Homogeneous Aptasensor with Electrochemical and Electrochemiluminescence Dual Detection Channels Enabled by Nanochannel-Based Probe Enrichment and DNase I Cleavage for Tumor Biomarker Detection
Abstract
:1. Introduction
2. Results and Discussion
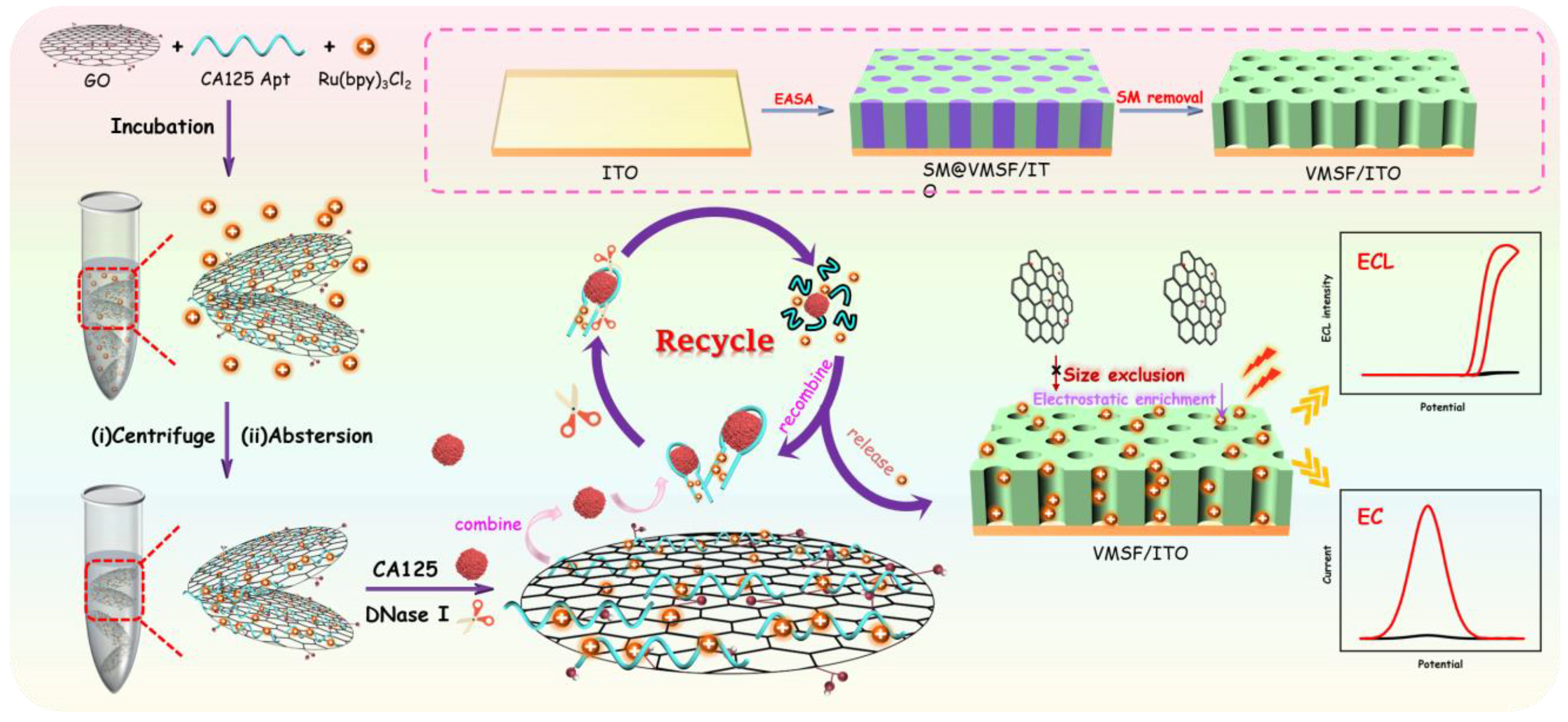
2.1. Strategies for Construction of Homogeneous Aptasensor and EC/ECL Dual-Mode Detection
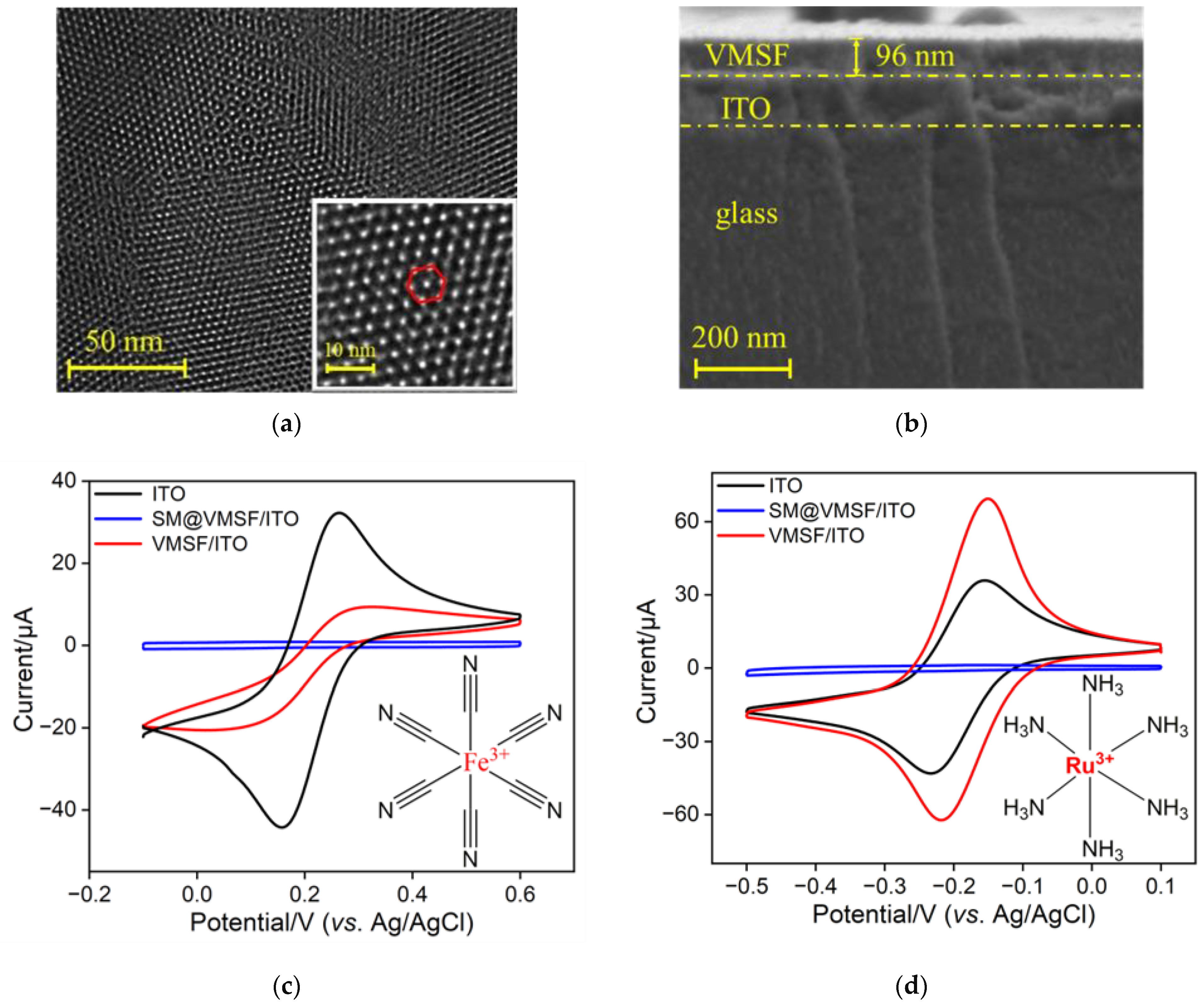
2.2. Characterization of VMSF and VMSF-Modified ITO Electrode
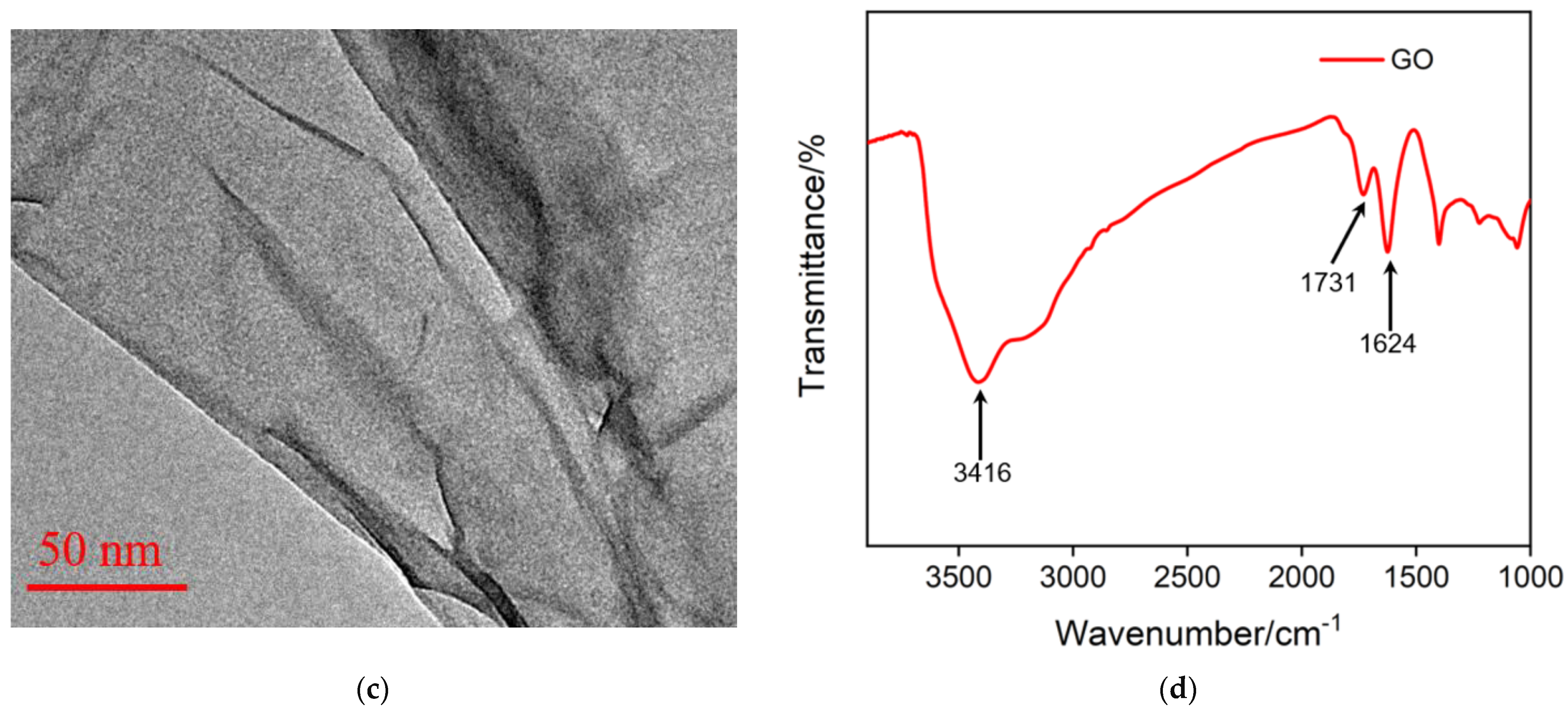
2.3. Characterization of Graphene Oxide
2.4. Feasibility of 2D Composite Probe for Homogeneous Sensor and EC/ECL Dual-Mode Detection
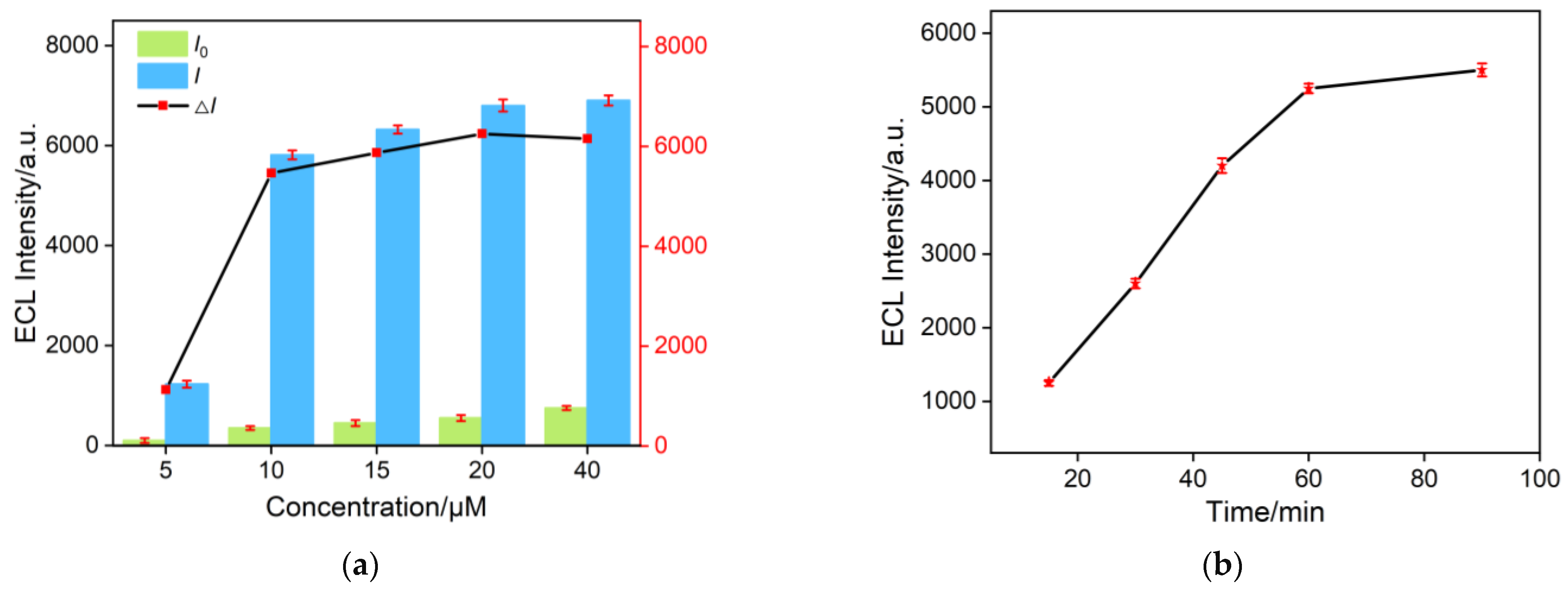
2.5. Optimization of Conditions
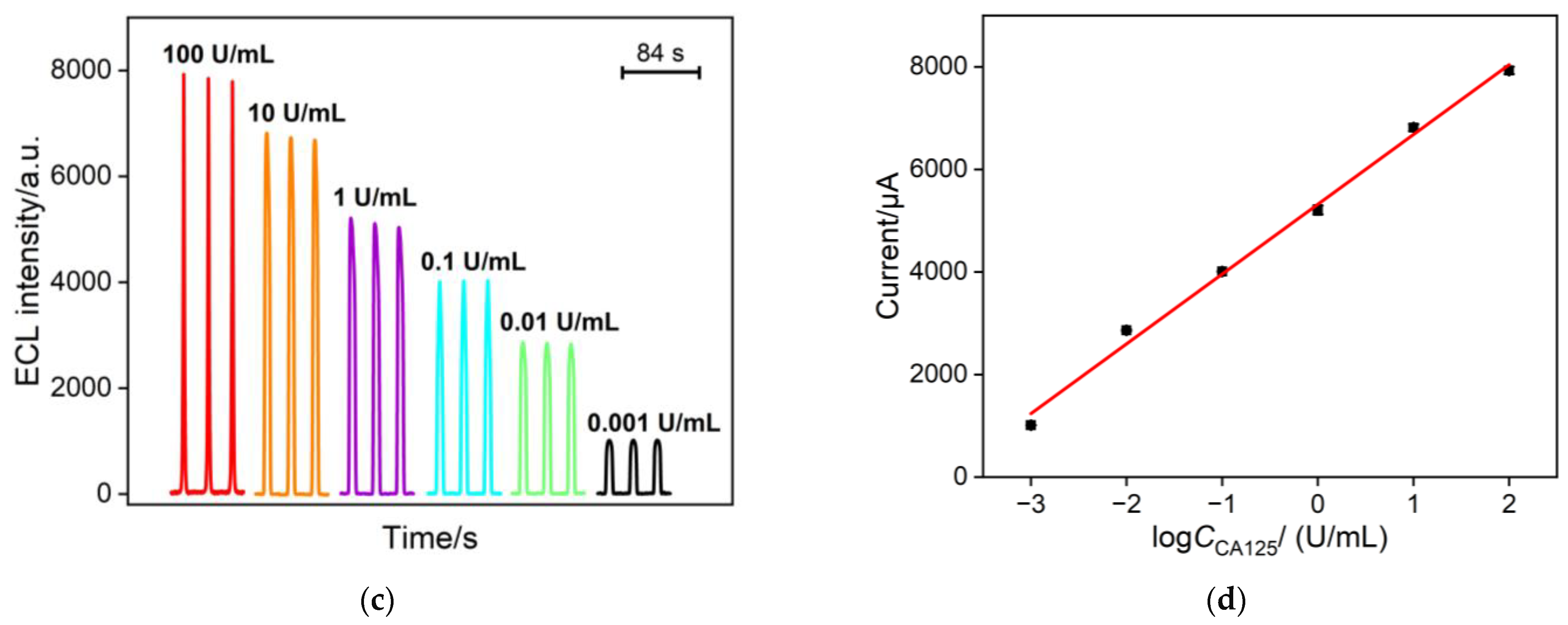
2.6. ECL Detection of CA 125
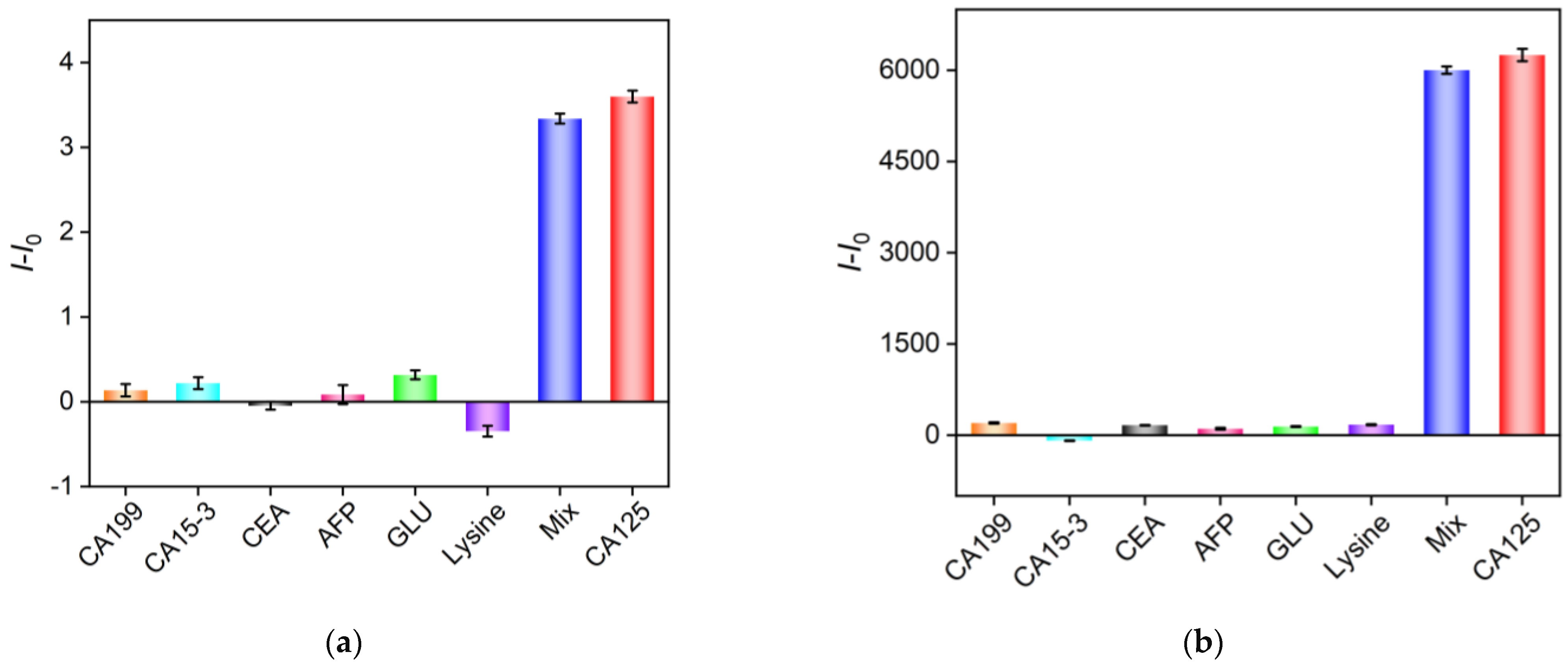
2.7. Selectivity of the Aptasensor
2.8. Analysis of Real Samples
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Measurements and Instrumentations
3.3. Preparation of VMSF-Modified ITO Electrode
3.4. Preparation of the Composite Probe Ru(bpy)32+/Apt@GO
3.5. EC/ECL Dual-Mode Detection of CA125
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S. World cancer report 2014. Geneva, switzerland: World health organization, international agency for research on cancer, who press, 2015. Adv. Nutr. 2016, 7, 418. [Google Scholar] [CrossRef]
- Sharifi, M.; Ezzati Nazhad Dolatabadi, J.; Fathi, F.; Zakariazadeh, M.; Barzegar, A.; Rashidi, M.; Tajalli, H.; Rashidi, M.-R. Surface plasmon resonance and molecular docking studies of bovine serum albumin interaction with neomycin: Kinetic and thermodynamic analysis. BioImpacts 2017, 7, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Hamd-Ghadareh, S.; Salimi, A.; Fathi, F.; Bahrami, S. An amplified comparative fluorescence resonance energy transfer immunosensing of CA125 tumor marker and ovarian cancer cells using green and economic carbon dots for bio-applications in labeling, imaging and sensing. Biosens. Bioelectron. 2017, 96, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Wang, Y.; Gao, X.; Du, S.; Cheng, Y.; Yu, S.; Zou, G.; Xue, F. Electrochemiluminescence ultrasensitive immunoassay for carbohydrate antigen 125 based on AgInS2/ZnS nanocrystals. Anal. Bioanal. Chem. 2021, 413, 2207–2215. [Google Scholar] [CrossRef]
- Pakchin, P.S.; Fathi, M.; Ghanbari, H.; Saber, R.; Omidi, Y. A novel electrochemical immunosensor for ultrasensitive detection of CA125 in ovarian cancer. Biosens. Bioelectron. 2020, 153, 112029. [Google Scholar]
- Hong, G.; Su, C.; Lai, M.; Huang, Z.; Weng, Z.; Chen, Y.; Deng, H.; Chen, W.; Peng, H. Co-reactant-mediated low-potential anodic electrochemiluminescence platform and its immunosensing application. Anal. Chem. 2022, 94, 12500–12506. [Google Scholar] [CrossRef]
- Gong, J.; Tang, H.; Wang, M.; Lin, X.; Wang, K.; Liu, J. Novel three-dimensional graphene nanomesh prepared by facile electro-etching for improved electroanalytical performance for small biomolecules. Mater. Des. 2022, 215, 110506. [Google Scholar] [CrossRef]
- Zhou, H.; Dong, G.; Sailjoi, A.; Liu, J. Facile pretreatment of three-dimensional graphene through electrochemical polarization for improved electrocatalytic performance and simultaneous electrochemical detection of catechol and hydroquinone. Nanomaterials 2022, 12, 65. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, T.; Dong, G.; Zhu, S.; Yan, F.; Liu, J. Direct and sensitive electrochemical detection of bisphenol a in complex environmental samples using a simple and convenient nanochannel-modified electrode. Front. Chem. 2022, 10, 900282. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, T.; Luo, T.; Luo, X.; Yan, F.; Tang, W.; Liu, J. Bipolar silica nanochannel array confined electrochemiluminescence for ultrasensitive detection of SARS-CoV-2 antibody. Biosens. Bioelectron. 2022, 215, 114563. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, Y.; Liu, J. Sensitive detection of biomarker in gingival crevicular fluid based on enhanced electrochemiluminescence by nanochannel-confined Co3O4 nanocatalyst. Biosensors 2025, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, C.; Liu, J.; Mou, Y. Nanochannel confined graphene quantum dots/platinum nanoparticles boosts electrochemiluminescence of luminal-O2 system for sensitive immunoassay. Talanta 2025, 285, 127223. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, Z.; Zheng, Y.; Liu, J. Solid-phase electrochemiluminescence enzyme electrodes based on nanocage arrays for highly sensitive detection of cholesterol. Biosensors 2024, 14, 403. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Lin, J.; Xie, L.; Tang, H.; Wang, K.; Liu, J. One-step preparation of nitrogen-doped graphene quantum dots with anodic electrochemiluminescence for sensitive detection of hydrogen peroxide and glucose. Front. Chem. 2021, 9, 688358. [Google Scholar]
- Gong, J.; Zhang, T.; Chen, P.; Yan, F.; Liu, J. Bipolar silica nanochannel array for dual-mode electrochemiluminescence and electrochemical immunosensing platform. Sens. Actuators B Chem. 2022, 368, 132086. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, T.; Zheng, Y.; Liu, J. Dual-mode sensing platform for cancer antigen 15-3 determination based on a silica nanochannel array using electrochemiluminescence and electrochemistry. Biosensors 2023, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gong, J.; Han, Q.; Hu, W.; Yan, F.; Liu, J. Nanogold amplified electrochemiluminescence/electrochemistry in bipolar silica nanochannel array for ultrasensitive detection of SARS-CoV-2 pseudoviruses. Talanta 2024, 277, 126319. [Google Scholar] [CrossRef] [PubMed]
- Foroozandeh, A.; Abdouss, M.; SalarAmoli, H.; Pourmadadi, M.; Yazdian, F. An electrochemical aptasensor based on g-C3N4/Fe3O4/PANI nanocomposite applying cancer antigen_125 biomarkers detection. Process Biochem. 2023, 127, 82–91. [Google Scholar] [CrossRef]
- Zhou, X.; Zou, Y.; Ru, H.; Yan, F.; Liu, J. Silica nanochannels as nanoreactors for the confined synthesis of Ag NPs to boost electrochemical stripping chemiluminescence of the luminol-O2 system for the sensitive aptasensor. Anal. Chem. 2024, 96, 10264–10273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, L.; Yan, F.; Wang, K. Vertically-ordered mesoporous silica film based electrochemical aptasensor for highly sensitive detection of alpha-fetoprotein in human serum. Biosensors 2023, 13, 628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, S.; Lin, X.; Liu, J.; Wang, K. Label-free electrochemical aptasensor based on the vertically-aligned mesoporous silica films for determination of aflatoxin B1. Biosensors 2023, 13, 661. [Google Scholar] [CrossRef]
- An, J.; Zhang, C.; Yan, F.; Ma, P. Nanochannel-confined platinum nanostructure for enhancement of luminol-dissolved oxygen electrochemiluminescence coupled with gated aptasensor for sensitive detection of carcinoembryonic antigen. Microchem. J. 2024, 206, 111413. [Google Scholar] [CrossRef]
- Chen, M.; Han, R.; Wang, W.; Li, Y.; Luo, X. Antifouling aptasensor based on self-assembled loop-closed peptides with enhanced stability for CA125 assay in complex biofluids. Anal. Chem. 2021, 93, 13555–13563. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Han, Q.; Zhou, J.; Liu, C.; Liu, J. Reagentless electrochemical detection of tumor biomarker based on stable confinement of electrochemical probe in bipolar silica nanochannel film. Nanomaterials 2023, 13, 1645. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Han, Q.; Liu, J.; Yan, Z. Electrochemical aptasensor fabricated by anchoring recognition aptamers and immobilizing redox probes on bipolar silica nanochannel array for reagentless detection of carbohydrate antigen 15-3. Front. Chem. 2023, 11, 1324469. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Wang, H.; Luo, T.; Yan, F.; Guo, J. Amino-functionalized vertically ordered mesoporous silica film on electrochemically polarized screen-printed carbon electrodes for the construction of gated electrochemical aptasensors and sensitive detection of carcinoembryonic antigens. Front. Chem. 2024, 12, 1490940. [Google Scholar] [CrossRef]
- Huang, Z.; Luo, X.; Yan, F.; Zhou, B. Homogeneous electrochemical aptasensor for sensitive detection of zearalenone using nanocomposite probe and silica nanochannel film. Molecules 2023, 28, 7241. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Moammeri, A.; Shamsabadipour, A.; Moghaddam, Y.F.; Rahdar, A.; Pandey, S. Application of various optical and electrochemical nanobiosensors for detecting cancer antigen 125 (CA-125): A review. Biosensors 2023, 13, 99. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, F.; Yang, Q.; Wu, W. Graphene oxide quantum dots based nanotree illuminates AFB1: Dual signal amplified aptasensor detection AFB1. Sens. Actuators B Chem. 2021, 345, 130387. [Google Scholar] [CrossRef]
- Yan, F.; Luo, T.; Jin, Q.; Zhou, H.; Sailjoi, A.; Dong, G.; Liu, J.; Tang, W. Tailoring molecular permeability of vertically-ordered mesoporous silica-nanochannel films on graphene for selectively enhanced determination of dihydroxybenzene isomers in environmental water samples. J. Hazard. Mater. 2021, 410, 124636. [Google Scholar] [CrossRef]
- Yan, F.; Wang, M.; Jin, Q.; Zhou, H.; Xie, L.; Tang, H.; Liu, J. Vertically-ordered mesoporous silica films on graphene for anti-fouling electrochemical detection of tert-butylhydroquinone in cosmetics and edible oils. J. Electroanal. Chem. 2021, 881, 114969. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, Z.; Chen, M.; Xi, F. Highly active nanozyme based on nitrogen-doped graphene quantum dots and iron ion nanocomposite for selective colorimetric detection of hydroquinone. Talanta 2025, 281, 126817. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, T.; Wang, M.; Yan, F.; Liu, J. Disposable electrochemical sensors for highly sensitive detection of chlorpromazine in human whole blood based on the silica nanochannel array modified screen-printed carbon electrode. Molecules 2022, 27, 8200. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, X.; Su, B. Molecular filtration by ultrathin and highly porous silica nanochannel membranes: Permeability and selectivity. Anal. Chem. 2016, 88, 10252–10258. [Google Scholar] [CrossRef]
- Vilà, N.; Ghanbaja, J.; Aubert, E.; Walcarius, A. Electrochemically assisted generation of highly ordered azide-functionalized mesoporous silica for oriented hybrid films. Angew. Chem. Int. Ed. 2014, 53, 2945–2950. [Google Scholar] [CrossRef]
- Zou, Y.; Zhou, X.; Xie, L.; Tang, H.; Yan, F. Vertically-ordered mesoporous silica films grown on boron nitride-graphene composite modified electrodes for rapid and sensitive detection of carbendazim in real samples. Front. Chem. 2022, 10, 939510. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zhao, Y.; Liu, J. Solid electrochemiluminescence sensor by immobilization of emitter ruthenium(ii)tris(bipyridine) in bipolar silica nanochannel film for sensitive detection of oxalate in serum and urine. Nanomaterials 2024, 14, 390. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, T.; Tang, H.; Liu, J. Novel electrochemical and electrochemiluminescence dual-modality sensing platform for sensitive determination of antimicrobial peptides based on probe encapsulated liposome and nanochannel array electrode. Front. Nutr. 2022, 9, 962736. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, T.; Zhou, H.; Yan, F.; Liu, Y. Silica nanochannels boosting Ru(bpy)32+-mediated electrochemical sensor for the detection of guanine in beer and pharmaceutical samples. Front. Nutr. 2022, 9, 987442. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, X.; Sailjoi, A.; Zou, Y.; Lin, X.; Yan, F.; Su, B.; Liu, J. Vertical silica nanochannels supported by nanocarbon composite for simultaneous detection of serotonin and melatonin in biological fluids. Sens. Actuators B Chem. 2022, 353, 131101. [Google Scholar] [CrossRef]
- Fan, X.; Wu, J.; Zhang, T.; Liu, J. Electrochemical/electrochemiluminescence sensors based on vertically-ordered mesoporous silica films for biomedical analytical applications. ChemBioChem 2024, e202400320. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, Y.; Zhou, X.; Yan, F.; Ding, Z. Vertically-ordered mesoporous silica films for electrochemical detection of Hg(II) ion in pharmaceuticals and soil samples. Front. Chem. 2022, 10, 952936. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, X.; Yan, F.; Lin, J. N-doped graphene quantum dots confined within silica nanochannels for enhanced electrochemical detection of doxorubicin. Molecules 2023, 28, 6443. [Google Scholar] [CrossRef]
- Huang, J.; Fan, X.; Yan, F.; Liu, J. Vertical Silica Nanochannels and o-Phenanthroline Chelator for the Detection of Trace Fe(II). ACS Appl. Nano Mater. 2024, 7, 7743–7752. [Google Scholar] [CrossRef]
- Huang, J.; Xu, S.; Yan, F.; Liu, J. Electrochemiluminescence enzyme biosensors for ultrasensitive determination of glucose using glucose dehydrogenase immobilized on vertical silica nanochannels. Sens. Actuators B Chem. 2024, 402, 135119. [Google Scholar] [CrossRef]
- Xing, J.; Wang, H.; Yan, F. Carbon nitride nanosheets as an adhesive layer for stable growth of vertically-ordered mesoporous silica film on a glassy carbon electrode and their application for CA15-3 immunosensor. Molecules 2024, 29, 4334. [Google Scholar] [CrossRef]
- Wang, M.; Lin, J.; Gong, J.; Ma, M.; Tang, H.; Liu, J.; Yan, F. Rapid and sensitive determination of doxorubicin in human whole blood by vertically-ordered mesoporous silica film modified electrochemically pretreated glassy carbon electrodes. RSC Adv. 2021, 11, 9021–9028. [Google Scholar] [CrossRef]
- Deng, X.; Lin, X.; Zhou, H.; Liu, J.; Tang, H. Equipment of vertically-ordered mesoporous silica film on electrochemically pretreated three-dimensional graphene electrodes for sensitive detection of methidazine in urine. Nanomaterials 2023, 13, 239. [Google Scholar] [CrossRef]



| Sample | Detection Mode | Added (ng mL−1) | Found (ng mL−1) | RSD (%, n = 3) | Recovery (%) |
|---|---|---|---|---|---|
| Fetal bovine serum a | EC | 0.500 | 0.48 | 3.6 | 96.0 |
| EC | 5.00 | 5.46 | 2.9 | 109 | |
| EC | 50.0 | 52.3 | 2.1 | 105 | |
| ECL | 0.100 | 0.0945 | 1.2 | 94.5 | |
| ECL | 1.00 | 1.09 | 1.5 | 109 | |
| ECL | 10.0 | 9.45 | 1.1 | 94.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Zhang, S.; Xi, F. Homogeneous Aptasensor with Electrochemical and Electrochemiluminescence Dual Detection Channels Enabled by Nanochannel-Based Probe Enrichment and DNase I Cleavage for Tumor Biomarker Detection. Molecules 2025, 30, 746. https://doi.org/10.3390/molecules30030746
Gao J, Zhang S, Xi F. Homogeneous Aptasensor with Electrochemical and Electrochemiluminescence Dual Detection Channels Enabled by Nanochannel-Based Probe Enrichment and DNase I Cleavage for Tumor Biomarker Detection. Molecules. 2025; 30(3):746. https://doi.org/10.3390/molecules30030746
Chicago/Turabian StyleGao, Jiong, Shiyue Zhang, and Fengna Xi. 2025. "Homogeneous Aptasensor with Electrochemical and Electrochemiluminescence Dual Detection Channels Enabled by Nanochannel-Based Probe Enrichment and DNase I Cleavage for Tumor Biomarker Detection" Molecules 30, no. 3: 746. https://doi.org/10.3390/molecules30030746
APA StyleGao, J., Zhang, S., & Xi, F. (2025). Homogeneous Aptasensor with Electrochemical and Electrochemiluminescence Dual Detection Channels Enabled by Nanochannel-Based Probe Enrichment and DNase I Cleavage for Tumor Biomarker Detection. Molecules, 30(3), 746. https://doi.org/10.3390/molecules30030746






