Exploring Bovine Serum Albumin (BSA) as a Facile Substrate for Testing of Osmolytes as Cosmetic Ingredients
Abstract
:1. Introduction
2. Results
2.1. Rationale for Setting the Testing Range Concentration
2.2. Evaluation of Protein Stability Under Stress Conditions
- Oxidation using 20 volumes of H2O2 (~6.00% w/v) to simulate a condition for hair dyeing, even if common commercial bleaching agents contain also persulfate salts [29]. The chosen concentration allows for lightening the hair by 1 to 2 levels of lift (results vary depending on the natural hair color) and is especially used by individuals with difficult-to-cover gray hair. Usually, this concentration is combined with bleach to enhance the powerlifting. Treatments using 30 (~9.00% w/v) or 40-volume (~12.00% w/v) developers are more extreme and suitable for bleaching thicker hair and achieving lighter colors [30,31]. Additionally, hair bleaching treatments are known to be extremely aggressive, damaging the cuticles and leaving hair weak and dull. Understanding whether a specific osmolyte can mitigate this damage under such stress conditions is crucial for developing a formulation that protects hair and prevents damage to the scalp.
- Thermal stress was applied using working temperatures of 70 °C and 4 °C. The first condition was selected to simulate hair drying, even though the transition in the amorphous phase of keratin occurs at 155 °C and the melting/denaturation of the α-crystalline phase at 233 °C. The temperature of 70 °C is significant because it marks the point at which loosely bound water is removed from the hair [35]. Additionally, the denaturation of BSA occurs at 65 °C making this protein a suitable substrate for studying stress-induced changes in protein conformation [36].
- ❖
- High-Temperature Stress: The thermal stress at 70 °C influences BSA negatively. Figure S2a–d shows that the protein had a significant conformational change, evidenced by a blue shift of about 10 nm, indicating that tryptophan resides in a more hydrophobic environment. The osmolytes did not have a significant protective effect on the protein structure, except for glycine (which caused a red shift of 5 nm), as shown by the spectra, which were similar to the treated control (referred to as ‘no osmolyte’, Figure S2a–d) [37,38]. Comparing the high-temperature stress effect on BSA with and without osmolytes (Figure 4), betaine and erythritol between 0.20 and 2.00% w/w seem to enhance this stress resulting in a worsened condition on tertiary structure.
- ❖
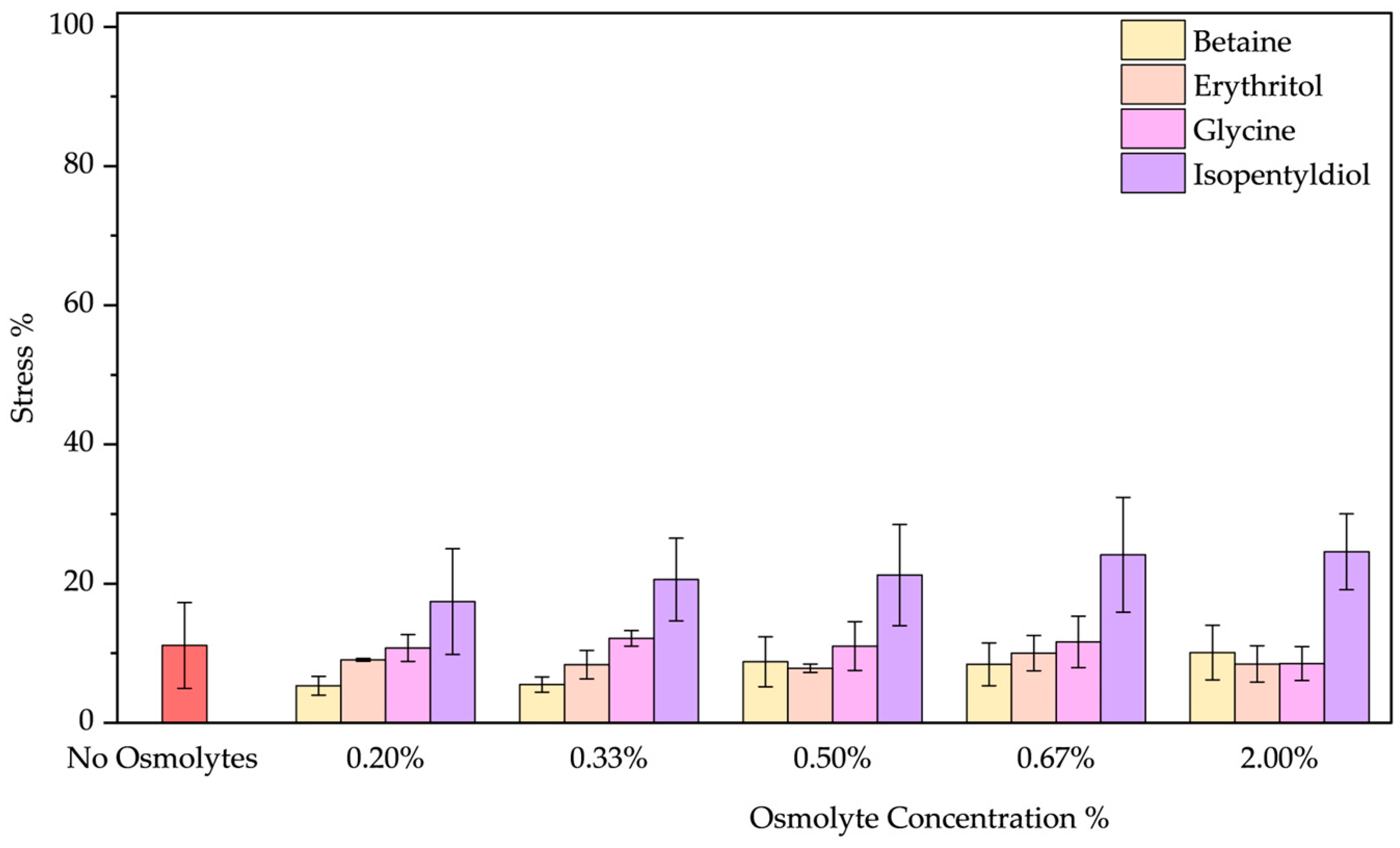
- Low-Temperature Stress: The thermal stress at 4 °C influences BSA negligibly showing a stress below 10%. However, only by adding betaine below 0.33%, the stress effect drops to 5–6%, by increasing the concentration no effect was noted (Figure 5). The presence of glycine and erythritol does not affect the stability as Figure S3a–d shows, but isopentyldiol allows a blue shift of 1–2 nm (increasing with concentration). In fact, isopentyldiol plays a negative role in increasing protein instability (stress 18–25%). Figure 5 shows better the effect of osmolytes by treating BSA at 4 °C. Among the data obtained, betaine addition appears beneficial at low concentrations (below 0.33% w/w).
- UV irradiation was applied using a UV lamp to simulate skin or hair exposure to sunlight. Ultraviolet (UV) rays are classified into three main categories based on wavelength: UVA (320–400 nm), UVB (280–320 nm), and UVC (100–280 nm) [39]. UVC rays do not reach the Earth’s surface due to absorption by the ozone layer. However, following the pandemic, the improper use of UVC lamps as germicidal agents for surface and hand sterilization, combined with the reduction in the ozone layer, has made this type of radiation a potential risk factor for the skin and eyes [40]. UVA and UVB rays remain the primary sources of exposure for humans and are responsible for both short-term effects, such as erythema and sunburn, and long-term effects, including photoaging and an increased risk of skin cancers. Although UVB rays are essential, in low doses, for vitamin D production, prolonged exposure to UV rays can also cause significant hair damage [39]. Specifically, UVA irradiation can degrade the cuticles, reducing moisture content and making hair dry, brittle, and frizzy, especially in individuals with curly or naturally dry hair. Also, melanin can be degraded, increasing hair discoloration and a dull appearance. The emission spectrum (Figure S4a–d) after UVA treatment shows a small reduction in intensity, suggesting a slight change in the environment around tryptophan and tyrosine residues, particularly in the presence of betaine [41,42]. The other osmolytes had similar effects, with the best results achieved by glycine above 0.50% w/w (Figure 6).
- pH stress was applied using a basic solution at pH 12 and an acid solution at pH 2 to simulate significant damage to the hair structure caused by applying harsh products. The pH level of hair products significantly affects the hair shaft. At an alkaline pH, hair’s water sorption increases, and hydrolysis temporarily breaks hydrogen bonds, making keratin more malleable and weakening the hair shaft. At pH levels above 10, hydroxyl group sorption rises, breaking salt bridges and disulfide bonds and hence damaging the hair structure [43,44]. Alkaline products, such as high-pH shampoos, increase the negative charge on fiber surfaces, leading to greater friction between fibers, breaking them, and damaging cuticles [45]. Conversely, at pH levels below 4, hydrogen ion sorption increases significantly. Carboxylic groups are protonated, substituting saline bridges with hydrogen bonds, further weakening the hair shaft. Peptide bonds may undergo hydrolysis below pH 2, causing irreversible changes [44]. Indeed, hair straightening products with pH values below 2 can cause considerable damage to the hair [46]. Moreover, the skin can also be affected by the pH of products that disturb this barrier, potentially leading to increased pH, weakened skin cohesion, dryness, irritation, and altered microflora [47].
- ❖
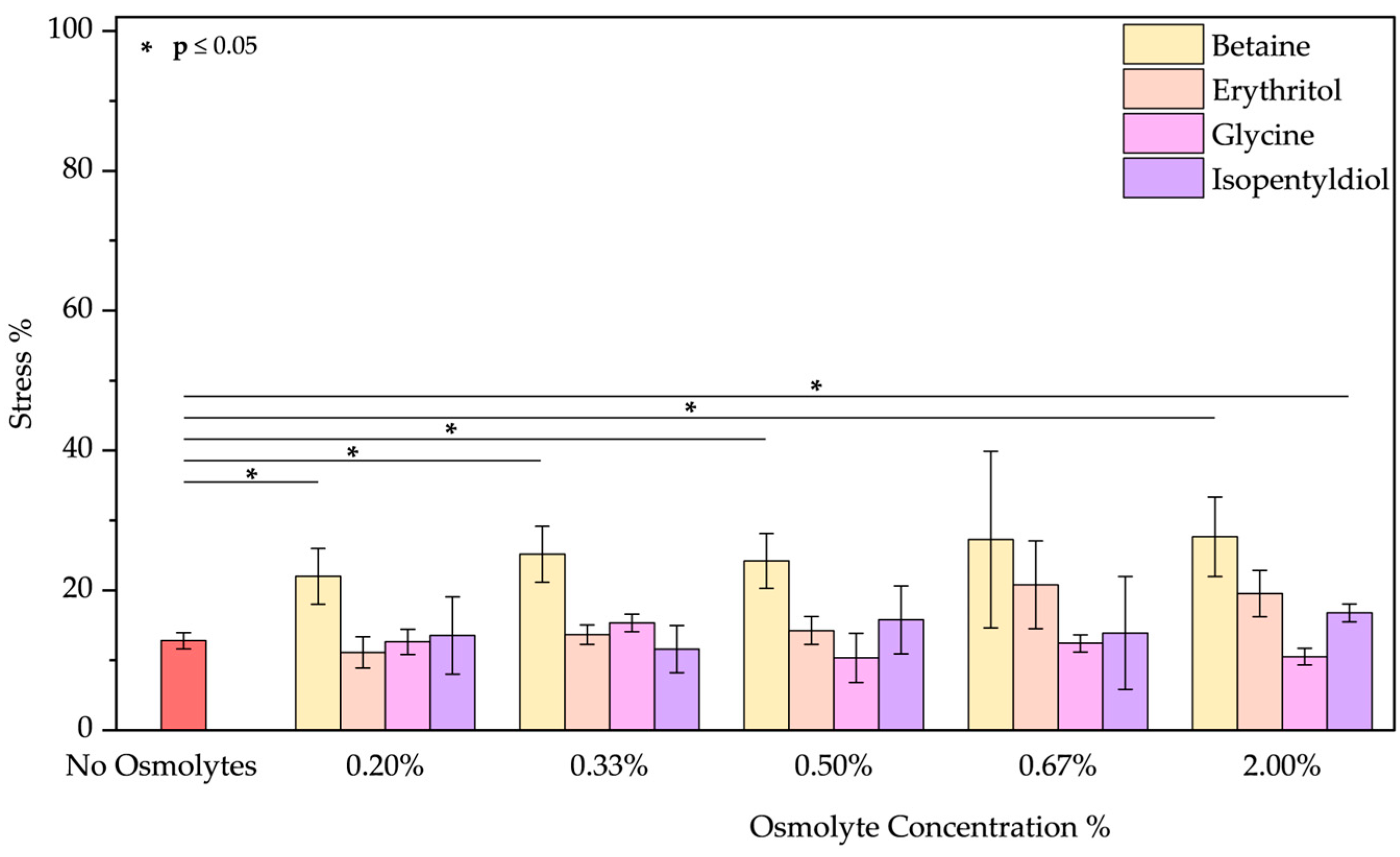
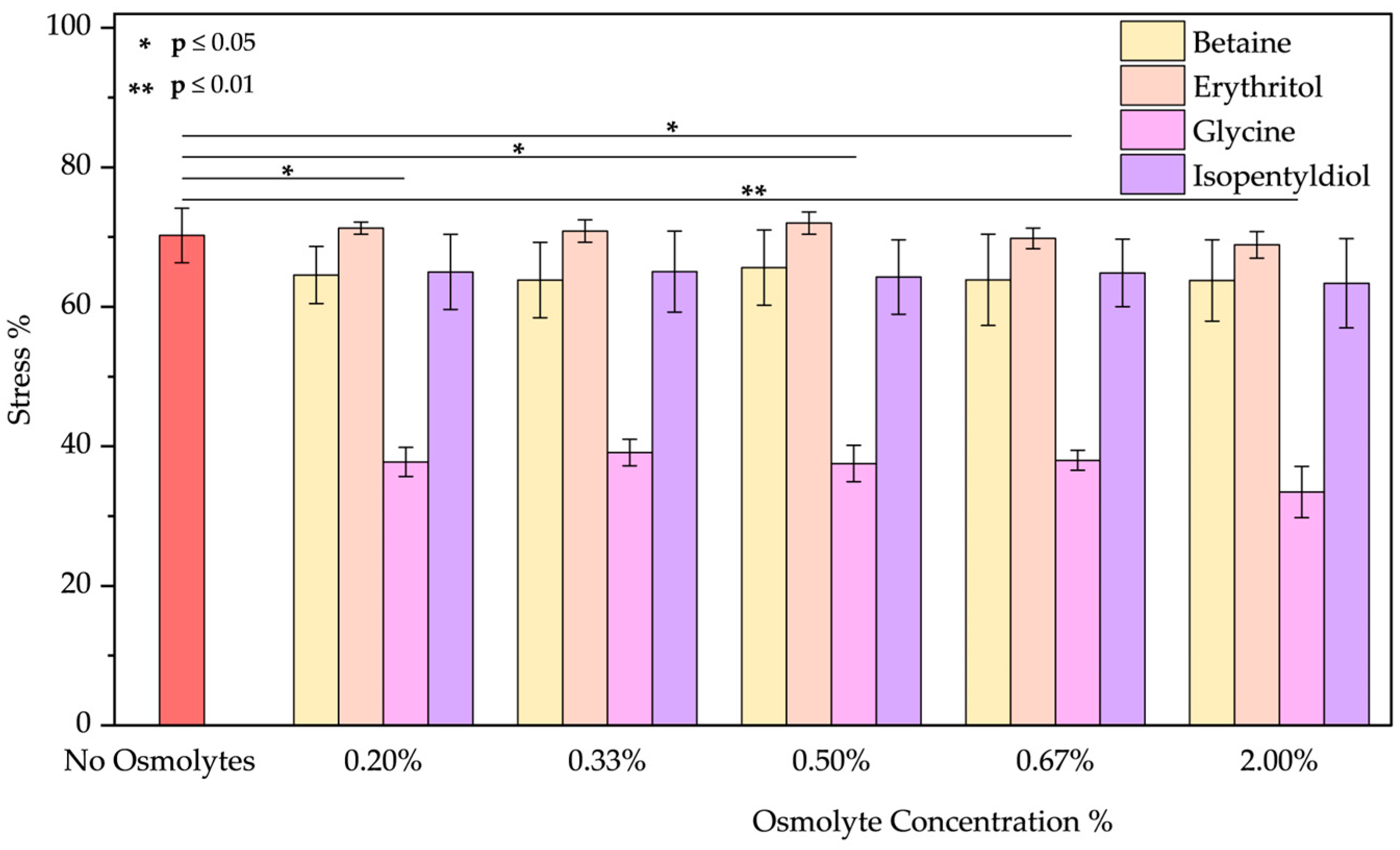
- Basic pH: As the emission spectrum shows, BSA adopts an “aged form” losing its tertiary structure at pH 12. The emission peak decreases due to the unfolding of the protein, as expected [48,49,50]. A blue shift of 12 nm suggests tryptophan is in a less polar environment. Only glycine plays a role in the unfolding of BSA. Upon detailed examination of all the spectra, it is evident that, except for glycine, the other osmolytes induced a blue shift (Figure S6a–d). Glycine showed the best results in reducing the stress % from 70.23 ± 3.90% to 33.45 ± 3.68% (value obtained using glycine at 2.00% w/w; applying lower concentrations the results are similar as Figure 7 demonstrates).
- ❖
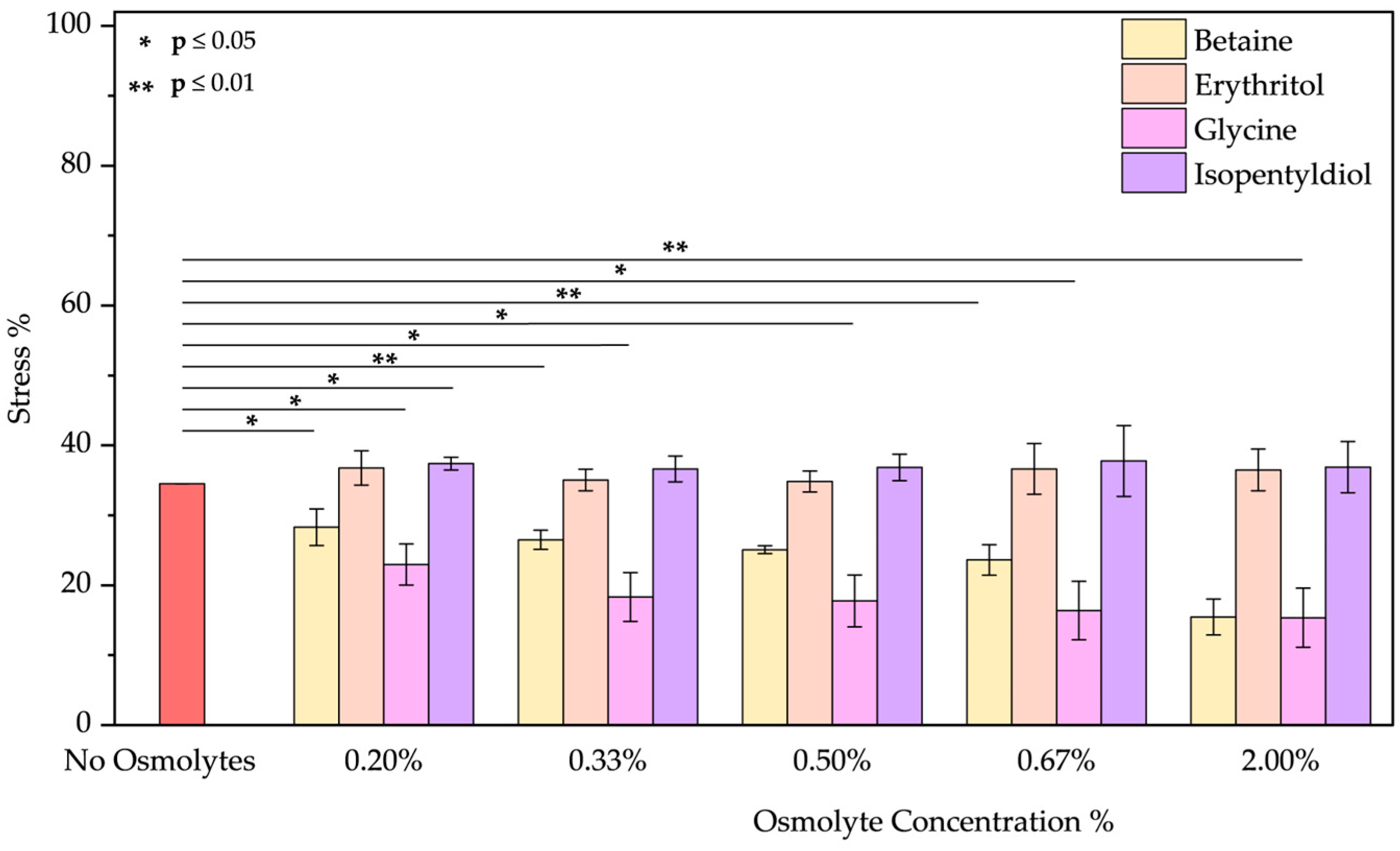
- Acid pH: As the emission spectrum shows, BSA assumes an “expanded form” due to a decrease in its negative charge at pH 2. Due to the exposure of hydrophobic groups, a blue shift of 10 nm was observed. Osmolytes slightly mitigated protein unfolding (Figure S7a–d) where a red shift was noted by increasing the glycine concentration (passing from 328 to 335 nm). Betaine and glycine show the most significant effects at 2.00% w/w reducing acid stress from 34.52 ± 0.01% to 15.45 ± 2.57% and 15.36 ± 4.23%, respectively (Figure 8). Even if, we tested extreme acid pH, these two molecules can be considered to diminish this stress.
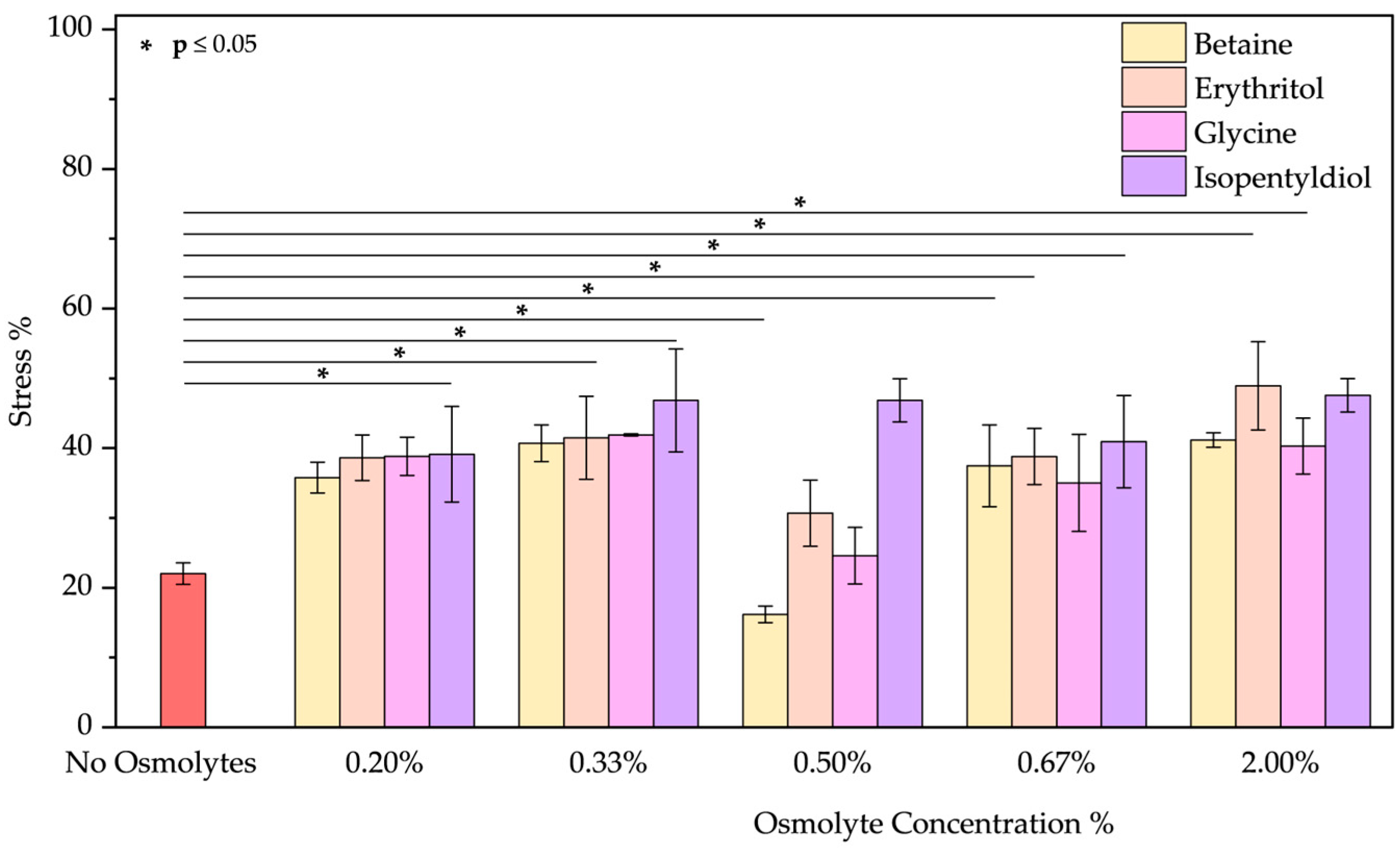
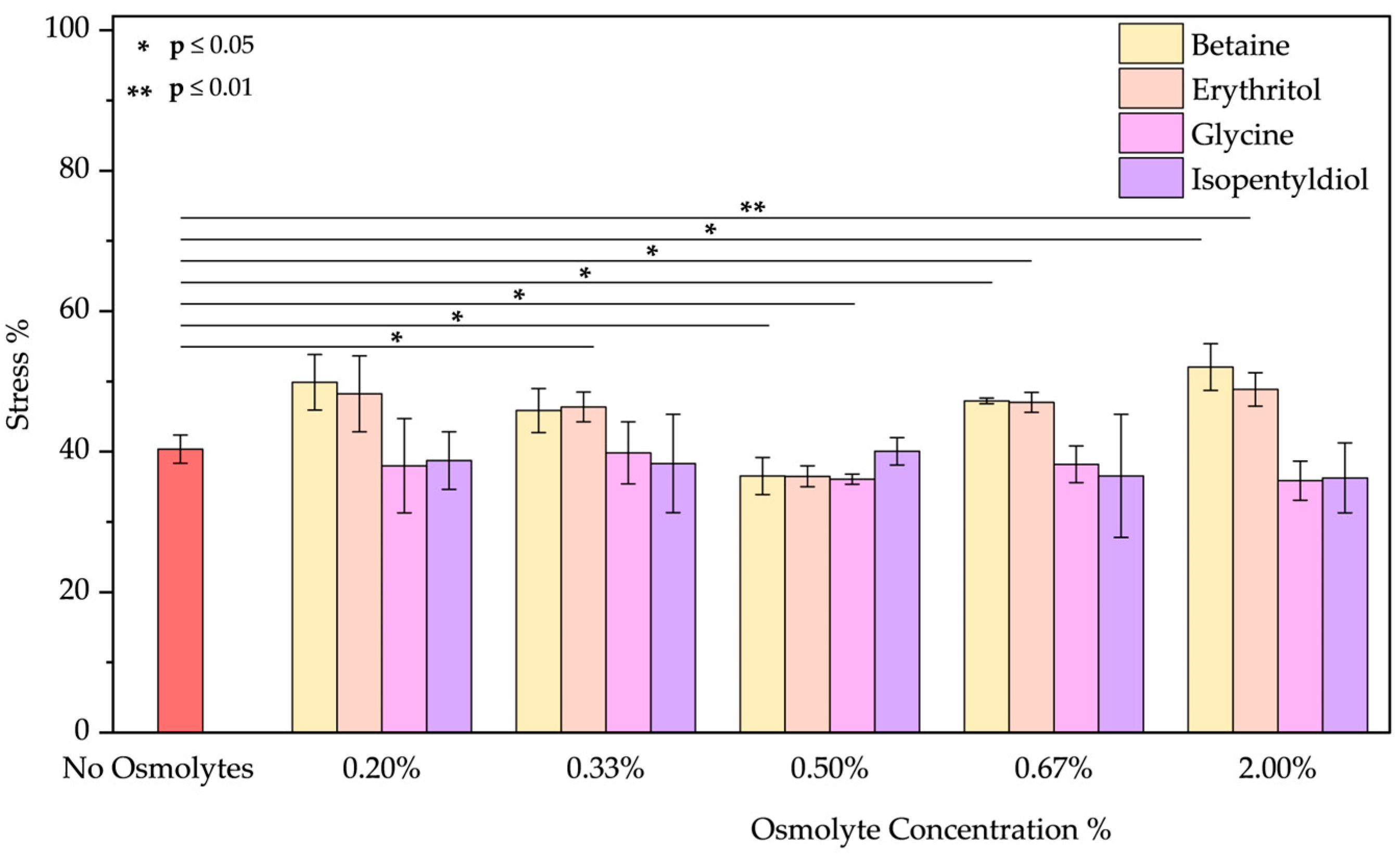
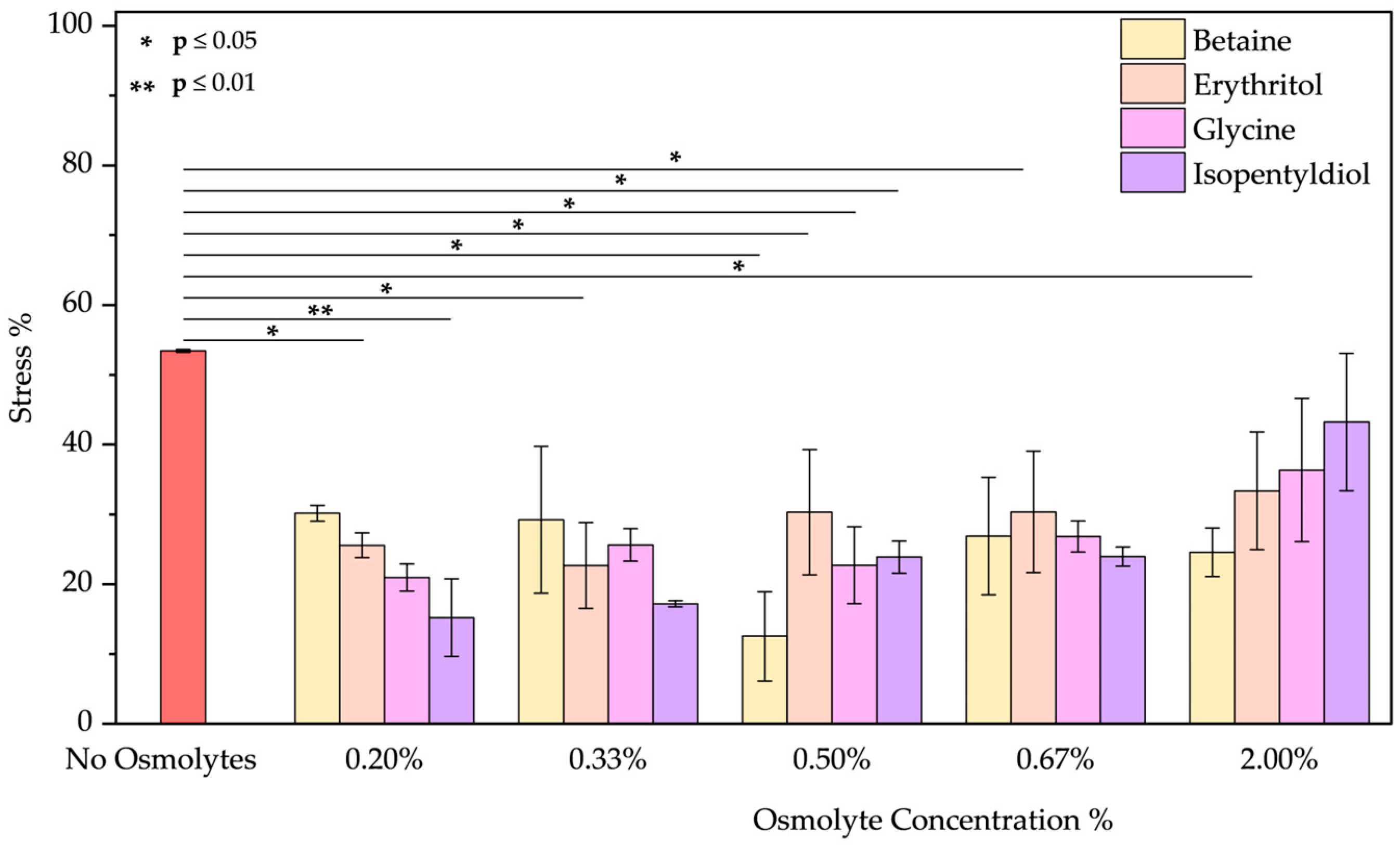
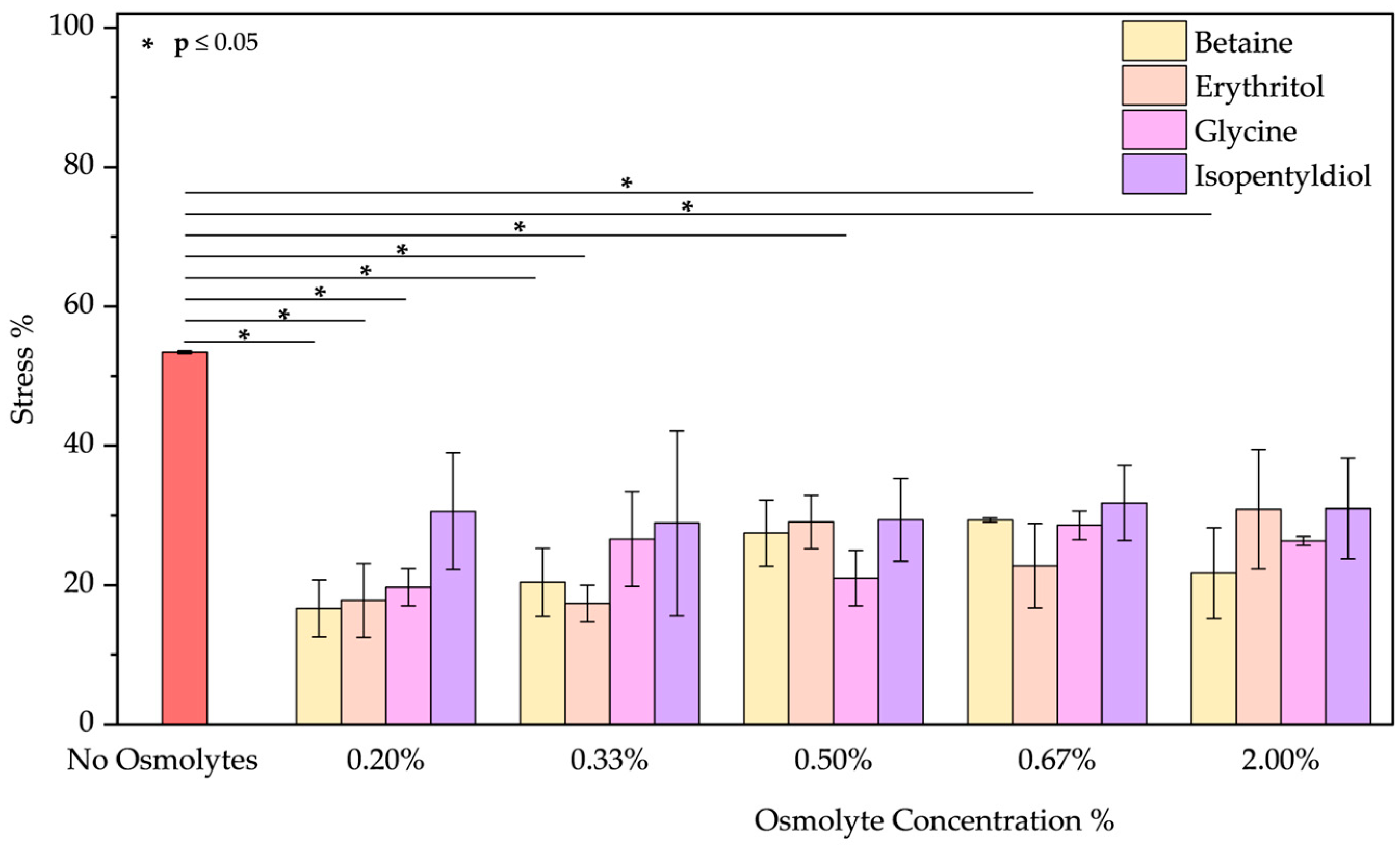
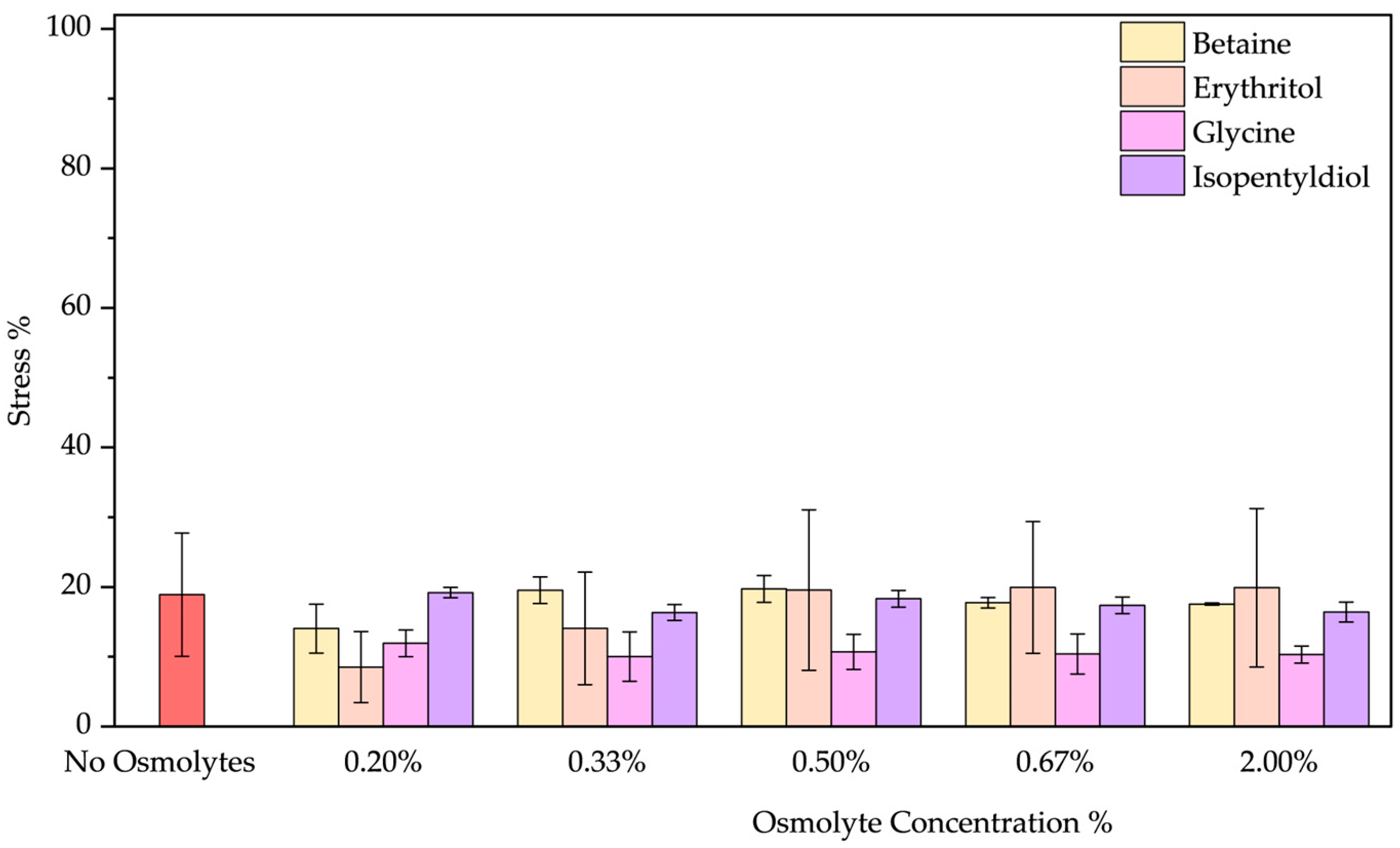
- The peeling treatment was simulated using an acid solution at pH 3.5, composed of typical molecules commonly found in skin acid peeling products. A chemical exfoliating agent is applied to stimulate rejuvenation, smoothness, and improvement of the skin’s superficial structure through controlled chemical damage. For instance, α-Hydroxy acids (AHAs), such as glycolic and lactic acid, are mild acids commonly used in these procedures. They promote exfoliation at low concentrations (<30%) by reducing sulfate and phosphate groups. However, at higher concentrations, they cause more significant damage to the epidermis [51,52]. To simulate the stress induced by these treatments, we performed two distinct protocols. In the first trial, BSA was treated with a solution of glycolic (5.70%), lactic (2.15%), and citric acid (2.15%) at pH 3.5 in the presence of osmolyte. In the second one, BSA was treated with this acid solution after the incubation with an osmolyte. These two approaches were designed to emulate common skincare routines: one where the protective component is included in the peeling solution and another where the peeling treatment is applied after a protective product. In both trials, the acid peeling treatment caused a blue shift of 10 nm and a decrease in peak intensity resulting in a stress of 53.43 ± 0.19%. However, osmolytes in both procedures provided significant benefits (Figures S7a–d and S8a–d). In the first treatment, where the protein is in the “fast form” [53] (similar situation in acid pH stress), isopentyldiol offered greater protection at lower concentrations. The other three osmolytes keep stress levels below 30% (Figure 9). As noted previously (Figure 3, in the Oxidative stress and Figure 4 in the High-Temperature Stress), betaine exhibits unusual behavior, showing a non-linear concentration dependence (V-shaped trend), with greater benefits observed at an intermediate concentration among those tested. In the second protocol, except for isopentyldiol, which shows benefits, lower osmolyte concentrations generally result in stress percentages ranging between 16% and 19% (Figure 10). It is crucial to provide the appropriate procedures to enhance the efficacy and prevent potential permanent damage. Overall, all four osmolytes demonstrated a positive effect, making them highly recommended for further in vitro testing.
- Surfactant treatment was performed by incubating BSA in a sodium lauryl ether sulfate (SLES) solution at 0.50 mg/mL. Surfactants are found in many cosmetic products and cleaning agents due to their ability to reduce interfacial tension between phases. In shampoos and conditioners, they can partially remove hair lipids, which exposes hair fibers to greater damage [54]. Since lipids are essential for protecting hair from environmental and chemical damage, surfactants may also interact negatively with epidermal proteins or lipids, leading to skin irritation [55]. SLES was chosen as a representative surfactant for this study. In Figure S10a–d, the emission spectrum shows a blue shift of 17 nm, indicating a more hydrophobic environment due to the application of SLES. None of the osmolytes significantly promote re-obtain initial protein conformation and not exposing the fluorescent residues (tryptophan or tyrosine) from the aqueous surroundings [56,57,58]. Among the osmolytes (Figure 11), betaine and erythritol seem to reduce stress when applied at a concentration of 0.20% (14.04 ± 3.49% and 8.52 ± 5.09%, respectively). Glycine displayed better results overall in terms of the range of applicable concentrations as showed reducing from stress of 18.89 ± 8.84% to values around 10–12%.
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Evaluation of Protein Stability Under Stress Conditions
4.2.1. Oxidative Stress
4.2.2. Thermal Stress
4.2.3. UV Radiation
4.2.4. pH Stress
4.2.5. Peeling
4.2.6. Surfactant Treatment
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Auton, M.; Rösgen, J.; Sinev, M.; Holthauzen, L.M.F.; Bolen, D.W. Osmolyte effects on protein stability and solubility: A balancing act between backbone and side-chains. Biophys. Chem. 2011, 159, 90–99. [Google Scholar] [CrossRef]
- Panuszko, A.; Pieloszczyk, M.; Kuffel, A.; Jacek, K.; Biernacki, K.A.; Demkowicz, S.; Stangret, J.; Bruździak, P. Hydration of simple model peptides in aqueous osmolyte solutions. Int. J. Mol. Sci. 2021, 22, 9350. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, G.; Choi, I. Roles of osmolytes in protein folding and aggregation in cells and their biotechnological applications. Int. J. Biol. Macromol. 2018, 109, 483–491. [Google Scholar] [CrossRef]
- Street, T.O.; Bolen, D.W.; Rose, G.D. A molecular mechanism for osmolyte-induced protein stability. Proc. Natl. Acad. Sci. USA 2006, 103, 13997–14002. [Google Scholar] [CrossRef] [PubMed]
- Canepa, J.; Torgerson, J.; Kim, D.; Lindahl, E.; Takahashi, R.; Whitelock, K.; Heying, M.; Wilkinson, S. Characterizing osmolyte chemical class hierarchies and functional group requirements for thermal stabilization of proteins. Biophys. Chem. 2020, 264, 106410. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Timasheff, S.N. Preferential interactions of proteins with solvent components in aqueous amino acid solutions. Arch. Biochem. Biophys. 1983, 224, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.N.; Shirley, B.A.; McNutt, M.; Gajiwala, K. Forces contributing to the conformational stability of proteins. FASEB J. 1996, 10, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Dill, K.A. Dominant forces in protein folding. Biochemistry 1990, 29, 7133–7155. [Google Scholar] [CrossRef]
- Zhou, H.X.; Pang, X. Electrostatic Interactions in Protein Structure, Folding, Binding, and Condensation. Chem. Rev. 2018, 118, 1691–1741. [Google Scholar] [CrossRef] [PubMed]
- Nick Pace, C.; Martin Scholtz, J.; Grimsley, G.R. Forces Stabilizing Proteins. FEBS Lett. 2014, 588, 2177. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.A.S. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.K.; Paal, M.C.; Donohue, T.M.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial Effects of Betaine: A Comprehensive Review. Biology 2021, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Alkyl Betaines as Used in Cosmetics. Int. J. Toxicol. 2018, 37 (Suppl. 1), 28S–46S. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.M.; Liu, Y.; Khan, S.M.A.; Hou, L.X.; Bolen, D.W. Increased thermal stability of proteins in the presence of naturally occurring osmolytes. Biochemistry 1992, 31, 5278–5283. [Google Scholar] [CrossRef]
- Roy, S.; Bhat, R. Effect of polyols on the structure and aggregation of recombinant human γ-Synuclein, an intrinsically disordered protein. Biochim. Biophys. Acta 2018, 1866, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.N.; Fiume, M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Alkane Diols as Used in Cosmetics. Int. J. Toxicol. 2024, 43 (Suppl. 2), 70S–131S. [Google Scholar] [CrossRef]
- Tecco, M.A.; Sanders, C. Personal Skin Care Compositions Containing Anti-Flammatory and Anti-Microbial Agents. U.S. Patent No 8,173,143, 5 August 2012. [Google Scholar]
- Øyaas, K.; Ellingsen, T.E.; Dyrset, N.; Levine, D.W. Utilization of osmoprotective compounds by hybridoma cells exposed to hyperosmotic stress. Biotechnol. Bioeng. 1994, 43, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Platts, L.; Falconer, R.J. Controlling protein stability: Mechanisms revealed using formulations of arginine, glycine and guanidinium HCl with three globular proteins. Int. J. Pharm. 2015, 486, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Burnett, C.L.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of α-Amino Acids as Used in Cosmetics. Int. J. Toxicol. 2013, 32, 41S–64S. [Google Scholar] [CrossRef]
- Dąbrowska, A.K.; Rotaru, G.-M.; Derler, S.; Spano, F.; Camenzind, M.; Annaheim, S.; Stämpfli, R.; Schmid, M.; Rossi, R.M. Materials used to simulate physical properties of human skin. Skin. Res. Technol. 2016, 22, 3–14. [Google Scholar] [CrossRef]
- Gekko, K.; Morikawa, T. Preferential hydration of bovine serum albumin in polyhydric alcohol-water mixtures. J. Biochem. 1981, 90, 39–50. [Google Scholar] [CrossRef]
- Kaushik, J.K.; Bhat, R. Thermal stability of proteins in aqueous polyol solutions: Role of the surface tension of water in the stabilizing effect of polyols. J. Phys. Chem. B 1998, 102, 7058–7066. [Google Scholar] [CrossRef]
- Brown, J.R. Serum Albumin: Amino acid sequence. In Albumin: Structure, Function and Uses; Pergamon Press: London, UK, 1977; pp. 27–40, 40a, 40b, 41–51, 52a, 52b. [Google Scholar] [CrossRef]
- Kronman, M.J.; Holmes, L.G. The fluorescence of native, denatured and reduced-denatured proteins. Photochem. Photobiol. 1971, 14, 113–134. [Google Scholar] [CrossRef]
- Determan, A.S.; Wilson, J.H.; Kipper, M.J.; Wannemuehler, M.J.; Narasimhan, B. Protein stability in the presence of polymer degradation products: Consequences for controlled release formulations. Biomaterials 2006, 27, 3312–3320. [Google Scholar] [CrossRef]
- Garidel, P.; Hegyi, M.; Bassarab, S.; Weichel, M. A rapid, sensitive and economical assessment of monoclonal antibody conformational stability by intrinsic tryptophan fluorescence spectroscopy. Biotechnol. J. 2008, 3, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Colzani, B.; Pandolfi, L.; Hoti, A.; Iovene, P.A.; Natalello, A.; Avvakumova, S.; Colombo, M.; Prosperi, D. Investigation of antitumor activities of trastuzumab delivered by PLGA nanoparticles. Int. J. Nanomed. 2018, 13, 957–973. [Google Scholar] [CrossRef]
- Jeong, M.S.; Lee, C.M.; Jeong, W.J.; Kim, S.J.; Lee, K.Y. Significant damage of the skin and hair following hair bleaching. J. Dermatol. 2010, 37, 882–887. [Google Scholar] [CrossRef]
- Gray, J. Hair care and hair care products. Clin. Dermatol. 2001, 19, 227–236. [Google Scholar] [CrossRef]
- Yuen, C.W.M.; Kan, C.W.; Lau, K.W.; Chow, Y.L. Effect of different human hair bleaching conditions on the hair coloration with hair boosting shampoo as colorant. Fibers Polym. 2009, 10, 709–715. [Google Scholar] [CrossRef]
- Payne, J.H.; Foster, L. The Action of Hydrogen Peroxide on Carbohydrates. J. Am. Chem. Soc. 1945, 67, 1654–1656. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, Z.Y. Density functional theory study of the hydrogen bonding interaction complexes of hydrogen peroxide with glycine. J. Mol. Struct. Theochem. 2004, 674, 113–119. [Google Scholar] [CrossRef]
- Tian, S.X. Quantum Chemistry Studies of Glycine−H2O2 Complexes. J. Phys. Chem. B. 2004, 108, 20388–20396. [Google Scholar] [CrossRef]
- Milczarek, P.; Zielinski, M.; Garcia, M.L. The mechanism and stability of thermal transitions in hair keratin. Colloid Polym. Sci. 1992, 270, 1106–1115. [Google Scholar] [CrossRef]
- Jiang, B.; Jain, A.; Lu, Y.; Hoag, S.W. Probing Thermal Stability of Proteins with Temperature Scanning Viscometer. Mol. Pharm. 2019, 16, 3687–3693. [Google Scholar] [CrossRef] [PubMed]
- Peña, I.; Domínguez, J.M. Thermally denatured BSA, a surrogate additive to replace BSA in buffers for high-throughput screening. J. Biomol. Screen. 2010, 15, 1281–1286. [Google Scholar] [CrossRef]
- Malarkani, K.; Sarkar, I.; Selvam, S. Denaturation studies on bovine serum albumin–bile salt system: Bile salt stabilizes bovine serum albumin through hydrophobicity. J. Pharm. Anal. 2018, 8, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Mohania, D.; Chandel, S.; Kumar, P.; Verma, V.; Digvijay, K.; Tripathi, D.; Choudhury, K.; Mitten, S.K.; Shah, D. Ultraviolet Radiations: Skin Defense-Damage Mechanism. Adv. Exp. Med. Biol. 2017, 996, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Yamano, N.; Kunisada, M.; Nishiaki-Sawada, A.; Ohashi, H.; Igarashi, T.; Nishigori, C. Evaluation of Acute Reactions on Mouse Skin Irradiated with 222 and 235 nm UV-C. Photochem. Photobiol. 2021, 97, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Sonthanasamy, R.S.A.; Sulaiman, N.M.N.; Tan, L.L.; Lazim, A.M. Comprehensive spectroscopic studies of synergism between Gadong starch based carbon dots and bovine serum albumin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 218, 85–96. [Google Scholar] [CrossRef]
- Pant, M.P.; Mariam, J.; Joshi, A.; Dongre, P.M. UV radiation sensitivity of bovine serum albumin bound to silver nanoparticles. J. Radiat. Res. Appl. Sci. 2014, 7, 399–405. [Google Scholar] [CrossRef]
- Dias, M.F.R.G.; De Almeida, A.M.; Cecato, P.M.R.; Adriano, A.R.; Pichler, J. The shampoo pH can affect the hair: Myth or Reality? Int. J. Trichol. 2014, 6, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Malinauskyte, E.; Cornwell, P.A.; Reay, L.; Shaw, N.; Petkov, J. Effect of equilibrium pH on the structure and properties of bleach-damaged human hair fibers. Biopolymers 2020, 111, 23401. [Google Scholar] [CrossRef] [PubMed]
- Zamani, N.A.N.; Md Heshamuddin, N.S.; Jafri, A.A.; Abdullah, S.S.; Ariff, E.A.R.E. The Effects of Temperature, pH and Moisture Exposure on Human Hair. In Intelligent Manufacturing and Mechatronics: Proceedings of SympoSIMM 2020; Springer Science and Business Media Deutschland GmbH: Berlin, Germany, 2021; pp. 1171–1183. [Google Scholar] [CrossRef]
- Velasco, M.V.R.; de Sá-Dias, T.C.; Dario, M.F.; Bedin, V.; Fileto, M.B.; de Oliveira, A.C.; Pinto, C.A.S.d.O.; Baby, A.R. Impact of acid (‘progressive brush’) and alkaline straightening on the hair fiber: Differential effects on the cuticle and cortex properties. Int. J. Trichol. 2022, 14, 197–203. [Google Scholar] [CrossRef]
- Blaak, J.; Staib, P. The Relation of pH and Skin Cleansing. Curr. Probl. Dermatol. 2018, 54, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.J.; Liu, Y.; Jiang, W.; Zhao, R.M.; Qu, S.S. Fluorometric investigation of the interaction of bovine serum albumin with surfactants and 6-mercaptopurine. J. Photochem. Photobiol. B 2005, 80, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Fehér, B.; Lyngsø, J.; Bartók, B.; Mihály, J.; Varga, Z.; Mészáros, R.; Pedersen, J.S.; Bóta, A.; Varga, I. Effect of pH on the conformation of bovine serume albumin—Gold bioconjugates. J. Mol. Liq. 2020, 309, 113065. [Google Scholar] [CrossRef]
- Varga, N.; Hornok, V.; Sebok, D.; Dékány, I. Comprehensive study on the structure of the BSA from extended-to aged form in wide (2–12) pH range. Int. J. Biol. Macromol. 2016, 88, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Landau, M. Chemical peels. Clin. Dermatol. 2008, 26, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Zakopoulou, N.; Kontochristopoulos, G. Superficial chemical peels. J. Cosmet. Dermatol. 2006, 5, 246–253. [Google Scholar] [CrossRef]
- Sadler, P.J.; Tucker, A. pH-induced structural transitions of bovine serum albumin. Eur. J. Biochem. 1993, 212, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Coderch, L.; Alonso, C.; García, M.T.; Pérez, L.; Martí, M. Hair Lipid Structure: Effect of Surfactants. Cosmetics 2023, 10, 107. [Google Scholar] [CrossRef]
- Seweryn, A. Interactions between surfactants and the skin—Theory and practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Ahmad, B.; Khan, R.H. Formation of a molten globule like state in bovine serum albumin at alkaline pH. Eur. Biophys. J. 2008, 37, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wu, Z.; Wangb, Y.; Ding, L.; Wang, Y. Role of pH-induced structural change in protein aggregation in foam fractionation of bovine serum albumin. Biotechnol. Rep. 2016, 9, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Wada, A.; Yamamoto, K.; Moriyama, Y.; Aoki, K. Conformational change of bovine serum albumin by heat treatment. J. Protein Chem. 1989, 8, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Virk, A.S.; Virk, S.S.; Akin-Ige, F.; Amin, S. Applications of artificial intelligence and machine learning on critical materials used in cosmetics and personal care formulation design. Curr. Opin. Colloid Interface Sci. 2024, 73, 101847. [Google Scholar] [CrossRef]
- Grech, V.S.; Kefala, V.; Rallis, E. Cosmetology in the Era of Artificial Intelligence. Cosmetics 2024, 11, 135. [Google Scholar] [CrossRef]

| Stress % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oxidation | 70 °C | 4 °C | UVA | pH 12 | pH 2 | Acid Peeling * | Acid Peeling ** | SLES | ||
| No Osmolyte | 22.01 ± 1.54 | 40.37 ± 2.00 | 11.13 ± 6.16 | 12.79 ± 1.16 | 70.23 ± 3.90 | 34.52 ± 0.01 | 53.43 ± 0.20 | 53.43 ± 0.20 | 18.89 ± 8.84 | |
| Betaine | 0.20% | 35.78 ± 2.21 | 49.88 ± 3.95 | 5.34 ± 1.35 | 22.01 ± 3.97 | 64.56 ± 4.10 | 28.29 ± 2.62 | 30.16 ± 1.14 | 16.64 ± 4.09 | 14.04 ± 3.50 |
| 0.33% | 40.71 ± 2.62 | 45.86 ± 3.14 | 5.51 ± 1.09 | 25.18 ± 3.99 | 63.84 ± 5.40 | 26.49 ± 1.37 | 29.23 ± 10.52 | 20.41 ± 4.86 | 19.53 ± 1.89 | |
| 0.50% | 16.20 ± 1.18 | 36.54 ± 2.65 | 8.79 ± 3.59 | 24.20 ± 3.92 | 65.63 ± 5.39 | 25.07 ± 0.56 | 12.54 ± 6.40 | 27.46 ± 4.76 | 19.72 ± 1.92 | |
| 0.67% | 37.48 ± 5.85 | 47.22 ± 0.40 | 8.41 ± 3.07 | 27.27 ± 12.62 | 63.86 ± 6.54 | 23.62 ± 2.18 | 26.89 ± 8.42 | 29.34 ± 0.32 | 17.73 ± 0.74 | |
| 2.00% | 41.17 ± 1.03 | 52.03 ± 3.32 | 10.10 ± 3.92 | 27.67 ± 5.68 | 63.77 ± 5.83 | 15.46 ± 2.57 | 24.56 ± 3.48 | 21.72 ± 6.49 | 17.54 ± 0.16 | |
| Erythritol | 0.20% | 38.62 ± 3.25 | 48.23 ± 5.39 | 9.05 ± 0.20 | 11.12 ± 2.22 | 71.27 ± 0.87 | 36.79 ± 2.45 | 25.57 ± 1.78 | 17.80 ± 5.30 | 8.52 ± 5.09 |
| 0.33% | 41.48 ± 5.94 | 46.37 ± 2.11 | 8.36 ± 2.06 | 13.66 ± 1.41 | 70.86 ± 1.59 | 35.06 ± 1.55 | 22.68 ± 6.14 | 17.37 ± 2.62 | 14.07 ± 8.06 | |
| 0.50% | 30.68 ± 4.75 | 36.50 ± 1.49 | 7.85 ± 0.61 | 14.25 ± 2.00 | 71.99 ± 1.59 | 34.85 ± 1.49 | 30.31 ± 8.97 | 29.04 ± 3.83 | 19.56 ± 11.51 | |
| 0.67% | 38.81 ± 4.03 | 47.01 ± 1.41 | 10.01 ± 2.54 | 20.80 ± 6.25 | 69.80 ± 1.47 | 36.65 ± 3.61 | 30.36 ± 8.69 | 22.77 ± 6.04 | 19.93 ± 9.43 | |
| 2.00% | 48.92 ± 6.32 | 48.86 ± 2.38 | 8.46 ± 2.61 | 19.53 ± 3.33 | 68.88 ± 1.91 | 36.50 ± 2.98 | 33.39 ± 8.43 | 30.88 ± 8.57 | 19.89 ± 11.35 | |
| Glycine | 0.20% | 38.84 ± 2.74 | 38.00 ± 6.70 | 10.75 ± 1.93 | 12.65 ± 1.80 | 37.77 ± 2.09 | 22.97 ± 2.94 | 20.95 ± 1.95 | 19.69 ± 2.67 | 11.93 ± 1.92 |
| 0.33% | 41.89 ± 0.16 | 39.84 ± 4.41 | 12.14 ± 1.12 | 15.34 ± 1.24 | 39.12 ± 1.91 | 18.32 ± 3.48 | 25.63 ± 2.32 | 26.61 ± 6.79 | 10.03 ± 3.53 | |
| 0.50% | 24.59 ± 4.06 | 36.09 ± 0.72 | 11.02 ± 3.50 | 10.36 ± 3.53 | 37.54 ± 2.62 | 17.76 ± 3.71 | 22.71 ± 5.49 | 20.99 ± 3.97 | 10.70 ± 2.50 | |
| 0.67% | 35.01 ± 6.95 | 38.21 ± 2.62 | 11.62 ± 3.69 | 12.43 ± 1.23 | 37.99 ± 1.43 | 16.38 ± 4.17 | 26.82 ± 2.22 | 28.58 ± 2.07 | 10.40 ± 2.87 | |
| 2.00% | 40.29 ± 4.01 | 35.87 ± 2.78 | 8.52 ± 2.44 | 10.53 ± 1.20 | 33.45 ± 3.68 | 15.36 ± 4.23 | 36.36 ± 10.26 | 26.34 ± 0.62 | 10.31 ± 1.22 | |
| Isopentyldiol | 0.20% | 39.13 ± 6.86 | 38.73 ± 4.10 | 17.42 ± 7.58 | 13.55 ± 5.52 | 65.00 ± 5.37 | 37.41 ± 0.91 | 15.21 ± 5.56 | 30.62 ± 8.38 | 19.20 ± 0.73 |
| 0.33% | 46.83 ± 7.38 | 38.32 ± 6.99 | 20.59 ± 5.95 | 11.60 ± 3.38 | 65.05 ± 5.80 | 36.64 ± 1.85 | 17.19 ± 0.45 | 28.90 ± 13.26 | 16.33 ± 1.14 | |
| 0.50% | 46.84 ± 3.08 | 40.06 ± 1.94 | 21.23 ± 7.27 | 15.78 ± 4.85 | 64.27 ± 5.34 | 36.86 ± 1.89 | 23.88 ± 2.32 | 29.36 ± 5.94 | 18.30 ± 1.21 | |
| 0.67% | 40.93 ± 6.62 | 36.54 ± 8.77 | 24.15 ± 8.25 | 13.90 ± 8.07 | 64.85 ± 4.83 | 37.78 ± 5.06 | 23.95 ± 1.37 | 31.78 ± 5.40 | 17.37 ± 1.18 | |
| 2.00% | 47.58 ± 2.40 | 36.27 ± 4.97 | 24.57 ± 5.44 | 16.78 ± 1.29 | 63.37 ± 6.41 | 36.89 ± 3.67 | 43.24 ± 9.84 | 31.00 ± 7.25 | 16.40 ± 1.43 | |
| Aggravate | * co-incubation | |||||||||
| Negligible (variation ≤ 10%) | ** pre-incubation | |||||||||
| Moderate (10% < Variation ≤ 50%) | ||||||||||
| Good (50% < Variation ≤ 75%) | ||||||||||
| Optimal (Variation > 75%) | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinesi, G.; Salvioni, L.; Ronchi, E.; Barbuzzi, E.M.G.; Prosperi, D.; Colombo, M.; Giustra, M.D. Exploring Bovine Serum Albumin (BSA) as a Facile Substrate for Testing of Osmolytes as Cosmetic Ingredients. Molecules 2025, 30, 664. https://doi.org/10.3390/molecules30030664
Sinesi G, Salvioni L, Ronchi E, Barbuzzi EMG, Prosperi D, Colombo M, Giustra MD. Exploring Bovine Serum Albumin (BSA) as a Facile Substrate for Testing of Osmolytes as Cosmetic Ingredients. Molecules. 2025; 30(3):664. https://doi.org/10.3390/molecules30030664
Chicago/Turabian StyleSinesi, Giulia, Lucia Salvioni, Elisabetta Ronchi, Elena Maria Gabriella Barbuzzi, Davide Prosperi, Miriam Colombo, and Marco Davide Giustra. 2025. "Exploring Bovine Serum Albumin (BSA) as a Facile Substrate for Testing of Osmolytes as Cosmetic Ingredients" Molecules 30, no. 3: 664. https://doi.org/10.3390/molecules30030664
APA StyleSinesi, G., Salvioni, L., Ronchi, E., Barbuzzi, E. M. G., Prosperi, D., Colombo, M., & Giustra, M. D. (2025). Exploring Bovine Serum Albumin (BSA) as a Facile Substrate for Testing of Osmolytes as Cosmetic Ingredients. Molecules, 30(3), 664. https://doi.org/10.3390/molecules30030664








