Evaluation of the Antibacterial Activity of Cinnamic Acid and Its Derivatives: Synergistic Effects with Cloxacillin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biofilm-Forming Ability
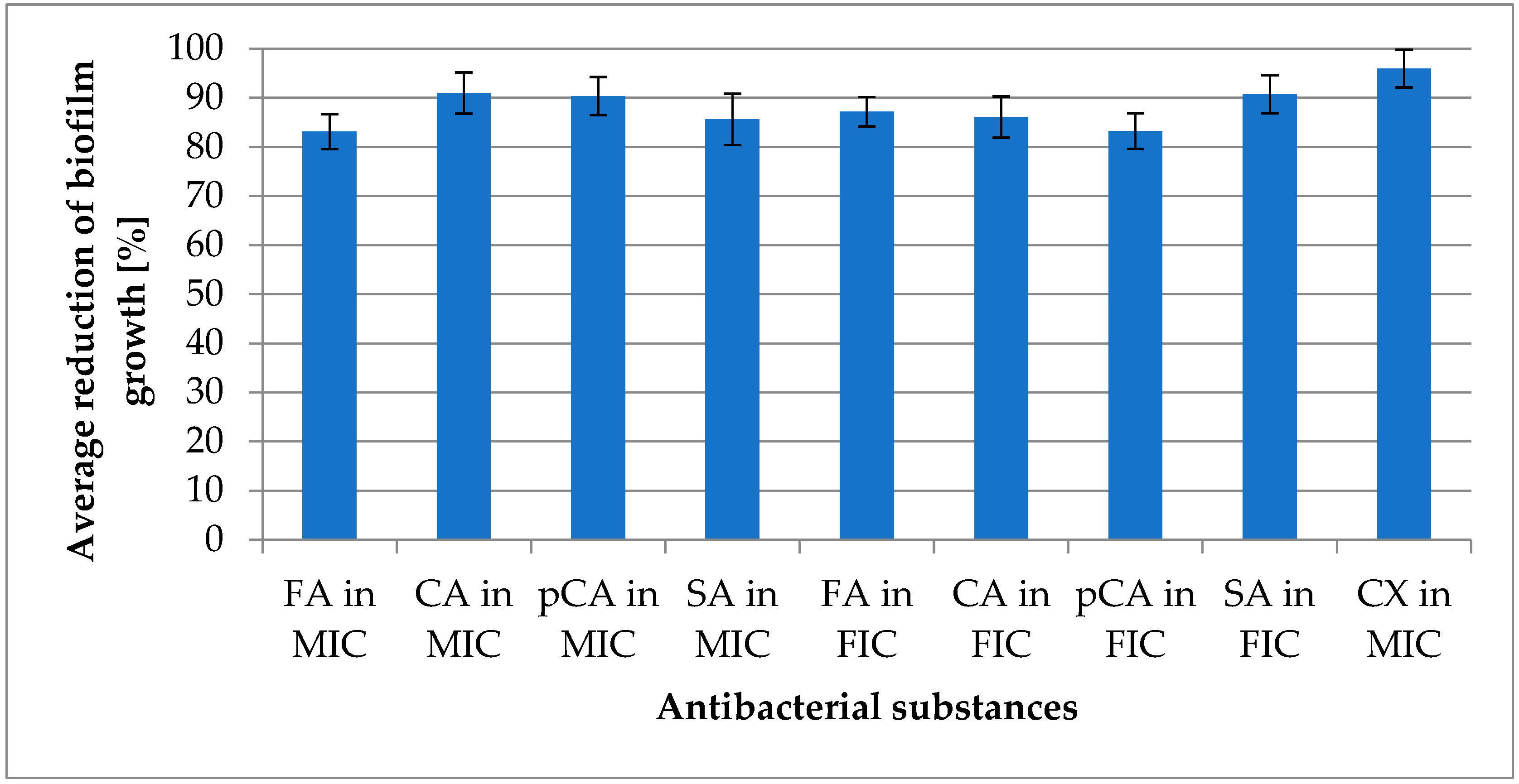
2.2. Antimicrobial and Antibiofilm Activity
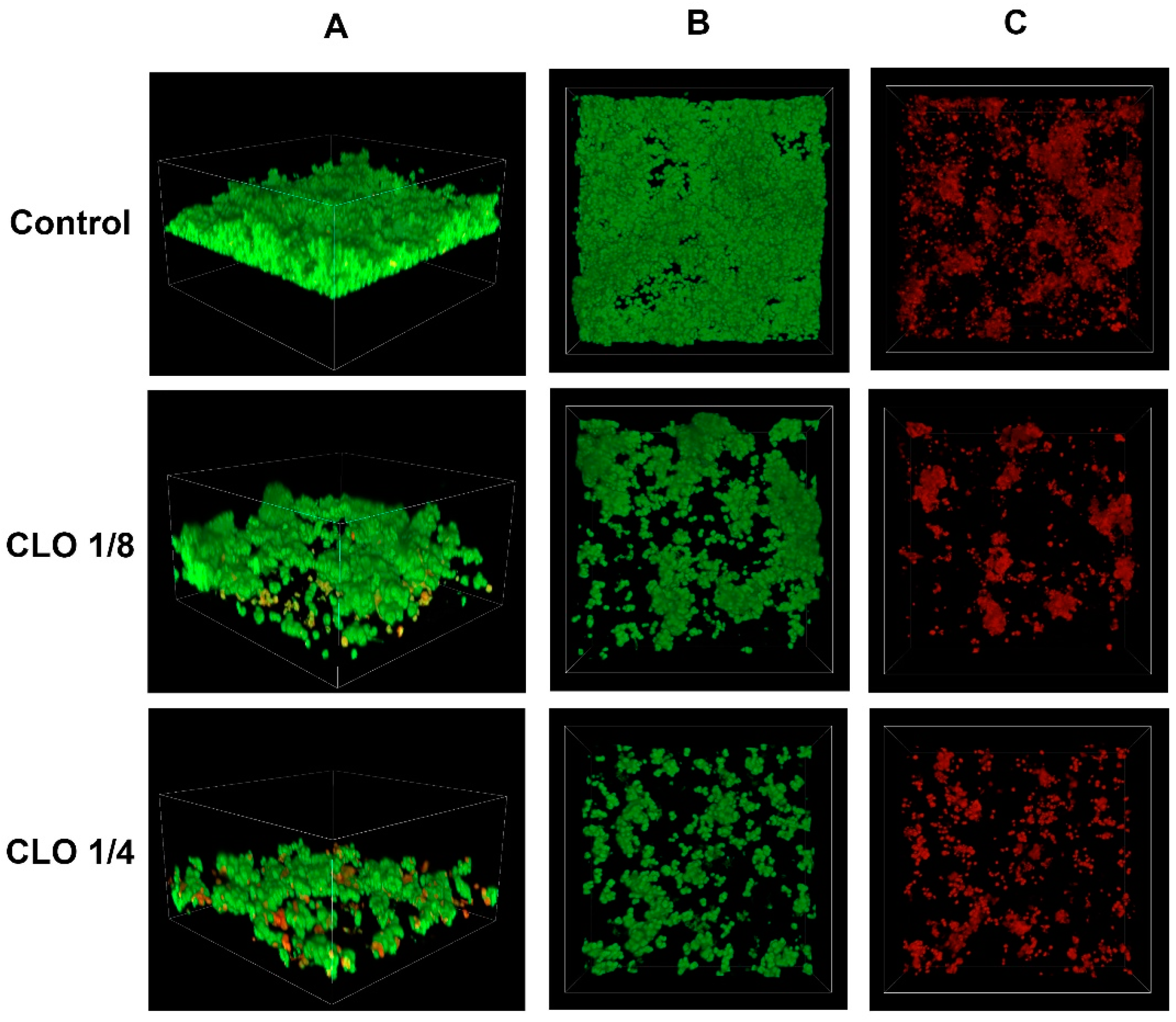
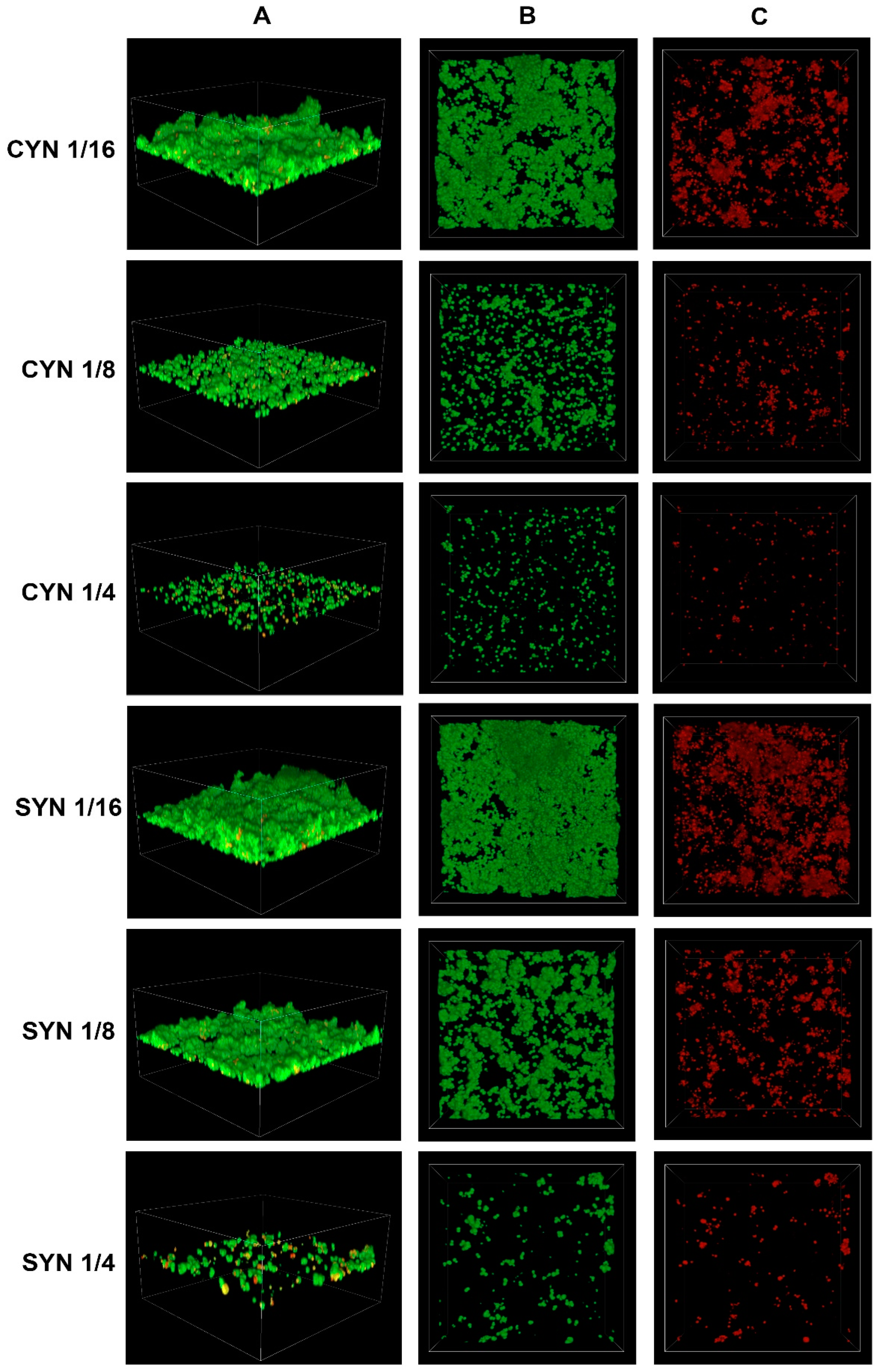
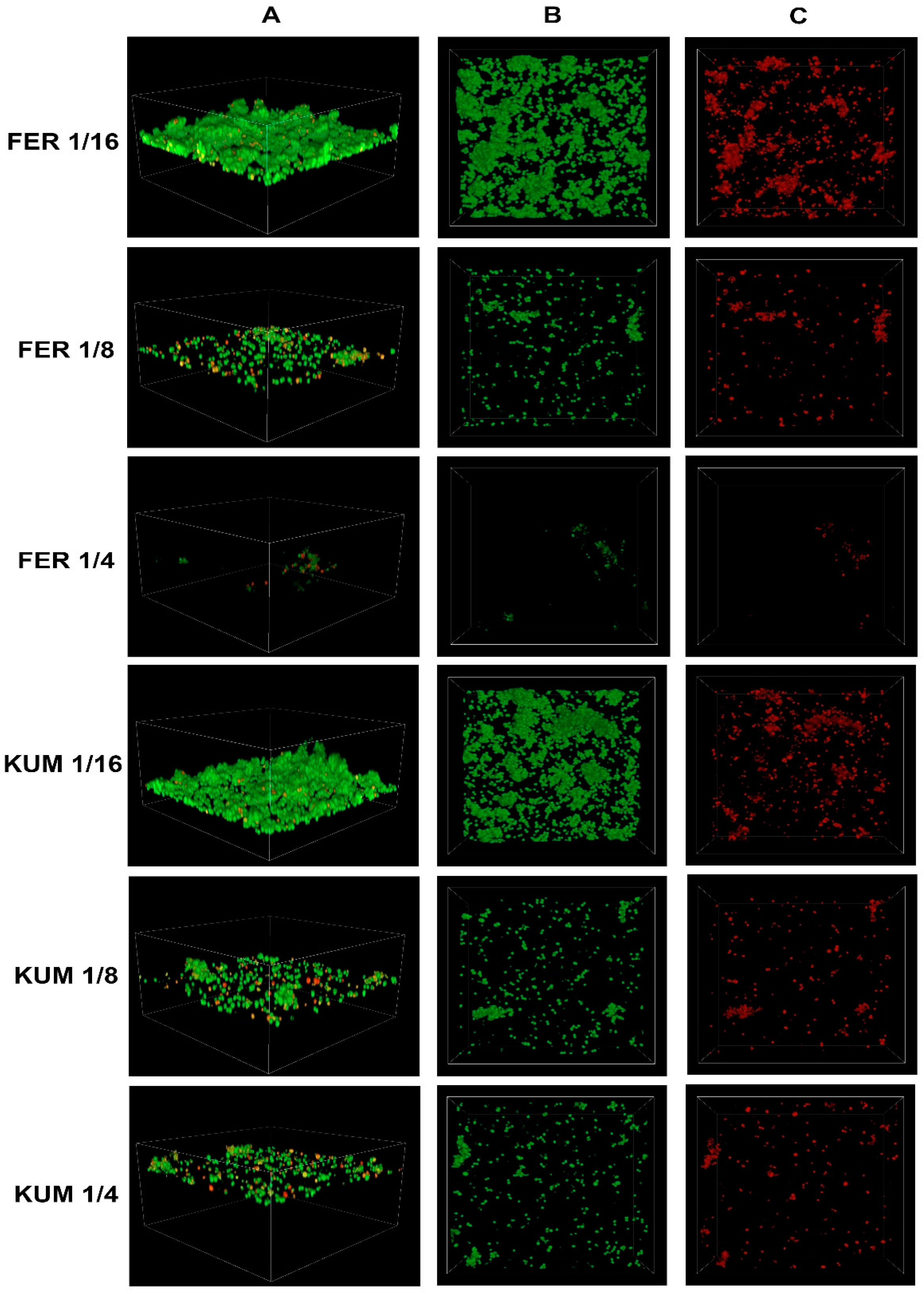
2.3. Confocal Imaging of Staphylococcus epidermidis Biofilm
3. Materials and Methods
3.1. Bacterial Cultures
3.2. Biofilm-Formation Assay
3.3. Assessment of Anti-Biofilm Activity of Combinations
3.4. Visualization of Bacterial Biofilm
4. Conclusions
- In the studied population of Staphylococcus epidermidis, 20% of the strains exhibited a high ability to form biofilm.
- The highest activity against planktonic forms was observed for cloxacillin, cinnamic acid, and p-coumaric acid.
- The most significant anti-biofilm activity was demonstrated by cloxacillin, cinnamic acid, and a combination of sinapic acid and cloxacillin, as assessed by the FIC (fractional inhibitory concentration) index.
- All tested substances exhibited strong activity against planktonic forms, and nearly all displayed the ability to reduce biofilm formation by over 80% compared to the growth control. The exception was the combination of p-coumaric acid and cloxacillin at the FIC value, which showed a reduction capacity of 79.65%.
- The integration of antibiotics with selected derivatives achieved up to a 16-fold decrease in growth-inhibitory concentrations. It is therefore recommended to consider further work on the combinations of cinnamic acid derivatives and antibiotics, which may potentially lead to the development of new combinations with therapeutic potential.
- Confocal laser scanning microscopy revealed an increase in the percentage of dead cells with the increasing concentration of cinnamic acid and its derivatives.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, M.M.; Horswill, A.R. Staphylococcus Epidermidis-Skin Friend or Foe? PLoS Pathog. 2020, 16, e1009026. [Google Scholar] [CrossRef]
- Namvar, A.E.; Bastarahang, S.; Abbasi, N.; Ghehi, G.S.; Farhadbakhtiarian, S.; Arezi, P.; Hosseini, M.; Baravati, S.Z.; Jokar, Z.; Chermahin, S.G. Clinical Characteristics of Staphylococcus Epidermidis: A Systematic Review. GMS Hyg. Infect. Control 2014, 9, Doc23. [Google Scholar] [CrossRef]
- Severn, M.M.; Horswill, A.R. Staphylococcus Epidermidis and Its Dual Lifestyle in Skin Health and Infection. Nat. Rev. Microbiol. 2023, 21, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Swolana, D.; Kępa, M.; Kabała-Dzik, A.; Dzik, R.; Wojtyczka, R.D. Sensitivity of Staphylococcal Biofilm to Selected Compounds of Plant Origin. Antibiotics 2021, 10, 607. [Google Scholar] [CrossRef]
- Wojtyczka, R.D.; Orlewska, K.; Kępa, M.; Idzik, D.; Dziedzic, A.; Mularz, T.; Krawczyk, M.; Miklasińska, M.; Wąsik, T.J. Biofilm Formation and Antimicrobial Susceptibility of Staphylococcus Epidermidis Strains from a Hospital Environment. Int. J. Environ. Res. Public Health 2014, 11, 4619–4633. [Google Scholar] [CrossRef] [PubMed]
- Bryers, J.D. Medical Biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.M.; da Silva, B.N.M.; Barbosa, G.; Barreiro, E.J. β-Lactam Antibiotics: An Overview from a Medicinal Chemistry Perspective. Eur. J. Med. Chem. 2020, 208, 112829. [Google Scholar] [CrossRef] [PubMed]
- Gunia-Krzyżak, A.; Słoczyńska, K.; Popiół, J.; Koczurkiewicz, P.; Marona, H.; Pękala, E. Cinnamic Acid Derivatives in Cosmetics: Current Use and Future Prospects. Int. J. Cosmet. Sci. 2018, 40, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.D. Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- de Morais, M.C.; de Oliveira Lima, E.; Perez-Castillo, Y.; de Sousa, D.P. Synthetic Cinnamides and Cinnamates: Antimicrobial Activity, Mechanism of Action, and In Silico Study. Molecules 2023, 28, 1918. [Google Scholar] [CrossRef]
- Carvalho, S.A.; da Silva, E.F.; de Souza, M.V.; Lourenço, M.C.; Vicente, F.R. Synthesis and antimycobacterial evaluation of new trans-cinnamic acid hydrazide derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, A. Evidence on the Health Benefits of Supplemental Propolis. Nutrients 2019, 11, 2705. [Google Scholar] [CrossRef] [PubMed]
- Riesmeier, M.; Mattonai, M.; Wong, S.S.; Veall, M.-A.; Betts, J.; Johnston, M.; Ribechini, E.; Devièse, T. Molecular Profiling of Peru Balsam Reveals Active Ingredients Responsible for Its Pharmaceutical Properties. Nat. Prod. Res. 2021, 35, 5311–5316. [Google Scholar] [CrossRef] [PubMed]
- Zawiła, T.; Swolana, D.; Zawiła, M.; Wojtyczka, R.D. Synergistic Interactions between Selected β-Lactam Antibiotics and Cinnamic Acid and Its Chosen Derivatives. Antibiotics 2024, 13, 710. [Google Scholar] [CrossRef]
- Cabrera-Contreras, R.; Morelos-Ramírez, R.; Galicia-Camacho, A.N.; Meléndez-Herrada, E. Antibiotic Resistance and Biofilm Production in Staphylococcus Epidermidis Strains, Isolated from a Tertiary Care Hospital in Mexico City. ISRN Microbiol. 2013, 2013, 918921. [Google Scholar] [CrossRef] [PubMed]
- Soumya, K.R.; Philip, S.; Sugathan, S.; Mathew, J.; Radhakrishnan, E.K. Virulence Factors Associated with Coagulase Negative Staphylococci Isolated from Human Infections. 3 Biotech 2017, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, T.D.L.; Pereira, E.M.; dos Santos, K.R.N.; Maciel, E.L.N.; Schuenck, R.P.; Nunes, A.P.F. Modification of the Congo Red Agar Method to Detect Biofilm Production by Staphylococcus Epidermidis. Diagn. Microbiol. Infect. Dis. 2013, 75, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Malheiro, J.; Gomes, I.; Borges, A.; Bastos, M.M.S.M.; Maillard, J.-Y.; Borges, F.; Simões, M. Phytochemical Profiling as a Solution to Palliate Disinfectant Limitations. Biofouling 2016, 32, 1007–1016. [Google Scholar] [CrossRef]
- Borges, A.; Saavedra, M.J.; Simões, M. The Activity of Ferulic and Gallic Acids in Biofilm Prevention and Control of Pathogenic Bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Ergün, B.Ç.; Çoban, T.; Onurdag, F.K.; Banoglu, E. Synthesis, Antioxidant and Antimicrobial Evaluation of Simple Aromatic Esters of Ferulic Acid. Arch. Pharm. Res. 2011, 34, 1251–1261. [Google Scholar] [CrossRef]
- Yue, L.; Wang, M.; Khan, I.M.; Xu, J.; Peng, C.; Wang, Z. Preparation, Characterization, and Antibiofilm Activity of Cinnamic Acid Conjugated Hydroxypropyl Chitosan Derivatives. Int. J. Biol. Macromol. 2021, 189, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Albano, M.; Crulhas, B.P.; Alves, F.C.B.; Pereira, A.F.M.; Andrade, B.F.M.T.; Barbosa, L.N.; Furlanetto, A.; da Silveira Lyra, L.P.; Rall, V.L.M.; Júnior, A.F. Antibacterial and Anti-Biofilm Activities of Cinnamaldehyde against S. Epidermidis. Microb. Pathog. 2019, 126, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Merck Life Science. p-Coumaric Acid; Safety Data Sheet According to Regulation (EC) No. 1907/2006, Product Number: C9008, Version 6.7, Revision Date 3 January 2023. Available online: https://www.sigmaaldrich.com/PL/de/sds/sigma/c9008?userType=undefined (accessed on 9 December 2024).
- Merck Life Science. Cinnamic Acid for Synthesis; Safety Data Sheet According to Regulation (EC) No. 1907/2006, Catalogue No.: 800235, Version 7.1, Revision Date 16 February 2024. Available online: https://www.sigmaaldrich.com/PL/en/sds/mm/8.00235?userType=anonymous (accessed on 9 December 2024).
- De Oliveira, A.; Cataneli Pereira, V.; Pinheiro, L.; Moraes Riboli, D.F.; Benini Martins, K.; Ribeiro de Souza da Cunha, M.D.L. Antimicrobial Resistance Profile of Planktonic and Biofilm Cells of Staphylococcus Aureus and Coagulase-Negative Staphylococci. Int. J. Mol. Sci. 2016, 17, 1423. [Google Scholar] [CrossRef] [PubMed]
- Kincses, A.; Ghazal, T.S.A.; Hohmann, J. Synergistic Effect of Phenylpropanoids and Flavonoids with Antibiotics against Gram-Positive and Gram-Negative Bacterial Strains. Pharm. Biol. 2024, 62, 659. [Google Scholar] [CrossRef] [PubMed]
- Vilarrasa, J.; Delgado, L.M.; Galofré, M.; Àlvarez, G.; Violant, D.; Manero, J.M.; Blanc, V.; Gil, F.J.; Nart, J. In Vitro Evaluation of a Multispecies Oral Biofilm over Antibacterial Coated Titanium Surfaces. J. Mater. Sci. Mater. Med. 2018, 29, 164. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Zhou, J.-W.; Zhang, P.-P.; Luo, H.-Z.; Tang, S.; Li, J.-J.; Deng, S.-M.; Jia, A.-Q. Quorum Sensing Inhibition and Tobramycin Acceleration in Chromobacterium Violaceum by Two Natural Cinnamic Acid Derivatives. Appl. Microbiol. Biotechnol. 2020, 104, 5025–5037. [Google Scholar] [CrossRef]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of Coagulase-Negative Staphylococci to Plastic Tissue Culture Plates: A Quantitative Model for the Adherence of Staphylococci to Medical Devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawiła, T.; Swolana, D.; Rok, J.; Rzepka, Z.; Wojtyczka, R.D. Evaluation of the Antibacterial Activity of Cinnamic Acid and Its Derivatives: Synergistic Effects with Cloxacillin. Molecules 2025, 30, 660. https://doi.org/10.3390/molecules30030660
Zawiła T, Swolana D, Rok J, Rzepka Z, Wojtyczka RD. Evaluation of the Antibacterial Activity of Cinnamic Acid and Its Derivatives: Synergistic Effects with Cloxacillin. Molecules. 2025; 30(3):660. https://doi.org/10.3390/molecules30030660
Chicago/Turabian StyleZawiła, Tomasz, Denis Swolana, Jakub Rok, Zuzanna Rzepka, and Robert D. Wojtyczka. 2025. "Evaluation of the Antibacterial Activity of Cinnamic Acid and Its Derivatives: Synergistic Effects with Cloxacillin" Molecules 30, no. 3: 660. https://doi.org/10.3390/molecules30030660
APA StyleZawiła, T., Swolana, D., Rok, J., Rzepka, Z., & Wojtyczka, R. D. (2025). Evaluation of the Antibacterial Activity of Cinnamic Acid and Its Derivatives: Synergistic Effects with Cloxacillin. Molecules, 30(3), 660. https://doi.org/10.3390/molecules30030660






