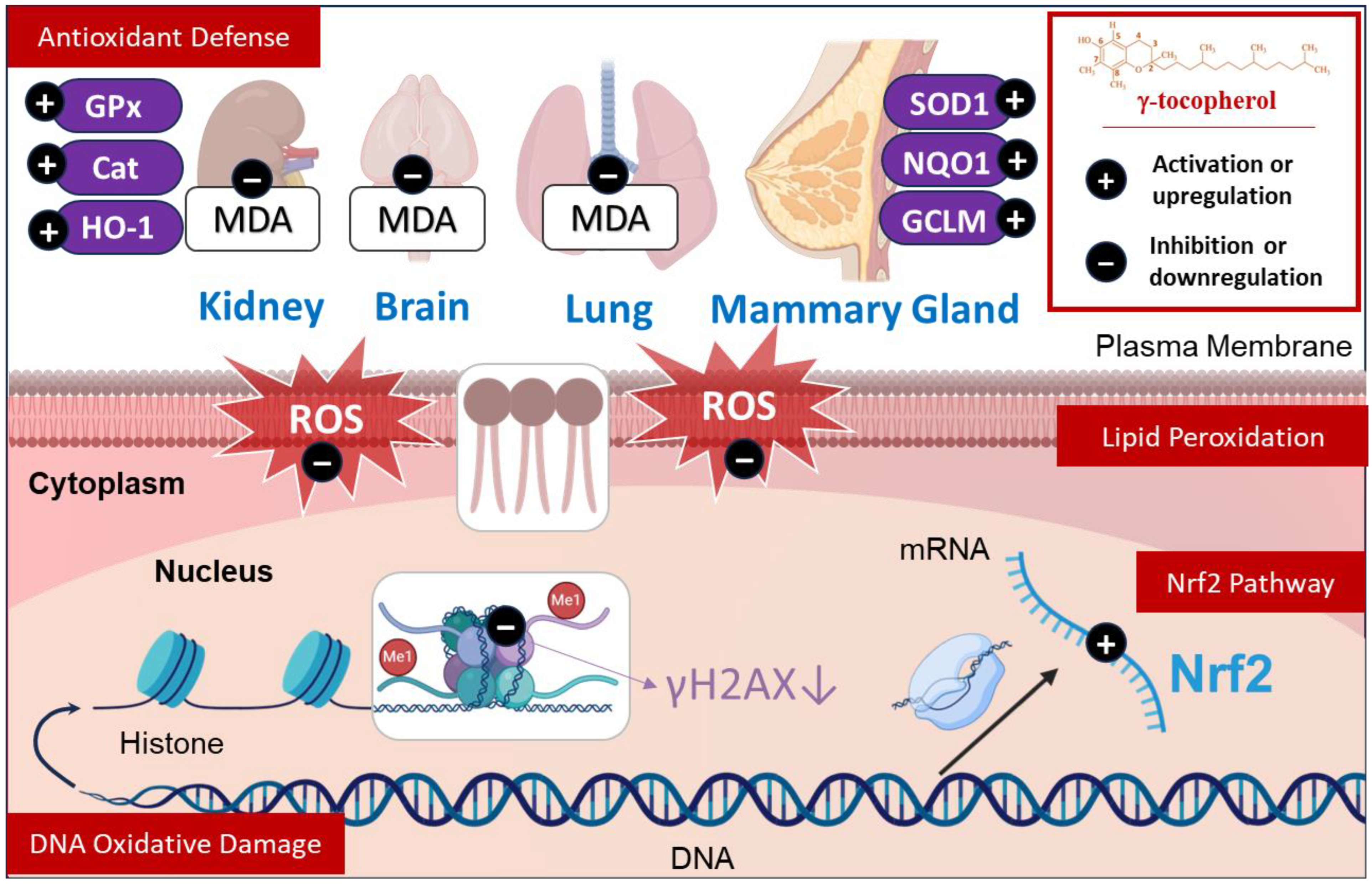
3.1. Effects Against Lipid Peroxidation
3.2. Effects Against DNA Oxidative Damage
3.3. Effect on Antioxidant Defense
3.4. Effect on Nrf2 Pathway
4. Anti-Inflammatory Activity
4.1. Carrageenan-Induced Inflammation
4.2. Diabetes-Induced Inflammation
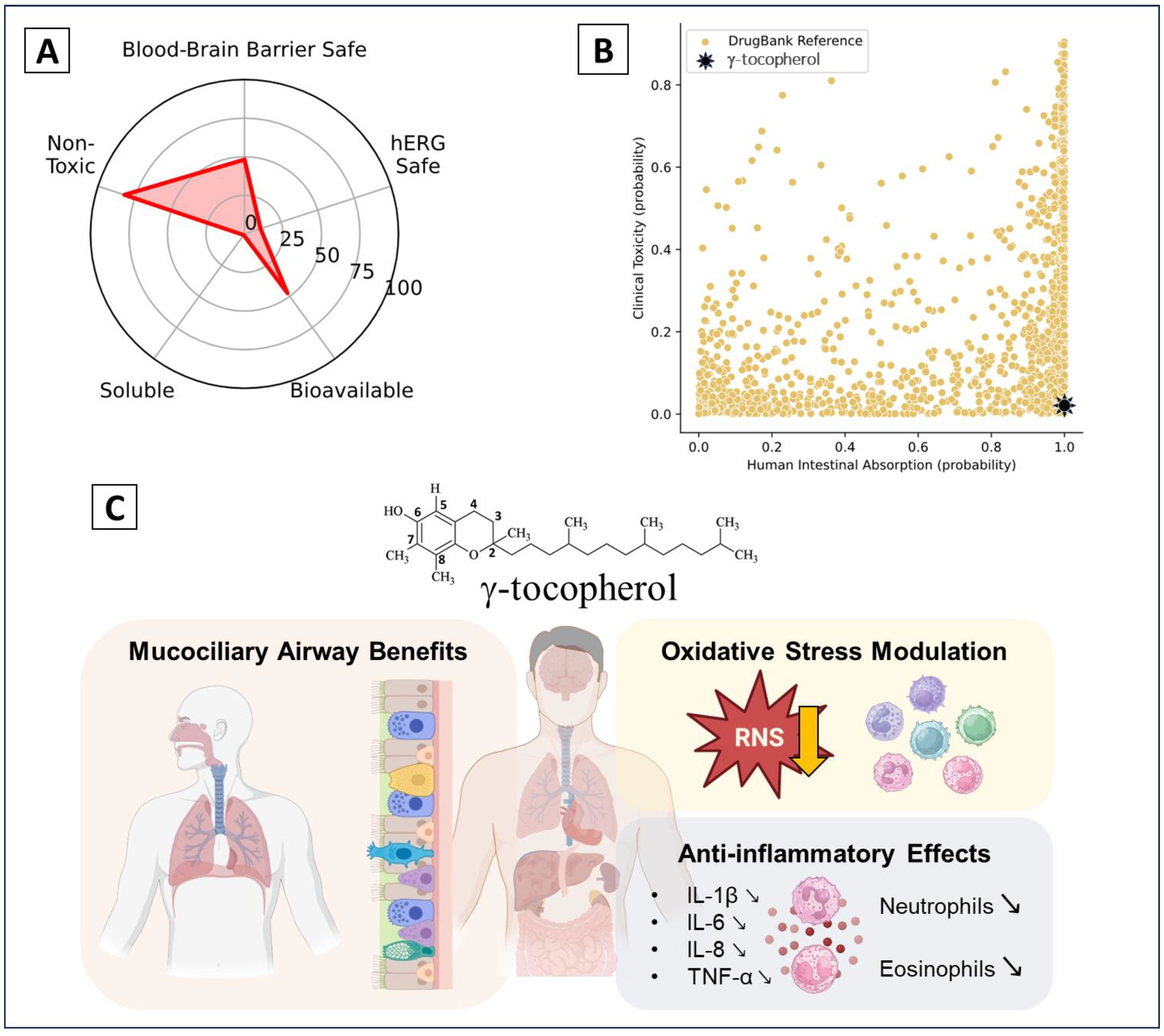
4.3. Asthmatic Disease
4.3.1. Non-Allergic Asthma Induced by LPS
4.3.2. Other Asthma Inducers
4.4. Nanoformulation
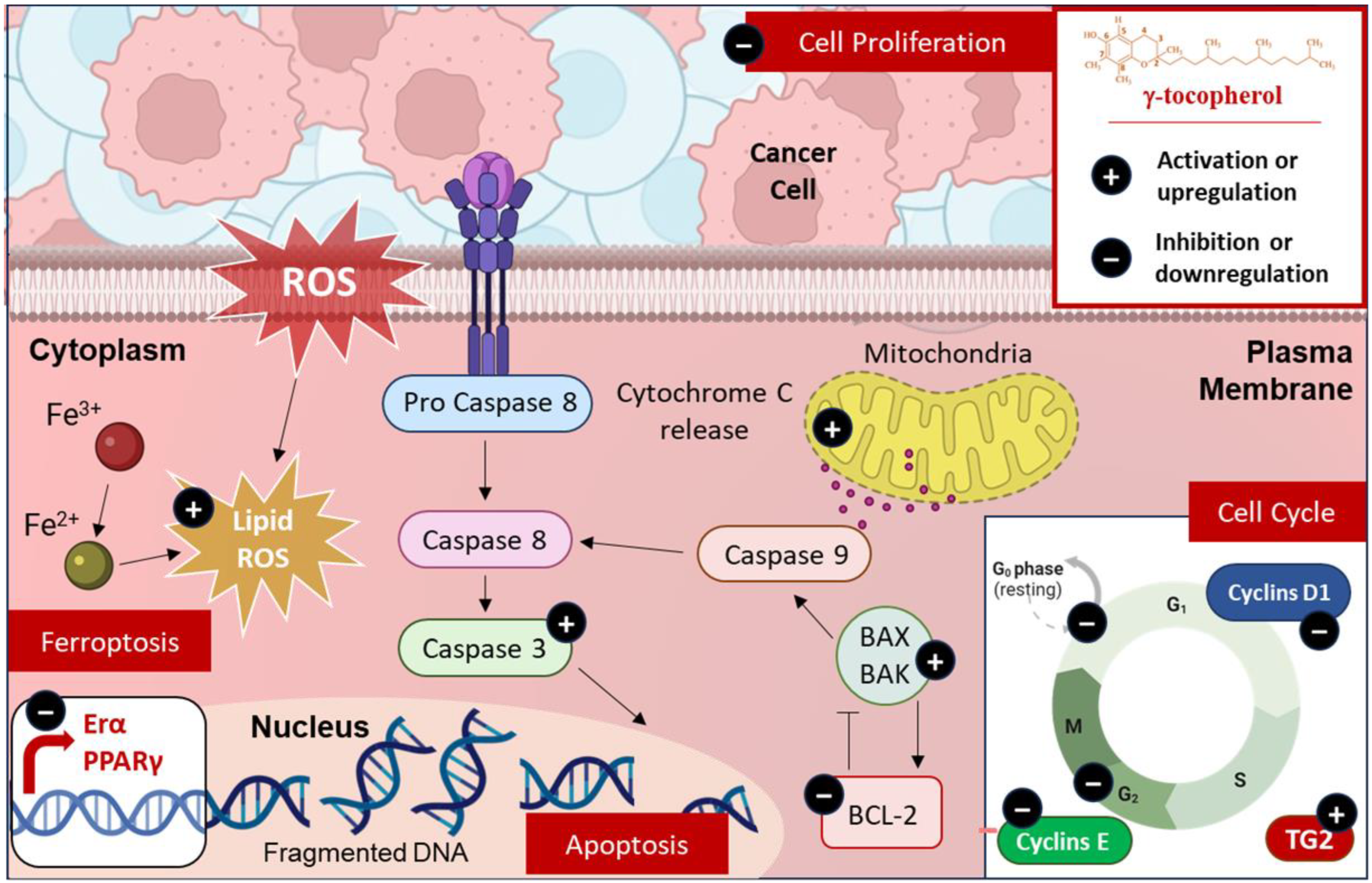
5. Anticancer Activity
5.1. Modulation of Cell Death
5.2. Modulation of Cell Cycle
5.3. Modulation of Cell Proliferation
6. Clinical Trials and Therapeutic Potential
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin e in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar] [PubMed]
- Jiang, Q.; Im, S.; Wagner, J.G.; Hernandez, M.L.; Peden, D.B. Gamma-tocopherol, a major form of vitamin E in diets: Insights into antioxidant and anti-inflammatory effects, mechanisms, and roles in disease management. Free Radic. Biol. Med. 2022, 178, 347–359. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- Hba, S.; Ghaddar, S.; Wahnou, H.; Pinon, A.; El Kebbaj, R.; Pouget, C.; Sol, V.; Liagre, B.; Oudghiri, M.; Limami, Y. Natural Chalcones and Derivatives in Colon Cancer: Pre-Clinical Challenges and the Promise of Chalcone-Based Nanoparticles. Pharmaceutics 2023, 15, 2718. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- McEvoy, L.; Carr, D.F.; Pirmohamed, M. Pharmacogenomics of NSAID-Induced Upper Gastrointestinal Toxicity. Front. Pharmacol. 2021, 12, 684162. [Google Scholar] [CrossRef]
- Wahnou, H.; Ndayambaje, M.; Ouadghiri, Z.; Benayad, S.; Elattar, H.; Chgari, O.; Naya, A.; Zaid, Y.; Oudghiri, M. Artemisia herba-alba: Antioxidant capacity and efficacy in preventing chronic arthritis in vivo. Inflammopharmacology 2024, 32, 1855–1870. [Google Scholar] [CrossRef] [PubMed]
- Pahrudin Arrozi, A.; Shukri, S.N.S.; Wan Ngah, W.Z.; Mohd Yusof, Y.A.; Ahmad Damanhuri, M.H.; Jaafar, F.; Makpol, S. Comparative Effects of Alpha- and Gamma-Tocopherol on Mitochondrial Functions in Alzheimer’s Disease In Vitro Model. Sci. Rep. 2020, 10, 8962. [Google Scholar] [CrossRef] [PubMed]
- Reiter, E.; Jiang, Q.; Christen, S. Anti-inflammatory properties of alpha- and gamma-tocopherol. Mol. Asp. Med. 2007, 28, 668–691. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Jialal, I. Failure of Vitamin E in Clinical Trials: Is Gamma-Tocopherol the Answer? Nutr. Rev. 2005, 63, 290–293. [Google Scholar] [CrossRef]
- Kuo, F.; Subramanian, B.; Kotyla, T.; Wilson, T.A.; Yoganathan, S.; Nicolosi, R.J. Nanoemulsions of an anti-oxidant synergy formulation containing gamma tocopherol have enhanced bioavailability and anti-inflammatory properties. Int. J. Pharm. 2008, 363, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Das Gupta, S.; Suh, N. Tocopherols in cancer: An update. Mol. Nutr. Food Res. 2016, 60, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.L. An Update on Vitamin E, Tocopherol and Tocotrienol—Perspectives. Molecules 2010, 15, 2103–2113. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Harvey, K.; Pavlina, T.; Zaloga, G.; Siddiqui, R. Tocopherol and Tocotrienol Homologs in Parenteral Lipid Emulsions. Eur. J. Lipid Sci. Technol. 2015, 117, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Charlton, N.C.; Mastyugin, M.; Török, B.; Török, M. Structural Features of Small Molecule Antioxidants and Strategic Modifications to Improve Potential Bioactivity. Molecules 2023, 28, 1057. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.; Harroun, T.; Wassall, S.R.; Stillwell, W.; Katsaras, J. The location and behavior of alpha-tocopherol in membranes. Mol. Nutr. Food Res. 2010, 54, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; de Camargo, A.C. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, G.; Schneider, R.G. Tocopherol degradation and lipid oxidation during storage of Chenopodium quinoa. J. Food Compos. Anal. 2023, 123, 105549. [Google Scholar] [CrossRef]
- Bruscatto, M.H.; Zambiazi, R.C.; Sganzerla, M.; Pestana, V.R.; Otero, D.; Lima, R.; Paiva, F. Degradation of Tocopherols in Rice Bran Oil Submitted to Heating at Different Temperatures. J. Chromatogr. Sci. 2009, 47, 762–765. [Google Scholar] [CrossRef]
- Aoyama, S.; Nishio, T.; Moriya, D.; Munemasa, S.; Murata, Y.; Nakamura, Y.; Nakamura, T. The Metabolite of γ-Tocopherol, 2,7,8-Trimethyl-2-(2′-Carboxyethyl)-6-Hydroxychroman, Exerts Intracellular Antioxidant Activity via Up-Regulation of Heme Oxygenase-1 in Hepatocytes. Nutraceuticals 2024, 4, 409–416. [Google Scholar] [CrossRef]
- Thbayh, D.K.; Mentes, D.; Boros, Z.R.; Palusiak, M.; Farkas, L.; Viskolcz, B.; Fiser, B. α-Tocopherol and Trolox as Effective Natural Additives for Polyurethane Foams: A DFT and Experimental Study. Molecules 2024, 29, 6037. [Google Scholar] [CrossRef]
- Amić, A.; Mastil’ák Cagardová, D. A DFT study of the antioxidant potency of α-tocopherol and its derivatives: PMHC, Trolox, and α-CEHC. J. Mol. Liq. 2024, 403, 124796. [Google Scholar] [CrossRef]
- Benbrahim, N.; Zeddour-Brahim, K.; Zizi, Z.; Bengharez, Z. Stability and Reactivity of Tocopherols: Theoretical Study. Chem. Proc. 2023, 14, 20. [Google Scholar] [CrossRef]
- Wagner, K.H.; Kamal-Eldin, A.; Elmadfa, I. Gamma-tocopherol—an underestimated vitamin? Ann. Nutr. Metab. 2004, 48, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, S.; Tsuchida, N.; Yamazaki, S. A DFT Study on Transition States of Inhibition of Oxidation by α-Tocopherol. ChemistrySelect 2020, 5, 9184–9194. [Google Scholar] [CrossRef]
- Abyar, F.; Farrokhpour, H. Ionization energies and photoelectron spectra of fat-soluble vitamins in the gas phase: A theoretical study. RSC Adv. 2014, 4, 35975–35987. [Google Scholar] [CrossRef]
- Warren, J.J.; Mayer, J.M. Predicting organic hydrogen atom transfer rate constants using the Marcus cross relation. Proc. Natl. Acad. Sci. USA 2010, 107, 5282–5287. [Google Scholar] [CrossRef] [PubMed]
- Povalishev, V.N.; Polozov, G.I.; Shadyro, O.I. Effects of α-tocopherol and related compounds on reactions involving various organic radicals. Bioorganic Med. Chem. Lett. 2006, 16, 1236–1239. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Eo, H.; Lim, Y. Similarities and differences between alpha-tocopherol and gamma-tocopherol in amelioration of inflammation, oxidative stress and pre-fibrosis in hyperglycemia induced acute kidney inflammation. Nutr. Res. Pract. 2016, 10, 33–41. [Google Scholar] [CrossRef]
- Chung, M.Y.; Yeung, S.F.; Park, H.J.; Volek, J.S.; Bruno, R.S. Dietary α- and γ-tocopherol supplementation attenuates lipopolysaccharide-induced oxidative stress and inflammatory-related responses in an obese mouse model of nonalcoholic steatohepatitis. J. Nutr. Biochem. 2010, 21, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Sousse, L.E.; Enkhbaatar, P.; Kraft, E.R.; Deyo, D.J.; Wright, C.L.; Taylor, A.; Traber, M.G.; Cox, R.A.; Hawkins, H.K.; et al. γ-tocopherol nebulization decreases oxidative stress, arginase activity, and collagen deposition after burn and smoke inhalation in the ovine model. Shock 2012, 38, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Das Gupta, S.; So, J.Y.; Wall, B.; Wahler, J.; Smolarek, A.K.; Sae-Tan, S.; Soewono, K.Y.; Yu, H.; Lee, M.J.; Thomas, P.E.; et al. Tocopherols inhibit oxidative and nitrosative stress in estrogen-induced early mammary hyperplasia in ACI rats. Mol. Carcinog. 2015, 54, 916–925. [Google Scholar] [CrossRef]
- Devaraj, S.; Leonard, S.; Traber, M.G.; Jialal, I. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic. Biol. Med. 2008, 44, 1203–1208. [Google Scholar] [CrossRef]
- Kowalska, M.; Piekut, T.; Prendecki, M.; Sodel, A.; Kozubski, W.; Dorszewska, J. Mitochondrial and Nuclear DNA Oxidative Damage in Physiological and Pathological Aging. DNA Cell Biol. 2020, 39, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Liu, A.; Lee, M.J.; Wang, H.; Yu, S.; Chi, E.; Reuhl, K.; Suh, N.; Yang, C.S. δ- and γ-tocopherols inhibit phIP/DSS-induced colon carcinogenesis by protection against early cellular and DNA damages. Mol. Carcinog. 2017, 56, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lee, M.J.; Liu, A.B.; Yang, Z.; Lin, Y.; Shih, W.J.; Yang, C.S. The antioxidant and anti-inflammatory activities of tocopherols are independent of Nrf2 in mice. Free Radic. Biol. Med. 2012, 52, 1151–1158. [Google Scholar] [CrossRef]
- Wiser, J.; Alexis, N.E.; Jiang, Q.; Wu, W.; Robinette, C.; Roubey, R.; Peden, D.B. In vivo gamma-tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects. Free Radic. Biol. Med. 2008, 45, 40–49. [Google Scholar] [CrossRef]
- Dey, P.; Mah, E.; Li, J.; Jalili, T.; Symons, J.D.; Bruno, R.S. Improved hepatic γ-tocopherol status limits oxidative and inflammatory stress-mediated liver injury in db/db mice with nonalcoholic steatohepatitis. J. Funct. Foods 2018, 40, 670–678. [Google Scholar] [CrossRef]
- Smolarek, A.K.; So, J.Y.; Thomas, P.E.; Lee, H.J.; Paul, S.; Dombrowski, A.; Wang, C.X.; Saw, C.L.; Khor, T.O.; Kong, A.N.; et al. Dietary tocopherols inhibit cell proliferation, regulate expression of ERα, PPARγ, and Nrf2, and decrease serum inflammatory markers during the development of mammary hyperplasia. Mol. Carcinog. 2013, 52, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Khor, T.O.; Shu, L.; Saw, C.L.; Wu, T.Y.; Suh, N.; Yang, C.S.; Kong, A.N. A γ-tocopherol-rich mixture of tocopherols maintains Nrf2 expression in prostate tumors of TRAMP mice via epigenetic inhibition of CpG methylation. J. Nutr. 2012, 142, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Korošec, T.; Tomažin, U.; Horvat, S.; Keber, R.; Salobir, J. The diverse effects of α- and γ-tocopherol on chicken liver transcriptome. Poult. Sci. 2017, 96, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.; Kharbach, M.; Vander Heyden, Y.; Doukkali, Z.; Ghchime, R.; Bouklouze, A.; Cherrah, Y.; Alaoui, K. In vivo anti-inflammatory response and bioactive compounds’ profile of polyphenolic extracts from edible Argan oil (Argania spinosa L.), obtained by two extraction methods. J. Food Biochem. 2019, 43, e13066. [Google Scholar] [CrossRef] [PubMed]
- Bouchab, H.; Ishaq, A.; El Kebbaj, R.; Nasser, B.; Saretzki, G. Protective effect of argan oil on DNA damage in vivo and in vitro. Biomarkers 2021, 26, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Bouchab, H.; Essadek, S.; El Kamouni, S.; Moustaid, K.; Essamadi, A.; Andreoletti, P.; Cherkaoui-Malki, M.; El Kebbaj, R.; Nasser, B. Antioxidant Effects of Argan Oil and Olive Oil against Iron-Induced Oxidative Stress: In Vivo and In Vitro Approaches. Molecules 2023, 28, 5924. [Google Scholar] [CrossRef]
- Haleng, J.; Pincemail, J.; Defraigne, J.O.; Charlier, C.; Chapelle, J.P. Oxidative stress. Rev. Medicale Liege 2007, 62, 628–638. [Google Scholar]
- El Faqer, O.; Rais, S.; Ouadghiri, Z.; El Faqer, A.; Benchama, Z.; El Ouaddari, A.; Dakir, M.; El Amrani, A.; Mtairag, E.M. Physicochemical properties, GC–MS profiling, and antibacterial potential of Allium sativum essential oil: In vitro and in silico approaches. Sci. Afr. 2024, 26, e02484. [Google Scholar] [CrossRef]
- El Faqer, O.; Elkoraichi, I.; Latif, M.; Debierre-Grockiego, F.; Ouadghiri, Z.; Rais, S.; Dimier-Poisson, I.; Mtairag, E.M. Pharmacological insights into Laurus nobilis: HPLC profiling and evaluation of its anti-Toxoplasma, antioxidant, and anti-hemolytic properties. Biochem. Syst. Ecol. 2024, 117, 104891. [Google Scholar] [CrossRef]
- Saldeen, T.; Li, D.; Mehta, J.L. Differential effects of alpha- and gamma-tocopherol on low-density lipoprotein oxidation, superoxide activity, platelet aggregation and arterial thrombogenesis. J. Am. Coll. Cardiol. 1999, 34, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- El Kebbaj, R.; Bouchab, H.; Tahri-Joutey, M.; Rabbaa, S.; Limami, Y.; Nasser, B.; Egbujor, M.C.; Tucci, P.; Andreoletti, P.; Saso, L.; et al. The Potential Role of Major Argan Oil Compounds as Nrf2 Regulators and Their Antioxidant Effects. Antioxidants 2024, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Wahnou, H.; Hmimid, F.; Errami, A.; Nait Irahal, I.; Limami, Y.; Oudghiri, M. Integrating ADMET, enrichment analysis, and molecular docking approach to elucidate the mechanism of Artemisia herba alba for the treatment of inflammatory bowel disease-associated arthritis. J. Toxicol. Environ. Health Part A 2024, 87, 836–854. [Google Scholar] [CrossRef]
- Jiang, Q.; Ames, B.N. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003, 17, 816–822. [Google Scholar] [CrossRef]
- Jiang, Q.; Moreland, M.; Ames, B.N.; Yin, X. A combination of aspirin and gamma-tocopherol is superior to that of aspirin and alpha-tocopherol in anti-inflammatory action and attenuation of aspirin-induced adverse effects. J. Nutr. Biochem. 2009, 20, 894–900. [Google Scholar] [CrossRef]
- Elisia, I.; Kitts, D.D. Tocopherol isoforms (α-, γ-, and δ-) show distinct capacities to control Nrf-2 and NfκB signaling pathways that modulate inflammatory response in Caco-2 intestinal cells. Mol. Cell. Biochem. 2015, 404, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Elson-Schwab, I.; Courtemanche, C.; Ames, B.N. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc. Natl. Acad. Sci. USA 2000, 97, 11494–11499. [Google Scholar] [CrossRef]
- Wells, S.R.; Jennings, M.H.; Rome, C.; Hadjivassiliou, V.; Papas, K.A.; Alexander, J.S. Alpha-, gamma- and delta-tocopherols reduce inflammatory angiogenesis in human microvascular endothelial cells. J. Nutr. Biochem. 2010, 21, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Mah, E.; Pei, R.; Guo, Y.; Ballard, K.D.; Barker, T.; Rogers, V.E.; Parker, B.A.; Taylor, A.W.; Traber, M.G.; Volek, J.S.; et al. γ-Tocopherol-rich supplementation additively improves vascular endothelial function during smoking cessation. Free Radic. Biol. Med. 2013, 65, 1291–1299. [Google Scholar] [CrossRef]
- Lee, H.; Lim, Y. Gamma-tocopherol ameliorates hyperglycemia-induced hepatic inflammation associated with NLRP3 inflammasome in alloxan-induced diabetic mice. Nutr. Res. Pract. 2019, 13, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Gans, M.D.; Gavrilova, T. Understanding the immunology of asthma: Pathophysiology, biomarkers, and treatments for asthma endotypes. Paediatr. Respir. Rev. 2020, 36, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.L.; Wagner, J.G.; Kala, A.; Mills, K.; Wells, H.B.; Alexis, N.E.; Lay, J.C.; Jiang, Q.; Zhang, H.; Zhou, H.; et al. Vitamin E, γ-tocopherol, reduces airway neutrophil recruitment after inhaled endotoxin challenge in rats and in healthy volunteers. Free Radic. Biol. Med. 2013, 60, 56–62. [Google Scholar] [CrossRef]
- Burbank, A.J.; Duran, C.G.; Pan, Y.; Burns, P.; Jones, S.; Jiang, Q.; Yang, C.; Jenkins, S.; Wells, H.; Alexis, N.; et al. Gamma tocopherol-enriched supplement reduces sputum eosinophilia and endotoxin-induced sputum neutrophilia in volunteers with asthma. J. Allergy Clin. Immunol. 2018, 141, 1231–1238.e1. [Google Scholar] [CrossRef] [PubMed]
- Peden, D.B.; Almond, M.; Brooks, C.; Robinette, C.; Wells, H.; Burbank, A.; Hernandez, M.; Hinderliter, A.; Caughey, M.; Jiang, Q.; et al. A pilot randomized clinical trial of γ-tocopherol supplementation on wood smoke-induced neutrophilic and eosinophilic airway inflammation. J. Allergy Clin. Immunol. Glob. 2023, 2, 100177. [Google Scholar] [CrossRef] [PubMed]
- Wahnou, H.; Liagre, B.; Sol, V.; El Attar, H.; Attar, R.; Oudghiri, M.; Duval, R.E.; Limami, Y. Polyphenol-Based Nanoparticles: A Promising Frontier for Enhanced Colorectal Cancer Treatment. Cancers 2023, 15, 3826. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Rodriguez-Torres, M.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta—Mol. Cell Res. 2013, 1833, 3448–3459. [Google Scholar] [CrossRef] [PubMed]
- Chgari, O.; Wahnou, H.; Ndayambaje, M.; Moukhfi, F.; Benkhnigue, O.; Marnissi, F.; Limami, Y.; Oudghiri, M. Orbea variegata (L.) Haw in skin carcinogenesis: Insights from an in vivo male Swiss mouse model study. J. Toxicol. Environ. Health Part A 2024, 87, 630–645. [Google Scholar] [CrossRef] [PubMed]
- Towers, C.G.; Wodetzki, D.; Thorburn, A. Autophagy and cancer: Modulation of cell death pathways and cancer cell adaptations. J. Cell Biol. 2020, 219, e201909033. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Jiang, T.; He, D. Emerging insights into the role of ferroptosis in the pathogenesis of autoimmune diseases. Front. Immunol. 2023, 14, 1120519. [Google Scholar] [CrossRef] [PubMed]
- Benayad, S.; Wahnou, H.; El Kebbaj, R.; Liagre, B.; Sol, V.; Oudghiri, M.; Saad, E.M.; Duval, R.E.; Limami, Y. The Promise of Piperine in Cancer Chemoprevention. Cancers 2023, 15, 5488. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Di Pardo, A.; Basit, A.; Armirotti, A.; Amico, E.; Castaldo, S.; Pepe, G.; Marracino, F.; Buttari, F.; Digilio, A.F.; Maglione, V. De novo Synthesis of Sphingolipids Is Defective in Experimental Models of Huntington’s Disease. Front. Neurosci. 2017, 11, 698. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Rao, X.; Kim, C.Y.; Freiser, H.; Zhang, Q.; Jiang, Z.; Li, G. Gamma-tocotrienol induces apoptosis and autophagy in prostate cancer cells by increasing intracellular dihydrosphingosine and dihydroceramide. Int. J. Cancer 2012, 130, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, A.; Yu, W.; Jiang, Q.; Jang, Y.; Sanders, B.G.; Kline, K. Involvement of de novo ceramide synthesis in gamma-tocopherol and gamma-tocotrienol-induced apoptosis in human breast cancer cells. Mol. Nutr. Food Res. 2012, 56, 1803–1811. [Google Scholar] [CrossRef]
- Jiang, Q.; Wong, J.; Fyrst, H.; Saba, J.D.; Ames, B.N. γ-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc. Natl. Acad. Sci. USA 2004, 101, 17825–17830. [Google Scholar] [CrossRef]
- Jang, Y.; Park, N.-Y.; Rostgaard-Hansen, A.L.; Huang, J.; Jiang, Q. Vitamin E metabolite 13′-carboxychromanols inhibit pro-inflammatory enzymes, induce apoptosis and autophagy in human cancer cells by modulating sphingolipids and suppress colon tumor development in mice. Free Radic. Biol. Med. 2016, 95, 190–199. [Google Scholar] [CrossRef]
- Yan, R.; Lin, B.; Jin, W.; Tang, L.; Hu, S.; Cai, R. NRF2, a Superstar of Ferroptosis. Antioxidants 2023, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Plati, J.; Bucur, O.; Khosravi-Far, R. Apoptotic cell signaling in cancer progression and therapy. Integr. Biol. 2011, 3, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Bazzaz, R.; Bijanpour, H.; Pirouzpanah, S.M.B.; Yaghmaei, P.; Rashtchizadeh, N. Adjuvant therapy with γ-tocopherol-induce apoptosis in HT-29 colon cancer via cyclin-dependent cell cycle arrest mechanism. J. Biochem. Mol. Toxicol. 2019, 33, e22399. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Zeidooni, L.; Hashemitabar, M.; Razzazzadeh, S.; Mahdavinia, M.; Ghasemi, K. Gamma-tocopherol enhances apoptotic effects of lovastatin in human colorectal carcinoma cell line (HT29). Nutr. Cancer 2014, 66, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Gysin, R.; Azzi, A.; Visarius, T. Gamma-tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. FASEB J. 2002, 16, 1952–1954. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, P.; Caraglia, M.; Abbruzzese, A.; Beninati, S. γ-Tocopherol inhibits human prostate cancer cell proliferation by up-regulation of transglutaminase 2 and down-regulation of cyclins. Amino Acids 2013, 44, 45–51. [Google Scholar] [CrossRef]
- Sato, C.; Kaneko, S.; Sato, A.; Virgona, N.; Namiki, K.; Yano, T. Combination Effect of δ-Tocotrienol and γ-Tocopherol on Prostate Cancer Cell Growth. J. Nutr. Sci. Vitaminol. 2017, 63, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.E.; Stone, W.L.; Whaley, S.G.; Qui, M.; Krishnan, K. Gamma (γ) tocopherol upregulates peroxisome proliferator activated receptor (PPAR) gamma (γ) expression in SW 480 human colon cancer cell lines. BMC Cancer 2003, 3, 25. [Google Scholar] [CrossRef] [PubMed]

| Model | Antioxidant Action | Mechanisms | References |
|---|---|---|---|
| Human (Plasma) Mice (Kidney, Liver) Rat (Plasma) Sheep (Lung) Chicken (Plasma, Liver) | Anti-lipid peroxidation | ↓ MDA ↓ MDA+HNE ↓ Lipid peroxide ↓ 8-isoprostane | [32,33,34,35,36] |
| Mice (Colon tumor and tissue adjacent, Colon) Rat (Mammary gland) | Anti-DNA damage | ↓ 8-oxo-dG ↓ γH2AX | [35,37,38,39] |
| Mice (Kidney, Liver, Prostate, mammary tumor) Rat (Plasma, Mammary gland, aorta) PBM Cells | Defense antioxidant | ↑ mRNA SOD1 ↑ SOD activity ↓ Superoxide generation ↓ ROS ↔/↑ Catalase ↔/↑ GPx ↔ HO-1 ↑ NQO1 ↑ GCLM ↑ HO-1 ↔/↑ Nrf2 Inhibition CpG hypermethylation in Nrf2 promoter | [32,35,40,41,42,43] |
| Intervention/Treatment | Phase | Actual Enrollment | Identifier | Responsible Party | Results |
|---|---|---|---|---|---|
| 3 doses of 1400 mg γ-tocopherol (2 capsules, each is 700 mg), at 12 h intervals | Not Applicable | 10 subjects 18 to 50 years | NCT02610829 | University of North Carolina, Chapel Hill |
|
| Oral doses 1200 mg of γ-tocopherol (2 capsules of the γ-tocopherol enriched vitamin E preparation) | Phase 1 | 25 subjects 18 to 50 years | NCT00836368 | Chapel Hill, North Carolina, United States | No Results Posted |
| 14 days of daily high dose (1200 mg) γ-tocopherol. Subjects will receive a 14 days supply (28 softgel capsules, approximately 600 g of γ-tocopherol each | Phase 1 | 8 subjects 18 to 50 years | NCT00466596 | University of North Carolina, Chapel Hill | No Results Posted |
| γ-tocopherol 1400 mg, taken as 2700 mg capsules every 12 h for a total of 4 doses | Phase 2 | 18 subjects 18 to 45 years | NCT02911688 | University of North Carolina, Chapel Hill |
|
| γ-tocopherol Maxi Gamma softgels 1200 mg | Phase 1 | 18 subjects 18 to 50 years | NCT00631085 | University of North Carolina, Chapel Hill |
|
| 1400 mg of γ-tocopherol -enriched supplement once daily for 7 days | Phase 2 | 16 subjects 18 to 45 years | NCT03444298 | University of North Carolina, Chapel Hill |
|
| 600 mg and 1200 mg of γ-tocopherol safety | Phase 1 | 16 subjects 18 to 50 years | NCT00386178 | University of North Carolina, Chapel Hill | No Results Posted |
| 1200 mg of γ-tocopherol daily for 14 days | Phase 1 Phase 2 | 23 subjects 18 to 50 years | NCT02104505 | University of North Carolina, Chapel Hill |
|
| One administration of 500 mg γ-tocopherol | Phase 1 Phase 2 | 67 subjects 18 to 60 years | NCT01314443 | University of Connecticut |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Es-Sai, B.; Wahnou, H.; Benayad, S.; Rabbaa, S.; Laaziouez, Y.; El Kebbaj, R.; Limami, Y.; Duval, R.E. Gamma-Tocopherol: A Comprehensive Review of Its Antioxidant, Anti-Inflammatory, and Anticancer Properties. Molecules 2025, 30, 653. https://doi.org/10.3390/molecules30030653
Es-Sai B, Wahnou H, Benayad S, Rabbaa S, Laaziouez Y, El Kebbaj R, Limami Y, Duval RE. Gamma-Tocopherol: A Comprehensive Review of Its Antioxidant, Anti-Inflammatory, and Anticancer Properties. Molecules. 2025; 30(3):653. https://doi.org/10.3390/molecules30030653
Chicago/Turabian StyleEs-Sai, Basma, Hicham Wahnou, Salma Benayad, Soufiane Rabbaa, Yassir Laaziouez, Riad El Kebbaj, Youness Limami, and Raphaël Emmanuel Duval. 2025. "Gamma-Tocopherol: A Comprehensive Review of Its Antioxidant, Anti-Inflammatory, and Anticancer Properties" Molecules 30, no. 3: 653. https://doi.org/10.3390/molecules30030653
APA StyleEs-Sai, B., Wahnou, H., Benayad, S., Rabbaa, S., Laaziouez, Y., El Kebbaj, R., Limami, Y., & Duval, R. E. (2025). Gamma-Tocopherol: A Comprehensive Review of Its Antioxidant, Anti-Inflammatory, and Anticancer Properties. Molecules, 30(3), 653. https://doi.org/10.3390/molecules30030653













