Beyond Static Tethering at Membrane Contact Sites: Structural Dynamics and Functional Implications of VAP Proteins
Abstract
:1. Introduction
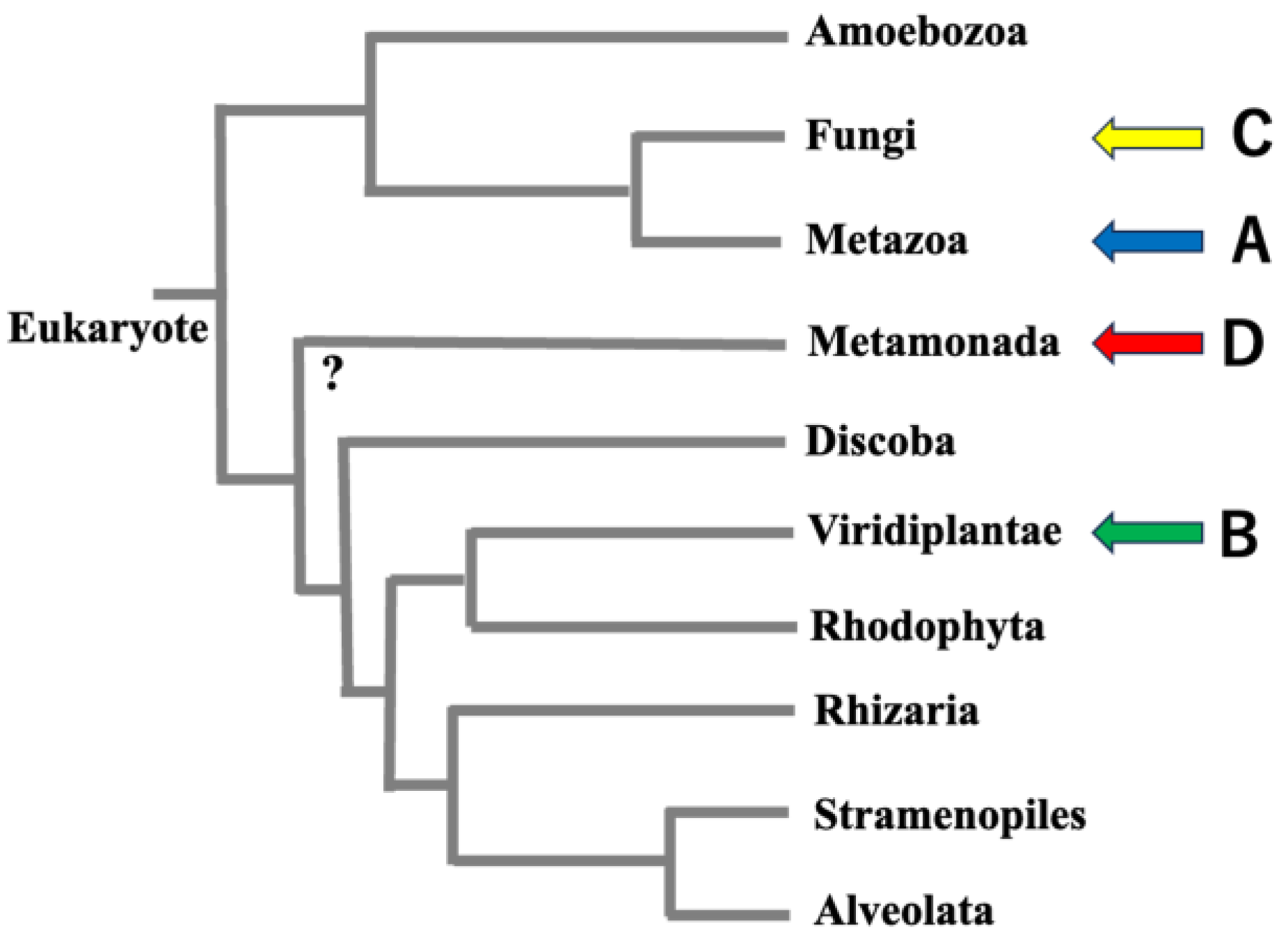
2. Origin of VAP Homologs and Paralogs
2.1. Origin of VAP Homologs
2.2. Origin and Significance of VAP Paralogs VAPA and VAPB
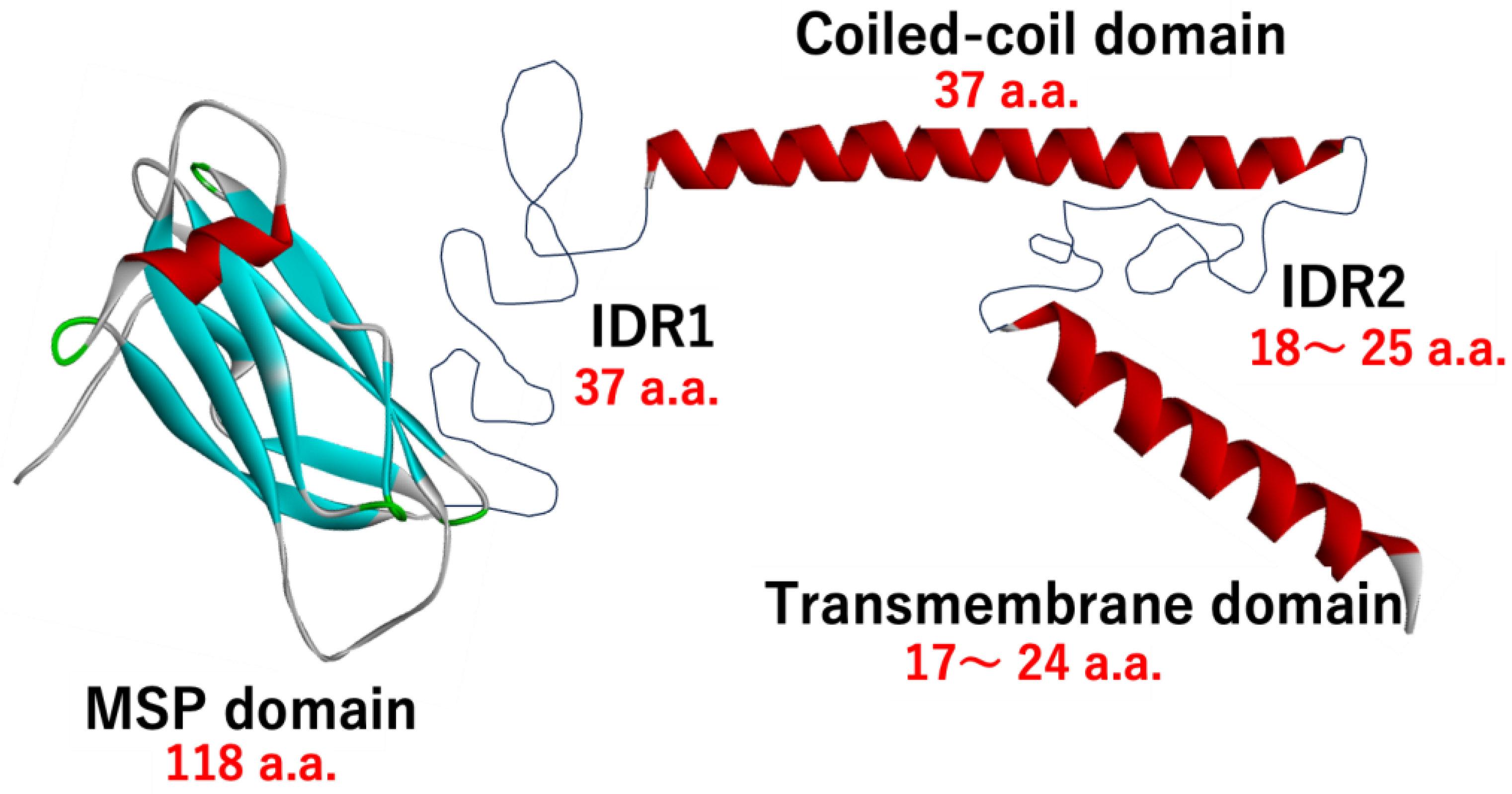
3. Domain Architecture and Domain Boundaries of VAPA and VAPB
3.1. Domain Architecture
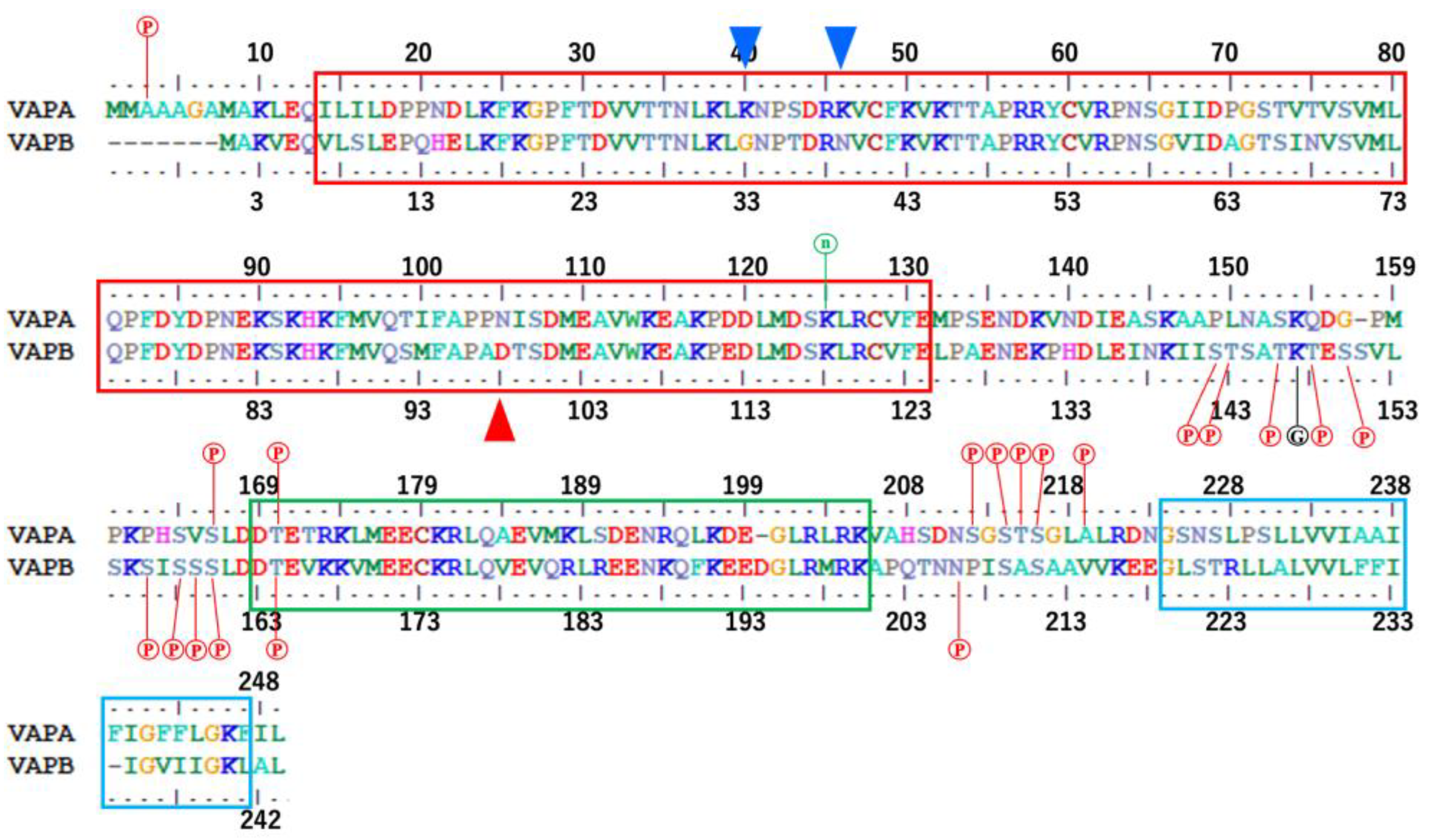
3.2. Domain Boundaries of Regions MSPd, IDR1, CCd, IDR2, and TMd
4. Molecular Phylogenetic Considerations
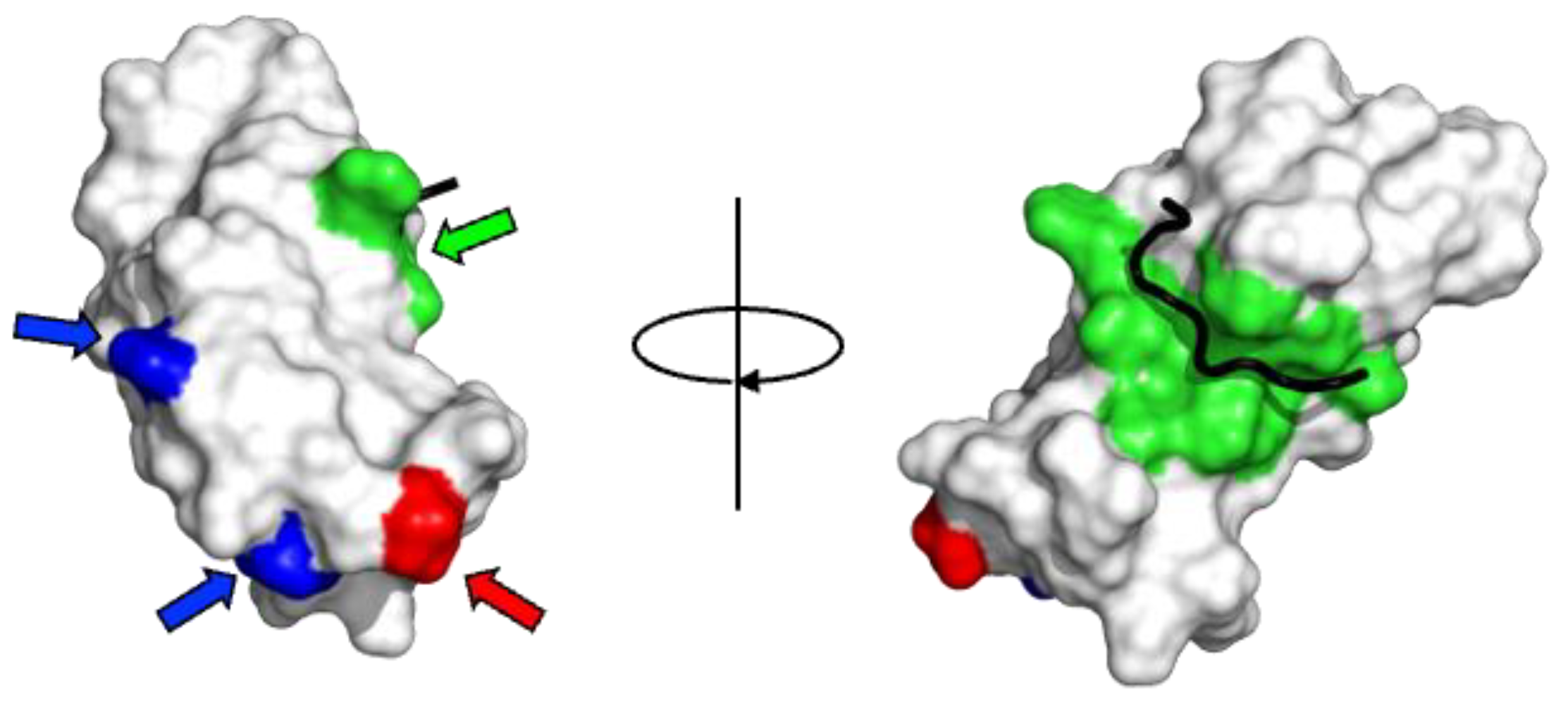
4.1. Molecular Phylogenetic Characteristics of the MSP Domain
4.2. Molecular Phylogenetic Characteristics in Differences Between VAPA and VAPB
4.3. Dimerization of MSPd
4.4. Differences in Structural and Dimerization Characteristics Between VAPA and VAPB
4.5. Phylogenetic Characteristics of IDR1
4.6. Phylogenetic Characteristics of the Coiled-coil Domain
4.7. The Phylogenetic Characteristics of IDR2
4.8. The Phylogenetic Characteristics of the Transmembrane Domain
5. Quantitative Image of Full-Length VAPA/B in Action
5.1. Segmental Distribution of IDRs as Unperturbed Flexible Chains
5.2. Multivalent Interactions and Local Effective Concentrations
6. Importance of IDRs
7. Relationship Between VAP and Disease
8. Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Butu, I.C.; An, D.; O’Shaughnessy, B. How SNARE proteins generate force to fuse membranes. Biophys. J. 2025, 124, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Perkins, H.T.; Allan, V. Intertwined and finely balanced: Endoplasmic reticulum morphology, dynamics, function, and diseases. Cells 2021, 10, 2341. [Google Scholar] [CrossRef] [PubMed]
- Jamecna, D.; Antonny, B. Intrinsically disordered protein regions at membrane contact sites. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 159020. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Meng, T.; Huang, H.; Zhuang, H.; He, Z.; Yang, H.; Feng, D. Molecular machineries and physiological relevance of ER-mediated membrane contacts. Theranostics 2020, 11, 974–995. [Google Scholar] [CrossRef]
- Jang, W.; Haucke, V. ER remodeling via lipid metabolism. Trends Cell Biol. 2024, 34, 942–954. [Google Scholar] [CrossRef]
- Prinz, W.A.; Toulmay, A.; Balla, T. The functional universe of membrane contact sites. Nat. Rev. Mol. Cell Biol. 2020, 21, 7–24. [Google Scholar] [CrossRef]
- Lev, S.; Halevy, D.B.; Peretti, D.; Dahan, N. The VAP protein family: From cellular functions to motor neuron disease. Trends Cell Biol. 2008, 18, 282–290. [Google Scholar] [CrossRef]
- Murphy, S.E.; Levine, T.P. VAP, a Versatile Access Point for the Endoplasmic Reticulum: Review and analysis of FFAT-like motifs in the VAPome. Biochim. Biophys. Acta 2016, 1861, 952–961. [Google Scholar] [CrossRef]
- Kamemura, K.; Chihara, T. Multiple functions of the ER-resident VAP and its extracellular role in neural development and disease. J. Biochem. 2019, 165, 391–400. [Google Scholar] [CrossRef]
- Zaman, M.F.; Nenadic, A.; Radojičić, A.; Rosado, A.; Beh, C.T. Sticking With It: ER-PM Membrane Contact Sites as a Coordinating Nexus for Regulating Lipids and Proteins at the Cell Cortex. Front. Cell Dev. Biol. 2020, 8, 675. [Google Scholar] [CrossRef]
- Corbeil, D.; Santos, M.F.; Karbanová, J.; Kurth, T.; Rappa, G.; Lorico, A. Uptake and fate of extracellular membrane vesicles: Nucleoplasmic reticulum-associated late endosomes as a new gate to intercellular communication. Cells 2020, 9, 1931. [Google Scholar] [CrossRef] [PubMed]
- Dudás, E.F.; Huynen, M.A.; Lesk, A.M.; Pastore, A. Invisible leashes: The tethering VAPs from infectious diseases to neurodegeneration. J. Biol. Chem. 2021, 296, 100421. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Kehlenbach, R.H. The interactome of the VAP family of proteins: An overview. Cells 2021, 10, 1780. [Google Scholar] [CrossRef]
- Kors, S.; Costello, J.L.; Schrader, M. VAP Proteins—From Organelle Tethers to Pathogenic Host Interactors and Their Role in Neuronal Disease. Front. Cell Dev. Biol. 2022, 10, 895856. [Google Scholar] [CrossRef]
- Nikawa, J.I.; Murakami, A.; Esumi, E.; Hosaka, K. Cloning and sequence of the SCS2 gene, which can suppress the defect of IN01 expression in an inositol auxotrophic mutant of saccharomyces cerevisiae. J. Biochem. 1995, 118, 39–45. [Google Scholar] [CrossRef]
- Skehel, P.A.; Martin, K.C.; Kandel, E.R.; Bartsch, D. A VAMP-Binding Protein from Aplysia Required for Neurotransmitter Release. Science 1995, 269, 1580–1583. [Google Scholar] [CrossRef]
- Kagiwada, S.; Hosaka, K.; Murata, M.; Nikawa, J.I.; Takatsuki, A. The Saccharomyces cerevisiae SCS2 gene product, a homolog of a synaptobrevin-associated protein, is an integral membrane protein of the endoplasmic reticulum and is required for inositol metabolism. J. Bacteriol. 1998, 180, 1700–1708. [Google Scholar] [CrossRef]
- Weir, M.L.; Klip, A.; Trimble, W.S. Identification of a human homologue of the vesicle-associated membrane protein (VAMP)-associated protein of 33 kDa (VAP-33): A broadly expressed protein that binds to VAMP. Biochem. J. 1998, 333, 247–251. [Google Scholar] [CrossRef]
- Nishimura, Y.; Hayashi, M.; Inada, H.; Tanaka, T. Molecular cloning and characterization of mammalian homologues of vesicle-associated membrane protein-associated (VAMP-associated) proteins. Biochem. Biophys. Res. Commun. 1999, 254, 21–26. [Google Scholar] [CrossRef]
- Costello, J.L.; Castro, I.G.; Hacker, C.; Schrader, T.A.; Metz, J.; Zeuschner, D.; Azadi, A.S.; Godinho, L.F.; Costina, V.; Findeisen, P.; et al. ACBD5 and VAPB mediate membrane associations between peroxisomes and the ER. J. Cell Biol. 2017, 216, 331–342. [Google Scholar] [CrossRef]
- Kawano, M.; Kumagai, K.; Nishijima, M.; Hanada, K. Efficient trafficking of ceramide from the endoplasmic reticulum to the golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT. J. Biol. Chem. 2006, 281, 30279–30288. [Google Scholar] [CrossRef] [PubMed]
- Ngo, M.; Ridgway, N.D. Oxysterol binding protein-related protein 9 (ORP9) is a cholesterol transfer protein that regulates golgi structure and function. Mol. Biol. Cell 2009, 20, 1388–1399. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Liu, N.; Miao, G.; Chen, Y.; Zhao, H.; Zhang, H. The ER Contact Proteins VAPA/B Interact with Multiple Autophagy Proteins to Modulate Autophagosome Biogenesis. Curr. Biol. 2018, 28, 1234–1245.e4. [Google Scholar] [CrossRef]
- De Vos, K.J.; Mórotz, G.M.; Stoica, R.; Tudor, E.L.; Lau, K.F.; Ackerley, S.; Warley, A.; Shaw, C.E.; Miller, C.C.J. VAPB interacts with the mitochondrial protein PTPIP51 to regulate calcium homeostasis. Hum. Mol. Genet. 2012, 21, 1299–1311. [Google Scholar] [CrossRef]
- Lindhout, F.W.; Cao, Y.; Kevenaar, J.T.; Bodzęta, A.; Stucchi, R.; Boumpoutsari, M.M.; Katrukha, E.A.; Altelaar, M.; MacGillavry, H.D.; Hoogenraad, C.C. VAP-SCRN1 interaction regulates dynamic endoplasmic reticulum remodeling and presynaptic function. EMBO J. 2019, 38, e101345. [Google Scholar] [CrossRef]
- Kanekura, K.; Nishimoto, I.; Aiso, S.; Matsuoka, M. Characterization of amyotrophic lateral sclerosis-linked P56S mutation of vesicle-associated membrane protein-associated protein B (VAPB/ALS8). J. Biol. Chem. 2006, 281, 30223–30233. [Google Scholar] [CrossRef]
- Gkogkas, C.; Middleton, S.; Kremer, A.M.; Wardrope, C.; Hannah, M.; Gillingwater, T.H.; Skehel, P. VAPB interacts with and modulates the activity of ATF6. Hum. Mol. Genet. 2008, 17, 1517–1526. [Google Scholar] [CrossRef]
- Nishimura, A.L.; Mitne-Neto, M.; Silva, H.C.A.; Richieri-Costa, A.; Middleton, S.; Cascio, D.; Kok, F.; Oliveira, J.R.M.; Gillingwater, T.; Webb, J.; et al. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am. J. Hum. Genet. 2004, 75, 822–831. [Google Scholar] [CrossRef]
- Nishimura, A.L.; Al-Chalabi, A.; Zatz, M. A common founder for amyotrophic lateral sclerosis type 8 (ALS8) in the Brazilian population. Hum. Genet. 2005, 118, 499–500. [Google Scholar] [CrossRef]
- Loewen, C.J.R.; Roy, A.; Levine, T.P. A conserved ER targeting motif in three families of lipid binding proteins and in Opi1p binds VAP. EMBO J. 2003, 22, 2025–2035. [Google Scholar] [CrossRef]
- Kaiser, S.E.; Brickner, J.H.; Reilein, A.R.; Fenn, T.D.; Walter, P.; Brunger, A.T. Structural basis of FFAT motif-mediated ER targeting. Structure 2005, 13, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Di Mattia, T.; Martinet, A.; Ikhlef, S.; McEwen, A.G.; Nominé, Y.; Wendling, C.; Poussin-Courmontagne, P.; Voilquin, L.; Eberling, P.; Ruffenach, F.; et al. FFAT motif phosphorylation controls formation and lipid transfer function of inter-organelle contacts. EMBO J. 2020, 39, e104369. [Google Scholar] [CrossRef] [PubMed]
- Furuita, K.; Hiraoka, M.; Hanada, K.; Fujiwara, T.; Kojima, C. Sequence requirements of the FFAT-like motif for specific binding to VAP-A are revealed by NMR. FEBS Lett. 2021, 595, 2248–2256. [Google Scholar] [CrossRef] [PubMed]
- Fasana, E.; Fossati, M.; Ruggiano, A.; Brambillasca, S.; Hoogenraad, C.C.; Navone, F.; Francolini, M.; Borgese, N. A VAPB mutant linked to amyotrophic lateral sclerosis generates a novel form of organized smooth endoplasmic reticulum. FASEB J. 2010, 24, 1419–1430. [Google Scholar] [CrossRef]
- Kamemura, K.; Kozono, R.; Tando, M.; Okumura, M.; Koga, D.; Kusumi, S.; Tamai, K.; Okumura, A.; Sekine, S.; Kamiyama, D.; et al. Secretion of endoplasmic reticulum protein VAPB/ALS8 requires topological inversion. Nat. Commun. 2024, 15, 8777. [Google Scholar] [CrossRef]
- Boratyn, G.M.; Camacho, C.; Cooper, P.S.; Coulouris, G.; Fong, A.; Ma, N.; Madden, T.L.; Matten, W.T.; McGinnis, S.D.; Merezhuk, Y.; et al. BLAST: A more efficient report with usability improvements. Nucleic Acids Res. 2013, 41, W29–W33. [Google Scholar] [CrossRef]
- Karnkowska, A.; Vacek, V.; Zubáčová, Z.; Treitli, S.C.; Petrželková, R.; Eme, L.; Novák, L.; Žárský, V.; Barlow, L.D.; Herman, E.K.; et al. A Eukaryote without a Mitochondrial Organelle. Curr. Biol. 2016, 26, 1274–1284. [Google Scholar] [CrossRef]
- Jewari, C.A.; Baldauf, S.L. An excavate root for the eukaryote tree of life. Sci. Adv. 2023, 9, eade4973. [Google Scholar] [CrossRef]
- Gomez-Suaga, P.; Paillusson, S.; Stoica, R.; Noble, W.; Hanger, D.P.; Miller, C.C.J. The ER-Mitochondria Tethering Complex VAPB-PTPIP51 Regulates Autophagy. Curr. Biol. 2017, 27, 371–385. [Google Scholar] [CrossRef]
- Paillusson, S.; Gomez-Suaga, P.; Stoica, R.; Little, D.; Gissen, P.; Devine, M.J.; Noble, W.; Hanger, D.P.; Miller, C.C.J. α-Synuclein binds to the ER–mitochondria tethering protein VAPB to disrupt Ca2+ homeostasis and mitochondrial ATP production. Acta Neuropathol. 2017, 134, 129–149. [Google Scholar] [CrossRef]
- Gómez-Suaga, P.; Pérez-Nievas, B.G.; Glennon, E.B.; Lau, D.H.W.W.; Paillusson, S.; Mórotz, G.M.; Calì, T.; Pizzo, P.; Noble, W.; Miller, C.C.J.J. The VAPB-PTPIP51 endoplasmic reticulum-mitochondria tethering proteins are present in neuronal synapses and regulate synaptic activity. Acta Neuropathol. Commun. 2019, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Suaga, P.; Mórotz, G.M.; Markovinovic, A.; Martín-Guerrero, S.M.; Preza, E.; Arias, N.; Mayl, K.; Aabdien, A.; Gesheva, V.; Nishimura, A.; et al. Disruption of ER-mitochondria tethering and signalling in C9orf72-associated amyotrophic lateral sclerosis and frontotemporal dementia. Aging Cell 2022, 21, e13549. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.H.W.; Paillusson, S.; Hartopp, N.; Rupawala, H.; Mórotz, G.M.; Gomez-Suaga, P.; Greig, J.; Troakes, C.; Noble, W.; Miller, C.C.J. Disruption of endoplasmic reticulum-mitochondria tethering proteins in post-mortem Alzheimer’s disease brain. Neurobiol. Dis. 2020, 143, 105020. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Leonzino, M.; Hancock-Cerutti, W.; Horenkamp, F.A.; Li, P.Q.; Lees, J.A.; Wheeler, H.; Reinisch, K.M.; De Camilli, P. VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J. Cell Biol. 2018, 217, 3625–3639. [Google Scholar] [CrossRef]
- Yeshaw, W.M.; van der Zwaag, M.; Pinto, F.; Lahaye, L.L.; Faber, A.I.E.; Gómez-Sánchez, R.; Dolga, A.M.; Poland, C.; Monaco, A.P.; van IJzendoorn, S.C.D.; et al. Human VPS13A is associated with multiple organelles and influences mitochondrial morphology and lipid droplet motility. Elife 2019, 8, e43561. [Google Scholar] [CrossRef]
- Freyre, C.A.C.; Rauher, P.C.; Ejsing, C.S.; Klemm, R.W. MIGA2 Links Mitochondria, the ER, and Lipid Droplets and Promotes De Novo Lipogenesis in Adipocytes. Mol. Cell 2019, 76, 811–825.e14. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Zhou, J.; Qiu, Y.; Shang, W.; Liu, J.P.; Wang, L.; Tong, C. Miga-mediated endoplasmic reticulum-mitochondria contact sites regulate neuronal homeostasis. Elife 2020, 9, e56584. [Google Scholar] [CrossRef]
- Roger, A.J. Reconstructing Early Events in Eukaryotic Evolution. Am. Nat. 1999, 154, S146–S163. [Google Scholar] [CrossRef]
- Rotte, C.; Henze, K.; Müller, M.; Martin, W. Origins of hydrogenosomes and mitochondria: Commentary. Curr. Opin. Microbiol. 2000, 3, 481–486. [Google Scholar] [CrossRef]
- Williams, B.A.P.; Hirt, R.P.; Lucocq, J.M.; Embley, T.M. A mitochondrial remnant in the microsporidian Trachipleistophora hominis. Nature 2002, 418, 865–869. [Google Scholar] [CrossRef]
- Embley, T.M.; Van Der Giezen, M.; Horner, D.S.; Dyal, P.L.; Foster, P.; Tielens, A.G.M.; Martin, W.; Tovar, J.; Douglas, A.E.; Cavalier-Smith, T.; et al. Mitochondria and hydrogenosomes are two forms of the same fundamental organelle. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2003, 358, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Tovar, J.; León-Avila, G.; Sánchez, L.B.; Sutak, R.; Tachezy, J.; Van Der Giezen, M.; Hernández, M.; Müller, M.; Lucocq, J.M. Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature 2003, 426, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Arisue, N.; Hasegawa, M.; Hashimoto, T. Root of the Eukaryota tree as inferred from combined maximum likelihood analyses of multiple molecular sequence data. Mol. Biol. Evol. 2005, 22, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C. Animal Evolution: Interrelationships of the Living Phyla; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Duff, M.O.; Olson, S.; Wei, X.; Garrett, S.C.; Osman, A.; Bolisetty, M.; Plocik, A.; Celniker, S.E.; Graveley, B.R. Genome-wide identification of zero nucleotide recursive splicing in Drosophila. Nature 2015, 521, 376–379. [Google Scholar] [CrossRef]
- Brown, G.R.; Hem, V.; Katz, K.S.; Ovetsky, M.; Wallin, C.; Ermolaeva, O.; Tolstoy, I.; Tatusova, T.; Pruitt, K.D.; Maglott, D.R.; et al. Gene: A gene-centered information resource at NCBI. Nucleic Acids Res. 2015, 43, D36–D42. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Sonnhammer, E.L.; von Heijne, G.; Krogh, A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1998, 6, 175–182. [Google Scholar]
- Hallgren, J.; Tsirigos, K.D.; Damgaard Pedersen, M.; Juan, J.; Armenteros, A.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv 2022. [Google Scholar] [CrossRef]
- Tsirigos, K.D.; Peters, C.; Shu, N.; Käll, L.; Elofsson, A. The TOPCONS web server for consensus prediction of membrane protein topology and signal peptides. Nucleic Acids Res. 2015, 43, W401–W407. [Google Scholar] [CrossRef]
- Bocharov, E.V.; Mineev, K.S.; Pavlov, K.V.; Akimov, S.A.; Kuznetsov, A.S.; Efremov, R.G.; Arseniev, A.S. Helix-helix interactions in membrane domains of bitopic proteins: Specificity and role of lipid environment. Biochim. Biophys. Acta Biomembr. 2017, 1859, 561–576. [Google Scholar] [CrossRef]
- Reid Alderson, T.; Pritišanac, I.; Kolaric, D.; Moses, A.M.; Forman-Kay, J.D.; Alderson, T.R.; Pritišanac, I.; Kolarić, Đ.; Moses, A.M.; Forman-Kay, J.D. Systematic identification of conditionally folded intrinsically disordered regions by AlphaFold2. Proc. Natl. Acad. Sci. USA 2023, 120, e2304302120. [Google Scholar] [CrossRef] [PubMed]
- Yariv, B.; Yariv, E.; Kessel, A.; Masrati, G.; Chorin, A.B.; Martz, E.; Mayrose, I.; Pupko, T.; Ben-Tal, N. Using evolutionary data to make sense of macromolecules with a “face-lifted” ConSurf. Protein Sci. 2023, 32, e4582. [Google Scholar] [CrossRef] [PubMed]
- Frishman, D.; Argos, P. Knowledge-based protein secondary structure assignment. Proteins Struct. Funct. Bioinforma. 1995, 23, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Furuita, K.; Jee, J.; Fukada, H.; Mishima, M.; Kojima, C. Electrostatic interaction between oxysterol-binding protein and VAMP-associated protein A revealed by NMR and mutagenesis studies. J. Biol. Chem. 2010, 285, 12961–12970. [Google Scholar] [CrossRef]
- Van De Peer, Y.; Maere, S.; Meyer, A. The evolutionary significance of ancient genome duplications. Nat. Rev. Genet. 2009, 10, 725–732. [Google Scholar] [CrossRef]
- Ohno, S. Evolution by Gene Duplication; Springer: Berlin/Heidelberg, Germany, 1970. [Google Scholar]
- Dehal, P.; Boore, J.L. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 2005, 3, e314. [Google Scholar] [CrossRef]
- Ohno, S. Gene duplication and the uniqueness of vertebrate genomes circa 1970–1999. Semin. Cell Dev. Biol. 1999, 10, 517–522. [Google Scholar] [CrossRef]
- Yu, D.; Ren, Y.; Uesaka, M.; Beavan, A.J.S.; Muffato, M.; Shen, J.; Li, Y.; Sato, I.; Wan, W.; Clark, J.W.; et al. Hagfish genome elucidates vertebrate whole-genome duplication events and their evolutionary consequences. Nat. Ecol. Evol. 2024, 8, 519–535. [Google Scholar] [CrossRef]
- Nakatani, Y.; Shingate, P.; Ravi, V.; Pillai, N.E.; Prasad, A.; McLysaght, A.; Venkatesh, B. Reconstruction of proto-vertebrate, proto-cyclostome and proto-gnathostome genomes provides new insights into early vertebrate evolution. Nat. Commun. 2021, 12, 4489. [Google Scholar] [CrossRef]
- Simakov, O.; Marlétaz, F.; Yue, J.X.; O’Connell, B.; Jenkins, J.; Brandt, A.; Calef, R.; Tung, C.H.; Huang, T.K.; Schmutz, J.; et al. Deeply conserved synteny resolves early events in vertebrate evolution. Nat. Ecol. Evol. 2020, 4, 820–830. [Google Scholar] [CrossRef]
- Peterson, K.J.; Beavan, A.; Chabot, P.J.; McPeek, M.A.; Pisani, D.; Fromm, B.; Simakov, O. MicroRNAs as Indicators into the Causes and Consequences of Whole-Genome Duplication Events. Mol. Biol. Evol. 2022, 39, msab344. [Google Scholar] [CrossRef] [PubMed]
- Haaf, A.; LeClaire, L.; Roberts, G.; Kent, H.M.; Roberts, T.M.; Stewart, M.; Neuhaus, D. Solution structure of the motile major sperm protein (MSP) of Ascaris suum-Evidence for two manganese binding sites and the possible role of bivalent cations in filament formation. J. Mol. Biol. 1998, 284, 1611–1624. [Google Scholar] [CrossRef]
- Shi, J.; Lua, S.; Tong, J.S.; Song, J. Elimination of the native structure and solubility of the hVAPB MSP domain by the Pro56Ser mutation that causes amyotrophic lateral sclerosis. Biochemistry 2010, 49, 3887–3897. [Google Scholar] [CrossRef]
- Neefjes, J.; Cabukusta, B. What the VAP: The Expanded VAP Family of Proteins Interacting With FFAT and FFAT-Related Motifs for Interorganellar Contact. Contact 2021, 4, 25152564211012250. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Takagi, M.; Kosako, H.; Hirano, T.; Yoshimura, S.H. Cell cycle-specific phase separation regulated by protein charge blockiness. Nat. Cell Biol. 2022, 24, 625–632. [Google Scholar] [CrossRef]
- Kim, A.S.; Kakalis, L.T.; Abdul-Manan, N.; Liu, G.A.; Rosen, M.K. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature 2000, 404, 151–158. [Google Scholar] [CrossRef]
- Simm, D.; Hatje, K.; Waack, S.; Kollmar, M. Critical assessment of coiled-coil predictions based on protein structure data. Sci. Rep. 2021, 11, 12439. [Google Scholar] [CrossRef]
- Simm, D.; Hatje, K.; Kollmar, M. Waggawagga: Comparative visualization of coiled-coil predictions and detection of stable single α-helices (SAH domains). Bioinformatics 2015, 31, 767–769. [Google Scholar] [CrossRef]
- Delorenzi, M.; Speed, T. An HMM model for coiled-coil domains and a comparison with PSSM-based predictions. Bioinformatics 2002, 18, 617–625. [Google Scholar] [CrossRef]
- Trigg, J.; Gutwin, K.; Keating, A.E.; Berger, B. Multicoil2: Predicting coiled coils and their oligomerization states from sequence in the twilight zone. PLoS ONE 2011, 6, e23519. [Google Scholar] [CrossRef]
- Lupas, A. Prediction and analysis of coiled-coil structures. Methods Enzymol. 1996, 266, 513–524. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, A.V.; Jiang, T.; Keating, A.E.; Berger, B. Paircoil2: Improved prediction of coiled coils from sequence. Bioinformatics 2006, 22, 356–358. [Google Scholar] [CrossRef]
- Armstrong, C.T.; Vincent, T.L.; Green, P.J.; Woolfson, D.N. SCORER 2.0: An algorithm for distinguishing parallel dimeric and trimeric coiled-coil sequences. Bioinformatics 2011, 27, 1908–1914. [Google Scholar] [CrossRef]
- Mahrenholz, C.C.; Abfalter, I.G.; Bodenhofer, U.; Volkmer, R.; Hochreiter, S. Complex networks govern coiled-coil oligomerization-Predicting and profiling by means of a machine learning approach. Mol. Cell. Proteom. 2011, 10, M110.004994. [Google Scholar] [CrossRef]
- Vincent, T.L.; Green, P.J.; Woolfson, D.N. LOGICOIL--multi-state prediction of coiled-coil oligomeric state. Bioinformatics 2013, 29, 69–76. [Google Scholar] [CrossRef]
- Cheng, J.; Randall, A.Z.; Sweredoski, M.J.; Baldi, P. SCRATCH: A protein structure and structural feature prediction server. Nucleic Acids Res. 2005, 33, W72–W76. [Google Scholar] [CrossRef]
- McGuffin, L.J.; Bryson, K.; Jones, D.T. The PSIPRED protein structure prediction server. Bioinformatics 2000, 16, 404–405. [Google Scholar] [CrossRef]
- Qureshi, T.; Goto, N.K. Impact of Differential Detergent Interactions on Transmembrane Helix Dimerization Affinities. ACS Omega 2016, 1, 277–285. [Google Scholar] [CrossRef]
- Russ, W.P.; Engelman, D.M. The GxxxG motif: A framework for transmembrane helix-helix association. J. Mol. Biol. 2000, 296, 911–919. [Google Scholar] [CrossRef]
- Strobl, G. The physics of Polymers: Concepts for Understanding Their Structures and Behavior, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 9783540252788. [Google Scholar]
- Creighton, T.E. Proteins: Structures and Molecular Properties; WH Freeman: New York, NY, USA, 1993. [Google Scholar]
- Evers, T.H.; Van Dongen, E.M.W.M.; Faesen, A.C.; Meijer, E.W.; Merkx, M. Quantitative understanding of the energy transfer between fluorescent proteins connected via flexible peptide linkers. Biochemistry 2006, 45, 13183–13192. [Google Scholar] [CrossRef]
- Kim, S.H.; Leal, S.S.; Halevy, D.B.; Gomes, C.M.; Lev, S. Structural requirements for VAP-B oligomerization and their implication in amyotrophic lateral sclerosis-associated VAP-B(P56S) neurotoxicity. J. Biol. Chem. 2010, 285, 13839–13849. [Google Scholar] [CrossRef] [PubMed]
- Jibiki, K.; Kodama, T.S.; Yasuhara, N. Importin alpha family NAAT/IBB domain: Functions of a pleiotropic long chameleon sequence. Adv. Protein Chem. Struct. Biol. 2023, 134, 175–209. [Google Scholar] [CrossRef] [PubMed]
- Williamson, M. How Proteins Work; Garland Science: New York, NY, USA, 2012. [Google Scholar]
- Huang, Q.; Szklarczyk, D.; Wang, M.; Simonovic, M.; Mering, C. von PaxDb 5.0: Curated Protein Quantification Data Suggests Adaptive Proteome Changes in Yeasts. Mol. Cell. Proteom. 2023, 22, 100640. [Google Scholar] [CrossRef] [PubMed]
- Milo, R. What is the total number of protein molecules per cell volume? A call to rethink some published values. BioEssays 2013, 35, 1050–1055. [Google Scholar] [CrossRef]
- Milo, R.; Phillips, R.; Orme, N. Cell Biology by the Numbers; Garland Science: New York, NY, USA; Taylor & Francis Group: Oxford, UK, 2016; ISBN 9780815345374. [Google Scholar]
- Skehel, P.A.; Fabian-Fine, R.; Kandel, E.R. Mouse VAP33 is associated with the endoplasmic reticulum and microtubules. Proc. Natl. Acad. Sci. USA 2000, 97, 1101–1106. [Google Scholar] [CrossRef]
- Tompa, P. Intrinsically disordered proteins: A 10-year recap. Trends Biochem. Sci. 2012, 37, 509–516. [Google Scholar] [CrossRef]
- Peng, Z.; Mizianty, M.J.; Kurgan, L. Genome-scale prediction of proteins with long intrinsically disordered regions. Proteins 2014, 82, 145–158. [Google Scholar] [CrossRef]
- Schad, E.; Tompa, P.; Hegyi, H. The relationship between proteome size, structural disorder and organism complexity. Genome Biol. 2011, 12, R120. [Google Scholar] [CrossRef]
- Bürgi, J.; Xue, B.; Uversky, V.N.; Van Der Goot, F.G. Intrinsic Disorder in Transmembrane Proteins: Roles in Signaling and Topology Prediction. PLoS ONE 2016, 11, e0158594. [Google Scholar] [CrossRef]
- Minezaki, Y.; Homma, K.; Nishikawa, K. Intrinsically disordered regions of human plasma membrane proteins preferentially occur in the cytoplasmic segment. J. Mol. Biol. 2007, 368, 902–913. [Google Scholar] [CrossRef]
- Latorraca, N.R.; Wang, J.K.; Bauer, B.; Townshend, R.J.L.; Hollingsworth, S.A.; Olivieri, J.E.; Xu, H.E.; Sommer, M.E.; Dror, R.O. Molecular mechanism of GPCR-mediated arrestin activation. Nature 2018, 557, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, P.; Bugge, K.; Nygaard, M.; Haxholm, G.W.; Martinsen, J.H.; Pedersen, M.N.; Arleth, L.; Boomsma, W.; Kragelund, B.B. Orchestration of signaling by structural disorder in class 1 cytokine receptors. Cell Commun. Signal. 2020, 18, 132. [Google Scholar] [CrossRef] [PubMed]
- Bush, M.; Alhanshali, B.M.; Qian, S.; Stanley, C.B.; Heller, W.T.; Matsui, T.; Weiss, T.M.; Nicholl, I.D.; Walz, T.; Callaway, D.J.E.; et al. An ensemble of flexible conformations underlies mechanotransduction by the cadherin–catenin adhesion complex. Proc. Natl. Acad. Sci. USA 2019, 116, 21545–21555. [Google Scholar] [CrossRef]
- Liao, Y.C.; Fernandopulle, M.S.; Wang, G.; Choi, H.; Hao, L.; Drerup, C.M.; Patel, R.; Qamar, S.; Nixon-Abell, J.; Shen, Y.; et al. RNA Granules Hitchhike on Lysosomes for Long-Distance Transport, Using Annexin A11 as a Molecular Tether. Cell 2019, 179, 147–164.e20. [Google Scholar] [CrossRef]
- Johnson, B.; Leek, A.N.; Solé, L.; Maverick, E.E.; Levine, T.P.; Tamkun, M.M. Kv2 potassium channels form endoplasmic reticulum/plasma membrane junctions via interaction with VAPA and VAPB. Proc. Natl. Acad. Sci. USA 2018, 115, 201805757. [Google Scholar] [CrossRef]
- Johnson, B.; Leek, A.N.; Tamkun, M.M. Kv2 channels create endoplasmic reticulum/plasma membrane junctions: A brief history of Kv2 channel subcellular localization. Channels 2019, 13, 88–101. [Google Scholar] [CrossRef]
- Kirmiz, M.; Gillies, T.E.; Dickson, E.J.; Trimmer, J.S. Neuronal ER-plasma membrane junctions organized by Kv2-VAP pairing recruit Nir proteins and affect phosphoinositide homeostasis. J. Biol. Chem. 2019, 294, 17735–17757. [Google Scholar] [CrossRef]
- Vullhorst, D.; Bloom, M.S.; Akella, N.; Buonanno, A. ER-PM Junctions on GABAergic Interneurons Are Organized by Neuregulin 2/VAP Interactions and Regulated by NMDA Receptors. Int. J. Mol. Sci. 2023, 24, 2908. [Google Scholar] [CrossRef]
- Serrano-Novillo, C.; Estadella, I.; Navarro-Pérez, M.; Oliveras, A.; de Benito-Bueno, A.; Socuéllamos, P.G.; Bosch, M.; Coronado, M.J.; Sastre, D.; Valenzuela, C.; et al. Routing of Kv7.1 to endoplasmic reticulum plasma membrane junctions. Acta Physiol. 2024, 240, e14106. [Google Scholar] [CrossRef]
- Hegyi, H.; Schad, E.; Tompa, P. Structural disorder promotes assembly of protein complexes. BMC Struct. Biol. 2007, 7, 65. [Google Scholar] [CrossRef]
- McCune, B.T.; Tang, W.; Lu, J.; Eaglesham, J.B.; Thorne, L.; Mayer, A.E.; Condiff, E.; Nice, T.J.; Goodfellow, I.; Krezel, A.M.; et al. Noroviruses Co-opt the Function of Host Proteins VAPA and VAPB for Replication via a Phenylalanine–Phenylalanine-Acidic-Tract-Motif Mimic in Nonstructural Viral Protein NS1/2. MBio 2017, 8, e00668-17. [Google Scholar] [CrossRef] [PubMed]
- Baron, Y.; Pedrioli, P.G.; Tyagi, K.; Johnson, C.; Wood, N.T.; Fountaine, D.; Wightman, M.; Alexandru, G. VAPB/ALS8 interacts with FFAT-like proteins including the p97 cofactor FAF1 and the ASNA1 ATPase. BMC Biol. 2014, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Ma-Lauer, Y.; Li, P.; Niemeyer, D.; Richter, A.; Pusl, K.; von Brunn, B.; Ru, Y.; Xiang, C.; Schwinghammer, S.; Liu, J.; et al. Oxysterole-binding protein targeted by SARS-CoV-2 viral proteins regulates coronavirus replication. Front. Cell. Infect. Microbiol. 2024, 14, 1383917. [Google Scholar] [CrossRef] [PubMed]
- Vormittag, S.; Hüsler, D.; Haneburger, I.; Kroniger, T.; Anand, A.; Prantl, M.; Barisch, C.; Maaß, S.; Becher, D.; Letourneur, F.; et al. Legionella- and host-driven lipid flux at LCV-ER membrane contact sites promotes vacuole remodeling. EMBO Rep. 2023, 24, e56007. [Google Scholar] [CrossRef] [PubMed]
- Ernst, W.L.; Shome, K.; Wu, C.C.; Gong, X.; Frizzell, R.A.; Aridor, M. VAMP-associated Proteins (VAP) as Receptors That Couple Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Proteostasis with Lipid Homeostasis. J. Biol. Chem. 2016, 291, 5206–5220. [Google Scholar] [CrossRef]
- Rimessi, A.; Pozzato, C.; Carparelli, L.; Rossi, A.; Ranucci, S.; de Fino, I.; Cigana, C.; Talarico, A.; Wieckowski, M.R.; Ribeiro, C.M.P.; et al. Pharmacological modulation of mitochondrial calcium uniporter controls lung inflammation in cystic fibrosis. Sci. Adv. 2020, 6, eaax9093. [Google Scholar] [CrossRef]
- Spires-Jones, T.L.; Attems, J.; Thal, D.R. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol. 2017, 134, 187–205. [Google Scholar] [CrossRef]
- Lu, C.; Ren, S.; Xie, W.; Zhao, Z.; Wu, X.; Guo, S.; Suo, A.; Zhou, N.; Yang, J.; Wu, S.; et al. Characterizing Relevant MicroRNA Editing Sites in Parkinson’s Disease. Cells 2022, 12, 75. [Google Scholar] [CrossRef]
- Mori, F.; Nakamura, Y.; Miki, Y.; Tanji, K.; Kon, T.; Tomiyama, M.; Kakita, A.; Wakabayashi, K. Alteration of Vesicle-Associated Membrane Protein-Binding Protein B in α-Synuclein Aggregates in Lewy Body Disease. J. Neuropathol. Exp. Neurol. 2022, 81, 807–815. [Google Scholar] [CrossRef]
- Markovinovic, A.; Martín-Guerrero, S.M.; Mórotz, G.M.; Salam, S.; Gomez-Suaga, P.; Paillusson, S.; Greig, J.; Lee, Y.; Mitchell, J.C.; Noble, W.; et al. Stimulating VAPB-PTPIP51 ER-mitochondria tethering corrects FTD/ALS mutant TDP43 linked Ca2+ and synaptic defects. Acta Neuropathol. Commun. 2024, 12, 32. [Google Scholar] [CrossRef]
- Mori, F.; Miki, Y.; Tanji, K.; Kon, T.; Tomiyama, M.; Kakita, A.; Wakabayashi, K. Role of VAPB and vesicular profiles in α-synuclein aggregates in multiple system atrophy. Brain Pathol. 2021, 31, e13001. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Anagnostou, G.; Chai, A.; Withers, J.; Morris, A.; Adhikaree, J.; Pennetta, G.; De Belleroche, J.S. Characterization of the properties of a novel mutation in VAPB in familial amyotrophic lateral sclerosis. J. Biol. Chem. 2010, 285, 40266–40281. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Dong, Y.; Wang, J.; Lu, J.H.; Chen, Y.; Wu, J. jun A novel mutation of VAPB in one Chinese familial amyotrophic lateral sclerosis pedigree and its clinical characteristics. J. Neurol. 2017, 264, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Kabashi, E.; El Oussini, H.; Bercier, V.; Gros-Louis, F.; Valdmanis, P.N.; Mcdearmid, J.; Mejier, I.A.; Dion, P.A.; Dupre, N.; Hollinger, D.; et al. Investigating the contribution of VAPB/ALS8 loss of function in amyotrophic lateral sclerosis. Hum. Mol. Genet. 2013, 22, 2350–2360. [Google Scholar] [CrossRef]
- van Blitterswijk, M.; van Es, M.A.; Koppers, M.; van Rheenen, W.; Medic, J.; Schelhaas, H.J.; van der Kooi, A.J.; de Visser, M.; Veldink, J.H.; van den Berg, L.H. VAPB and C9orf72 mutations in 1 familial amyotrophic lateral sclerosis patient. Neurobiol. Aging 2012, 33, 2950.e1–2950.e4. [Google Scholar] [CrossRef]
- Landers, J.E.; Leclerc, A.L.; Shi, L.; Virkud, A.; Cho, T.; Maxwell, M.M.; Henry, A.F.; Polak, M.; Glass, J.D.; Kwiatkowski, T.J.; et al. New VAPB deletion variant and exclusion of VAPB mutations in familial ALS. Neurology 2008, 70, 1179–1185. [Google Scholar] [CrossRef]
- Landrum, M.J.; Chitipiralla, S.; Kaur, K.; Brown, G.; Chen, C.; Hart, J.; Hoffman, D.; Jang, W.; Liu, C.; Maddipatla, Z.; et al. ClinVar: Updates to support classifications of both germline and somatic variants. Nucleic Acids Res. 2025, 53, D1313–D1321. [Google Scholar] [CrossRef]
- Rhizobium, G.E. UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2025, 53, 13–14. [Google Scholar] [CrossRef]
- Donadio, V.; Sturchio, A.; Rizzo, G.; Abu Rumeileh, S.; Liguori, R.; Espay, A.J. Pathology vs pathogenesis: Rationale and pitfalls in the clinicopathology model of neurodegeneration. Handb. Clin. Neurol. 2023, 192, 35–55. [Google Scholar] [CrossRef]
- Chin, I.M.; Gardell, Z.A.; Corces, M.R. Decoding polygenic diseases: Advances in noncoding variant prioritization and validation. Trends Cell Biol. 2024, 34, 465–483. [Google Scholar] [CrossRef]
- Alpy, F.; Rousseau, A.; Schwab, Y.; Legueux, F.; Stoll, I.; Wendling, C.; Spiegelhalter, C.; Kessler, P.; Mathelin, C.; Rio, M.C.; et al. STARD3 or STARD3NL and VAP form a novel molecular tether between late endosomes and the ER. J. Cell Sci. 2013, 126, 5500–5512. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Busnadiego, R.; Saheki, Y.; De Camilli, P. Three-dimensional architecture of extended synaptotagmin-mediated endoplasmic reticulum-plasma membrane contact sites. Proc. Natl. Acad. Sci. USA 2015, 112, E2004–E2013. [Google Scholar] [CrossRef] [PubMed]
- Subra, M.; Dezi, M.; Bigay, J.; Lacas-Gervais, S.; Di Cicco, A.; Araújo, A.R.D.; Abélanet, S.; Fleuriot, L.; Debayle, D.; Gautier, R.; et al. VAP-A intrinsically disordered regions enable versatile tethering at membrane contact sites. Dev. Cell 2023, 58, 121–138.e9. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Kumagai, K.; Yasuda, S.; Miura, Y.; Kawano, M.; Fukasawa, M.; Nishijima, M. Molecular machinery for non-vesicular trafficking of ceramide. Nature 2003, 426, 803–809. [Google Scholar] [CrossRef]
- Goto, A.; Egawa, D.; Tomishige, N.; Yamaji, T.; Shimasaki, K.; Kumagai, K.; Hanada, K. Involvement of a Cluster of Basic Amino Acids in Phosphorylation-Dependent Functional Repression of the Ceramide Transport Protein CERT. Int. J. Mol. Sci. 2022, 23, 8576. [Google Scholar] [CrossRef]
- Gehin, C.; Lone, M.A.; Lee, W.; Capolupo, L.; Ho, S.; Adeyemi, A.M.; Gerkes, E.H.; Stegmann, A.P.A.; López-Martín, E.; Bermejo-Sánchez, E.; et al. CERT1 mutations perturb human development by disrupting sphingolipid homeostasis. J. Clin. Investig. 2023, 133, e165019. [Google Scholar] [CrossRef]
- de la Mora, E.; Dezi, M.; Di Cicco, A.; Bigay, J.; Gautier, R.; Manzi, J.; Polidori, J.; Castaño-Díez, D.; Mesmin, B.; Antonny, B.; et al. Nanoscale architecture of a VAP-A-OSBP tethering complex at membrane contact sites. Nat. Commun. 2021, 12, 3459. [Google Scholar] [CrossRef]
- Wilhelm, L.P.; Wendling, C.; Védie, B.; Kobayashi, T.; Chenard, M.; Tomasetto, C.; Drin, G.; Alpy, F. STARD3 mediates endoplasmic reticulum-to-endosome cholesterol transport at membrane contact sites. EMBO J. 2017, 36, 1412–1433. [Google Scholar] [CrossRef]
- Kumagai, K.; Hanada, K. Structure, functions and regulation of CERT, a lipid-transfer protein for the delivery of ceramide at the ER-Golgi membrane contact sites. FEBS Lett. 2019, 593, 2366–2377. [Google Scholar] [CrossRef]
- Unterreitmeier, S.; Fuchs, A.; Schafler, T.; Heym, R.G.; Frishman, D.; Langosch, D. Phenylalanine promotes interaction of transmembrane domains via GxxxG motifs. J Mol. Biol. 2007, 374, 705–718. [Google Scholar] [CrossRef]
- Teese, M.G.; Langosch, D. Role of GxxxG Motifs in Transmembrane Domain Interactions. Biochemistry 2015, 54, 5125–5135. [Google Scholar] [CrossRef] [PubMed]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]

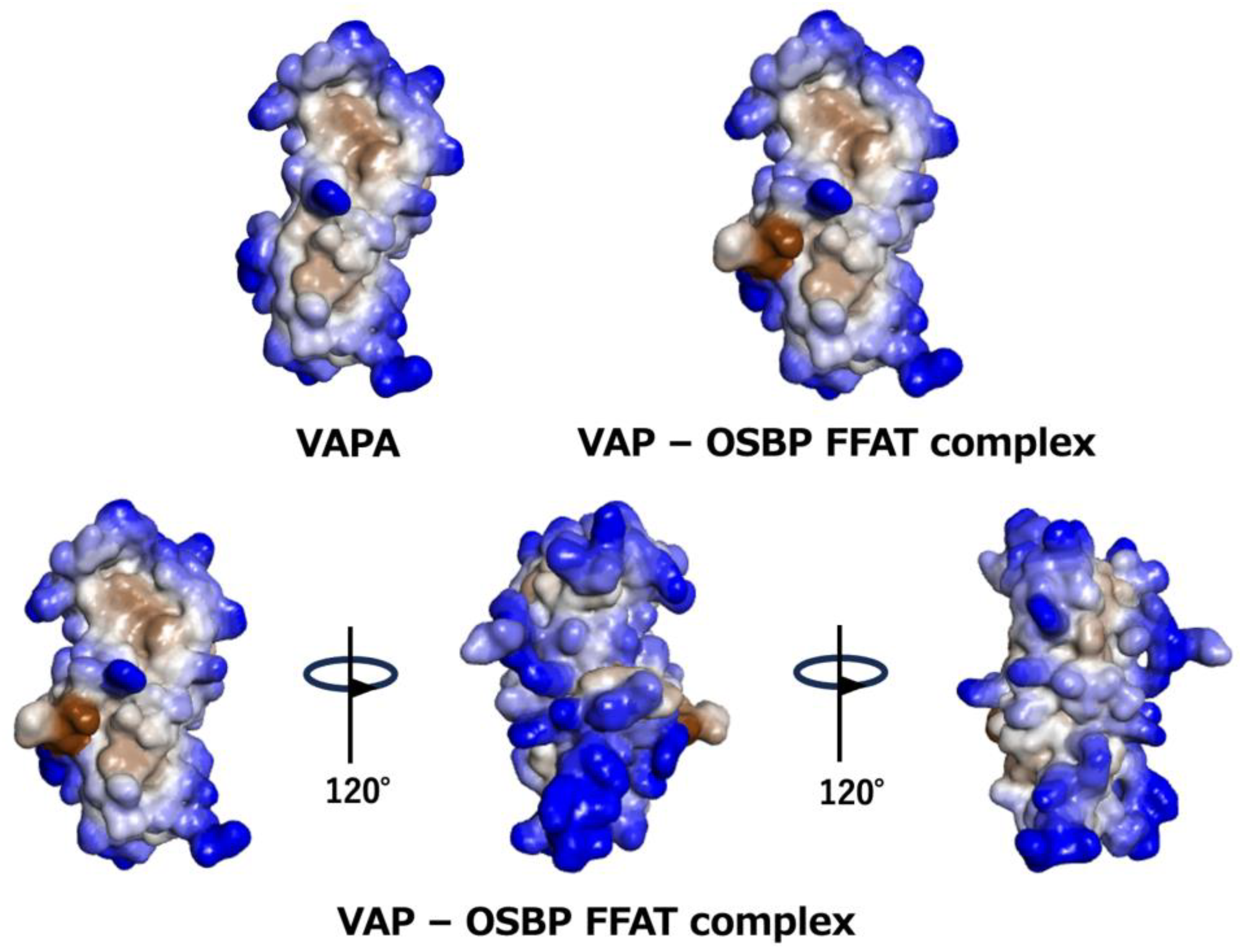
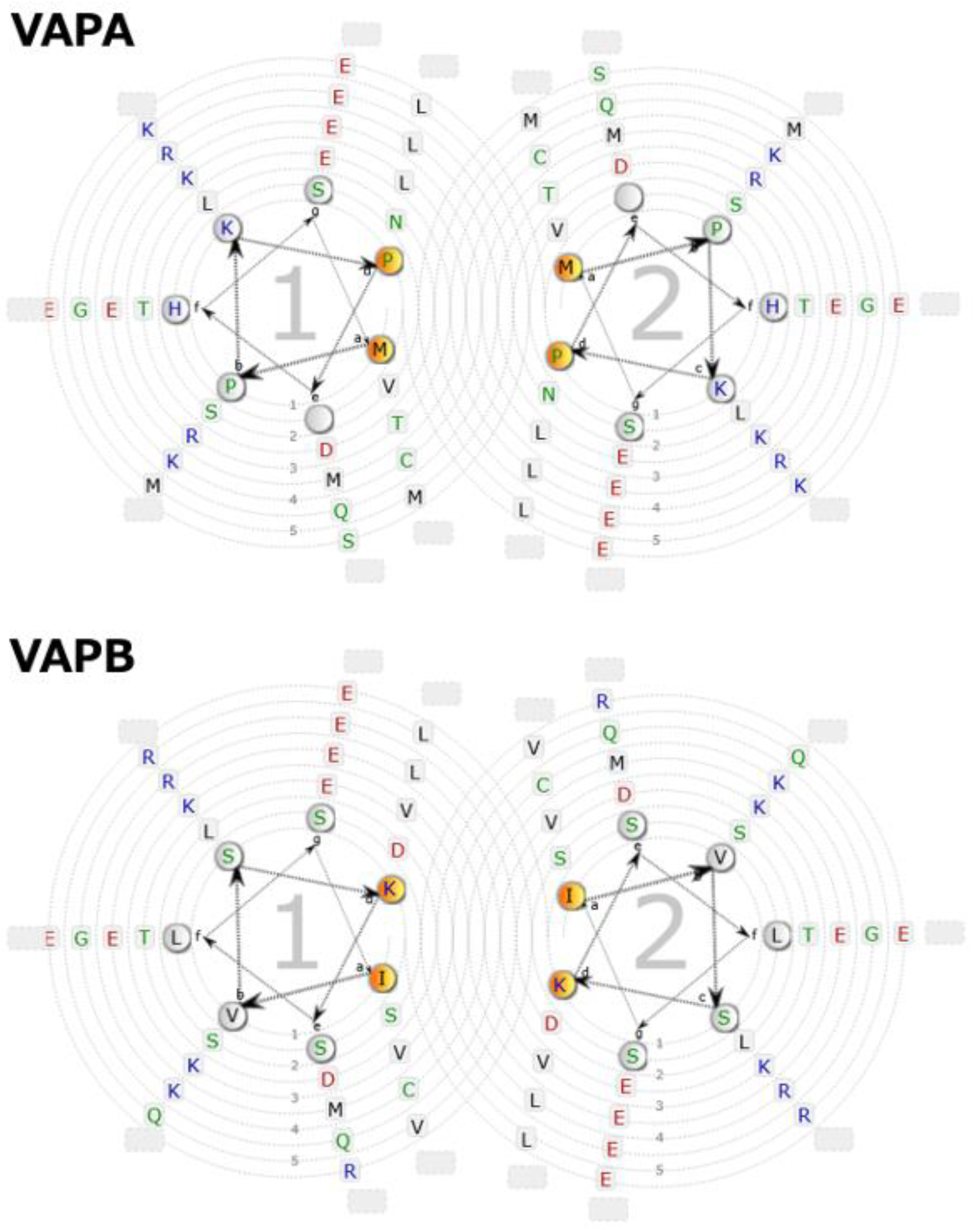
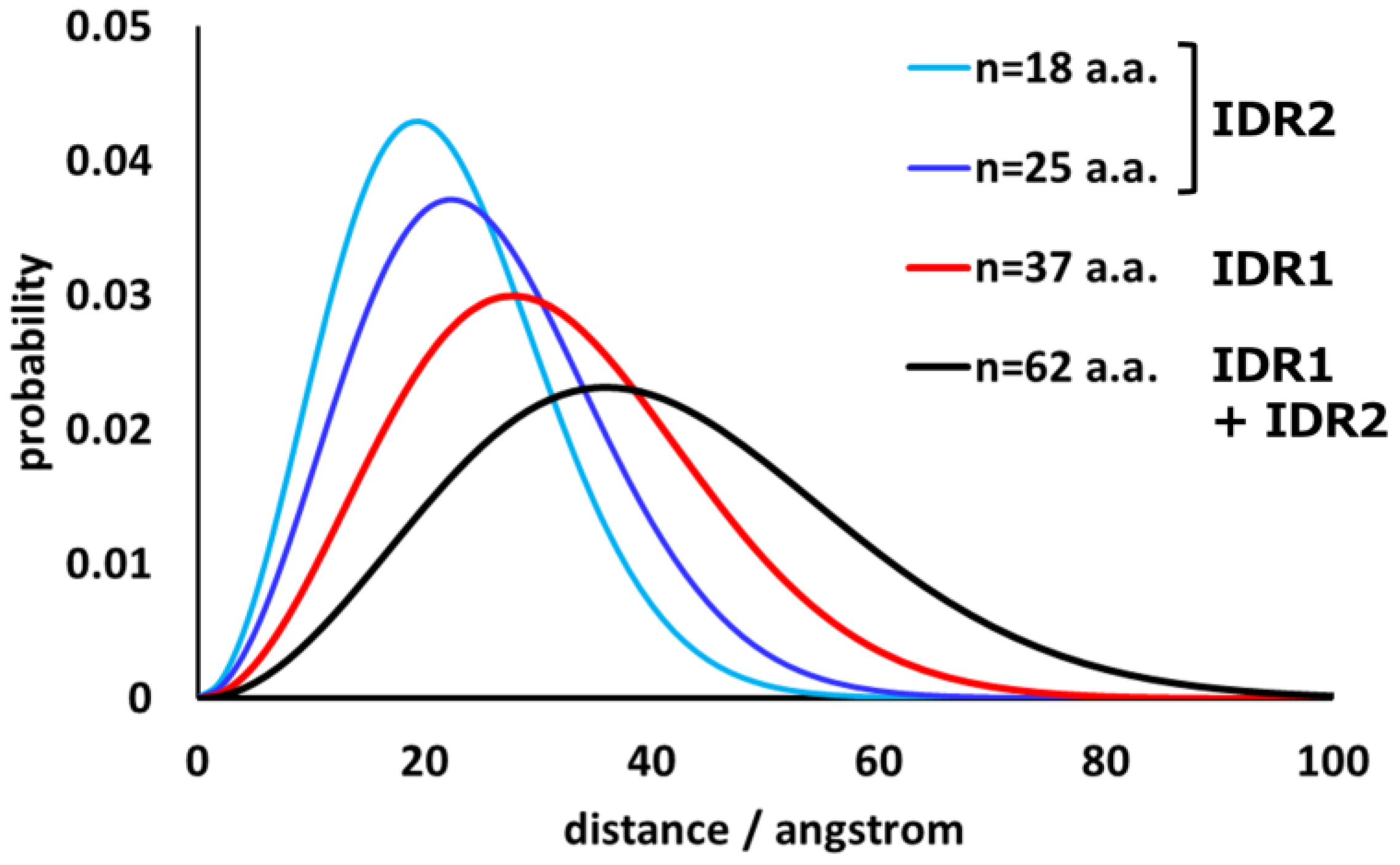

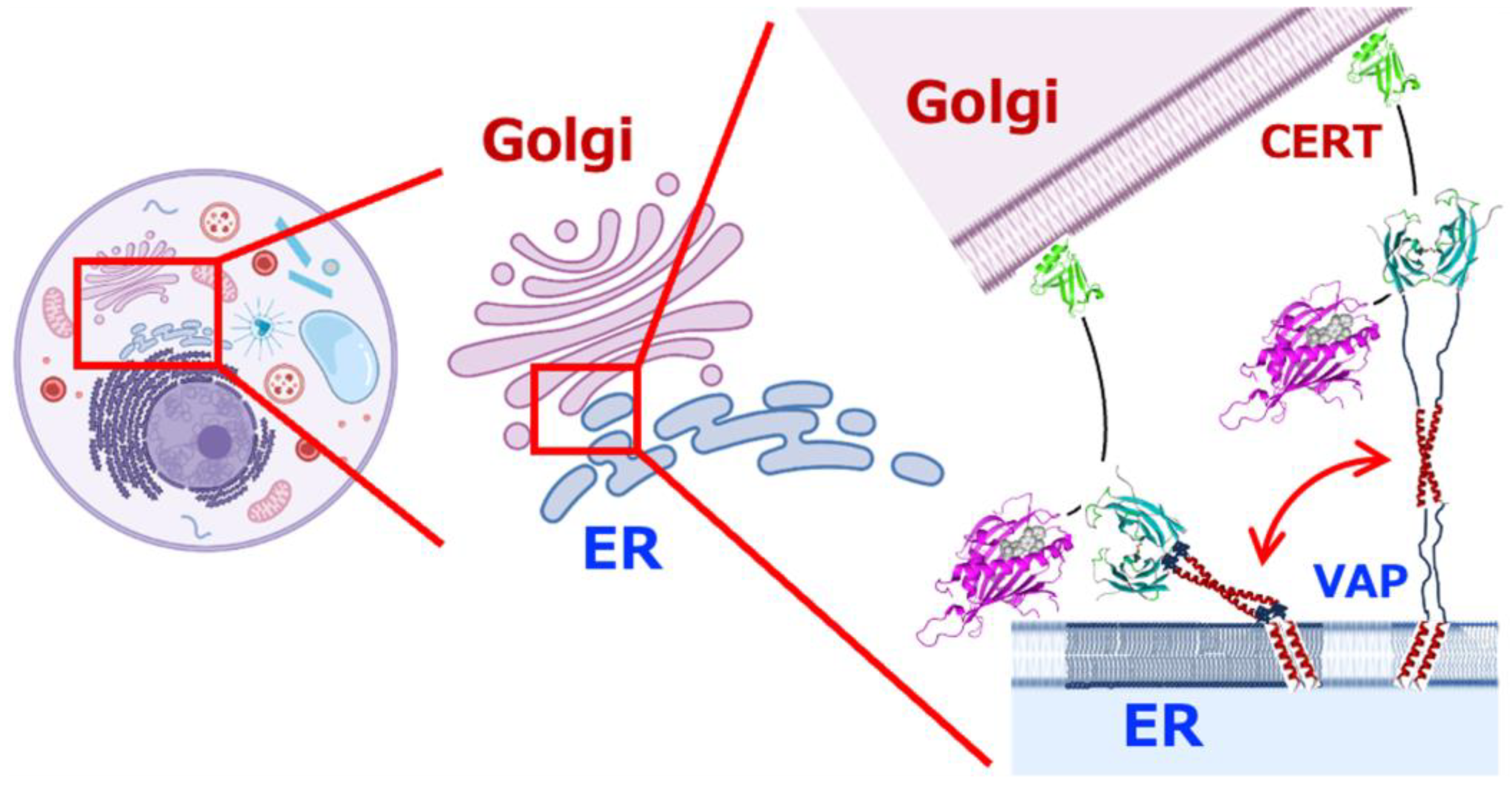
| PDB ID | Molecule | Species | MSPd Chain | FFAT Chain | Method | Resolution | Comments |
|---|---|---|---|---|---|---|---|
| 6lp4 | Scs2p | yeast | A | X-ray | 2.049 Å | ||
| 3ikk | VAP-B | human | A-B | X-ray | 2.5 Å | S-S dimer | |
| 2mdk | VAP-B | human | A | NMR | P56S in DPC | ||
| 7x14 | VAP-B | mouse | A | B | X-ray | 1.675 Å | |
| 2cri | VAP-A | mouse | A | NMR | |||
| 1z9l | VAP-A | rat | A | X-ray | 1.7 Å | ||
| 1z9o | VAP-A | rat | A, B, C, D, E, F | G, H, I, J, K, L | X-ray | 1.9 Å | include non S-S |
| 2rr3 | VAP-A | human | A | B | NMR | ||
| 6tqr | VAP-A | human | A, B, C, D | E, F | X-ray | 1.85 Å | S-S dimer |
| 6tqs | MOSPD2 | human | A, B, C, D, E, F | G, H, I, J, K | X-ray | 2.25 Å | |
| 6tqt | MOSPD2 | human | A | X-ray | 1.5 Å | ||
| 6tqu | MOSPD2 | human | A, B | C, D | X-ray | 2.4 Å | one Cys |
| 1wic | MOSPD2 | mouse | A | NMR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodama, T.S.; Furuita, K.; Kojima, C. Beyond Static Tethering at Membrane Contact Sites: Structural Dynamics and Functional Implications of VAP Proteins. Molecules 2025, 30, 1220. https://doi.org/10.3390/molecules30061220
Kodama TS, Furuita K, Kojima C. Beyond Static Tethering at Membrane Contact Sites: Structural Dynamics and Functional Implications of VAP Proteins. Molecules. 2025; 30(6):1220. https://doi.org/10.3390/molecules30061220
Chicago/Turabian StyleKodama, Takashi S., Kyoko Furuita, and Chojiro Kojima. 2025. "Beyond Static Tethering at Membrane Contact Sites: Structural Dynamics and Functional Implications of VAP Proteins" Molecules 30, no. 6: 1220. https://doi.org/10.3390/molecules30061220
APA StyleKodama, T. S., Furuita, K., & Kojima, C. (2025). Beyond Static Tethering at Membrane Contact Sites: Structural Dynamics and Functional Implications of VAP Proteins. Molecules, 30(6), 1220. https://doi.org/10.3390/molecules30061220






