Abstract
Multifunctional materials bearing photoluminescence, single-molecule magnet (SMM) behavior, and proton conduction have been particularly attractive for various promising applications in optics, molecular spintronics, high-density data storage, and fuel cells. However, these kinds of multifunctional systems have rarely been reported. Herein, a DyIII-SMM together with luminescent and proton-conducting properties, [Dy2(1-tza)4(phen)4]∙(ClO4)2∙(H2O)2 (1, 1-tza = 2-(1H-tetrazol-1-yl)acetic, phen = 1,10-phenanthroline), was prepared and structurally characterized. Complex 1 features a dinuclear structure bridged by carboxylate oxygen atoms of the 1-tza− ligands, and its supramolecular network contains a 1D stacking channel. Complex 1 exhibits strong room-temperature DyIII characteristic emissions and SMM behaviors. In addition, complex 1 shows a moderate proton conductivity with 4.00 × 10−6 S cm−1 at 37 °C and 100% R.H. (R.H. = Relative Humidity), which may be ascribed to the 1D-extended H-bonds in the 1D stacking channel of 1.
1. Introduction
The design and construction of multifunctional coordination complexes (CCs) built by metal ions/clusters and organic ligands have attracted considerable interest because different functions, such as catalysis, sensing, optical, electrical, and magnetic properties, can exist separately or act in synergy, resulting in more efficient, flexible and smart applications [1,2,3,4]. Moreover, CCs provide a powerful platform for achieving multifunctional materials in which a variety of functions can be realized by judicious selection of metal ions and organic ligands as well as by pre-designing structures [5,6]. Within this context, the use of lanthanide ions (Ln) as the metal center is appealing as these trivalent ions possess amazing physical properties owing to their unique 4f electrons. On the one hand, lanthanide ions are known to present exceptional luminescent properties such as long-lived emissions, high quantum yields, and large Stokes shifts, resulting in numerous applications in white-light emission, lasers, sensing, color displays, and luminescent thermometers [7,8,9]. Moreover, Ln-based complexes show a wide range of emission from UV (e.g., Dy3+, Gd3+) and visible (e.g., Eu3+, Tb3+, Sm3+) to the near-infrared region (e.g., Nd3+, Yb3+, Er3+) [10,11,12]. In this field, the use of suitable ligands that can absorb light and transfer energy to LnIII ions (so-called “antenna effect”) to enhance the luminescence of LnIII ions is of importance [13]. On the other hand, the large magnetic moment and substantial magnetic anisotropy of LnIII ions, especially for the DyIII ion, make them very useful for the construction of high-performance single-molecule magnets (SMMs) [14,15]. Such magnets as a class of nanomagnets, which have well-defined magnetic bistability, are promising for applications in high-density data storage, quantum computing, and spintronics [16,17]. Numerous lanthanide-based polynuclear and mononuclear SMMs have been reported [18,19,20]. Previous studies showed that the magnetic anisotropy of LnIII ions mainly originates from spin–orbit coupling and crystal field effects [21,22,23]. For example, the axial crystal field is necessary for a Dy-based SMM, and thus, some high-performance Dy-based complexes displaying high-energy barriers [24] or magnetic blocking temperature (TB) values near liquid nitrogen temperature have been documented [25,26]. However, these Dy organometallic complexes suffer from poor air stability. Therefore, the construction of SMMs with air stability and high performance is highly desired. Moreover, magnetic interactions in SMMs can powerfully suppress the quantum tunneling of magnetization (QTM) to enhance the effective barrier (Ueff) [27]; therefore, the construction of the simple polynuclear Ln2 SMMs is particularly pursued because they can provide a good platform for studying magnetic exchange. Multifunctional SMMs combined with other properties are an emerging research hotspot to deeply understand magnetic behavior in SMMs and realize their applications as soon as possible [28]. A number of bifunctional SMMs, such as electrical-conducting [29,30], chiral [31], and proton-conductive−SMM [32], have been reported. Luminescent SMMs [33,34,35] are of special interest because not only the association between magnetic anisotropy and luminescence observed on the basis of the electronic structure of 4f metal ions [36] but also their potential uses in novel opto-magnetic devices [37,38]. Tri- or more functional SMMs have been explored to expand multifunctionality in this field [39]; however, reasonable design and preparation of more functional SMMs remains challenging.
Proton conducting materials fabricated based on CCs, which have promising applications in fuel cells, smart grids, and information processing devices inspired by biological systems [40,41,42,43], have attracted immense attention because of their unique features, such as low cost, moderate operating temperature, and an enormous structural and chemical variety [44,45]. These crystalline solids can also offer deep insight into the proton transport mechanism, which is conducive to designing and constructing high-performance proton conductors [46,47,48]. Previous studies revealed that the density of H-bonding networks in CCs is one of the key factors for proton transportation. To date, a number of CCs-based water-assisted low-temperature proton conductors with high conductivity have been prepared [49,50,51,52,53,54]; meanwhile, various CPs-based high-performance anhydrous proton conductors have been fabricated by loading guest proton carriers (e.g., imidazole, triazole, histamine) into frameworks at temperatures higher than 100 °C [55,56,57,58]. In addition, the combination of proton conduction and magnetism has attracted much attention because possible synergy may result in a new phenomenon, namely, spinprotonics [59,60]. Although many bifunctional proton-conducting magnets and luminescent magnets have been reported, trifunctional proton-conducting fluorescent magnets are extremely limited because incorporating luminescence and magnetism into porous structures with proton conduction is still challenging. Thus far, only five works on such trifunctional materials have been documented [61,62,63,64,65].
In recent years, our group has focused on investigating the luminescent, electrical, and magnetic properties of coordination complexes [66,67,68]. As part of our continuing research, in this work, the DyIII ion and the 2-(1H-tetrazol-1-yl)acetic acid (1-Htza) ligand, as well as the 1,10-phenanthroline (phen) auxiliary ligand, become our first choice to construct trifunctional luminescent SMMs with proton conduction based on the following reasons: (1) the phen molecule as a N,N’bulky auxiliary ligand can block the DyIII center to form low dimensionality with a nanomagnet behavior; (2) the phen ligand as an aromatic conjugate molecule can sensitize DyIII emission; (3) the 1-Htza ligand possessing a tetrazole ring is an excellent proton acceptor, and has advantages to achieve a proton conductor. Herein, we report a new dinuclear trifunctional complex, namely, [Dy2(1-tza)4(phen)4]∙(ClO4)2∙(H2O)2 (1, 1-tza = 2-(1H-tetrazol-1-yl)acetic, phen = 1,10-phenanthroline), which exhibits emissive, single-molecule magnetic and proton-conductive properties.
2. Experimental Section
2.1. Materials and Instruments
2-(1H-tetrazol-1-yl)acetic acid, 1,10-phenanthroline, Dy(ClO4)3∙6H2O, and CH3CN were employed commercially without further purification. The FT-IR spectra were collected on a Nicolet Magna 750 FT-IR spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) in the region of 4000–400 cm−1 using KBr pellets as bases. Elements analyses of C, H, and N were conducted on an Elementar Vario EL III microanalyzer (Elementar Analysensysteme GmbH, Langenselbold, Germany). PXRD patterns at room temperature were acquired on a Rigaku Miniflex II diffractometer (Rigaku Holdings Corporation, Tokyo, Japan) using Mo-K radiation (λ = 1.540598 Å). Simulated PXRD patterns were measured from the Mercury Version 1.4 software (https://www.ccdc.cam.ac.uk/solutions/software/mercury/ (accessed on 8 August 2024)). TGA measurements have been carried out on polycrystalline samples under a nitrogen atmosphere at a heating rate of 10 °C/min with the temperature ranging from 25 °C to 800 °C by Mettler Toledo thermo gravimetric analyzer (Mettler Toledo, Hongkong, China). Solid-state absorption spectra were collected on Cary 5000 UV-Vis-NIR (Agilent Technologies, Inc., Santa Clara, CA, USA) in the region of 200–800 nm. Photoluminescence analyses were conducted on an Edinburgh FL S920 fluorescence spectrometer (Edinburgh Instruments Ltd., Livingston, UK). Proton conductivity measurements were conducted via a quasi-four-electrode AC impedance technique equipped with a Solartron 1260 impedance/gain-phase analyzer (AMETEK Scientific Instruments, Berwyn, PA, USA). The ground microcrystalline samples were compressed to 0.78 mm in thickness and 2.5 mm in diameter with a pressure of 0.1 GPa. Two sides of the pellet were attached to a couple of gold wires with gold paste. The sample pellets were recorded as the temperatures increased from 25 to 37 °C and/or as the relative humidities (RH) rose from 60% to 100% over a frequency range of 1–107 Hz. The proton conductivity of the samples was deduced from the Debye semicircle by the equation σ = L/(RS), where L (cm) is the thickness, R (Ω) is the resistance, and S (cm2) is the cross-sectional area. Magnetic susceptibilities were collected by means of a Quantum Design PPMS model 6000 magnetometer (Quantum Design, San Diego State, CA, USA), and the diamagnetic contributions of the empty container have been deducted. The experimental susceptibilities were corrected for diamagnetism by using Pascal’s constants.
2.2. Synthesis of [Dy2(1-tza)4(phen)4]∙(ClO4)2∙(H2O)2 (1)
Compound 1 was synthesized by a solvothermal approach. A mixture of Dy(ClO4)3∙6H2O (0.2 mmol, 0.092 g), 2-(1H-tetrazol-1-yl)acetic acid (0.40 mmol, 0.052 g), 1,10-phenanthroline (0.40 mmol, 0.072 g), and CH3CN (10 mL) was sealed into a 25 mL Teflon-lined stainless container under autogenous pressure. The reaction mixture was kept at 180 °C for 3 days and then cooled to room temperature at a rate of 3 °C/h. Colorless column crystals of 1 appropriate for X-ray analyses were prepared. Yield: 38% (based on Dy) for 1. Anal. Calcd (C60H48Cl2Dy2N24O18, %): C, 40.22; H, 2.70; N, 18.77; Found (%): C, 41.08; H, 2.79; N, 18.42. FT-IR peaks (KBr, cm−1) for 1: 3855 w, 3636 w, 3136 w, 1670 vs, 1517 m, 1438 s, 1395 s, 1323 m, 1255 w, 1105 vs, 973 w, 848 s, 803 m, 727 s, 686 m, 627 m, 581 w, 439 w.
2.3. X-Ray Single-Crystal Structure Determination
A single crystal of complex 1, mounted on a Bruker SMART APEX CCD diffractometer (Bruker, Billerica, MA, USA), was collected by adopting graphite-monochromated Mo-K radiation (λ = 0.71073 Å) at 293 K. The diffraction datasets were collected and reduced by CrysAlisPro (version 1.171.38.46) [69]. The structures were solved by a direct method by using SHELX-2014 within Olex2 [70] and refined on F2 with full-matrix least-squares techniques [71]. Hydrogen atoms were added geometrically and refined using a riding model. All non-H atoms were located by different Fourier maps and subjected to anisotropic refinement. No higher space groups for 1 were available, adopting the Platon software 81024 from the IUcr website (http://www.iucr.org/ (accessed on 16 October 2024)). The crystallographic data, selected bond distances, and bond angles are summarized in Tables S1 and S2 in ESI, respectively.
3. Results and Discussion
3.1. Structural Description and Discussion
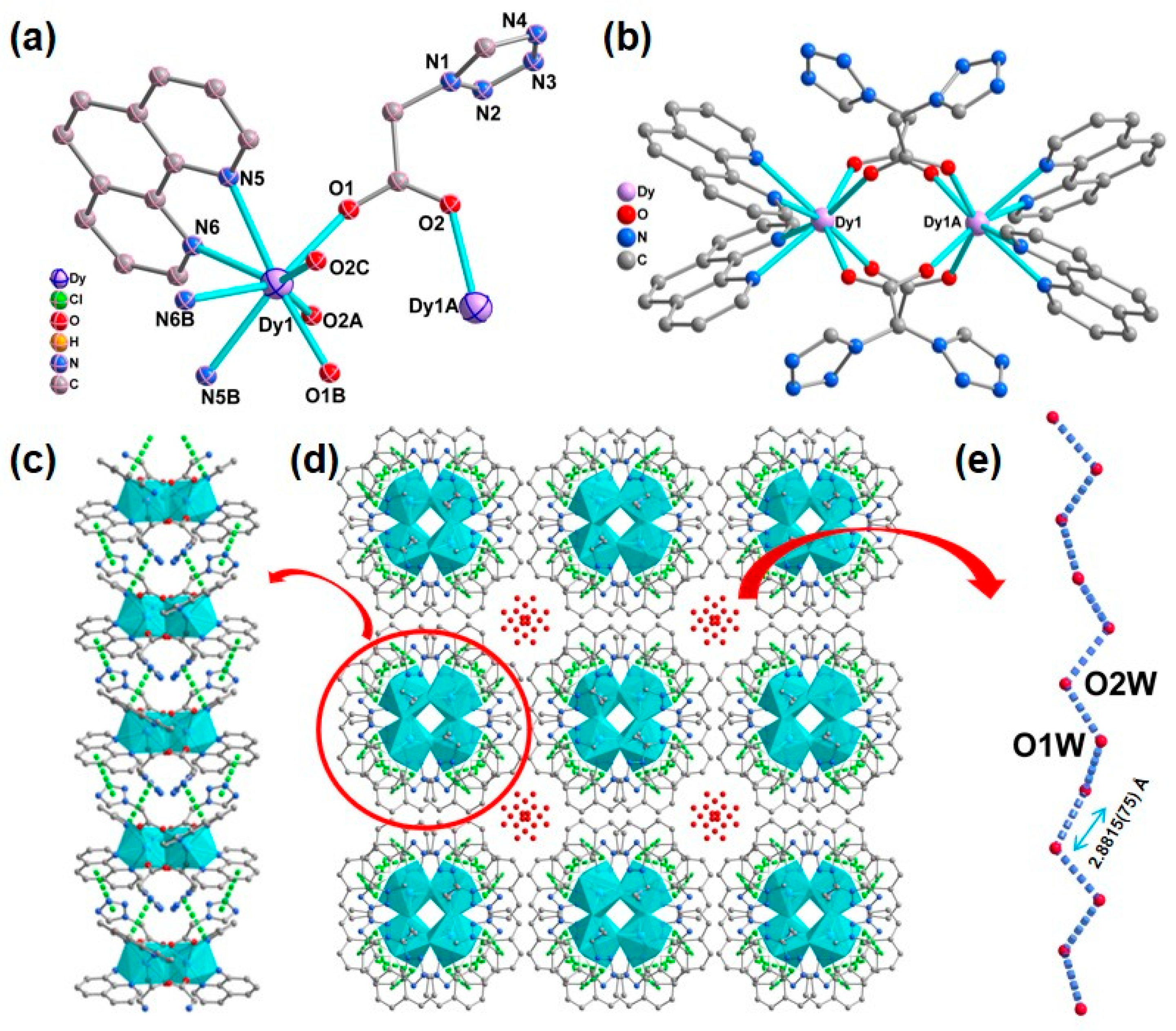
X-ray crystallographic analysis revealed that complex 1 crystallizes in the I41/acd space group with a dinuclear structure; ¼ part of the binuclear molecule of 1 comprises half of DyIII ions, one phen molecule, one 1-tza− ligand, half of the disordered perchlorate anion, and half of disordered lattice water molecule. The DyIII center of 1 is eight-coordinated via four carboxylate oxygen atoms from four different 1-tza− ligands (O1, O1B, O2A, O2C) and four nitrogen atoms from two different chelated phen molecules (N5, N5B, N6, N6B). Using the SHAPE 2.1 program [72], the coordination geometry of the DyIII center can be described as a triangular dodecahedron coordinated geometry with a continuous shape measurement (CShM) value of 0.353 (Figure 1 and Figure S1 and Table S3 in ESI). In 1, the Dy−O/N bond lengths are within 2.2880(19)−2.562(2) Å, which are similar to those Dy−O/N based compounds [73,74]. Two neighboring DyIII cations are bridged via four carboxyl groups from four μ2-η1:η1-based 1-tza− ligands, leading to a lanthanide dimer (Figure 1b). These adjacent dimers are connected via face-to-face π-π stacking interactions through phenyl rings of phen ligands and tetrazole rings of 1-tza− ligands, forming a 1D supramolecular chain with the centroid-to-centroid distances of 3.841(3) and 3.708(3) Å, respectively (Figure 1c). The lattice water molecules fill voids in the supramolecular framework of 1 (Figure 1d). Moreover, as shown in Figure 1d,e, a 1D extended hydrogen bonding chain via water molecules (O1W⋯O2W = 2.8815(75) Å) is formed within the stacking pores of supramolecular network 1. In 1, the ClO4− anions fill in the accessible voids of these 1D supramolecular chains along the ac plane for charge balance (Figure S2 in the ESI).
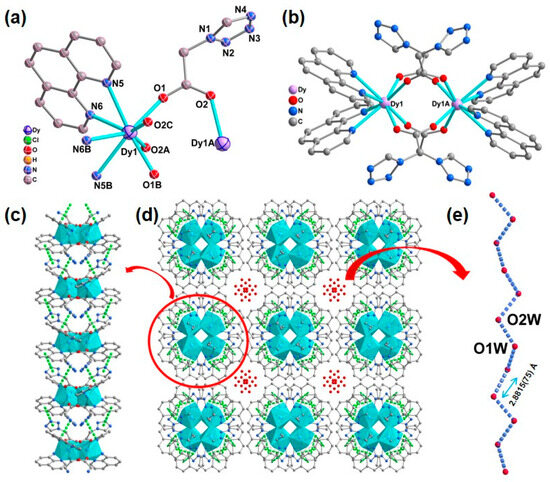
Figure 1.
(a) ¼ part of the binuclear molecule of 1 with 40% thermal ellipsoids. Symmetry codes: A: − x, − y + 1/2, + z; B: − x + 1/4, − y + 1/4, − z + 1/4; C: x +1/4, y − 1/4, − z + 1/4. The H atoms, perchlorate anion, and lattice water molecules are omitted for clarity; (b) Drawing of the dinuclear structure of 1; (c) One-dimensional supramolecular chains constructed by π⋯π interactions (green dashed lines) in 1 along a direction; (d) The stacking supramolecular framework constructed via π⋯π interactions (green dashed lines) in 1 in the ab plane, perchlorate anion are omitted for clarity; (e) The 1D extended hydrogen bonding chain in 1.
3.2. Thermal Stability and UV Spectra
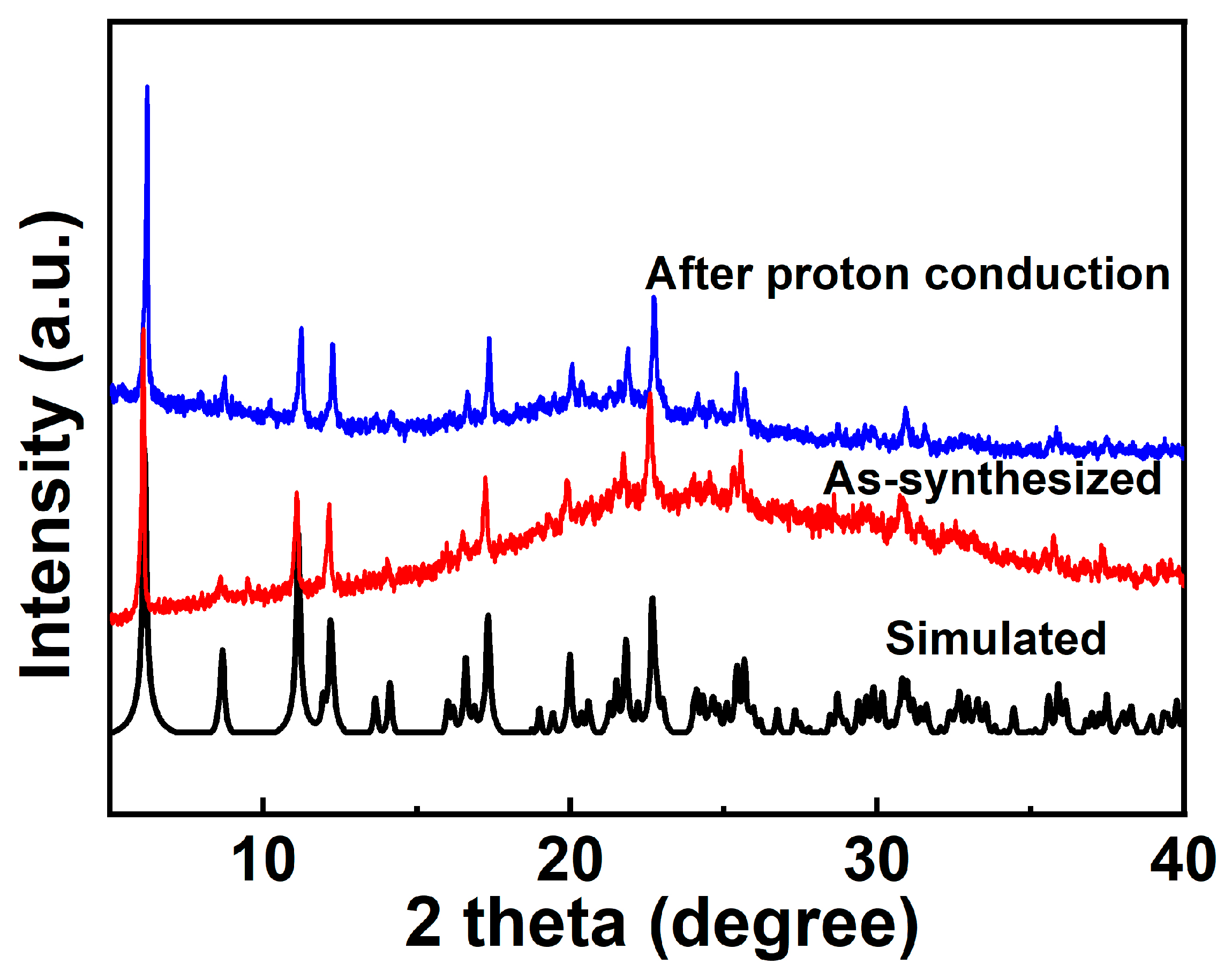
The measured PXRD patterns of the as-synthesized 1 well match the simulated patterns generated from the results of single-crystal diffraction data, indicating the purity of the product (Figure 2). Thermogravimetric analysis (TGA) was carried out to characterize the thermal stability of complex 1. As shown in Figure S3, the TGA curve shows that the weight is reduced by 1.98% within 25–78 °C, which could be attributed to the loss of two lattice waters (calcd. 2.01%). With further increase in the temperature, the framework gradually decomposes, and the weight decreases by 40.85% (calcd. 40.32%) at 78–395 °C, which is assigned to the removal of four 1,10-phenanthroline molecules in 1. Pyrolysis occurs above 480 °C due to the collapse of the network. In the DTA diagram of 1, there are three exothermic peaks near 50 °C, 299 °C, and 341 °C, which correspond to the release of two lattice water molecules and the loss of four phen molecules, respectively. As shown in Figure S4, the absorption spectra of complex 1 was consistent with that of 1,10-phenanthroline (phen).
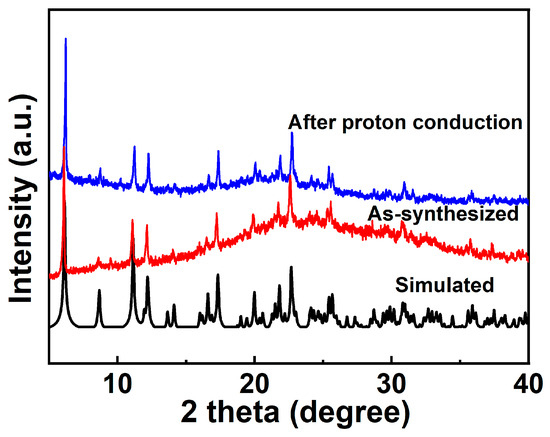
Figure 2.
PXRD of compound 1 after proton conduction.
3.3. Magnetic Properties
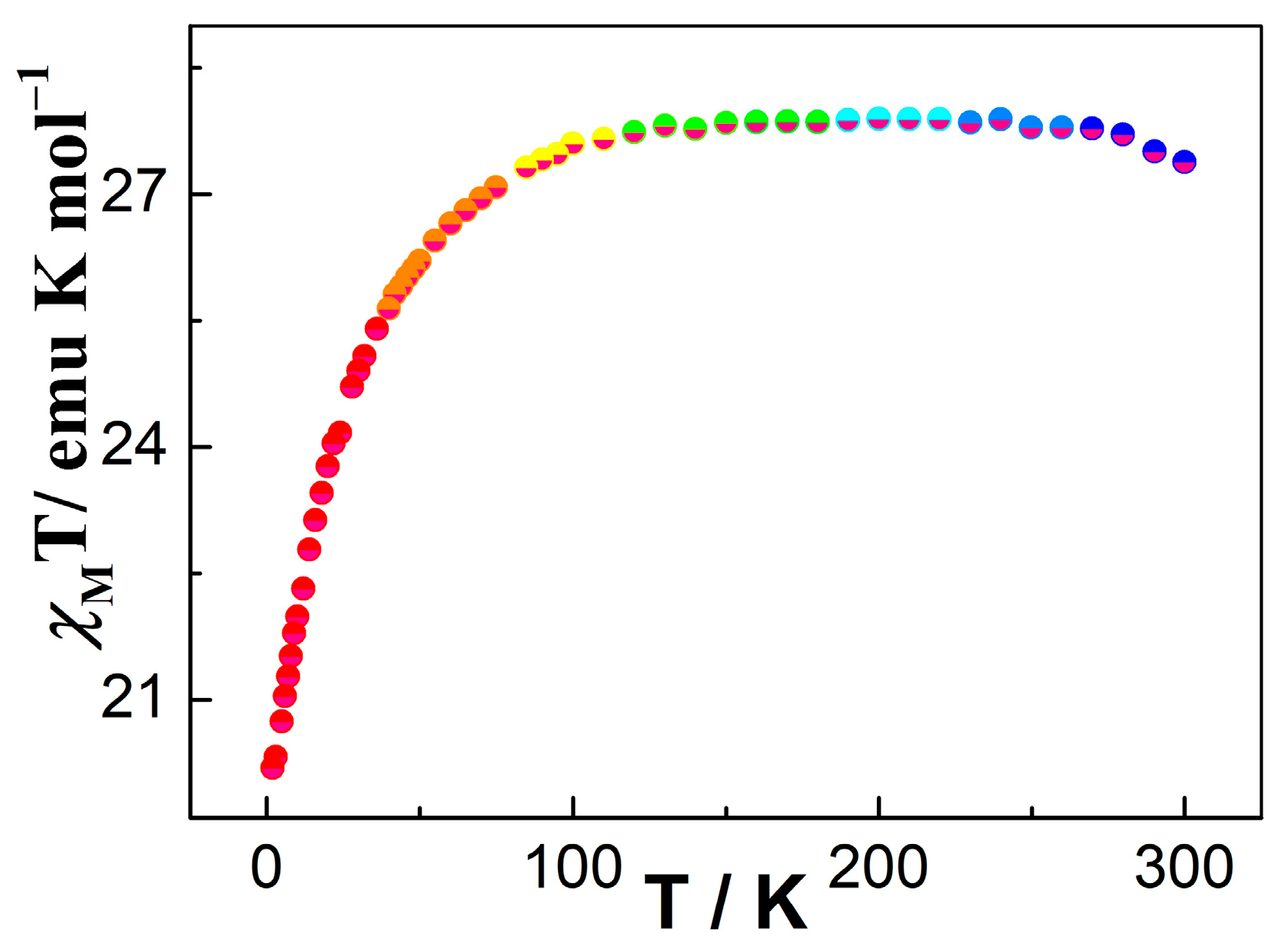
Variable-temperature dc magnetic susceptibility measurements were performed for 1 in the temperature range of 2 to 300 K under an applied magnetic field of 1000 Oe (Figure 3). The χMT value at 280 K for 1 is 27.32 cm3 K mol−1, which is close to two isolated DyIII ions at room temperature (6H15/2, S = 5/2, L = 5, J = 15/2, g = 4/3, C = 28.34 cm3 K mol−1) [75]. In the 300–100 K range, the χMT product reduces smoothly; at below 100 K, the χMT for 1 further decreases to the lowest value of 20.19 cm3 K mol−1 at 2 K. This behavior can be ascribed to possible weak inter-complex antiferromagnetic coupling and/or the thermal depopulation of Stark sublevels of DyIII ion [76]. For complex 1, the magnetization value was investigated within 2–6 K (Figure S5b). The plot of M versus H displays a steady increase with increasing field to reach the values of 10.56 Nβ for 1 at 6 K, which is lower than the theoretical value of 20 Nβ for two DyIII ions (Figure S5a) [77]. Moreover, as shown in Figure S5b, the curve of M vs. H/T displayed a non-superimposable nature, suggesting that significant magnetic anisotropies exist in 1 [78].
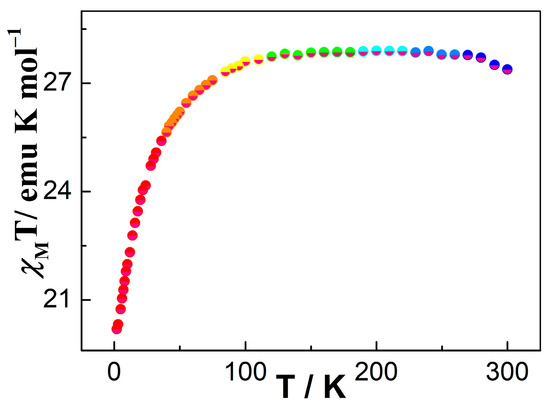
Figure 3.
Temperature dependence of the magnetic susceptibility for complex 1 between 2 and 300 K.
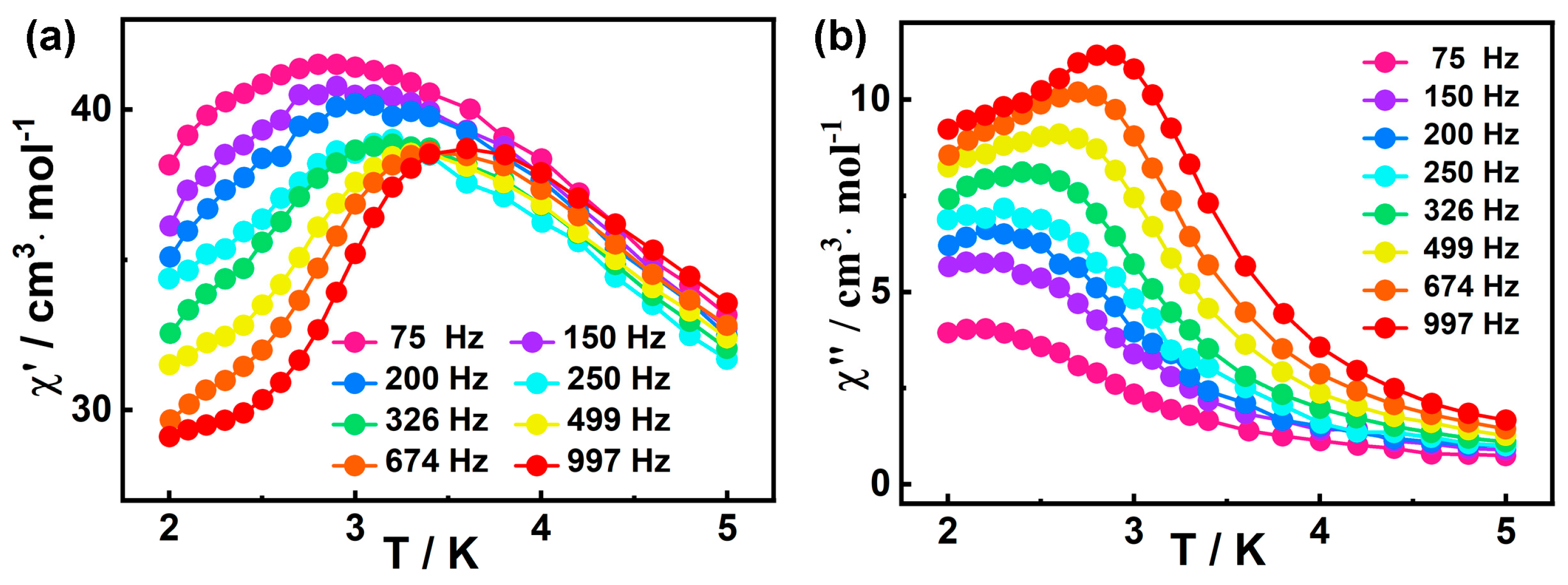
The frequency dependence of ac susceptibilities were also measured to investigate their dynamic magnetic behavior. Based on the field scan results (Figure S6), the optimal field for studying the slow magnetic relaxation of 1 appears to be 1400 Oe. However, the out-of-phase (χ″) peak of ac magnetic susceptibility was not observed at 3 K and Hdc = 1400 Oe (Figure S7), indicating 1400 Oe is not the optimal external magnetic field. Therefore, we tried to choose a common 2000 Oe external field to eliminate QTM. Fortunately, as shown in Figure 4a,b, the out-of-phase (χ″) peaks at all frequencies (10 Hz−1399 Hz) appear under this external magnetic field, so the external magnetic field is appropriate. The in-phase (χ′) and out-of-phase (χ″) signals of 1 show frequency dependencies within 2.0–5.0 K, indicating the SMM behavior of 1. [79].
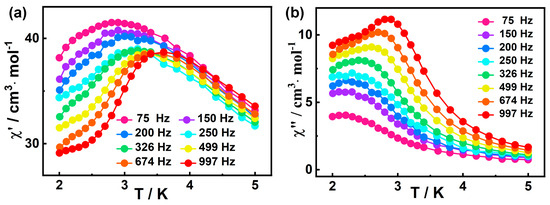
Figure 4.
Frequency-dependent χ′ (a) and χ″ (b) signals for 1 at 2000 Oe dc field.
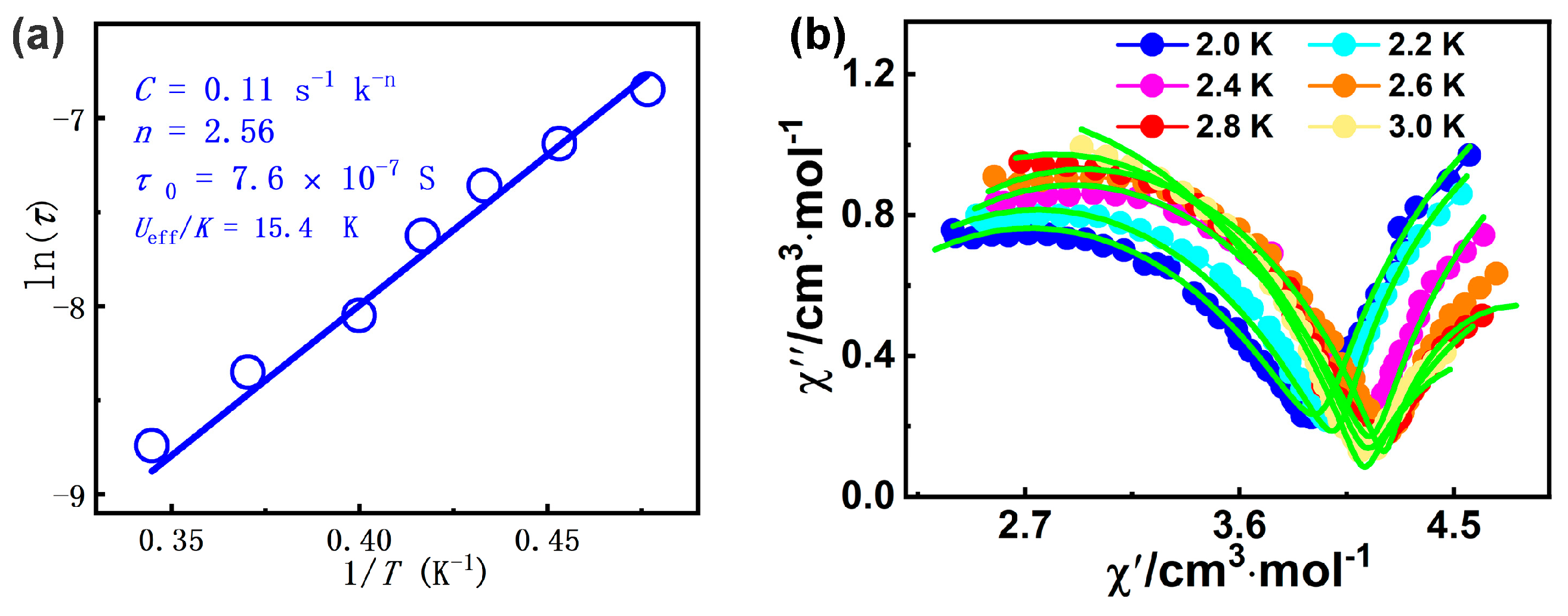
The ln(τ)-1/T curve could be fitted with the equation including Raman and Orbach processes, τ−1 = CTn + τ0−1 exp(−Ueff/kT) [80], giving n = 2.56, C = 0.11 s−1 K−2.56, Ueff/k = 15.4 K, and τ0 = 7.6 × 10−7 s for 1 (Figure 5a). The energy barrier of 1 can be comparable to that of the complex [Dy4(μ4-O)-(HL3)4(H2L3)2]∙3H2O∙EtOH∙CH3CN (H3L3 = 3-(((2-hydroxynaphthaen-1-yl)methylene)amino)-propane-1,2-diol, Ueff = 2.6 K) [81]. The obtained τ0 value falls in the characteristic range for SMMs (10−5 to 10−11 s) [61]. Furthermore, the Cole–Cole diagram (χ′M vs. χ′′M) of complex 1 between 2.0 and 3.0 K exhibits dual magnetic relaxation characteristics (Figure 5b), which the left and the right semi-circular curves correspond to the fast relaxation (FR) phase and the slow relaxation (SR) phase, respectively. By using CC–FIT2 software (https://www.nfchilton.com/ (accessed on 5 October 2024)), Table S4 summarizes the least-squares fitting parameters. The α1 and α2 values are not small, and the α1 value (0.203–0.326) is slightly larger than the α2 values (0.000–0.274); the results show that the SR phase has a relatively narrow distribution of relaxation time with respect to the FR phase.
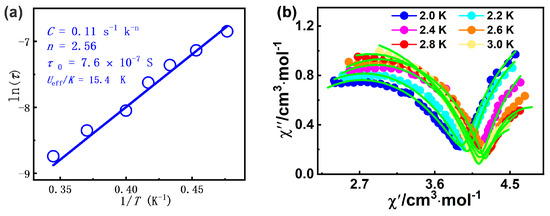
Figure 5.
(a) The ln τ vs. 1/T graph for 1; (b) Cole-Cole plot for 1 under 2000 Oe dc field (solid lines: fitting results).
3.4. Proton Conduction
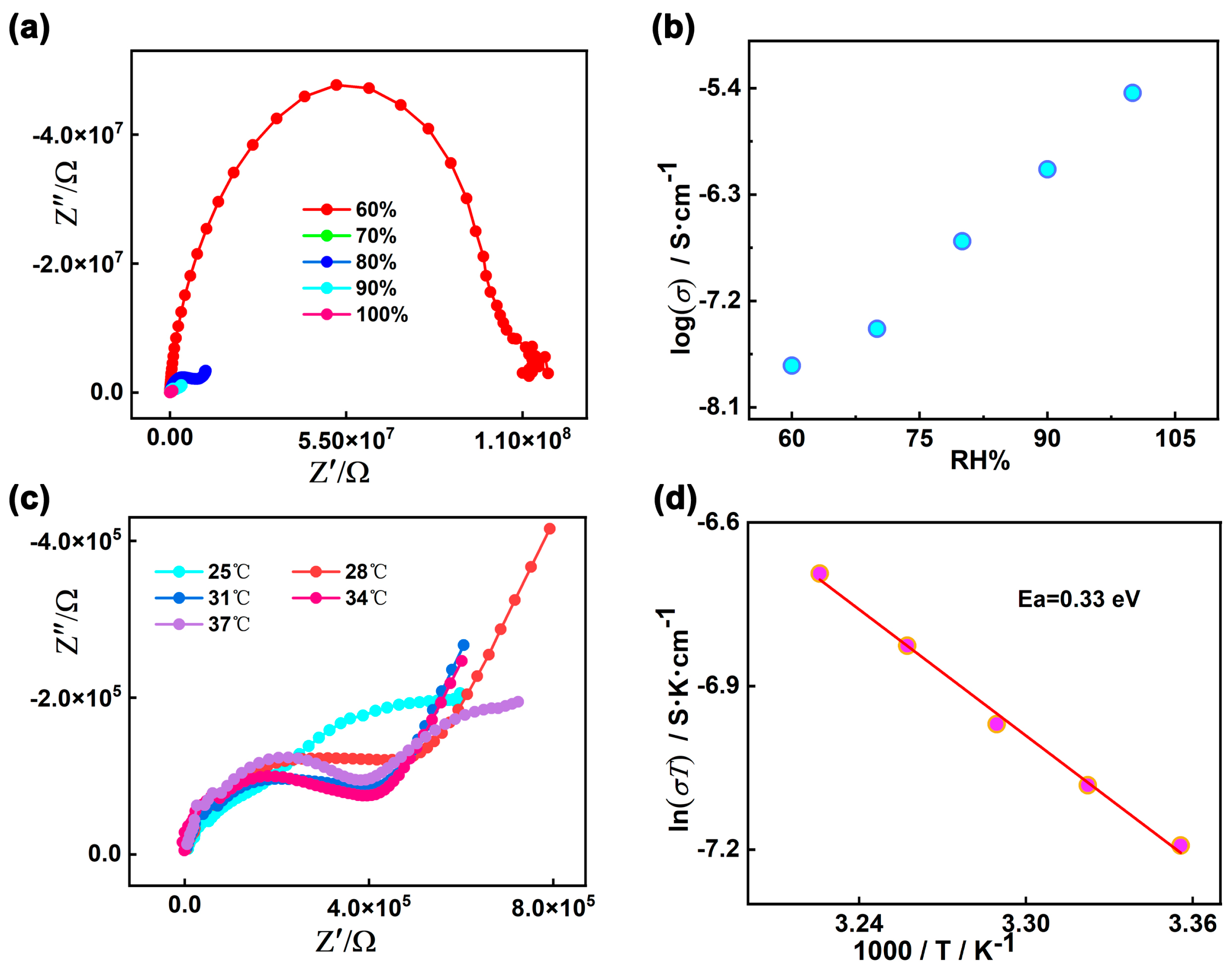
The proton conductivities of 1 were carried out using Alternating-current impedance measurement at different RH levels (60−100%) and varied temperatures (25–37 °C). The semicircles in the high-frequency region and inclined tails in the low-frequency range in the Nyquist plots are displayed in Figure 6a,c, indicating that 1 is a typical proton conductive behavior [82,83,84]. At the constant temperature of 35 °C, the diameters of the semicircles notably decreased with increasing in RH from 60% to 100%, suggesting the proton conductivity values (σ) are closely dependent on humidity (Figure 6b and Figure S8). As demonstrated in Figure S9 and Table S5, the conductivity (σ) of complex 1 increases by two orders of magnitude with the σ from 1.79 × 10−8 S cm−1 at 60% RH to 3.63 × 10−6 S cm−1 at 100% RH under 35 °C. The behavior is similar with other many water-mediated proton-conductive CPs [85,86]. The temperature-dependent proton conductivitywas investigated under different temperatures and fixed humidity. Under 100% RH, the conductivity (σ) of complex 1 slowly increased from 2.52 × 10−6 S cm−1 at 25 °C to reach a maximum value of 4.00 × 10−6 S cm−1 at 37 °C (Figure 6c, Figures S10 and S11, and Table S6). The optimal proton conductivity for complex 1 is comparable to those of previously reported CPs-based proton conductor, for instance, UiO-66-NH2 (3.5 × 10−6 S cm−1 at 80 °C and 98% RH) [87], [Zn3(ssa)2(1,4-bib)3∙4H2O]n (H3ssa = 5-sulfosalicylic acid; 1,4-bib =1,4-bis (1H-imidazol-1-yl)benzene], 6.26 × 10−6 S cm−1, At 60 °C and 95% RH) [88], 5,10,15, 20-tetrakis[p-phenylphosphonic acid] (GTUB5, 3.00 × 10−6 S cm−1 at 75 °C and 75% RH) [89]. To further explore the proton migration mechanism of 1, Ea was calculated by fitting to the Arrhenius equation: σT = A exp(−Ea/kBT), where Ea, σ0, kB and T are the activation energy for conduction, pre-exponential factor, Boltzmann’s constant and the absolute temperature, respectively. The previous reports showed thatthe Grotthuss mechanism (usually Ea < 0.4 eV) involves the proton transport along the hydrogen bonding network [90,91] and the vehicle mechanism (usually Ea > 0.5 eV) is governed by the migration of protons through the assistance of a moveable species (e.g., NH3, H2O) [92,93,94]. The Ea value of 1 is 0.33 eV, indicating that the proton transfer of 1 is mediated via the Grotthuss mechanism (Figure 6d). The proton conduction is related to the extended 1D H-bonds formed by the lattice water molecules within the pores of supramolecular 1. Furthermore, the structural stability of 1 was confirmed by the PXRD spectra before and after the conductivity measurements, and there are no obvious changes and no fade on the crystal surface (Figure 2 and Figure S12).
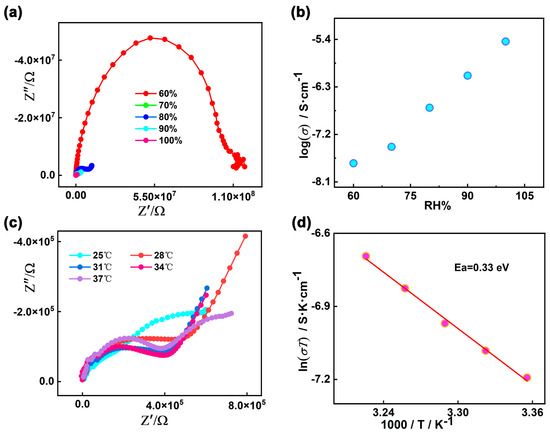
Figure 6.
(a) Nyquist plot for 1 at 35 °C under different RH levels; (b) Plot of proton conductivity for 1 vs. RH at 35 °C; (c) Nyquist plot for 1 at different temperatures under 100% RH; (d) Plots of ln(σT) vs. 1000/T for 1 under 100% RH.
3.5. Photoluminescent Properties
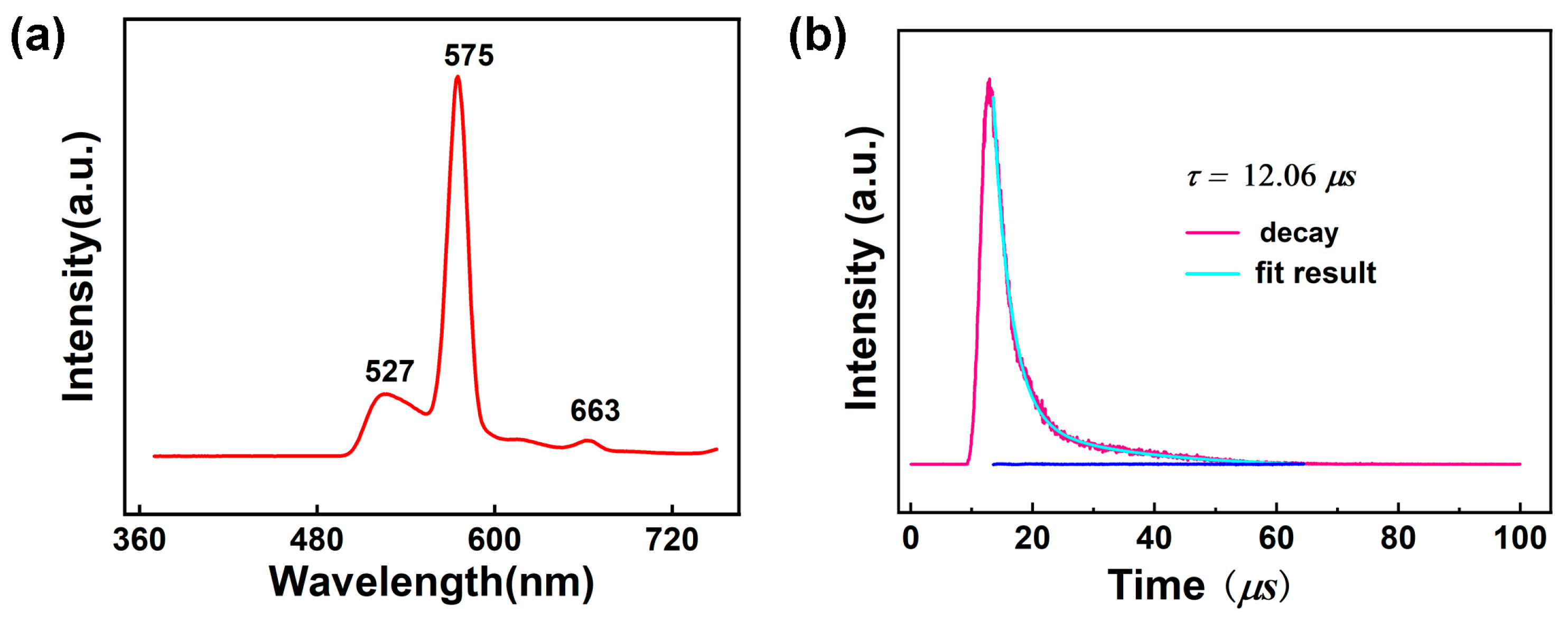
Lanthanide complexes display intriguing photoluminescent properties originating from unique 4f electrons of these trivalent cations [95,96]. Consequently, luminescent Ln-based complexes have gained extensive attention because of their wide applications in sensors, biolabeling, solid-state lasers, light-emitting diodes, optical switches, and so on [97,98]. The free 1-Htza ligand shows photoluminescent emission bands at 423 nm upon photoexcitation at 278 nm, which can be assigned to the ligand-centered π-π* transitions (Figure S13). The solid-state luminescence spectrum of 1 was tested at room temperature to explore the luminescence behavior of 1. As illustrated in Figure 7a and Figure S14, upon the excitation at 350 nm, 1 displays an emission spectrum containing three peaks at 527 nm, 573, and 662 nm. The round peak of 527 nm may be ascribed to the emission of the 1-tza– ligand because the free 1-tza– ligand shows emission bands at 525 nm upon photoexcitation at 350 nm (Figure S15). The peaks of 575 and 663 nm are attributed to 4F9/2→6H13/2 and 4F9/2→6H11/2 transitions of the DyIII ion, respectively. According to the literature [99], the singlet energy level (1ππ*) and the lowest triplet energy level of phen ligand (3ππ*) are 31,000 cm−1 and 22,100 cm−1, respectively. The energy difference (∆E = 3ππ*–5D4) between the lowest triplet level of phen and the resonant energy level of the DyIII ions was calculated to be 1397 cm−1, indicating that the value matches well with the 4f levels DyIII ions. Thus, the 1,10-phen can sensitize DyIII emission effectively by “antenna effect”. The luminescence lifetime of 1 was analyzed using the strongest emission (573 nm) and excitation (350 nm) within the visible region by fitting with the double-exponential function: I = A + B1exp(−t/τ1) + B2exp(−t/τ2) (τ1 and τ2 represent the fast and slow components of the luminescent lifetimes, and A, B1, and B2 are the fitting parameters) [100] (Figure 7b), and the average lifetime value is around 12.06 μs for 1 based on τ = (B1τ12 + B2τ22)/(B1τ1 + B2τ2) (τ1 = 2915.22 ns, τ2 = 20,721.68 ns, B1 = 1702.45 and B2 = 252.79) [101], which can be comparable to those reported for DyIII complexes [102]. In addition, the luminescence quantum yield of 1 was measured but is extremely low and almost negligible, which is consistent with the reported Dy-based complex {[Ln(μ4-pmdc)(NO3)(H2O)]∙H2O}n (pmdc = pyrimidine-4,6-dicarboxylic acid) [103].
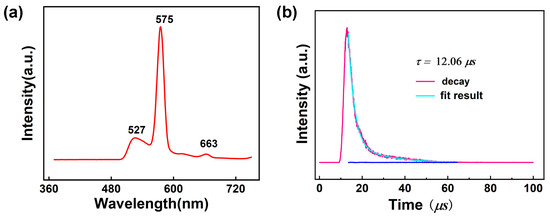
Figure 7.
(a) Solid-state emission spectra for 1 at room temperature; (b) Decay curves of 1.
4. Conclusions
In summary, a new multifunctional DyIII complex with a dinuclear structure was successfully obtained by using simple ligands with 2-(1H-tetrazol-1-yl)acetate ligand and 1,10-phenanthroline auxiliary ligand. The molecular materials exhibit trifunctionalities with DyIII characteristic emissions and a field-induced SMM behavior, together with a moderate proton conductivity related to the 1D extended H-bonds constructed by the lattice water molecules. This work provides new insights into the design of multifunctional materials that simultaneously exhibit photoluminescence, SMM, and proton conduction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30051086/s1. Table S1. Crystal data for 1. Table S2. Selected Bond Lengths (Å) and Bond Angles (o) for 1. Table S3. Summary of SHAPE analysis of Dy1 in 1. Figure S1. The triangular dodecahedron (TDD-8) coordination geometry of DyIII in 1. Figure S2. The 3D network of 1 in which the perchloric acid filled in one channel along c axes (H at oms are omitted for clarity). Figure S3. TGA of 1 from 25 °C to 800 °C. Figure S4. Solid-state absorption spectra of complex 1, and phen and 1-Htza ligands. Figure S5. (a) M versus H plot of 1 at 2 K; (b) Experimental M versus H/T plots of 1 Figure S6 The in-phase (χ′) and out-of-phase (χ″) components of ac magnetic susceptibility under variable dc fields for 1 at 3 K and frequency with 1399 Hz. Figure S7. ac susceptibility measurements at frequency with 1399 Hz for 1 at 3 K and Hdc = 1400 Oe. Table S4. Linear combination of two modified debye model fitting parameters from 3 to 7 K at Hdc = 2000 Oe. Figure S8. Nyquist plot for 1 Aat 35 °C under (a) 60%, (b) 70%, (c) 80%, (d) 90%, (e) 100% RH. Figure S9. The best-fit result of Nyquist plot for 1 at 35 °C under different RH levels. Table S5. The proton conductivity of 1 at 35 °C under variable relative humidity (RH). Figure S10. Nyquist plot for 1 at (a)25 °C, (b) 28 °C, (c) 31 °C, (d) 34 °C and (e) 37 °C under 100% RH. Figure S11. The best-fit result of Nyquist plot for 1 at different temperatures under 100% RH. Table S6. The proton conductivity of 1 at 100% RH under variable temperature. Figure S12. The photograph of crystals of complex 1 after exposed to 25−37 °C and 60−100% RH conditions during the whole proton conductivity measurements. Figure S13. Solid-state excited (a) and emission (b) spectra free Htza ligand. Figure S14. Solid-state excitation spectra for 1 at room temperature. Figure S15. Soild-state emission spectra of the free 1-Htza ligand at room-temperature. Figure S16. IR spectra for 1.
Author Contributions
Conceptualization, Y.L. (Yingbing Lu) and Y.L. (Yu Lei); methodology, H.W., S.Z. and C.L.; validation, S.L. and C.L.; investigation, D.C., L.L., Y.L. (Yu Lei) and X.H.; writing—original draft preparation, S.Z.; writing—review and editing, Y.L. (Yingbing Lu) and C.L.; project administration, S.Z. and Y.L. (Yingbing Lu); funding acquisition, Y.L. (Yingbing Lu) and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
The NSF of China: 21871274, 22161002 and 22461001;the NSF of Jiangxi Provincial Education Department: GJJ2201223.
Data Availability Statement
X-ray crystallographic data file in CIF format, PXRD, IR spectra, selected bond distances, and angles for 1. The TGA plot of 1. Solid-state fluorescence excited spectra of 1. Magnetic data of 1. The proton conductivity of 1 under different RHs and temperatures. Solid-state fluorescence emission spectra of the free 1-Htza ligand. CCDC number: 2190704 for 1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Surbella, R.G.; Reilly, D.D.; Sinnwell, M.A.; McNamara, B.K.; Sweet, L.E.; Schwantes, J.M.; Thallapally, P.K. Multifunctional Two-Dimensional Metal-Organic Frameworks for Radionuclide Sequestration and Detection. ACS Appl. Mater. Interfaces 2021, 13, 45696–45707. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.P.; Subudhi, S.; Parida, K. Inter-MOF hybrid (IMOFH): A concise analysis on emerging core-shell based hierarchical and multifunctional nanoporous materials. Coord. Chem. Rev. 2021, 434, 213786. [Google Scholar] [CrossRef]
- Sherman, D.A.; Murase, R.; Duyker, S.G.; Gu, Q.Y.; Lewis, W.; Lu, T.; Liu, Y.; D’Alessandro, D.M. Reversible single crystal-to-single crystal double [2+2] cycloaddition induces multifunctional photo-mechano-electrochemical properties in framework materials. Nat. Commun. 2020, 11, 2808. [Google Scholar] [CrossRef] [PubMed]
- Lysova, A.A.; Samsonenko, D.G.; Dorovatovskii, P.V.; Lazarenko, V.A.; Khrustalev, V.N.; Kovalenko, K.A.; Dybtsev, D.N.; Fedin, V.P. Tuning the Molecular and Cationic Affinity in a Series of Multifunctional Metal-Organic Frameworks Based on Dodecanuclear Zn(II) Carboxylate Wheels. J. Am. Chem. Soc. 2019, 141, 17260–17269. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Chen, Z.J.; Dong, J.Q.; Liu, Y.; Cui, Y. Chiral Metal-Organic Frameworks. Chem. Rev. 2022, 122, 9078–9144. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.L.; Xu, Q. Metal-organic framework composites, Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.Y.; Yu, Q.; Wei, H.J.; Liu, S.J.; Zhao, Q.; Huang, W. Long-Lived Emissive Probes for Time-Resolved Photoluminescence Bioimaging and Biosensing. Chem. Rev. 2018, 118, 1770–1839. [Google Scholar] [CrossRef] [PubMed]
- Galico, D.A.; Kitos, A.; Ovens, J.S.; Sigoli, F.A.; Murugesu, M. Lanthanide-Based Molecular Cluster-Aggregates: Optical Barcoding and White-Light Emission with Nanosized Ln20 Compounds. Angew. Chem. Int. Ed. 2021, 60, 6130–6136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.B.; Muhtadi, A.; Iwahara, N.; Ungur, L.; Chibotaru, L.F. Magnetic anisotropy in divalent lanthanide compounds. Angew. Chem. Int. Ed. 2020, 59, 12720–12724. [Google Scholar] [CrossRef]
- Su, J.; Xu, N.; Murase, R.; Yang, Z.M.; D’Alessandro, D.M.; Zuo, J.L.; Zhu, J. Persistent Radical Tetrathiafulvalene-Based 2D Metal-Organic Frameworks and Their Application in Efficient Photothermal Conversion. Angew. Chem. Int. Ed. 2021, 60, 4789–4795. [Google Scholar] [CrossRef]
- Kovacs, D.; Lu, X.L.; Meszaros, S.M.; Ott, M.; Andres, J.; Borbas, K.E. Photophysics of Coumarin and Carbostyril-Sensitized Luminescent Lanthanide Complexes: Implications for Complex Design in Multiplex Detection. J. Am. Chem. Soc. 2017, 139, 5756–5767. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.K.; Li, F.; Wu, Y.N.; Wang, H.; Gu, L.T.; Zhang, J.Y.; Qi, Y.K.; Meng, L.K.; Kong, N.; Chai, Y.J.; et al. Lanthanide luminescence nanothermometer with working wavelength beyond 1500 nm for cerebrovascular temperature imaging in vivo. Nat. Commun. 2024, 15, 2341. [Google Scholar] [CrossRef]
- Luo, T.Y.; Das, P.; White, D.L.; Liu, C.A.; Star and Rosi, N.L. Luminescence “Turn-On” Detection of Gossypol Using Ln3+-Based Metal-Organic Frameworks and Ln3+ Salts. J. Am. Chem. Soc. 2020, 142, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Harriman, K.L.M.; Brosmer, J.L.; Ungur, L.; Diaconescu, P.L.; Murugesu, M. Pursuit of Record Breaking Energy Barriers: A Study of Magnetic Axiality in Diamide Ligated DyIII Single-Molecule Magnets. J. Am. Chem. Soc. 2017, 139, 1420–1423. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Xie, J.; Kong, X.J.; Long, L.S.; Zheng, L.S. Recent advances in the assembly of high-nuclearity lanthanide clusters. Coord. Chem. Rev. 2019, 378, 222–236. [Google Scholar] [CrossRef]
- Da Cunha, T.T.; Jung, J.M.; Boulon, E.; Campo, G.; Pointillart, F.; Pereira, C.L.M.; Le Guennic, B.; Cador, O.; Bernot, K.; Pineider, F.; et al. Magnetic Poles Determinations and Robustness of Memory Effect upon Solubilization in a DyIII-Based Single Ion Magnet. J. Am. Chem. Soc. 2013, 135, 16332–16335. [Google Scholar] [CrossRef]
- Wang, H.L.; Wang, B.W.; Bian, Y.Z.; Gao, S.; Jiang, J.Z. Single-molecule magnetism of tetrapyrrole lanthanide compounds with sandwich multiple-decker structures. Coord. Chem. Rev. 2016, 306, 195–216. [Google Scholar] [CrossRef]
- Thomas-Hargreaves, L.R.; Hunger, D.; Kern, M.; Wooles, A.J.; van Slageren, J.; Chilton, N.F.; Liddle, S.T. Insights into D4h@metal-symmetry single-molecule magnetism: The case of a dysprosium-bis(boryloxide) complex. Chem. Commun. 2021, 57, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Latendresse, T.P.; Bhuvanesh, N.S.; Nippe, M. Hard Single-Molecule Magnet Behavior by a Linear Trinuclear Lanthanide-[1]Metallocenophane Complex. J. Am. Chem. Soc. 2017, 139, 14877–14880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Perfetti, M.; Kern, M.; Hallmen, P.P.; Ungur, L.; Lenz, S.; Ringenberg, M.R.; Frey, W.; Stoll, H.; Rauhut, G.; et al. Exchange coupling and single molecule magnetism in redox-active tetraoxolene-bridged dilanthanide complexes. Chem. Sci. 2018, 9, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Hilgar, J.D.; Bernbeck, M.G.; Rinehart, J.D. Million-fold Relaxation Time Enhancement across a Series of Phosphino-Supported Erbium Single-Molecule Magnets. J. Am. Chem. Soc. 2019, 141, 1913–1917. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.H.; Guo, M.; Li, X.Y.; Tang, J.K. Molecular magnetism of lanthanide: Advances and perspectives. Coord. Chem. Rev. 2019, 378, 350–364. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Ariciu, A.M.; McAdams, S.; Weihe, H.; Bendix, J.; Tuna, F.; Piligkos, S. Toward Molecular 4f Single-Ion Magnet Qubits. J. Am. Chem. Soc. 2016, 138, 5801–5804. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.C.; Liu, J.L.; Vieru, V.; Ungur, L.; Jia, J.H.; Chibotaru, L.F.; Lan, Y.H.; Wernsdorfer, W.; Gao, S.; et al. A Stable Pentagonal Bipyramidal Dy(III) Single-Ion Magnet with a Record Magnetization Reversal Barrier over 1000 K. J. Am. Chem. Soc. 2016, 138, 5441–5450. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.S.; Day, B.M.; Chen, Y.C.; Tong, M.L.; Mansikkamaki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Guo, Y.N.; Tang, J.K. Recent advances in dysprosium-based single molecule magnets: Structural overview and synthetic strategies. Coord. Chem. Rev. 2013, 257, 1728–1763. [Google Scholar] [CrossRef]
- Marin, R.; Brunet, G.; Murugesu, M. Shining new light on multifunctional lanthanide single-molecule magnets. Angew. Chem. Int. Ed. 2021, 60, 1728–1746. [Google Scholar] [CrossRef]
- Sato, T.; Breedlove, B.K.; Yamashita, M.; Katoh, K. Electro-Conductive Single-Molecule Magnet Composed of a Dysprosium(III)-Phthalocyaninato Double-Decker Complex with Magnetoresistance. Angew. Chem. Int. Ed. 2021, 60, 21179–21183. [Google Scholar] [CrossRef]
- Shen, Y.B.; Cosquer, G.; Zhang, H.T.; Breedlove, B.K.; Cui, M.X.; Yamashita, M. 4f-π Molecular Hybrid Exhibiting Rich Conductive Phases and Slow Relaxation of Magnetization. J. Am. Chem. Soc. 2021, 143, 9543–9550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.H.; Zhao, C.; Feng, T.T.; Liu, X.D.; Ying, X.; Li, X.L.; Zhang, Y.Q.; Tang, J.K. Air-Stable Chiral Single-Molecule Magnets with Record Anisotropy Barrier Exceeding 1800 K. J. Am. Chem. Soc. 2021, 143, 10077–10082. [Google Scholar] [CrossRef]
- Qin, L.; Yu, Y.Z.; Liao, P.Q.; Xue, W.; Zheng, Z.P.; Chen, X.M.; Zheng, Y.Z. A “Molecular Water Pipe”: A Giant Tubular Cluster {Dy72} Exhibits Fast Proton Transport and Slow Magnetic Relaxation. Adv. Mater. 2016, 28, 10772–10779. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Guari, Y.; Ferreira, R.A.S.; Carlos, L.D.; Larionova, J. Recent advances in luminescent lanthanide based Single-Molecule Magnets. Coord. Chem. Rev. 2018, 363, 57–79. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, J.H.; Zychowicz, M.; Zakrzewski, J.J.; Nakabayashi, K.; Sieklucka, B.; Chorazy, S.; Ohkoshi, S. Dehydration–Hydration Switching of Single-Molecule Magnet Behavior and Visible Photoluminescence in a Cyanido-Bridged DyIIICoIII Framework. J. Am. Chem. Soc. 2019, 141, 18211–18220. [Google Scholar] [CrossRef]
- Corredoira-Vazquez, J.; Gonzalez-Barreira, C.; Garcia-Deibe, A.M.; Sanmartin-Matalobos, J.; Hernandez-Rodriguez, M.A.; Brites, C.D.S.; Carlos, L.D.; Fondo, M. Harnessing ligand design to develop primary and self-calibrated luminescent thermometers with field-induced single ion magnet behaviour in Dy3+ complexes. Inorg. Chem. Front. 2024, 11, 1087–1098. [Google Scholar] [CrossRef]
- Long, J.; Rouquette, J.; Thibaud, J.M.; Ferreira, R.A.S.; Carlos, L.D.; Donnadieu, B.; Vieru, V.; Chibotaru, L.F.; Konczewicz, L.; Haines, J.; et al. A High-Temperature Molecular Ferroelectric Zn/Dy Complex Exhibiting Single-Ion-Magnet Behavior and Lanthanide Luminescence. Angew. Chem. Int. Ed. 2015, 54, 2236–2240. [Google Scholar] [CrossRef]
- Brunet, G.; Marin, R.; Monk, M.J.; Resch-Genger, U.; Gálico, D.A.; Sigoli, F.A.; Suturina, E.A.; Hemmer, E.; Murugesu, M. Exploring the dual functionality of an ytterbium complex for luminescence thermometry and slow magnetic relaxation. Chem. Sci. 2019, 10, 6799–6808. [Google Scholar] [CrossRef]
- Errulat, D.R.; Marin, D.A.; Gálico, K.L.M.; Harriman, A.; Pialat, B.; Gabidullin, F.; Iikawa, O.D.D., Jr.; Couto, J.O.; Moilanen, E.; Hemmer, F.; et al. A luminescent thermometer exhibiting slow relaxation of the magnetization: Toward selfmonitored building blocks for next-generation optomagnetic devices. ACS Cent. Sci. 2019, 5, 1187–1198. [Google Scholar] [CrossRef]
- Guo, P.H.; Meng, Y.; Chen, Y.C.; Li, Q.W.; Wang, B.Y.; Leng, J.D.; Bao, D.H.; Jia, J.H.; Tong, M.L. A zigzag DyIII4 cluster exhibiting single-molecule magnet, ferroelectric and white-light emitting properties. J. Mater. Chem. C 2014, 42, 8858–8864. [Google Scholar] [CrossRef]
- Lim, D.W.; Kitagawa, H. Proton Transport in Metal–Organic Frameworks. Chem. Rev. 2020, 120, 8416–8467. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.Z.; Pan, L.; Liu, L.Z.; Zhang, J.D.; Lin, Q.J.; Ye, Y.X.; Zhang, Z.J.; Xiang, S.C.; Chen, B.L. Simultaneous implementation of resistive switching and rectifying effects in a metal-organic framework with switched hydrogen bond pathway. Sci. Adv. 2019, 5, eaaw4515. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; He, W.; Li, X.M.; Sun, L.; Wang, H.Y.; Lan, Y.Q.; Ding, M.; Zuo, J.L. High Electrical Conductivity in a 2D MOF with Intrinsic Superprotonic Conduction and Interfacial Pseudo-capacitance. Matter 2020, 2, 711–722. [Google Scholar] [CrossRef]
- Shinde, D.B.; Aiyappa, H.B.; Bhadra, M.; Biswal, B.P.; Wadge, P.; Kandambeth, S.; Garai, B.; Kundu, T.; Kurungot, S.; Banerjee, R. A mechanochemically synthesized covalent organic framework as a proton-conducting solid electrolyte. J. Mater Chem. A. 2016, 4, 2682–2690. [Google Scholar] [CrossRef]
- Liu, S.S.; Liu, Q.Q.; Huang, S.Z.; Zhang, C.; Dong, X.Y.; Zang, S.Q. Sulfonic and phosphonic porous solids as proton conductors. Coord. Chem. Rev. 2022, 451, 214241. [Google Scholar] [CrossRef]
- Ma, X.; Wang, S.Y.; Fan, Q.H.; Wang, P.; Wang, L.; Luo, Y.J.; Du, L.; Zhao, Q.H.A. Highly Stable Multifunctional Bi-Based MOF for Rapid Visual Detection of S2- and H2S Gas with High Proton Conductivity. ACS Appl. Mater. Interfaces 2024, 16, 33865–33876. [Google Scholar] [CrossRef]
- Lee, H.K.; Oruganti, Y.; Lee, J.; Han, S.; Kim, J.; Moon, D.; Kim, M.; Lim, D.W.; Moon, H.R. Moisture-triggered proton conductivity switching in metal-organic frameworks: Role of coordinating solvents. J. Mater. Chem. A. 2024, 12, 795–801. [Google Scholar] [CrossRef]
- Kallem, P.; Drobek, M.; Julbe, A.; Vriezekolk, E.J.; Mallada, R.; Pina, M.P. Hierarchical Porous Polybenzimidazole Microsieves: An Efficient Architecture for Anhydrous Proton Transport via Polyionic Liquids. ACS Appl. Mater. Interfaces 2017, 9, 14844–14857. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.Q.; Sun, R.; Xiong, J.; Sun, H.L.; Song, S. Tuning the dynamic magnetic behaviour and proton conductivity via water-induced reversible single-crystal to single-crystal structural transformation. J. Mater Chem. C 2021, 9, 15858–15867. [Google Scholar] [CrossRef]
- Li, S.J.; Zhao, Y.; Knoll, S.; Liu, R.J.; Li, G.; Peng, Q.P.; Qiu, P.T.; He, D.F.; Streb, C.; Chen, X.N.A. High Proton-Conductivity in Covalently Linked Polyoxometalate Organoboronic Acid-Polymers. Angew. Chem. Int. Ed. 2021, 60, 16953–16957. [Google Scholar] [CrossRef]
- Lim, D.W.; Sadakiyo, M.; Kitagawa, H. Proton Transfer in Hydrogen-Bonded Degenerate Systems of Water and Ammonia in Metal-Organic Frameworks. Chem. Sci. 2019, 10, 16–33. [Google Scholar] [CrossRef]
- Pal, S.C.; Das, M.C. Superprotonic Conductivity of MOFs and Other Crystalline Platforms Beyond 10−1 S c m−1. Adv. Funct. Mater. 2021, 31, 2101584. [Google Scholar] [CrossRef]
- Ye, Y.; Guo, W.; Wang, L.; Li, Z.; Song, Z.; Chen, J.; Zhang, Z.; Xiang, S.; Chen, B. Straightforward Loading of Imidazole Molecules into Metal-Organic Framework for High Proton Conduction. J. Am. Chem. Soc. 2017, 139, 15604–15607. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.S.; Shimizu, G.K.H.; Zheng, L.M. Proton conductive metal phosphonate frameworks. Coord. Chem. Rev. 2019, 378, 577–594. [Google Scholar] [CrossRef]
- Xie, X.X.; Yang, Y.C.; Dou, B.H.; Li, Z.F.; Li, G. Proton conductive carboxylate-based metal-organic frameworks. Coord. Chem. Rev. 2020, 403, 213100–213132. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, L.Q.; Peng, Q.F.; Wang, G.E.; Shen, Y.; Li, Z.; Wang, L.H.; Ma, X.Y.; Chen, Q.H.; Zhang, Z.J.; et al. High Anhydrous Proton Conductivity of Imidazole-Loaded Meso-porous Polyimides over a Wide Range from Subzero to Moderate Temperature. J. Am. Chem. Soc. 2015, 137, 913–918. [Google Scholar] [CrossRef]
- Luo, H.B.; Ren, Q.; Wang, P.; Zhang, J.; Wang, L.F.; Ren, X.M. High Proton Conductivity Achieved by Encapsulation of Imidazole Molecules into Proton-Conducting MOF-808. ACS Appl. Mater. Interfaces 2019, 11, 9164–9171. [Google Scholar] [CrossRef]
- Nguyen, M.V.; Lo, T.H.N.; Luu, L.C.; Nguyen, H.T.T.; Tu, T.N. Enhancing proton conductivity in a metal-organic framework at T > 80 °C by an anchoring strategy. J. Mater. Chem. A 2018, 6, 1816–1821. [Google Scholar] [CrossRef]
- Dong, X.Y.; Li, J.J.; Han, Z.; Duan, P.G.; Li, L.K.; Zang, S.Q. Tuning the Functional Substituent Group and Guest of Metal-Organic Frameworks in Hybrid Membranes for Improved Interface Compatibility and Proton Conduction. J. Mater. Chem. A 2017, 5, 3464–3474. [Google Scholar] [CrossRef]
- Sadakiyo, M.; Ōkawa, H.; Shigematsu, A.; Ohba, M.; Yamada, T.; Kitagawa, H. Promotion of Low-Humidity Proton Conduction by Controlling Hydrophilicity in Layered Metal-Organic Frameworks. J. Am. Chem. Soc. 2012, 134, 5472–5475. [Google Scholar] [CrossRef] [PubMed]
- Okawa, H.; Sadakiyo, M.; Yamada, T.; Maesato, M.; Ohba, M.; Kitagawa, H. Proton-Conductive Magnetic Metal–Organic Frameworks, {NR3(CH2COOH)}[MaIIMbIII(ox)3]: Effect of Carboxyl Residue upon Proton Conduction. J. Am. Chem. Soc. 2013, 135, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Zakrzewski, J.J.; Heczko, M.; Zychowicz, M.; Nakagawa, K.; Nakabayashi, K.; Sieklucka, B.; Chorazy, S.; Ohkoshi, S. Proton Conductive Luminescent Thermometer Based on Near Infrared Emissive {YbCo2} Molecular Nanomagnets. J. Am. Chem. Soc. 2020, 142, 3970–3979. [Google Scholar] [CrossRef]
- Zhu, S.D.; Hu, J.J.; Dong, L.; Wen, H.R.; Liu, S.J.; Lu, Y.B.; Liu, C.M. Multifunctional Zn(II)–Yb(III) complex enantiomers showing second-harmonic generation, near-infrared luminescence, single-molecule magnet behaviour and proton conduction. J. Mater. Chem. C 2020, 8, 16032–16041. [Google Scholar] [CrossRef]
- Zhang, X.N.; Hu, J.J.; Zhang, J.L.; Liu, S.J.; Liao, J.S.; Wen, H.R. Multifunctional ZnII-LnIII (Ln = Tb, Dy) complexes based on the amine-phenol ligand with field-induced slow magnetic relaxation, luminescence, and proton conduction. New J. Chem. 2021, 45, 3392–3399. [Google Scholar] [CrossRef]
- Chen, J.F.; Ge, Y.L.; Wu, D.H.; Cui, H.T.; Mu, Z.L.; Xiao, H.P.; Li, X.H.; Ge, J.Y. Two-dimensional dysprosium(III) coordination polymer: Structure, single-molecule magnetic behavior, proton conduction, and luminescence. Front. Chem. 2022, 10, 974914. [Google Scholar] [CrossRef]
- Lu, Y.B.; Huang, J.; Liao, Y.Q.; Lin, X.L.; Huang, S.Y.; Liu, C.M.; Wen, H.R.; Liu, S.J.; Wang, F.Y.; Zhu, S.D. Multifunctional Dinuclear Dy-Based Coordination Complex Showing Visible Photoluminescence Single-Molecule Magnet Behavior and Proton Conduction. Inorg. Chem. 2022, 61, 18545–18553. [Google Scholar] [CrossRef]
- Zhu, S.D.; Zhou, Y.L.; Lei, Y.; Wen, H.R.; Liu, S.J.; Liu, C.M.; Zhang, S.Y.; Lu, Y.B. Combined performance of circularly polarized luminescence and proton conduction in homochiral cadmium(II)-terbium(III) complexes. Inorg. Chem. Front. 2024, 11, 1531–1539. [Google Scholar] [CrossRef]
- Zhu, S.D.; Zhou, Y.L.; Liu, F.; Lei, Y.; Liu, S.J.; Wen, H.R.; Shi, B.; Zhang, S.Y.; Liu, C.M.; Lu, Y.B. A Pair of Multifunctional Cu(II)-Dy(III) Enantiomers with Zero-Field Single-Molecule Magnet Behaviors, Proton Conduction Properties and Magneto-Optical Faraday Effects. Molecules 2023, 28, 7506. [Google Scholar] [CrossRef]
- Lu, Y.B.; Liao, Y.Q.; Dong, L.; Zhu, S.D.; Wen, H.R.; Huang, J.; Dai, X.X.; Lian, P.; Jiang, X.M.; Li, R.; et al. Ultra-Stable Metal-Organic Framework with Concurrent High Proton Conductivity and Fluorescence Sensing for Nitrobenzene. Chem. Mater. 2021, 33, 7858–7868. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System, version v40.67a; Rigaku Corporation: Oxford, UK, 2019.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE, version 2.1; Electronic Structure Group, Universitat de Barcelona: Barcelona, Spain, 2013.
- Litvinova, Y.M.; Gayfulin, Y.M.; van Leusen, J.; Samsonenko, D.G.; Lazarenko, V.A.; Zubavichus, Y.V.; Kogerler, P.; Mironov, Y.V. Metal-organic frameworks based on polynuclear lanthanide complexes and octahedral rhenium clusters. Inorg. Chem. Front. 2019, 6, 1518–1526. [Google Scholar] [CrossRef]
- Canaj, A.B.; Dey, S.; Mart, E.R.; Wilson, C.; Rajaraman, G.; Murrie, M. Insight into D6h Symmetry: Targeting Strong Axiality in Stable Dysprosium(III) Hexagonal Bipyramidal Single-Ion Magnets. Angew. Chem. Int. Ed. 2019, 58, 14146–14151. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, S.; Hernández–Paredes, A.; Bayona–Andrés, M.; Gómez–García, C.J. Slow Relaxation of the Magnetization in Anilato–Based Dy(III) 2D Lattices. Molecules 2021, 26, 1190. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.; Reta, D.; Chilton, G.F.S.N.F.; Mills, D.P. A Bis-Monophospholyl Dysprosium Cation Showing Magnetic Hysteresis at 48 Kelvin. J. Am. Chem. Soc. 2020, 142, 19935–19940. [Google Scholar]
- Zhang, S.; Wu, H.P.; Sun, L.; Ke, H.S.; Chen, S.P.; Wei, Q.; Yang, D.S.; Gao, S.L. Ligand field fine-tuning on the modulation of the magnetic properties and relaxation dynamics of dysprosium(III) single-ion magnets (SIMs): Synthesis, structure, magnetism and ab initio calculations. J. Mater. Chem. C 2017, 5, 1369–1382. [Google Scholar] [CrossRef]
- Ma, Y.J.; Hu, J.X.; Han, S.D.; Pan, J.; Li, J.H.; Wang, G.M. Manipulating On/Off Single-Molecule Magnet Behavior in a Dy(III)-Based Photochromic Complex. J. Am. Chem. Soc. 2020, 142, 2682–2689. [Google Scholar] [CrossRef]
- Sun, L.; Wei, S.L.; Zhang, J.; Wang, W.Y.; Chen, S.P.; Zhang, Y.Q.; Wei, Q.; Xie, G.; Gao, S.L. Isomeric ligand enhancing the anisotropy barrier within nine-coordinated {Dy2} Compounds. J. Mater. Chem. C 2017, 5, 9488–9495. [Google Scholar] [CrossRef]
- Blagg, R.J.; Muryn, C.A.; McInnes, E.J.L.; Tuna, F.; Winpenny, R.E.P. Single Pyramid Magnets: Dy5 Pyramids with Slow Magnetic Relaxation to 40 K. Angew. Chem. Int. Ed. 2011, 50, 6530–6533. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Hu, H.C.; Chen, Y.K.; Chen, Z.L.; Zhang, Z.Y.; Ding, M.M.; Zhang, Y.Q.; Liu, D.C.; Liang, Y.N.; Liang, F.P. Acid and alkali-resistant Dy4 coordination clusters: Synthesis, structure and slow magnetic relaxation behaviors. J. Mater. Chem. C 2021, 9, 3854–3862. [Google Scholar] [CrossRef]
- Umeyama, D.; Horike, S.; Inukai, M.; Itakura, T.; Kitagawa, S. Inherent Proton Conduction in a 2D Coordination Framework. J. Am. Chem. Soc. 2012, 134, 12780–12785. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Nakagawa, K.; Imoto, K.; Tokoro, H.; Shibata, Y.; Okamoto, K.; Miyamoto, Y.; Komine, M.; Yoshikiyo, M.; Namai, A. A photoswitchable polar crystal that exhibits superionic conduction. Nat. Chem. 2020, 12, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Sadakiyo, M.; Yamada, T.; Honda, K.; Matsui, H.; Kitagawa, H. Control of Crystalline Proton-Conducting Pathways by Water-Induced Transformations of Hydrogen-Bonding Networks in A Metal-Organic Framework. J. Am. Chem. Soc. 2014, 136, 7701–7707. [Google Scholar] [CrossRef]
- Kim, S.; Joarder, B.; Hurd, J.; Zhang, J.; Dawson, K.W.; Gelfand, B.S.; Wong, N.E.; Shimizu, G.K.H. Achieving Superprotonic Conduction in Metal-Organic Frameworks through Iterative Design Advances. J. Am. Chem. Soc. 2018, 140, 1077–1082. [Google Scholar] [CrossRef]
- Zhang, F.M.; Dong, L.Z.; Qin, J.S.; Guan, W.; Liu, J.; Li, S.L.; Lu, M.; Lan, Y.Q.; Su, Z.M.; Zhou, H.C. Effect of Imidazole Arrangements on Proton-Conductivity in Metal-Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 6183–6189. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.N.; Zhang, D.S.; Xie, Y.L.; Luan, T.X.; Li, W.C.; Li, L.; Li, P.Z. High Enhancement in Proton Conductivity by Incorporating Sulfonic Acids into a Zirconium-Based Metal-Organic Framework via “Click” Reaction. Inorg. Chem. 2021, 60, 10089–10094. [Google Scholar] [CrossRef]
- Xu, T.Y.; Nie, H.J.; Li, J.M.; Shi, Z.F. Highly selective sensing of Fe3+/Hg2+ and proton conduction using two fluorescent Zn(II) coordination polymers. Dalton Trans. 2020, 49, 11129–11141. [Google Scholar] [CrossRef]
- Tholen, P.; Peeples, C.A.; Schaper, R.; Bayraktar, C.; Erkal, T.S.; Ayhan, M.M.; Cosut, B.; Beckmann, J.; Yazaydin, A.O.; Wark, M.; et al. Semiconductive microporous hydrogen-bonded organophosphonic acid frameworks. Nat. Commun. 2020, 11, 3180. [Google Scholar] [CrossRef]
- Ogawa, T.; Aonuma, T.; Tamaki, T.; Ohashi, H.; Ushiyama, H.; Yamashitac, K.; Yamaguchi, T. The proton conduction mechanism in a material consisting of packed acids. Chem. Sci. 2014, 5, 4878–4887. [Google Scholar] [CrossRef]
- Huang, Y.G.; Wu, S.Q.; Deng, W.H.; Xu, G.; Hu, F.L.; Hill, J.P.; Wei, W.; Su, S.Q.; Shrestha, L.K.; Sato, O.; et al. Selective CO2 Capture and High Proton Conductivity of a Functional Star-of-David Catenane Metal-Organic Framework. Adv. Mater. 2017, 29, 1703301. [Google Scholar] [CrossRef] [PubMed]
- Gui, D.X.; Dai, X.; Tao, Z.T.; Zheng, T.; Wang, X.X.; Silver, M.A.; Shu, J.; Chen, L.H.; Wang, Y.L.; Zhang, T.T.; et al. Unique Proton Transportation Pathway in a Robust Inorganic Coordination Polymer Leading to Intrinsically High and Sustainable Anhydrous Proton Conductivity. J. Am. Chem. Soc. 2018, 140, 6146–6155. [Google Scholar] [CrossRef] [PubMed]
- Pili, S.; Argent, S.P.; Morris, C.G.; Rought, P.; Garcia-Sakai, V.; Silverwood, I.P.; Easun, T.L.; Li, M.; Warren, M.R.; Murray, C.A.; et al. Proton Conduction in a Phosphonate-Based Metal-Organic Framework Mediated by Intrinsic “Free Diffusion inside a Sphere”. J. Am. Chem. Soc. 2016, 138, 6352–6355. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.Q.; Zhang, F.; Feng, W.; Zou, X.Q.; Zhao, C.J.; Na, H.; Liu, C.; Sun, F.X.; Zhu, G.S. From metal-organic framework (MOF) to MOF-polymer composite membrane: Enhancement of low-humidity proton conductivity. Chem. Sci. 2013, 4, 983–992. [Google Scholar] [CrossRef]
- Ananias, D.; Paz, F.A.A.; Yufit, D.S.; Carlos, L.D.; Rocha, J. Photoluminescent Thermometer Based on a Phase-TransitionLanthanide Silicate with Unusual Structural Disorder. J. Am. Chem. Soc. 2015, 137, 3051–3058. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, Y.H.; Zhang, Y.; Liu, Y.F.; Chen, L.J.; Zhao, J.W. Rare-Earth and Antimony-Oxo Clusters Simultaneously Connecting Antimonotungstates Comprising Divacant and Tetravacant Keggin Fragments. Inorg. Chem. 2019, 58, 11636–11648. [Google Scholar] [CrossRef]
- Martínez-Calvo, M.; Kotova, O.; Mobius, M.E.; Bell, A.P.; McCabe, T.; Boland, T.; Gunnlaugsson, T. Healable Luminescent Self-Assembly Supramolecular Metallogels Possessing Lanthanide (Eu/Tb) Dependent Rheological and Morphological Properties. J. Am. Chem. Soc. 2015, 137, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, E.R.; Eliseeva, S.V.; Jankolovits, J.; Olmstead, M.M.; Petoud, S.; Pecoraro, V.L. Highly Emitting Near-Infrared Lanthanide “Encapsulated Sandwich” Metallacrown Complexes with Excitation Shifted Toward Lower Energy. J. Am. Chem. Soc. 2014, 136, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Biju, S.; Ambili Raj, D.B.; Reddy, M.L.P.; Kariuki, B.M. Synthesis, Crystal Structure, and Luminescent Properties of Novel Eu3+ Heterocyclic β-Diketonate Complexes with Bidentate Nitrogen Donors. Inorg. Chem. 2006, 45, 10651–10660. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Wang, W.F.; Wang, Y.L.; Shan, Z.M.; Wang, M.S.; Tang, J. Diversity of Lanthanide(III)–Organic Extended Frameworks with a 4,8-Disulfonyl-2,6-naphthalenedicarboxylic Acid Ligand: Syntheses, Structures, and Magnetic and Luminescent Properties. Inorg. Chem. 2012, 51, 2381–2392. [Google Scholar] [CrossRef]
- Fujii, T.; Kodaira, K.; Kawauchi, O.; Tanaka, N.; Yamashita, H.; Anpo, M. Photochromic behavior in the fluorescence spectra of 9-anthrol encapsulated in Si-Al glasses prepared by the sol-gel method. J. Phys. Chem. B 1997, 101, 10631–10637. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.M.; Pang, J.J.; Liu, Y.F.; Li, P.; Chen, L.J.; Zhao, J.W. Two Penta-REIII Encapsulated Tetravacant Dawson Selenotungstates and Nanoscale Derivatives and Their Luminescence Properties. Inorg. Chem. 2019, 58, 7078–7090. [Google Scholar] [CrossRef]
- Cepeda, J.; Pérez-Yáñez, S.; Beobide, G.; Castillo, O.; García, J.Á.; Lanchasa, M.; Luque, A. Enhancing luminescence properties of lanthanide(III)/pyrimidine-4,6-dicarboxylato system by solvent-free approach. Dalton Trans. 2015, 44, 6972–6986. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).