Spray-Drying Synthesis of Na4Fe3(PO4)2P2O7@CNT Cathode for Ultra-Stable and High-Rate Sodium-Ion Batteries
Abstract
1. Introduction
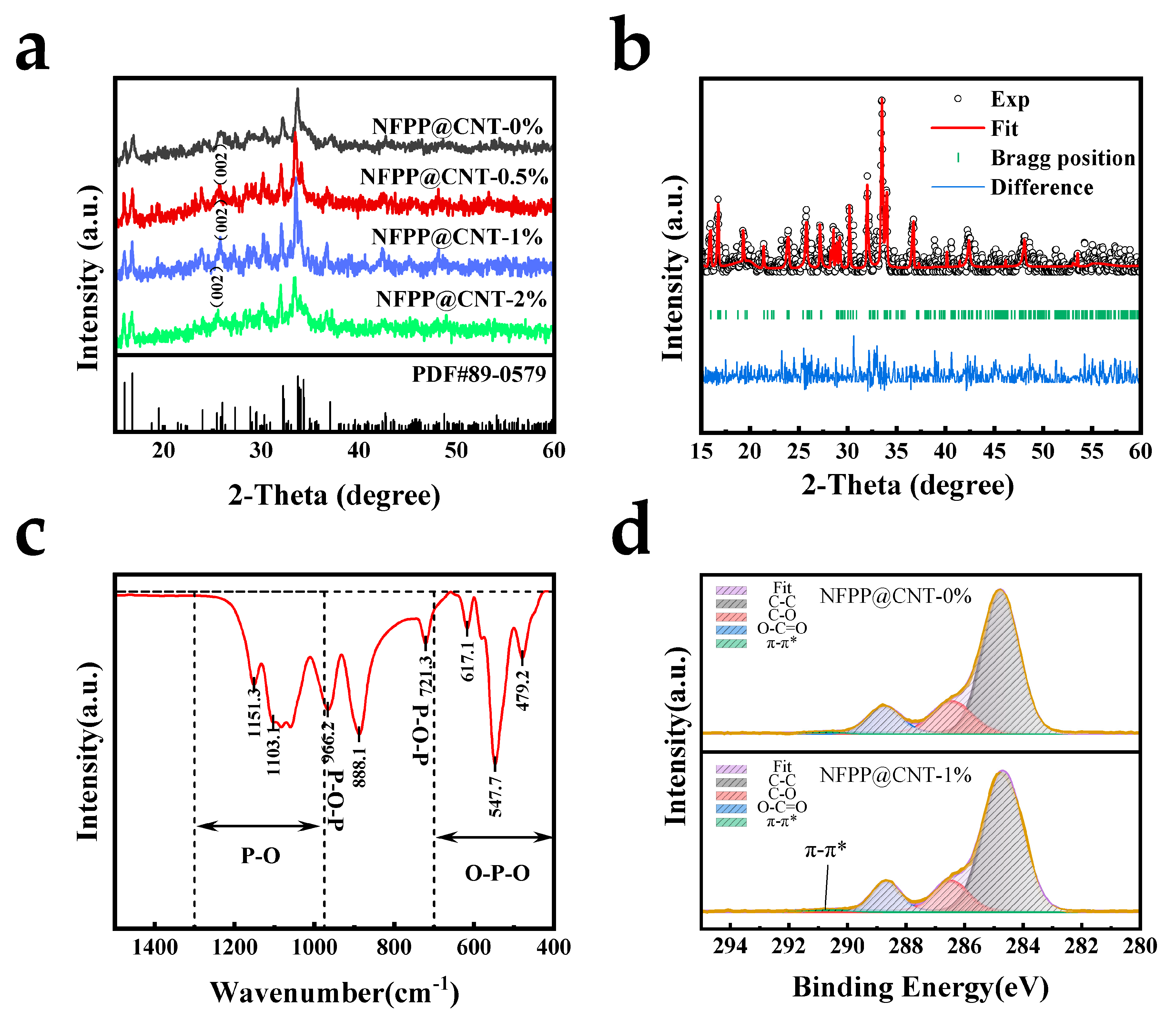
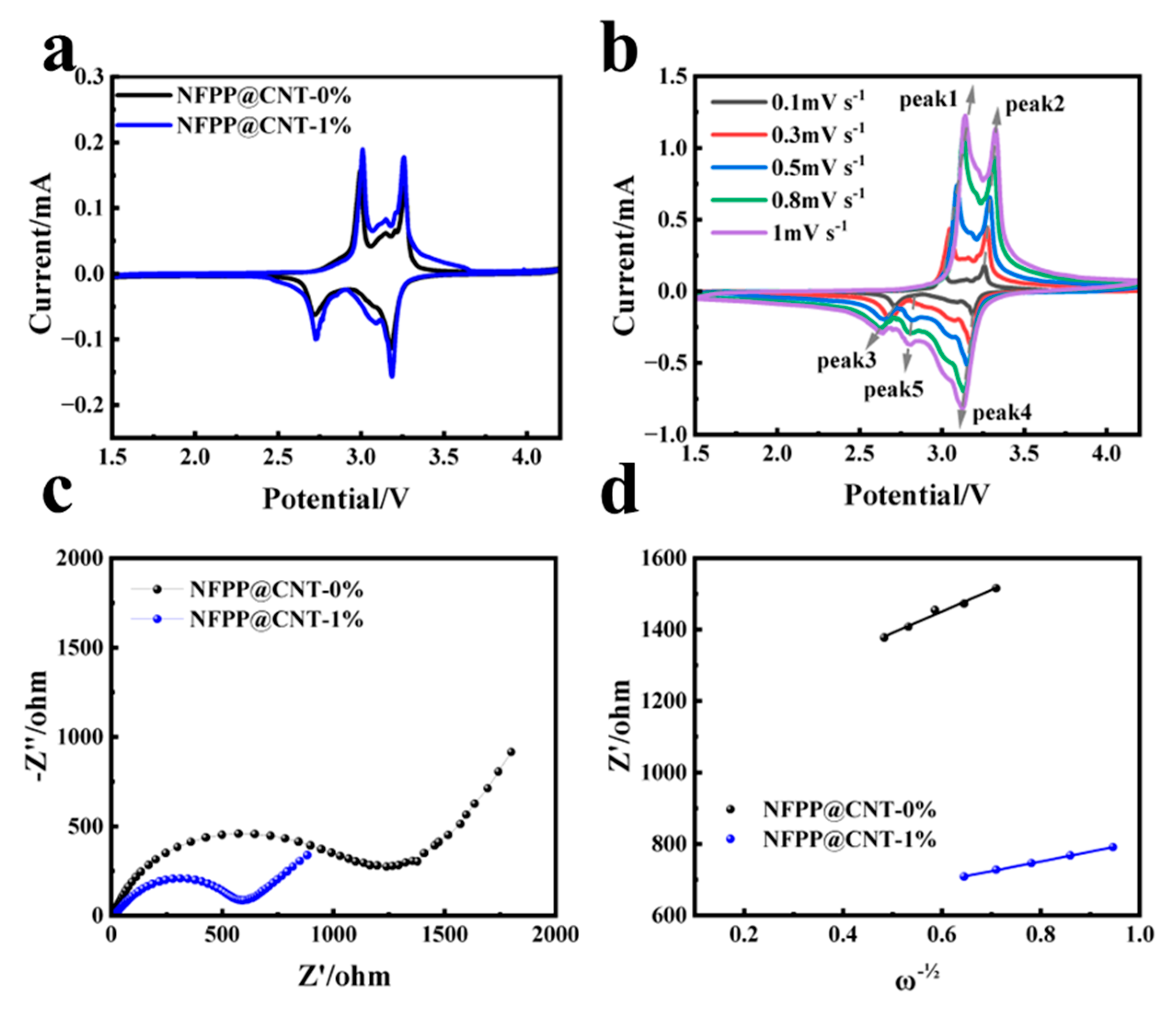
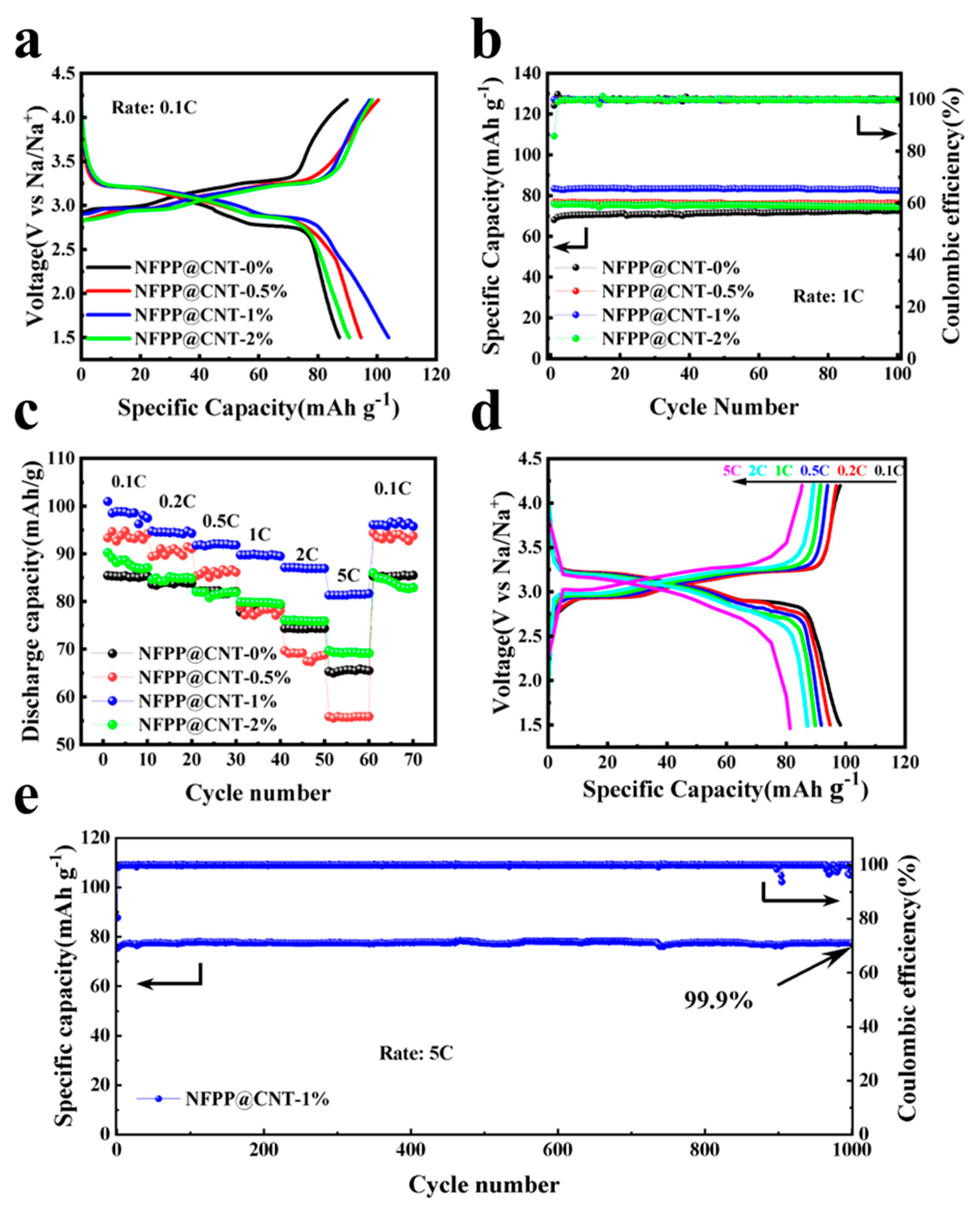
2. Results and Discussion
3. Materials and Methods
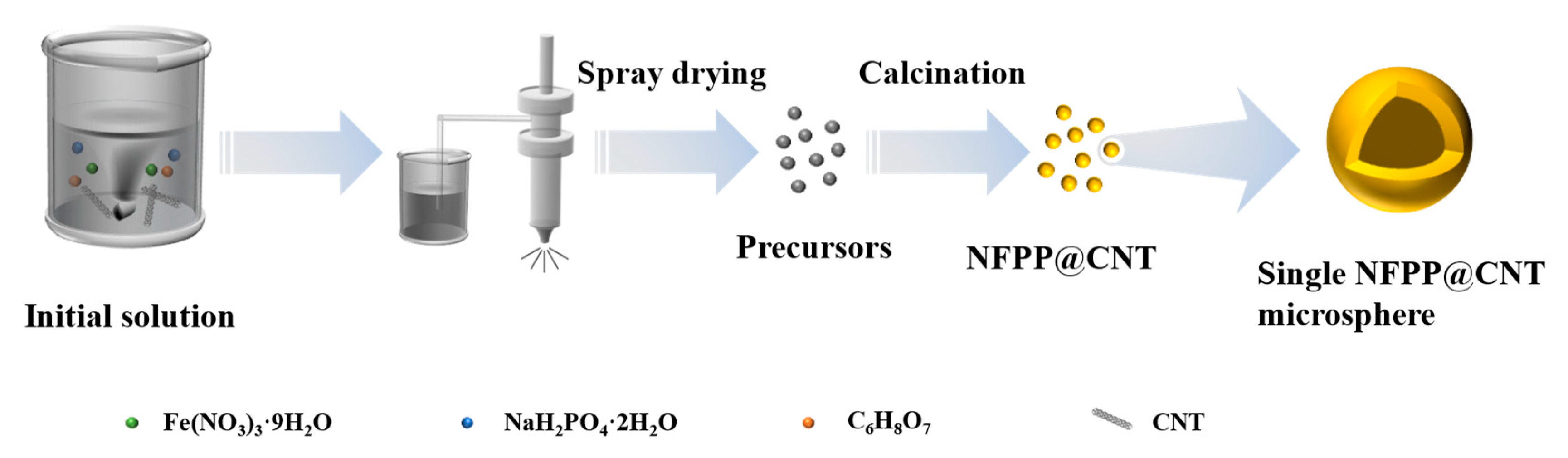
3.1. Material Synthesis
3.2. Characterizations
3.3. Electrochemical Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Zhang, W.; Ren, L.; Li, Y.; Wang, J.; Wang, C. Hierarchically cobalt phosphide nanostructures embedded in N, P co-doped porous carbon networks assembled by ultrathin sheets for high-performance Li/Na-ion batteries. Chem. Eng. Sci. 2023, 280, 119089. [Google Scholar] [CrossRef]
- Liu, Y.-K.; Zhao, C.-Z.; Du, J.; Zhang, X.-Q.; Chen, A.-B.; Zhang, Q. Research Progresses of Liquid Electrolytes in Lithium-Ion Batteries. Small 2023, 19, 2205315. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Chen, M.; Wu, W.; Qiu, S.; Wu, H.; Zheng, M.; Zhang, X.; Wu, X. Suppressing vacancies and crystal water of sodium manganese iron-based Prussian blue analogue by potassium doping for advanced sodium-ion batteries. Chem. Eng. Sci. 2025, 302, 120848. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Fang, C.; Huang, Y.; Zhang, W.; Han, J.; Deng, Z.; Cao, Y.; Yang, H. Routes to High Energy Cathodes of Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1501727. [Google Scholar] [CrossRef]
- Chen, L.; Dong, Y.; Jiang, H.; Hu, Y.; Li, C. Metal-cation-directed self-assembly of hierarchical MoS2 nanotubes as high-performance anode for Na-ion batteries. Chem. Eng. Sci. 2022, 261, 117953. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Y.; Guo, Y.; Li, C.; Liu, Y.; Yang, M.; Zhao, B.; Wu, W.; Wu, X. Highly stabilized single-crystal P2-type layered oxides obtained via rational crystal orientation modulation for sodium-ion batteries. Chem. Eng. J. 2023, 458, 141515. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Peng, J.; Fan, Y.; Zhao, L.; Li, L.; Xiao, Y.; Pang, W.K.; Wang, J.; Chou, S.-L. Prussian Blue Analogues with Optimized Crystal Plane Orientation and Low Crystal Defects toward 450 Wh kg−1 Alkali-Ion Batteries. Angew. Chem.-Int. Ed. 2023. Early Access. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem.-Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Liang, J.; Wei, Z.; Wang, C.; Ma, J. Vacancy-induced sodium-ion storage in N-doped carbon Nanofiber@MoS2 nanosheet arrays. Electrochim. Acta 2018, 285, 301–308. [Google Scholar] [CrossRef]
- Huang, J.; Wei, Z.; Liao, J.; Ni, W.; Wang, C.; Ma, J. Molybdenum and tungsten chalcogenides for lithium/sodium-ion batteries: Beyond MoS2. J. Energy Chem. 2019, 33, 100–124. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Jian, Z.; Ji, X. Potassium Secondary Batteries. ACS Appl. Mater. Interfaces 2017, 9, 4404–4419. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Ding, Z.; Lee, M.H.; Lim, K.; Yoon, G.; Kang, K. Recent Progress in Electrode Materials for Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600943. [Google Scholar] [CrossRef]
- Kundu, D.; Talaie, E.; Duffort, V.; Nazar, L.F. The Emerging Chemistry of Sodium Ion Batteries for Electrochemical Energy Storage. Angew. Chem.-Int. Ed. 2015, 54, 3431–3448. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.P.; Sougrati, M.T.; Feng, Z.; Leconte, Y.; Fisher, A.; Srinivasan, M.; Xu, Z. A Review on Design Strategies for Carbon Based Metal Oxides and Sulfides Nanocomposites for High Performance Li and Na Ion Battery Anodes. Adv. Energy Mater. 2017, 7, 1601424. [Google Scholar] [CrossRef]
- Zhang, S.; Steubing, B.; Potter, H.K.; Hansson, P.-A.; Nordberg, A. Future climate impacts of sodium-ion batteries. Resour. Conserv. Recycl. 2024, 202, 107362. [Google Scholar] [CrossRef]
- Senthilkumar, B.; Rambabu, A.; Murugesan, C.; Krupanidhi, S.; Barpanda, P. Iron-Based Mixed Phosphate Na4Fe3(PO4)2P2O7 Thin Films for Sodium-Ion Microbatteries. Acs Omega 2020, 5, 7219–7224. [Google Scholar] [CrossRef]
- Gao, J.; Tian, Y.; Mei, Y.; Ni, L.; Wang, H.; Liu, H.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Robust NASICON-type iron-based Na4Fe3(PO4)2(P2O7) cathode for high temperature sodium-ion batteries. Chem. Eng. J. 2023, 458, 141385. [Google Scholar] [CrossRef]
- Hu, X.; Li, H.; Wang, Z.; Liu, M.; Lu, Y.; Zhang, Y.; Li, J.; Ding, K.; Liu, H.; Ma, Z.-F.; et al. High Entropy Helps Na4Fe3(PO4)2P2O7 Improve Its Sodium Storage Performance. Adv. Funct. Mater. 2024. Early Access. [Google Scholar] [CrossRef]
- Xia, X.; Cao, Y.; Yao, L.; Yang, H.; Zhang, J. MCNT-Reinforced Na3Fe2(PO4)3 as Cathode Material for Sodium-Ion Batteries. Arab. J. Sci. Eng. 2020, 45, 143–151. [Google Scholar] [CrossRef]
- Xiong, F.; Li, J.; Zuo, C.; Zhang, X.; Tan, S.; Jiang, Y.; An, Q.; Chu, P.K.K.; Mai, L. Mg-Doped Na4Fe3(PO4)2(P2O7)/C Composite with Enhanced Intercalation Pseudocapacitance for Ultra-Stable and High-Rate Sodium-Ion Storage. Adv. Funct. Mater. 2023, 33, 2211257. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Zhang, B.; Qin, K.; Liu, H.; Ma, Z.-F. Mn-doped Na4Fe3(PO4)2(P2O7) facilitating Na+ migration at low temperature as a high performance cathode material of sodium ion batteries. J. Power Sources 2022, 521, 230922. [Google Scholar] [CrossRef]
- Wu, H.; Wen, T.; Chen, L.; Ding, Y.; Pu, X.; Cao, Y.; Chen, Z. Understanding the Role of Mn Substitution for Boosting High-Voltage Na4Fe3-xMnx(PO4)2P2O7 Cathode in Sodium-Ion Batteries. Small Methods 2024. Early Access. [Google Scholar] [CrossRef]
- Wu, F.; Ma, H.; Ye, X.; Wu, S. Structural modulation of Na4Fe3(PO4)2P2O7 via cation engineering towards high-rate and long-cycling sodium-ion batteries. J. Colloid Interface Sci. 2025, 679, 132–140. [Google Scholar] [CrossRef]
- You, Y.; Manthiram, A. Progress in High-Voltage Cathode Materials for Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1701785. [Google Scholar] [CrossRef]
- Wu, X.; Zhong, G.; Yang, Y. Sol-gel synthesis of Na4Fe3(PO4)2(P2O7)/C nanocomposite for sodium ion batteries and new insights into microstructural evolution during sodium extraction. J. Power Sources 2016, 327, 666–674. [Google Scholar] [CrossRef]
- Yuan, T.; Wang, Y.; Zhang, J.; Pu, X.; Ai, X.; Chen, Z.; Yang, H.; Cao, Y. 3D graphene decorated Na4Fe3(PO4)2(P2O7) microspheres as low-cost and high-performance cathode materials for sodium-ion batteries. Nano Energy 2019, 56, 160–168. [Google Scholar] [CrossRef]
- Zeng, Y.; Ying, Z.; Du, J.; Cheng, H.-M. Effects of carbon nanotubes on processing stability of polyoxymethylene in melt-mixing process. J. Phys. Chem. C 2007, 111, 13945–13950. [Google Scholar] [CrossRef]
- Pu, X.; Wang, H.; Yuan, T.; Cao, S.; Liu, S.; Xuc, L.; Yang, H.; Ai, X.; Chen, Z.; Cao, Y. Na4Fe3(PO4)2P2O7/C nanospheres as low-cost, high-performance cathode material for sodium-ion batteries. Energy Storage Mater. 2019, 22, 330–336. [Google Scholar] [CrossRef]
- Ge, X.; Li, H.; Li, J.; Guan, C.; Wang, X.; He, L.; Li, S.; Lai, Y.; Zhang, Z. High-Entropy Doping Boosts Ion/Electronic Transport of Na4Fe3(PO4)2(P2O7)/C Cathode for Superior Performance Sodium-Ion Batteries. Small 2023, 19, 2302609. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-R.; Wu, Z.-G.; Xiang, W.; Wang, E.-H.; Wu, C.-J.; Chen, M.-Z.; Guo, X.-D.; Zhong, B.-H. The influences of sodium sources on the structure evolution and electrochemical performances of layered-tunnel hybrid Na0.6MnO2 cathode. Ceram. Int. 2017, 43, 6303–6311. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, X.; Zhou, H.; Wang, Y.; Sun, S.; Yang, C.; Liu, Y. Achieving Fast and Stable Sodium Storage in Na4Fe3(PO4)2(P2O7) via Entropy Engineering. Small 2024, 20, 8681. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Meng, Y.; Xiao, D. Three-Dimensional Holey Graphene Modified Na4Fe3(PO4)2(P2O7)/C as a High-Performance Cathode for Rechargeable Sodium-Ion Batteries. Chem. Eur. J. 2023, 29. [Google Scholar] [CrossRef]
- Shi, K.; Yang, W.; Wu, Q.; Yang, X.; Zhao, R.; She, Z.; Xie, Q.; Ruan, Y. Boosting the fast electrochemical kinetics of Na4Fe3(PO4)2(P2O7) via a 3D graphene network as a cathode material for potassium-ion batteries. New J. Chem. 2023, 47, 10153–10161. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, B.; Zhang, H.; Zheng, Q.; Wu, X.; Yan, Y.; Li, Z.; Tang, Y.; Hao, W.; Liu, G.; et al. Full-Dimensional Analysis of Electrolyte Decomposition on Cathode-Electrolyte Interface: Establishing Characterization Paradigm on LiNi0.6Co0.2Mn0.2O2 Cathode with Potential Dependence. J. Phys. Chem. Lett. 2023, 14, 4565–4574. [Google Scholar] [CrossRef]
- Wang, D.; Du, X.; Chen, G.; Song, F.; Du, J.; Zhao, J.; Ma, Y.; Wang, J.; Du, A.; Cui, Z.; et al. Cathode Electrolyte Interphase (CEI) Endows Mo6S8 with Fast Interfacial Magnesium-Ion Transfer Kinetics. Angew. Chem.-Int. Ed. 2023, 62, e202217709. [Google Scholar] [CrossRef]
- Ye, L.; Liao, M.; Zhao, T.; Sun, H.; Zhao, Y.; Sun, X.; Wang, B.; Peng, H. A Sodiophilic Interphase-Mediated, Dendrite-Free Anode with Ultrahigh Specific Capacity for Sodium-Metal Batteries. Angew. Chem.-Int. Ed. 2019, 58, 17054–17060. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zhang, Z.; Chen, D.; Yu, H.; Wu, Y.; Chen, Y. Spray-Drying Synthesis of Na4Fe3(PO4)2P2O7@CNT Cathode for Ultra-Stable and High-Rate Sodium-Ion Batteries. Molecules 2025, 30, 753. https://doi.org/10.3390/molecules30030753
Huang J, Zhang Z, Chen D, Yu H, Wu Y, Chen Y. Spray-Drying Synthesis of Na4Fe3(PO4)2P2O7@CNT Cathode for Ultra-Stable and High-Rate Sodium-Ion Batteries. Molecules. 2025; 30(3):753. https://doi.org/10.3390/molecules30030753
Chicago/Turabian StyleHuang, Jinri, Ziheng Zhang, Daiqian Chen, Hesheng Yu, Yu Wu, and Yuanfu Chen. 2025. "Spray-Drying Synthesis of Na4Fe3(PO4)2P2O7@CNT Cathode for Ultra-Stable and High-Rate Sodium-Ion Batteries" Molecules 30, no. 3: 753. https://doi.org/10.3390/molecules30030753
APA StyleHuang, J., Zhang, Z., Chen, D., Yu, H., Wu, Y., & Chen, Y. (2025). Spray-Drying Synthesis of Na4Fe3(PO4)2P2O7@CNT Cathode for Ultra-Stable and High-Rate Sodium-Ion Batteries. Molecules, 30(3), 753. https://doi.org/10.3390/molecules30030753






