Fragmentation and Isomerization Pathways of Natural and Synthetic Cannabinoids Studied via Higher Collisional Energy Dissociation Profiles
Abstract
1. Introduction
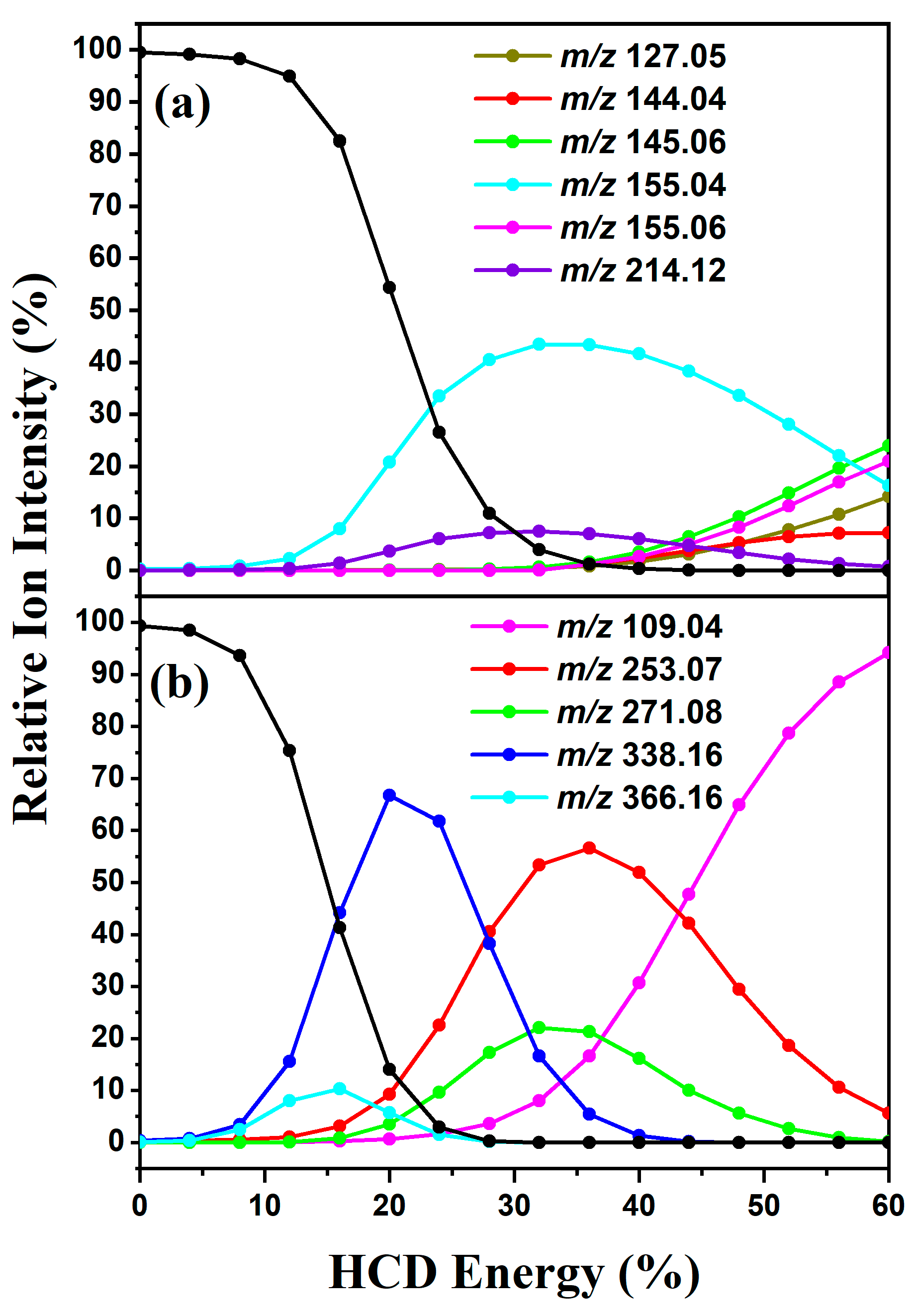
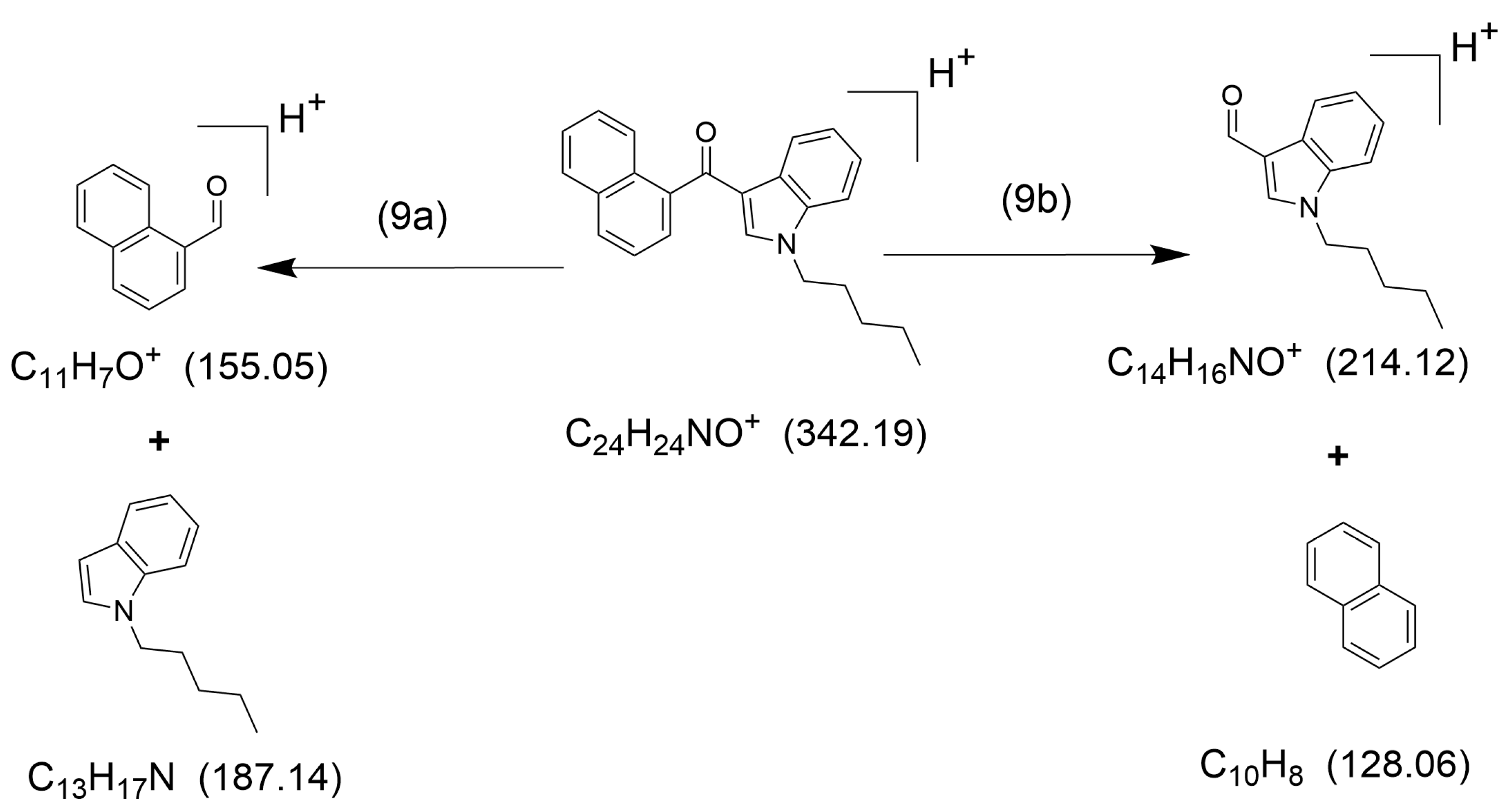
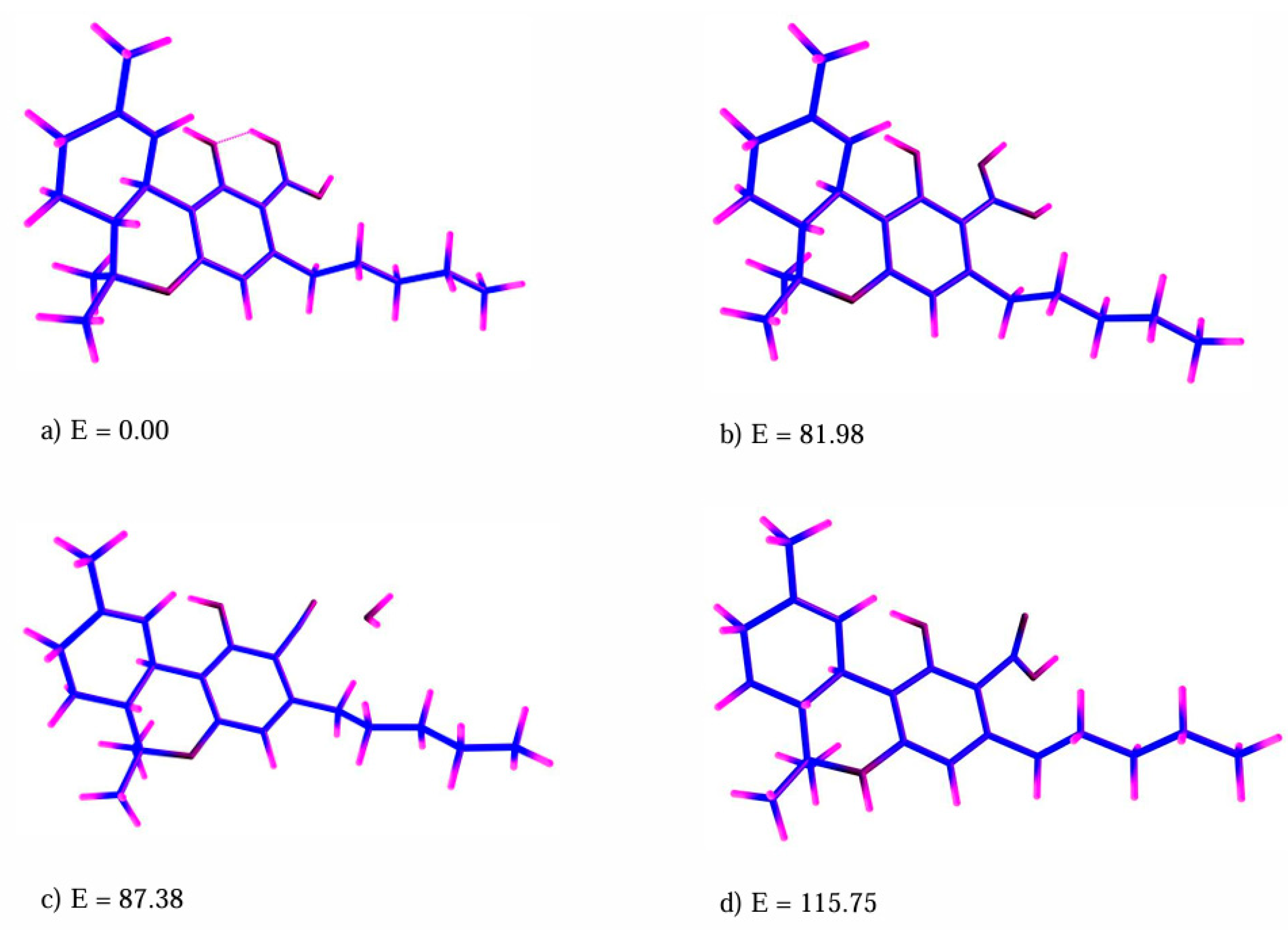
2. Results
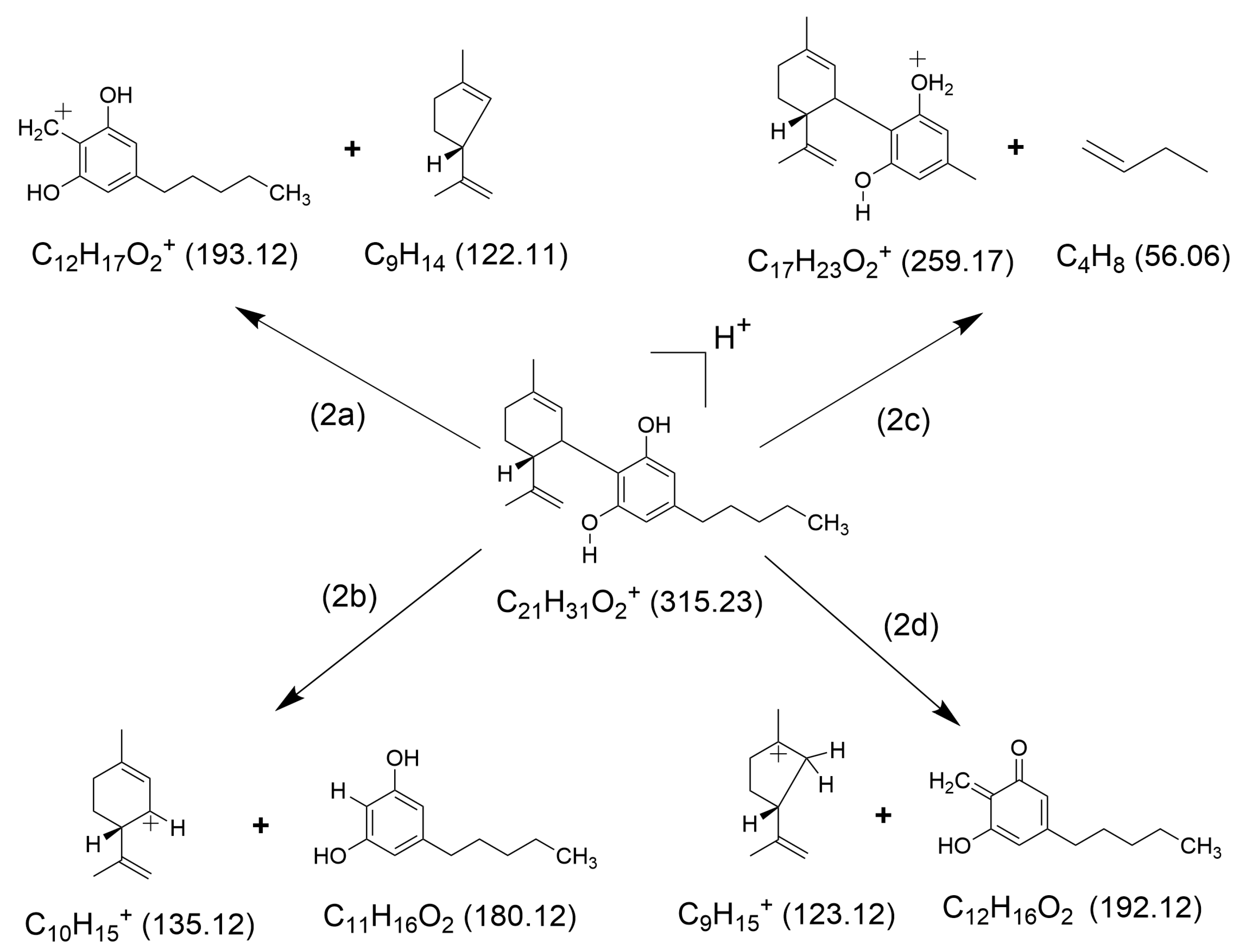
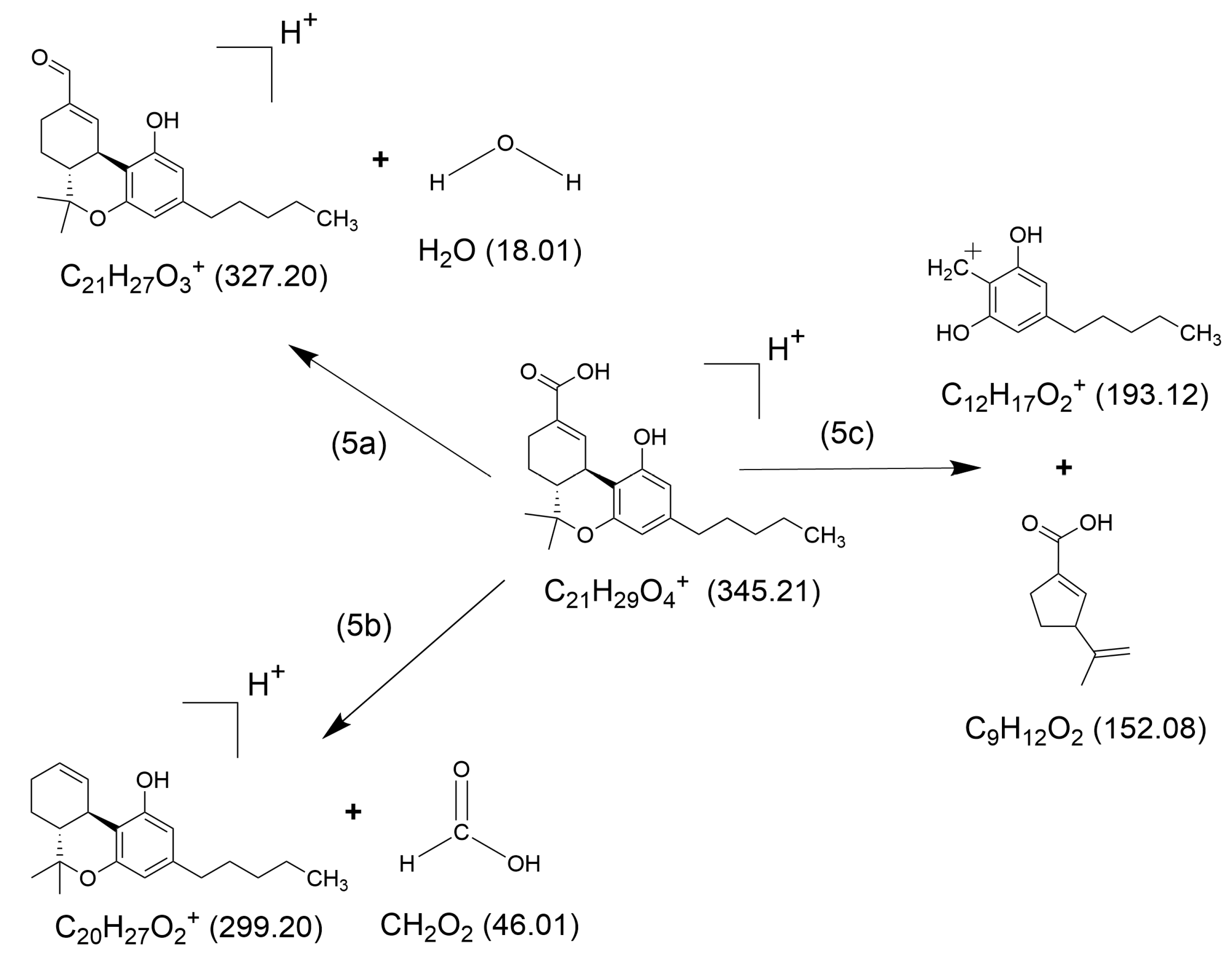
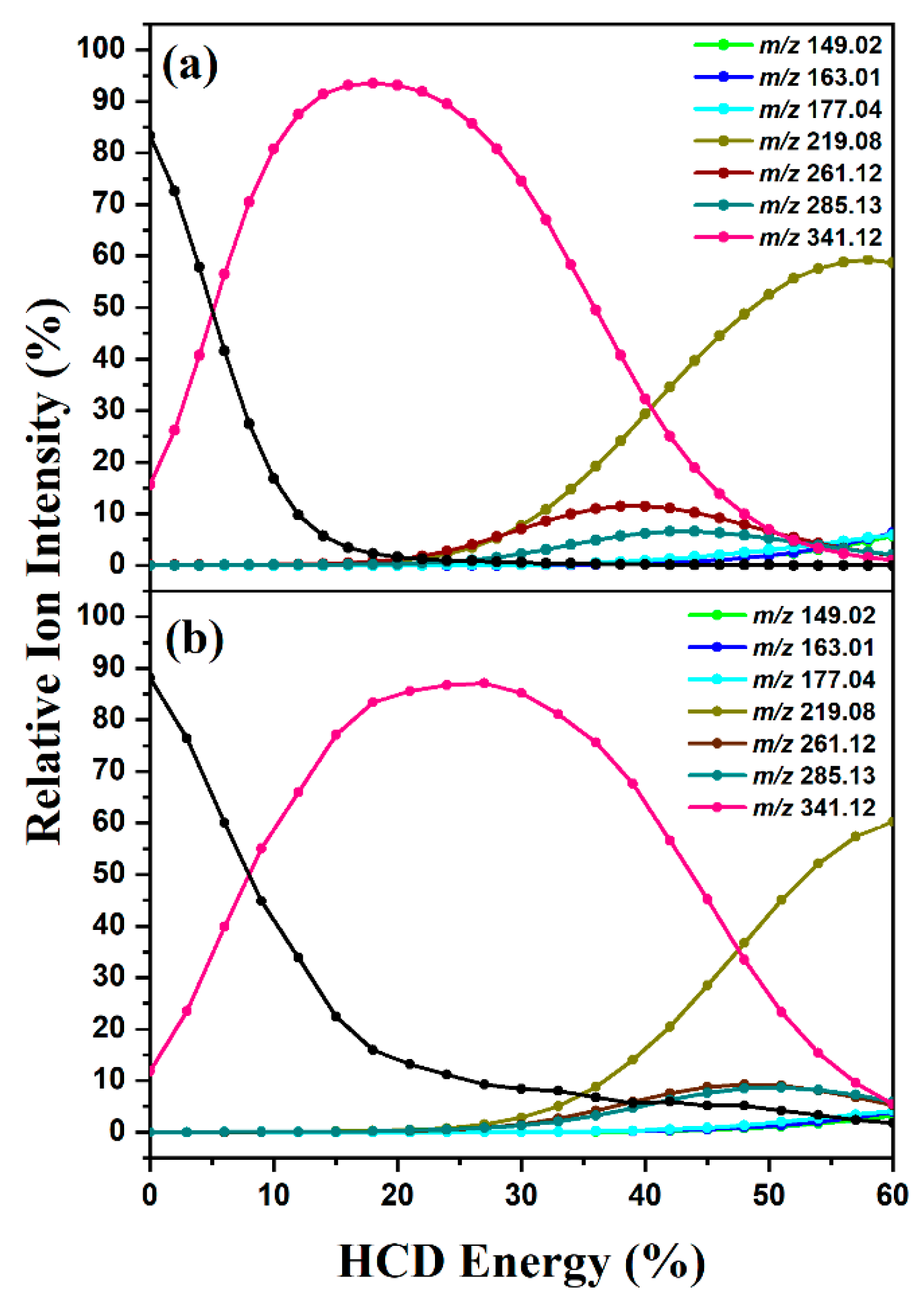
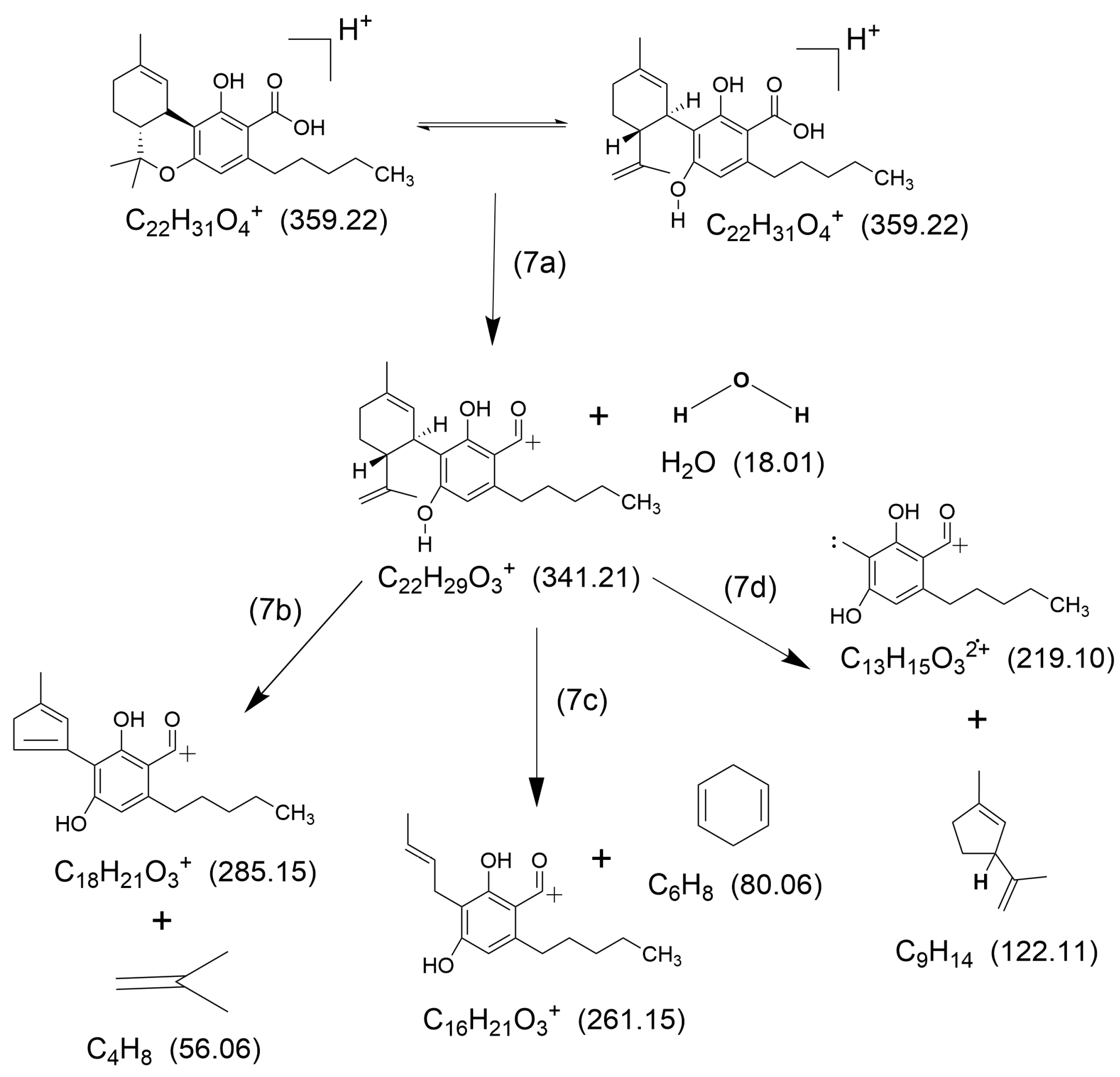
2.1. HCD Fragmentation
2.2. Quantum Chemistry Calculations
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duczmal, D.; Bazan-Wozniak, A.; Niedzielska, K.; Pietrzak, R. Cannabinoids—Multifunctional Compounds, Applications and Challenges—Mini Review. Molecules 2024, 29, 4923. [Google Scholar] [CrossRef] [PubMed]
- How, Z.T.; El-Din, M.G. A critical review on the detection, occurrence, fate, toxicity, and removal of cannabinoids in the water system and the environment. Environ. Pollut. 2021, 268, 115642. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, X.; Liu, X.; Xu, B.; Ma, Q.; Lei, H. Study on the mass fragmentation pathway of the synthetic cannabinoids JWH-018 and JWH-073. Int. J. Mass Spectrom. 2015, 379, 209–214. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Battistini, A.; Materazzi, S. Monitoring of cannabinoids in hemp flours by MicroNIR/Chemometrics. Talanta 2020, 211, 120672. [Google Scholar] [CrossRef]
- Antoniou, T.; Juurlink, D.N. Synthetic cannabinoids. Can. Med. Assoc. J. 2014, 186, 210. [Google Scholar] [CrossRef]
- Castiglioni, S.; Griffiths, P. Assessing Illicit Drugs in Wastewater: Advances in Wastewater-Based Drug Epidemiology; Publications Office: Washington, DC, USA, 2016. [Google Scholar]
- Burgard, D.A.; Williams, J.; Westerman, D.; Rushing, R.; Carpenter, R.; LaRock, A.; Sadetsky, J.; Clarke, J.; Fryhle, H.; Pellman, M.; et al. Using wastewater-based analysis to monitor the effects of legalized retail sales on cannabis consumption in Washington State, USA. Addiction 2019, 114, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- USA Food & Drug Administration. Real World Evidence; USA Food & Drug Administration: Silver Spring, MD, USA, 2018.
- Bade, R.; Tscharke, B.J.; O’Brien, J.W.; Magsarjav, S.; Humphries, M.; Ghetia, M.; Thomas, K.V.; Mueller, J.F.; White, J.M.; Gerber, C. Impact of COVID-19 Controls on the Use of Illicit Drugs and Alcohol in Australia. Environ. Sci. Technol. Lett. 2021, 8, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Löve, A.S.C.; Ásgrímsson, V.; Ólafsdóttir, K. Illicit drug use in Reykjavik by wastewater-based epidemiology. Sci. Total Environ. 2022, 803, 149795. [Google Scholar] [CrossRef]
- Apul, O.G.; Rowles, L.S.; Khalid, A.; Karanfil, T.; Richardson, S.D.; Saleh, N.B. Transformation potential of cannabinoids during their passage through engineered water treatment systems: A perspective. Environ. Int. 2020, 137, 105586. [Google Scholar] [CrossRef]
- Rodayan, A.; Majewsky, M.; Yargeau, V. Impact of approach used to determine removal levels of drugs of abuse during wastewater treatment. Sci. Total Environ. 2014, 487, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.B.; Apul, O.; Karanfil, T. The Genesis of a Critical Environmental Concern: Cannabinoids in Our Water Systems. Environ. Sci. Technol. 2019, 1746–1747. [Google Scholar] [CrossRef] [PubMed]
- Pires, B.; Oliveira, P.; Simao, A.Y.; Reis, J.; Ramos, S.; Duarte, A.P.; Margalho, C.; Rosado, T.; Barroso, M.; Gallardo, E. Characterisation of Cannabis-Based Products Marketed for Medical and Non-Medical Use Purchased in Portugal. Molecules 2024, 29, 2737. [Google Scholar] [CrossRef]
- Lazarjani, M.P.; Torres, S.; Hooker, T.; Fowlie, C.; Young, O.; Seyfoddin, A. Methods for quantification of cannabinoids: A narrative review. J. Cannabis Res. 2020, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Musah, R.A.; Domin, M.A.; Walling, M.A.; Shepard, J.R.E. Rapid identification of synthetic cannabinoids in herbal samples via direct analysis in real time mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Grabenauer, M.; Krol, W.L.; Wiley, J.L.; Thomas, B.F. Analysis of synthetic cannabinoids using high-resolution mass spectrometry and mass defect filtering: Implications for nontargeted screening of designer drugs. Anal. Chem. 2012, 84, 5574–5581. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zong, X.; Liu, J.; Ke, X.; Huang, Z.; Xu, Y. Development of a fragmentation pattern of synthetic cannabinoids based on electrospray ionization mass spectrometry in positive ion mode to screen synthetic cannabinoids in illicit products. J. Pharm. Biomed. Anal. 2021, 193, 113723. [Google Scholar] [CrossRef]
- Büttenbender, S.L.; Carvalho, Â.R.; De Souza Barbosa, F.; Scorsatto Ortiz, R.; Limberger, R.P.; Mendez, A.S.L. Fragmentation of Cannabinoids by Flow Injection Analysis Tandem Mass Spectrometry (FIA-MS/MS). J. AOAC Int. 2022, 105, 915–927. [Google Scholar] [CrossRef] [PubMed]
- González-Mariño, I.; Thomas, K.V.; Reid, M.J. Determination of cannabinoid and synthetic cannabinoid metabolites in wastewater by liquid–liquid extraction and ultra-high performance supercritical fluid chromatography-tandem mass spectrometry. Drug Test. Anal. 2018, 10, 222–228. [Google Scholar] [CrossRef]
- Lelario, F.; Pascale, R.; Bianco, G.; Scrano, L.; Bufo, S.A. Hemp chemotype definition by cannabinoids characterization using lc-esi(+)-ltq-fticr ms and infrared multiphoton dissociation. Separations 2021, 8, 245. [Google Scholar] [CrossRef]
- Broecker, S.; Pragst, F. Isomerization of cannabidiol and Δ9-tetrahydrocannabinol during positive electrospray ionization. In-source hydrogen/deuterium exchange experiments by fl ow injection hybrid quadrupole-time-of- fl ight mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 1407–1414. [Google Scholar] [CrossRef]
- Zubarev, R.A.; Makarov, A. Orbitrap Mass Spectrometry. Anal. Chem. 2013, 85, 5288–5296. [Google Scholar] [CrossRef]
- Cercola, R.; Matthews, E.; Dessent, C.E.H. Photoexcitation of Adenosine 5′-Triphosphate Anions in Vacuo: Probing the Influence of Charge State on the UV Photophysics of Adenine. J. Phys. Chem. B 2017, 121, 5553–5561. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.G.K.; Rankine, C.D.; Dessent, C.E.H. Measurement of the population of electrosprayed deprotomers of coumaric acids using UV–vis laser photodissociation spectroscopy. J. Phys. Chem. A 2021, 125, 6703–6714. [Google Scholar] [CrossRef]
- Whitaker, W.; Moncrieff, K.E.; Anstoter, C.S.; Wong, N.G.K.; Berenbeim, J.A.; Dessent, C.E.H. Probing the electronic relaxation pathways and photostability of the synthetic nucleobase Z via laser interfaced mass spectrometry. Phys. Chem. Chem. Phys. 2022, 24, 27836–27846. [Google Scholar] [CrossRef] [PubMed]
- Evans-Newman, K.C.; Schneider, G.L.; Perera, N.T. Classification of Mass Spectral Data to Assist in the Identification of Novel Synthetic Cannabinoids. Molecules 2024, 29, 4646. [Google Scholar] [CrossRef] [PubMed]
- Boleda, M.R.; Galceran, M.T.; Ventura, F. Trace determination of cannabinoids and opiates in wastewater and surface waters by ultra-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2007, 1175, 38–48. [Google Scholar] [CrossRef]
- Matthews, E.; Dessent, C.E.H. Locating the proton in nicotinamide protomers via low-Resolution UV action spectroscopy of electrosprayed solutions. J. Phys. Chem. A 2016, 120, 9209–9216. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.; Dessent, C.E.H. Experiment and theory confirm that UV laser photodissociation spectroscopy can distinguish protomers formed: Via electrospray. Phys. Chem. Chem. Phys. 2017, 19, 17434–17440. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Sen, A.; Harvey, A.J.A.; Dessent, C.E.H. Complexation of anions to gas-phase amino acids: Conformation is critical in determining if the global minimum is canonical or zwitterionic. Chem. Phys. Lett. 2013, 588, 43–46. [Google Scholar] [CrossRef]
- Selwe, K. Contaminants of Emerging Concern in Botswana’s Aquatic Systems. Ph.D. Thesis, University of York, York, UK, 2024. [Google Scholar]
- Uleanya, K.O.; Anstöter, C.S.; Dessent, C.E.H. Photodissociative decay pathways of the flavin mononucleotide anion and its complexes with tryptophan and glutamic acid. Phys. Chem. Chem. Phys. 2023, 25, 30697–30707. [Google Scholar] [CrossRef]
- Wong, N.G.K.; Rankine, C.D.; Dessent, C.E.H. Linking Electronic Relaxation Dynamics and Ionic Photofragmentation Patterns for the Deprotonated UV Filter Benzophenone-4. J. Phys. Chem. Lett. 2021, 12, 2831–2836. [Google Scholar] [CrossRef]
- Olsen, J.V.; Macek, B.; Lange, O.; Makarov, A.; Horning, S.; Mann, M. Higher-energy C-trap dissociation for peptide modification analysis. Nat. Methods 2007, 4, 709–712. [Google Scholar] [CrossRef]
- Kostiainen, R.; Kauppila, T.J. Effect of eluent on the ionization process in liquid chromatography—Mass spectrometry. J. Chromatogr. A 2009, 1216, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.B. Electrospray and MALDI Mass Spectrometry: Fundamentals, Instrumentation, Practicalities, and Biological Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Walker, M.; Harvey, A.J.A.; Sen, A.; Dessent, C.E.H. Performance of M06, M06-2X, and M06-HF Density Functionals for Conformationally Flexible Anionic Clusters: M06 Functionals Perform Better than B3LYP for a Model System with Dispersion and Ionic Hydrogen-Bonding Interactions. Phys. Chem. 2013, 117, 12590–12600. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Righetti, L.; Claasen, F.W.; Krishna, A.; Ma, M.; van Beek, T.A.; Chen, B.; Zuilhof, H.; Salentij, G.I. Ultrafast, Selective, and Highly Sensitive Nonchromatographic Analysis of Fourteen Cannabinoids in Cannabis Extracts, Δ8-Tetrahydrocannabinol Synthetic Mixtures, and Edibles by Cyclic Ion Mobility Spectrometry-Mass Spectrometry. Anal. Chem. 2024, 96, 10170–10181. [Google Scholar] [CrossRef]
- Tose, L.V.; Santos, N.A.; Rodrigues, R.R.T.; Murgu, M.; Gomes, A.F.; Vasconcelos, G.A.; Souza, P.C.T.; Vaz, B.G.; Romao, W. Isomeric separation of cannabinoids by UPLC combined with ionic mobility mass spectrometry (TWIM-MS)—Part I. Int. J. Mass Spectrom. 2017, 418, 112–121. [Google Scholar] [CrossRef]
- Kiselak, T.D.; Koerber, R.; Verbeck, G.F. Synthetic route sourcing of illicit at home cannabidiol (CBD) isomerization to psychoactive cannabinoids using ion mobility-coupled-LC–MS/MS. Forensic Sci. Int. 2020, 308, 110173. [Google Scholar] [CrossRef]
- Mashmoushi, N.; Campbell, J.L.; Lorenzo, R.; Hopkins, W.S. Rapid Separation of cannabinoid isomer sets using differential mobility spectrometry and mass spectrometry. Analyst 2022, 147, 2198–2206. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selwe, K.P.; Shaikh, A.S.A.; Uleanya, K.O.; Dessent, C.E.H. Fragmentation and Isomerization Pathways of Natural and Synthetic Cannabinoids Studied via Higher Collisional Energy Dissociation Profiles. Molecules 2025, 30, 717. https://doi.org/10.3390/molecules30030717
Selwe KP, Shaikh ASA, Uleanya KO, Dessent CEH. Fragmentation and Isomerization Pathways of Natural and Synthetic Cannabinoids Studied via Higher Collisional Energy Dissociation Profiles. Molecules. 2025; 30(3):717. https://doi.org/10.3390/molecules30030717
Chicago/Turabian StyleSelwe, Kgato P., Ambar S. A. Shaikh, Kelechi O. Uleanya, and Caroline E. H. Dessent. 2025. "Fragmentation and Isomerization Pathways of Natural and Synthetic Cannabinoids Studied via Higher Collisional Energy Dissociation Profiles" Molecules 30, no. 3: 717. https://doi.org/10.3390/molecules30030717
APA StyleSelwe, K. P., Shaikh, A. S. A., Uleanya, K. O., & Dessent, C. E. H. (2025). Fragmentation and Isomerization Pathways of Natural and Synthetic Cannabinoids Studied via Higher Collisional Energy Dissociation Profiles. Molecules, 30(3), 717. https://doi.org/10.3390/molecules30030717









