Anion-π Type Polymeric Nanoparticle Dispersants for Enhancing the Dispersion Stability of Organic Pigments in Water
Abstract
1. Introduction
2. Results and Discussion
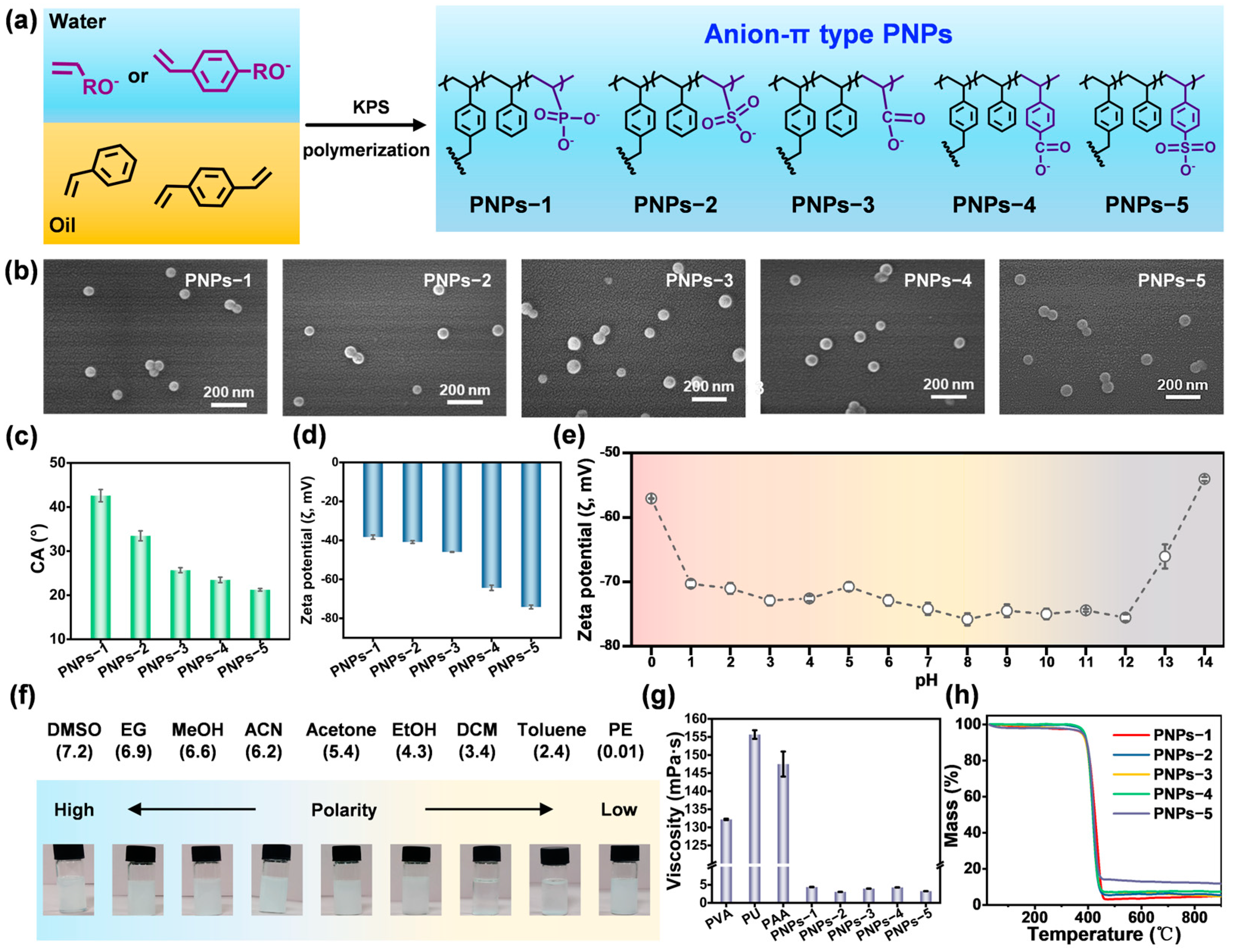
2.1. Synthesis and Characterization of Anion-π Type PNPs
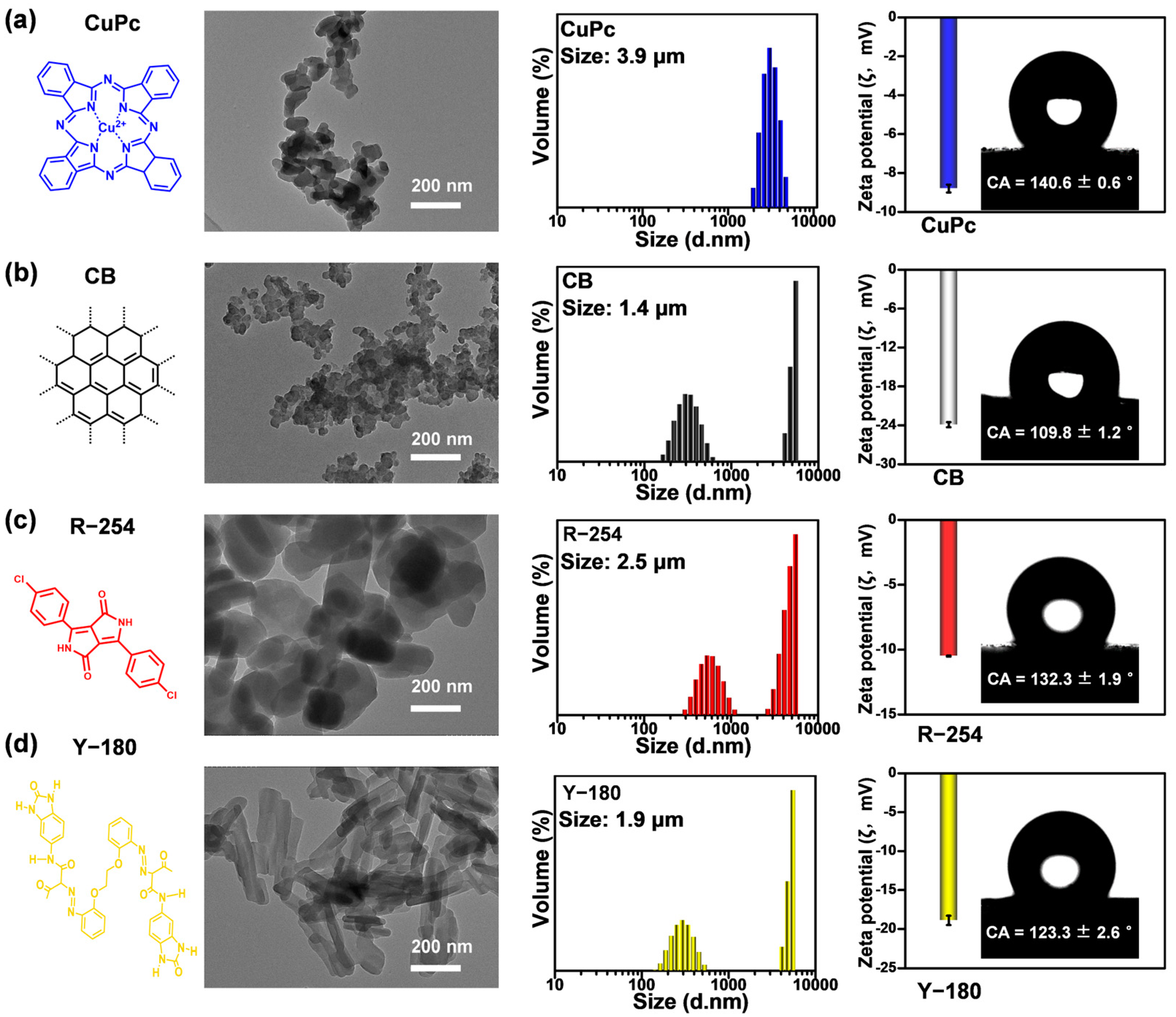
2.2. Characterization of Pigments with Different Types of Intermolecular Forces
2.3. Interactions and Dispersion Stability of Anion-π Type PNPs with CuPc and CB
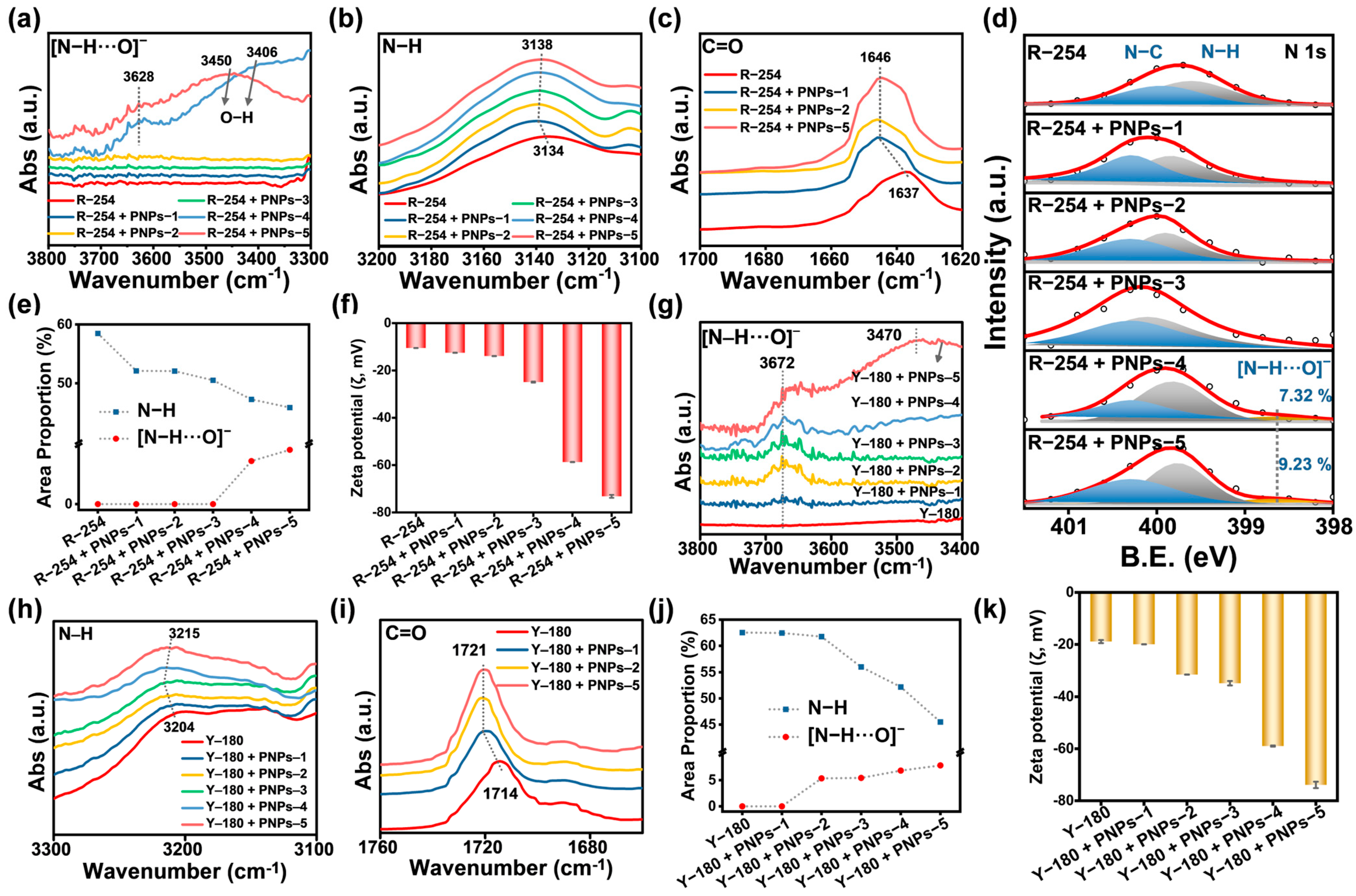
2.4. Interactions and Dispersion Stability of Anion-π Type PNPs with R-254 and Y-180
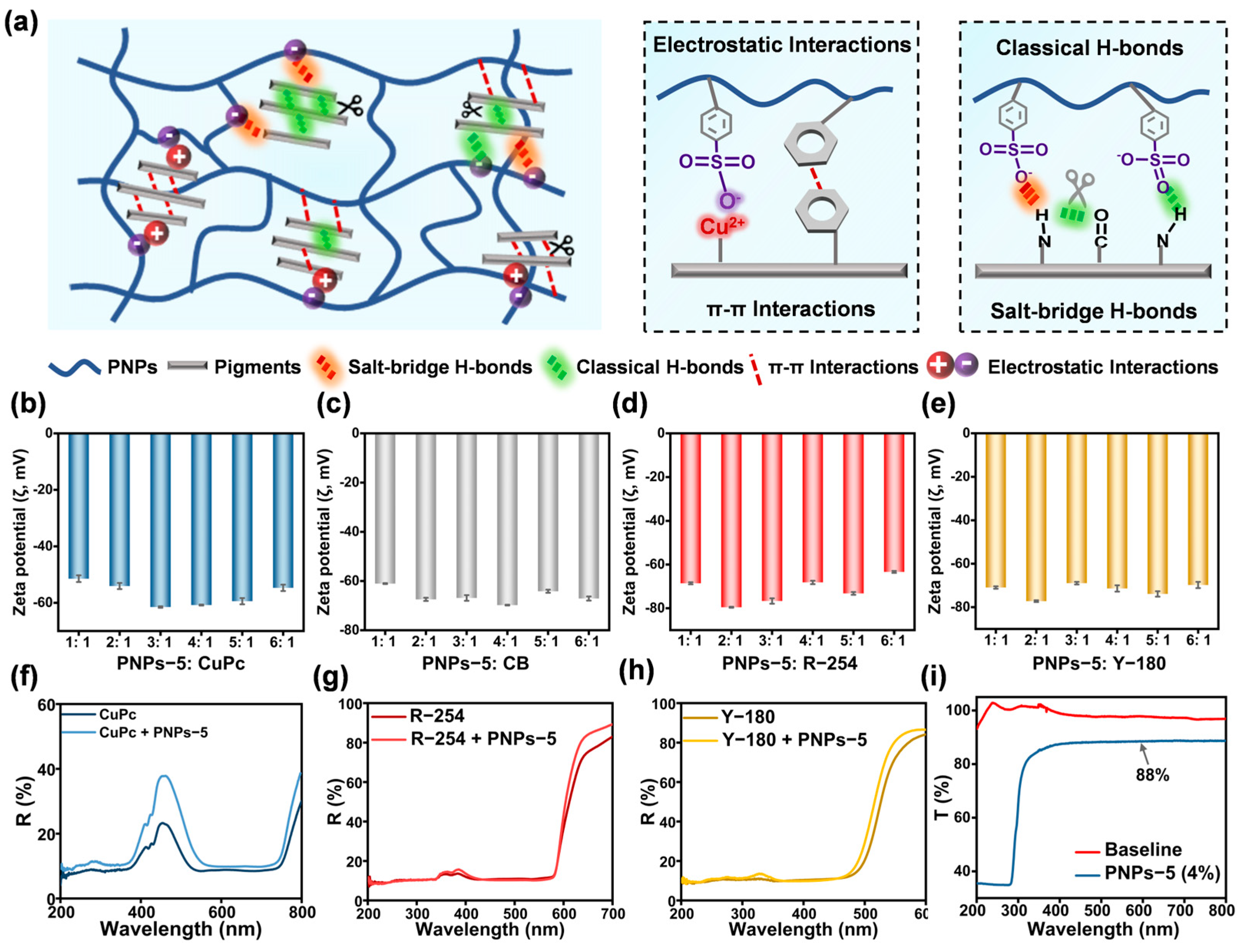
2.5. Interaction Mechanism of Anion-π Type PNPs with Pigments and Effect of Mixing Ratios of PNPs-5 with Four Pigments
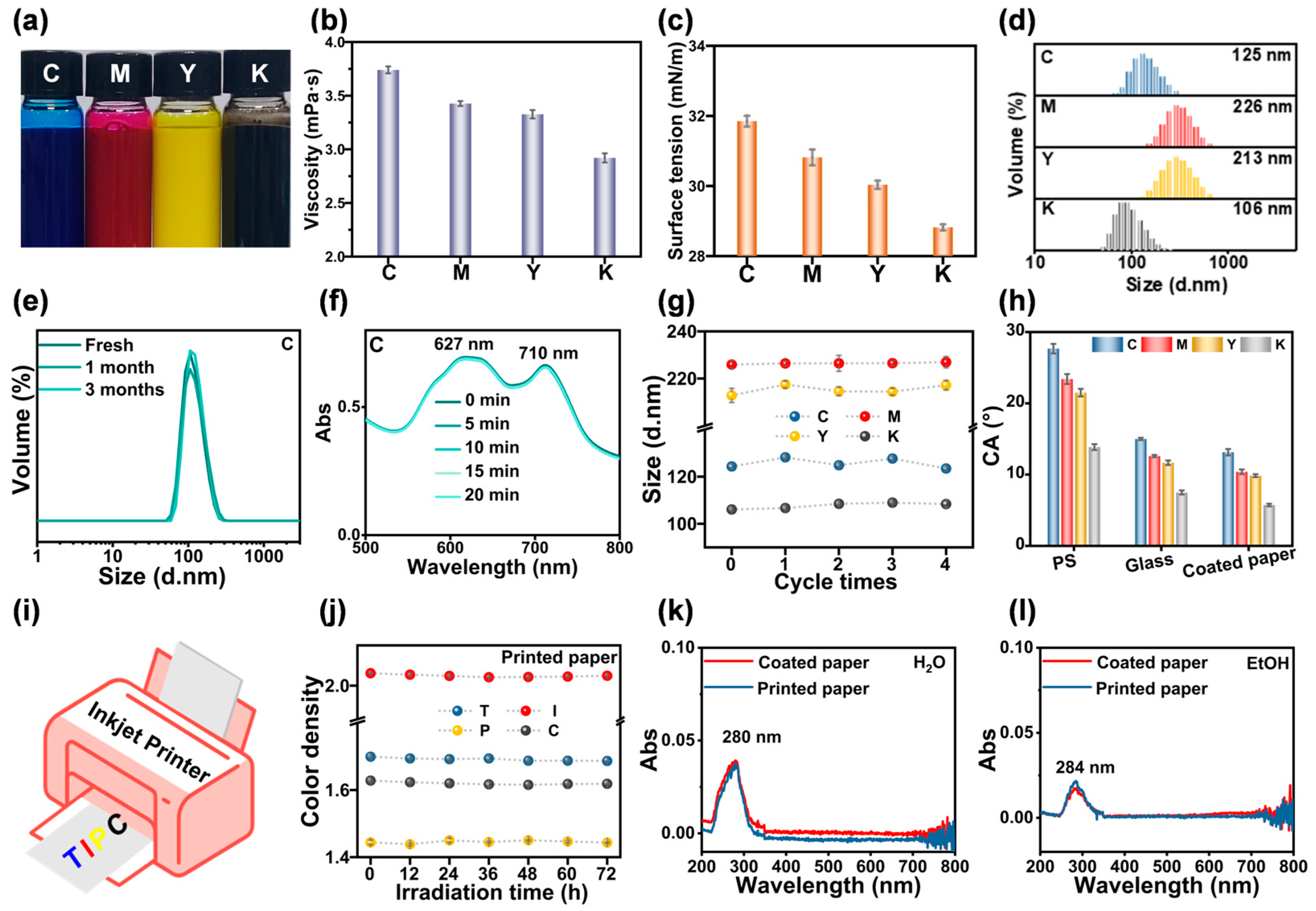
2.6. Performance of Anion-π Type PNPs in Water-Based Inkjet Inks
3. Materials and Methods
3.1. Materials
3.2. Instruments and Equipment
3.3. Synthesis of Anion-π Type PNPs
3.4. Preparation of CMYK Water-Based Inkjet Inks
3.5. Characterization of Morphologies
3.6. Characterization of FTIR Spectra
3.7. Characterization of UV-Vis Spectra
3.8. Characterization of XPS Spectra
3.9. Characterization of Contact Angles
3.10. Characterization of ζ Values
3.11. Characterization of Particle Size Distribution
3.12. Characterization of TGA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
- The following abbreviations are used in this manuscript:
| PNPs | Polymeric Nanoparticles |
| H-bond | Hydrogen Bonding |
| CMYK | Cyan Magenta Yellow Key |
| PU | Polyurethane |
| PAA | Polyacrylic Acid |
| PVA | Polyvinyl Alcohol |
| CPT | Controlled Polymerization Technology |
References
- Kozake, K.; Egawa, T.; Kunii, S.; Kawaguchi, H.; Okada, T.; Sakata, Y. Environmental impact assessment of flexible package printing with the “LUNAJET®” aqueous inkjet ink using nanodispersion technology. Sustainability 2021, 13, 9851. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, H.; Wang, M.; Fang, K.; Song, Y.; Liu, Q. Controlled micro-scale ink droplet spreading on cotton fabrics via cellulose-based coatings for greener textile inkjet printing. Ind. Crops. Prod. 2024, 213, 118488. [Google Scholar] [CrossRef]
- Rishi, K.; Mulderig, A.; Beaucage, G.; Vogtt, K.; Jiang, H. Thermodynamics of hierarchical aggregation in pigment dispersions. Langmuir 2019, 35, 13100–13109. [Google Scholar] [CrossRef] [PubMed]
- Mather, R.R. The degree of crystal aggregation in organic pigments. Dye. Pigment. 1999, 42, 103–106. [Google Scholar] [CrossRef]
- Daescu, C. Dispersability of organic pigments. Dye. Pigment. 1998, 38, 173–180. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Huang, K.-T.; Huang, C.-J. Polymerizable fatty acid surfactant: Encapsulation of organic pigments for excellent colloidal stability in aqueous solution and water-repellent property. Dye. Pigment. 2024, 232, 112488. [Google Scholar] [CrossRef]
- Haramagatti, C.R.; Dhande, P.; Bhavsar, R.; Umbarkar, A.; Joshi, A. Role of surfactants on stability of iron oxide yellow pigment dispersions. Prog. Org. Coat. 2018, 120, 260–265. [Google Scholar] [CrossRef]
- Yoon, C.; Choi, J.H. Synthesising polymeric dispersants to apply to carbon black pigmented mill bases for use in ink-jet inks. Color. Technol. 2019, 136, 60–74. [Google Scholar] [CrossRef]
- Asada, M.; Tanaka, H.; Suwa, Y.; Osawa, S.; Otsuka, H. Improved pigment dispersibility in thick inks based on increased molecular dispersion of poorly water-soluble block copolymers. Dye. Pigment. 2024, 226, 112140. [Google Scholar] [CrossRef]
- Zhao, L.; Hong, C.; Wang, C.; Li, J.; Ren, H.; Zhou, C. Enhancement of the adhesion strength of water-based ink binder based on waterborne polyurethane. Prog. Org. Coat. 2023, 183, 107765. [Google Scholar] [CrossRef]
- Lamminmäki, T.T.; Kettle, J.P.; Puukko, P.J.T.; Gane, P.A.C. Absorption capability and inkjet ink colorant penetration into binders commonly used in pigmented paper coatings. Ind. Eng. Chem. Res. 2011, 50, 3287–3294. [Google Scholar] [CrossRef]
- Jiao, C.; Sun, L.; Shao, Q.; Song, J.; Hu, Q.; Naik, N. Advances in waterborne acrylic resins: Synthesis principle, modification strategies, and their applications. ACS Omega 2021, 6, 12443–12449. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Liu, H. Lignins and lignin derivatives as dispersants for copper phthalocyanine pigment nanoparticles. ACS Sustain. Chem. Eng. 2023, 11, 8199–8207. [Google Scholar] [CrossRef]
- Hakeim, O.A.; Abdelghaffar, F.; Haroun, A.A. UV-curable hyperbranched polyester acrylate encapsulation of phthalocyanine pigments for high performance synthetic fabrics printing. Dye. Pigment. 2020, 177, 108307. [Google Scholar] [CrossRef]
- Xu, Q.; Long, S.; Liu, G.; Zhang, X.; Zhou, Y. Synthesis and dispersion of organic pigments by amphiphilic hyperbranched polyesteramides dispersant. IOP Conf. Ser. Mater. Sci. Eng. 2019, 611, 012036. [Google Scholar] [CrossRef]
- Pu, Z.; Fan, X.; Su, J.; Zhu, M.; Jiang, Z. Aqueous dispersing mechanism study of nonionic polymeric dispersant for organic pigments. Colloid. Polym. Sci. 2022, 200, 167–176. [Google Scholar] [CrossRef]
- Song, Y.; Fang, K.; Ren, Y.; Tang, Z.; Wang, R.; Chen, W. Inkjet printable and self-curable disperse dyes/P(St-BA-MAA) nanosphere inks for both hydrophilic and hydrophobic fabrics. Polymers 2018, 10, 1402. [Google Scholar] [CrossRef] [PubMed]
- Nsib, F.; Ayed, N.; Chevalier, Y. Selection of dispersants for the dispersion of C.I. Pigment Violet 23 in organic medium. ACS Appl. Polym. Mater. 2007, 74, 133–140. [Google Scholar] [CrossRef]
- Lu, L.; Duan, H.; Li, J.; Qi, D. Film-formation and binder-free pigment printing of fluorosilicone-modified polyacrylate/pigment hybrid latex: Effect of cross-linking degree. Dye. Pigment. 2023, 5, 1871–1881. [Google Scholar] [CrossRef]
- Luan, M.; Shen, D.; Zhou, P.; Li, D.; Li, P.; Shi, B. One-pot synthesis of block copolymer dispersant by ICAR ATRP with ppm copper catalyst and the dispersibility on pigment. Prog. Org. Coat. 2022, 169, 106914. [Google Scholar] [CrossRef]
- Cao, X.; Cai, Z.; Huang, Y.; Wang, D.; Zhang, L.; Huang, K. Preparation and utilization of a comb-like polycarboxylate dispersant for organic pigment. ChemistrySelect 2023, 8, e202302249. [Google Scholar] [CrossRef]
- Tian, X.; Lv, S.; Li, J.; Zhang, J.; Yu, L.; Liu, X. Recent advancement in synthesis and modification of water-based acrylic emulsion and their application in water-based ink: A comprehensive review. Prog. Org. Coat. 2024, 189, 108320. [Google Scholar] [CrossRef]
- Qiao, H.; Wu, B.; Sun, S.; Wu, P. Entropy-driven design of highly impact-stiffening supramolecular polymer networks with salt-bridge hydrogen bonds. J. Am. Chem. Soc. 2024, 146, 7533–7542. [Google Scholar] [CrossRef]
- He, Y.; Zhang, J.; Cai, Y.; Yi, L. Encapsulation of organic pigment via a facile dispersion approach and soap-free miniemulsion polymerization. Prog. Org. Coat. 2021, 159, 106403. [Google Scholar] [CrossRef]
- Dong, T.; Zhang, Z.; Liu, H.; Deng, Y.; Liu, W.; Li, Y. Phosphorylated poly (ethylene-co-vinyl alcohol) doping for enhanced proton conductivity and mechanical properties of side chain-type sulfonated poly (aryl ether ketone) proton-exchange membranes. J. Membr. Sci. 2024, 692, 122298. [Google Scholar] [CrossRef]
- Balding, P.; Borrelli, R.; Volkovinsky, R.; Russo, P.S. Physical properties of sodium poly (styrene sulfonate): Comparison to incompletely sulfonated polystyrene. Macromolecules 2022, 55, 1747–1762. [Google Scholar] [CrossRef]
- Huang, G.; Pan, Z.; Wang, Y. Synthesis of sodium polyacrylate copolymers as water-based dispersants for ultrafine grinding of praseodymium zirconium silicate. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 591–599. [Google Scholar] [CrossRef]
- Wang, X.; Chen, G.-X.; Han, R.; Zhou, Z.; Li, Q. Controlled synthesis of non-functionalized POSS nanoparticles with hydrophobic to hydrophilic wettability transition. Prog. Org. Coat. 2024, 191, 108398. [Google Scholar] [CrossRef]
- Song, Y.; Dong, X.; Shang, D.; Zhang, X.; Li, X.; Liang, X.; Wang, S. Unusual nanofractal microparticles for rapid protein capture and release. Small 2021, 17, e2102802. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, Z.; Xing, T.; Hou, X.; Chen, G. Controlling the micro-structure of disperse water-based inks for ink-jet printing. J. Mol. Liq. 2020, 297, 111783. [Google Scholar] [CrossRef]
- Strzałka, A.M.; Lubczak, J. Polyols and polyurethane foams based on water-soluble chitosan. Polymers 2023, 15, 1488. [Google Scholar] [CrossRef]
- Xue, W.; Guang, C.; Ruo, H.; Zheng, Z.; Qi, L. Water-soluble grafted sodium polyacrylate with low concentration: Synthesis and thermal properties. J. Mol. Liq. 2022, 345, 117837. [Google Scholar] [CrossRef]
- Zhou, C.; Mu, Z. Organic Pigment Chemistry and Technology, 3rd ed.; China Petrochemical Press: Beijing, China, 2014. [Google Scholar]
- Reznickova, A.; Kolska, Z.; Orendac, M.; Cizmar, E.; Sajdl, P.; Svorcik, V. Structural and magnetic characterization of copper sulfonated phthalocyanine grafted onto treated polyethylene. Appl. Surf. Sci. 2016, 379, 259–263. [Google Scholar] [CrossRef]
- Li, Y. Pigment Chemistry and Technology; Chemical Industry Press: Beijing, China, 2020. [Google Scholar]
- Wang, J.; Zeng, S.; Liu, H.; Zheng, Y.; Ma, Y. Thermochromic behavior of pigment red 254 in nylon 6 polymer for high-chromaticity engineering plastics. Dye. Pigment. 2025, 233, 112507. [Google Scholar] [CrossRef]
- Kang, J.; Wang, C.; Liu, Z.; Wang, L.; Meng, Y.; Zhai, Z. Electron-outflowing heterostructure hosts for high-voltage aqueous zinc-iodine batteries. Energy Storage Mater. 2024, 68, 103367. [Google Scholar] [CrossRef]
- Wang, S.; Walker-Gibbons, R.; Watkins, B.; Flynn, M.; Krishnan, M. A charge-dependent long-ranged force drives tailored assembly of matter in solution. Nat. Nanotechnol. 2024, 19, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Du, H.; Wickramasinghe, S.; Qian, X. The Effects of chemical substitution and polymerization on the pKa values of sulfonic acids. J. Phys. Chem. B 2009, 113, 14094–14101. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Y.; Yang, J.D.; Liu, Y.; Zhang, L.; Luo, S. Holistic prediction of the pKa in diverse solvents based on a machine-learning approach. Angew. Chem. Int. Ed. Engl. 2020, 59, 19282–19291. [Google Scholar] [CrossRef]
- Zheng, D.; Gao, Z.; He, X.; Zhang, F.; Liu, L. Surface and interface analysis for copper phthalocyanine (CuPc) and indium-tin-oxide (ITO) using X-ray photoelectron spectroscopy (XPS). Appl. Surf. Sci. 2003, 211, 24–30. [Google Scholar] [CrossRef]
- Mattioli, G.; Avaldi, L.; Bolognesi, P.; Bozek, J.D.; Castrovilli, M.C.; Chiarinelli, J. Unravelling molecular interactions in uracil clusters by XPS measurements assisted by ab initio and tight-binding simulations. Sci. Rep. 2020, 10, 13081. [Google Scholar] [CrossRef] [PubMed]
- Pike, S.J.; Hutchinson, J.J.; Hunter, C.A. H-bond acceptor parameters for anions. J. Am. Chem. Soc. 2017, 139, 6700–6706. [Google Scholar] [CrossRef]
- Zheng, S.; Xu, S.; Wang, G.; Tang, Q.; Jiang, X.; Li, Z. Proposed hydrogen-bonding index of donor or acceptor reflecting its intrinsic contribution to hydrogen-bonding strength. J. Chem. Inf. Model. 2017, 57, 1535–1547. [Google Scholar] [CrossRef] [PubMed]
- Godiya, C.B.; Kumar, S.; Xiao, Y. Amine functionalized egg albumin hydrogel with enhanced adsorption potential for diclofenac sodium in water. J. Hazard. Mater. 2020, 393, 122417. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, H.; Li, X.; Li, N.; Liu, Y.; Li, T. Direct spectroscopic evidence for charge-assisted hydrogen-bond formation between ionizable organic chemicals and carbonaceous materials. Environ. Sci. Technol. 2022, 56, 9356–9366. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Du, C.; Jin, Y.; Wu, X.; He, K. Effects of charge-assisted hydrogen bond on sorption and co-sorption of pharmaceutical contaminants on carbonaceous materials: Spectroscopic and theoretical studies. Sci. Total. Environ. 2024, 908, 168375. [Google Scholar] [CrossRef]
- Jiang, J.; Tang, Q.; Zhao, L.; Xi, Z.; Yuan, W. Molecular motion and hydrogen bond of long-chain PA1212 elastomer under thermal field. CIESC J. 2022, 73, 425–433. [Google Scholar] [CrossRef]
- Kenny, P.W. Hydrogen-bond donors in drug design. J. Med. Chem. 2022, 65, 14261–14275. [Google Scholar] [CrossRef] [PubMed]
- Grusser, M.; Waugh, D.G.; Lawrence, J.; Langer, N.; Scholz, D. On the droplet size and application of wettability analysis for the development of ink and printing substrates. Langmuir 2019, 35, 12356–12365. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Yang, S.; Qin, Z. The roles of wettability and surface tension in droplet formation during inkjet printing. Sci. Rep. 2017, 7, 11841. [Google Scholar] [CrossRef] [PubMed]
- Porntapin, P.; Meshaya, P.; Worawan, B. Surface modification of cotton fabrics by gas plasmas for color strength and adhesion by inkjet ink printing. Appl. Surf. Sci. 2016, 364, 208–220. [Google Scholar] [CrossRef]
- Mardani, H.; Bayrak, E.; Özçelik, Ş.; Babazadeh-Mamaqani, M.; Kahveci, M.U.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Anti-counterfeiting ink based on polymer nanoparticles containing spiropyran and Aza-BODIPY for artificial industries. React. Funct. Polym. 2023, 187, 105593. [Google Scholar] [CrossRef]
- Ren, L.; Qin, L.; Lei, Z.; Jian, Y.; Zhong, L. Detection of primary aromatic amines content in food packaging ink and migration from printed plastic bags. Food Packag. Shelf Life 2022, 32, 100820. [Google Scholar] [CrossRef]
- Zhuo, L.; Guang, C.; Yuan, Y.; Liu, H. Preparation and properties of alcohol-resistant thermal transfer printing inks for multifunctional label printing via physical blending of resins. J. Appl. Polym. Sci 2024, 141, e54930. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Li, L.; Sun, C.; Fixler, D.; Xiao, S.; Zhou, S. Anion-π Type Polymeric Nanoparticle Dispersants for Enhancing the Dispersion Stability of Organic Pigments in Water. Molecules 2025, 30, 975. https://doi.org/10.3390/molecules30050975
Li N, Li L, Sun C, Fixler D, Xiao S, Zhou S. Anion-π Type Polymeric Nanoparticle Dispersants for Enhancing the Dispersion Stability of Organic Pigments in Water. Molecules. 2025; 30(5):975. https://doi.org/10.3390/molecules30050975
Chicago/Turabian StyleLi, Na, Lulu Li, Chenghua Sun, Dror Fixler, Shizhuo Xiao, and Shuyun Zhou. 2025. "Anion-π Type Polymeric Nanoparticle Dispersants for Enhancing the Dispersion Stability of Organic Pigments in Water" Molecules 30, no. 5: 975. https://doi.org/10.3390/molecules30050975
APA StyleLi, N., Li, L., Sun, C., Fixler, D., Xiao, S., & Zhou, S. (2025). Anion-π Type Polymeric Nanoparticle Dispersants for Enhancing the Dispersion Stability of Organic Pigments in Water. Molecules, 30(5), 975. https://doi.org/10.3390/molecules30050975







