One-Component Catalytic Electrodes from Metal–Organic Frameworks Covalently Linked to an Anion Exchange Ionomer
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis
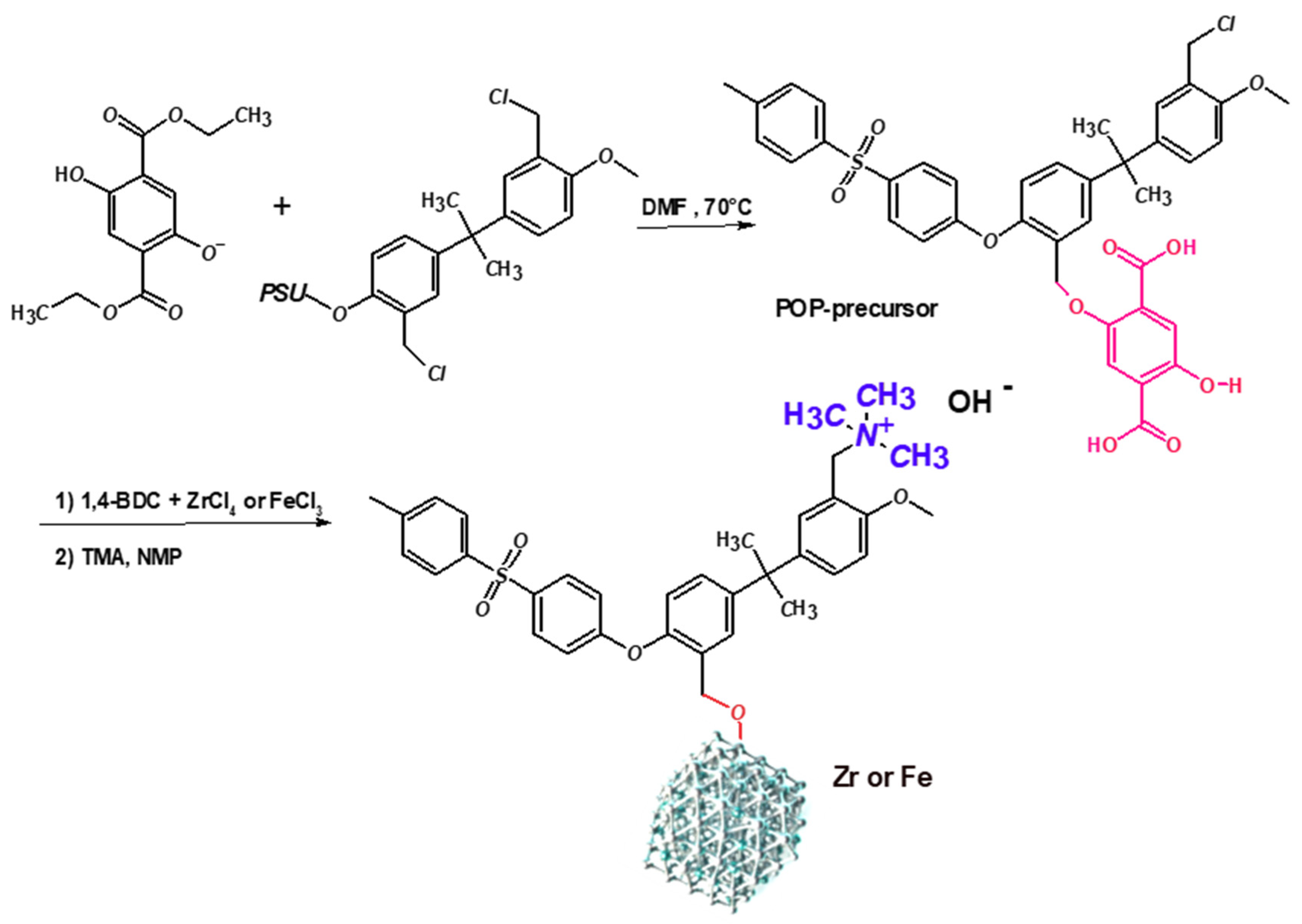
3.2.1. POP-Precursor
3.2.2. Hybrid Organic–Inorganic Polymers (Zr- and Fe-POP)
3.2.3. Quaternization of Zr- and Fe-POP
3.2.4. Electrode Fabrication
3.3. Characterization Techniques
3.3.1. Ion Exchange Capacity
3.3.2. 1H NMR Spectroscopy
3.3.3. FTIR Spectroscopy
3.3.4. X-Ray Powder Diffraction (XRD)
3.3.5. X-Ray Photoelectron Spectroscopy (XPS)
3.3.6. Thermogravimetric Analysis (TGA)
3.3.7. Brunauer–Emmett–Teller (BET) Analysis
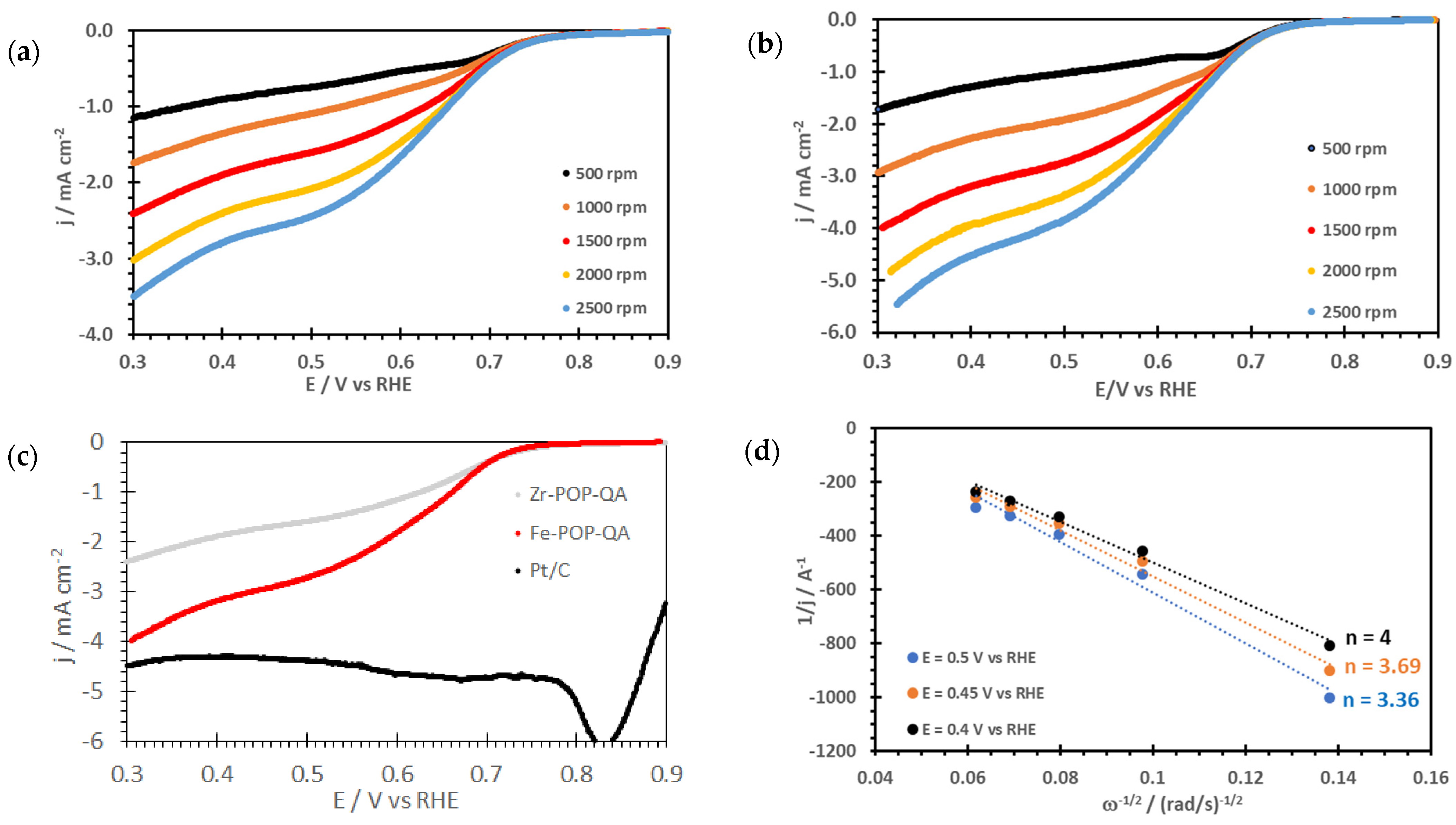
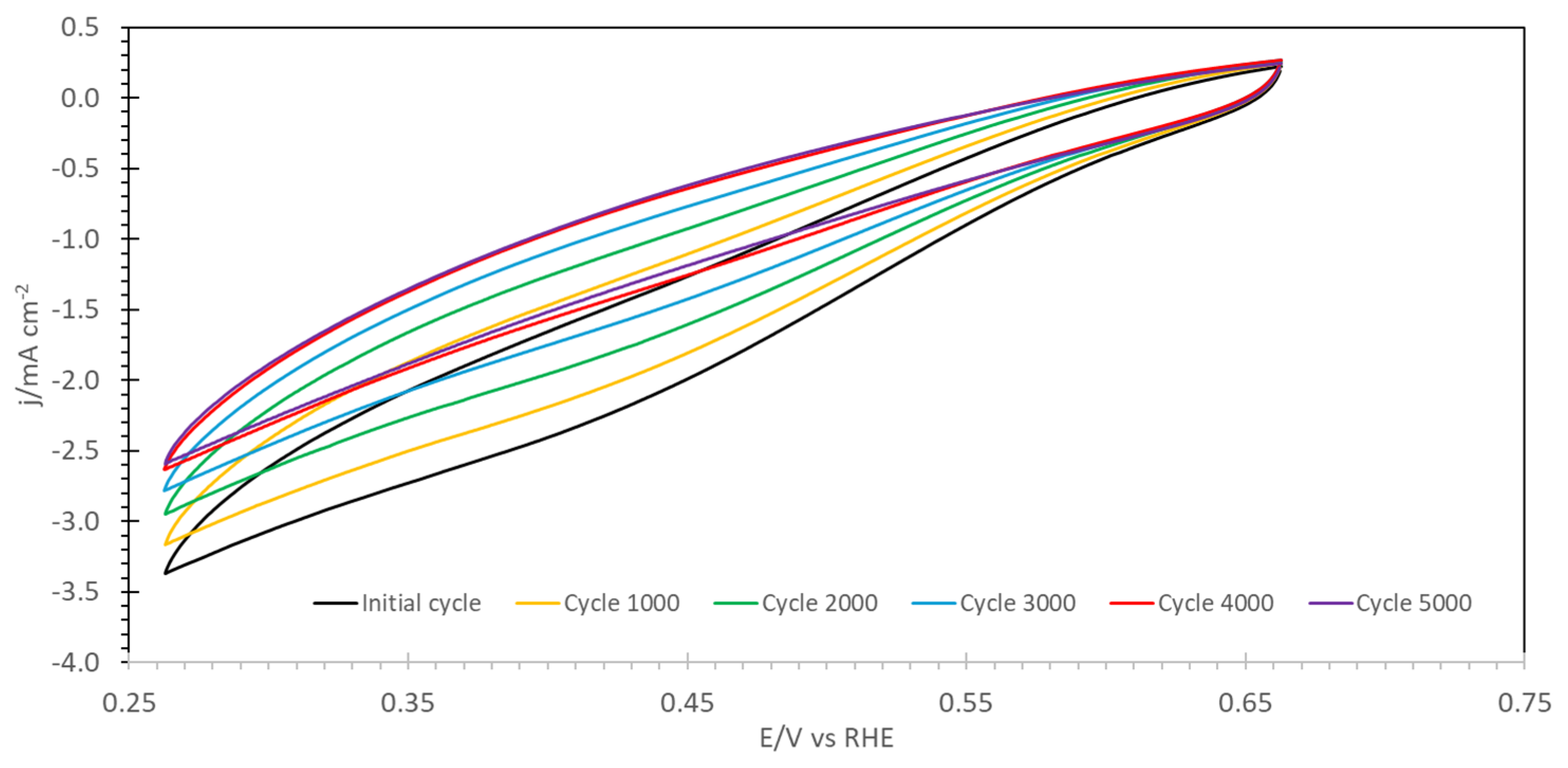
3.3.8. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalaj, M.; Bentz, K.C.; Ayala, S., Jr.; Palomba, J.M.; Barcus, K.S.; Katayama, Y.; Cohen, S.M. MOF-Polymer Hybrid Materials: From Simple Composites to Tailored Architectures. Chem. Rev. 2020, 120, 8267–8302. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-H.; Tao, Y.; Ding, X.; Han, B.-H. Porous organic polymers for electrocatalysis. Chem. Soc. Rev. 2022, 51, 761–791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xing, G.; Chen, W.; Chen, L. Porous organic polymers: A promising platform for efficient photocatalysis. Mater. Chem. Front. 2020, 4, 332–353. [Google Scholar] [CrossRef]
- Mohamed, M.G.; El-Mahdy, A.F.M.; Kotp, M.G.; Kuo, S.-W. Advances in porous organic polymers: Syntheses, structures, and diverse applications. Mater. Adv. 2022, 3, 707–733. [Google Scholar] [CrossRef]
- Naz, N.; Manzoor, M.H.; Naqvi, S.M.G.; Ehsan, U.; Aslam, M.; Verpoort, F. Porous organic polymers; an emerging material applied in energy, environmental and biomedical applications. Appl. Mater. Today 2024, 38, 102198. [Google Scholar] [CrossRef]
- Song, W.; Zhang, Y.; Tran, C.H.; Choi, H.K.; Yu, D.-G.; Kim, I. Porous organic polymers with defined morphologies: Synthesis, assembly, and emerging applications. Prog. Polym. Sci. 2023, 142, 101691. [Google Scholar] [CrossRef]
- Chen, W.; Chen, P.; Zhang, G.; Xing, G.; Feng, Y.; Yang, Y.-W.; Chen, L. Macrocycle-derived hierarchical porous organic polymers: Synthesis and applications. Chem. Soc. Rev. 2021, 50, 11684–11714. [Google Scholar] [CrossRef]
- Nallayagari, A.R.; Sgreccia, E.; Pasquini, L.; Sette, M.; Knauth, P.; Di Vona, M.L. Impact of Anion Exchange Ionomers on the Electrocatalytic Performance for the Oxygen Reduction Reaction of B-N Co-doped Carbon Quantum Dots on Activated Carbon. ACS Appl. Mater. Interfaces 2022, 14, 46537–46547. [Google Scholar] [CrossRef]
- Nallayagari, A.R.; Sgreccia, E.; Pasquini, L.; Vacandio, F.; Kaciulis, S.; Di Vona, M.L.; Knauth, P. Catalytic electrodes for the oxygen reduction reaction based on co-doped (B-N, Si-N, S-N) carbon quantum dots and anion exchange ionomer. Electrochim. Acta 2022, 427, 140861. [Google Scholar] [CrossRef]
- Knauth, P.; Sgreccia, E.; Nallayagari, A.R.; Pasquini, L.; Narducci, R.; Di Vona, M.L. Electrocatalytic composites of carbon quantum dots and anion exchange ionomers for the oxygen reduction reaction. Eur. J. Mater. 2023, 3, 2240350. [Google Scholar] [CrossRef]
- Jinnouchi, R.; Kudo, K.; Kodama, K.; Kitano, N.; Suzuki, T.; Minami, S.; Shinozaki, K.; Hasegawa, N.; Shinohara, A. The role of oxygen-permeable ionomer for polymer electrolyte fuel cells. Nat. Commun. 2021, 12, 4956. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pan, F.; Qin, C.; Wang, T.; Chen, K.-J. Porous Organic Polymers-Based Single-Atom Catalysts for Sustainable Energy-Related Electrocatalysis. Adv. Energy Mater. 2023, 13, 2301378. [Google Scholar] [CrossRef]
- Park, H.; Oh, S.; Lee, S.; Choi, S.; Oh, M. Cobalt- and nitrogen-codoped porous carbon catalyst made from core–shell type hybrid metal–organic framework (ZIF-L@ZIF-67) and its efficient oxygen reduction reaction (ORR) activity. Appl. Catal. B Environ. 2019, 246, 322–329. [Google Scholar] [CrossRef]
- Jahan, M.; Bao, Q.; Loh, K.P. Electrocatalytically Active Graphene–Porphyrin MOF Composite for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 6707–6713. [Google Scholar] [CrossRef]
- Xia, B.Y.; Yan, Y.; Li, N.; Wu, H.B.; Lou, X.W.; Wang, X. A metal–organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy 2016, 1, 15006. [Google Scholar] [CrossRef]
- Wang, H.-F.; Chen, L.; Pang, H.; Kaskel, S.; Xu, Q. MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev. 2020, 49, 1414–1448. [Google Scholar] [CrossRef]
- Mártire, A.P.; Segovia, G.M.; Azzaroni, O.; Rafti, M.; Marmisollé, W. Layer-by-layer integration of conducting polymers and metal organic frameworks onto electrode surfaces: Enhancement of the oxygen reduction reaction through electrocatalytic nanoarchitectonics. Mol. Syst. Des. Eng. 2019, 4, 893–900. [Google Scholar] [CrossRef]
- Guo, J.N.; Lin, C.Y.; Xia, Z.H.; Xiang, Z.H. A Pyrolysis-Free Covalent Organic Polymer for Oxygen Reduction. Angew. Chem.-Int. Ed. 2018, 57, 12567–12572. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, L.; Yang, Z.; Li, X.; Wen, Z.; Chen, L. Porous Organic Polymer Gel Derived Electrocatalysts for Efficient Oxygen Reduction. ChemElectroChem 2019, 6, 485–492. [Google Scholar] [CrossRef]
- Zhou, B.L.; Liu, L.Z.; Cai, P.W.; Zeng, G.; Li, X.Q.; Wen, Z.H.; Chen, L. Ferrocene-based porous organic polymer derived high-performance electrocatalysts for oxygen reduction. J. Mater. Chem. A 2017, 5, 22163–22169. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.-Y.; Day, G.S.; Ryder, M.R.; Zhou, H.-C. Destruction of Metal–Organic Frameworks: Positive and Negative Aspects of Stability and Lability. Chem. Rev. 2020, 120, 13087–13133. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.; Kim, J.Y.; Kim, M. Covalent connections between metal–organic frameworks and polymers including covalent organic frameworks. Chem. Soc. Rev. 2023, 52, 6379–6416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Nguyen, H.T.; Miller, S.A.; Cohen, S.M. polyMOFs: A Class of Interconvertible Polymer-Metal-Organic-Framework Hybrid Materials. Angew. Chem. Int. Ed. Engl. 2015, 54, 6152–6157. [Google Scholar] [CrossRef] [PubMed]
- Ayala, S.; Zhang, Z.; Cohen, S.M. Hierarchical structure and porosity in UiO-66 polyMOFs. Chem. Commun. 2017, 53, 3058–3061. [Google Scholar] [CrossRef]
- MacLeod, M.J.; Johnson, J.A. Block co-polyMOFs: Assembly of polymer–polyMOF hybrids via iterative exponential growth and “click” chemistry. Polym. Chem. 2017, 8, 4488–4493. [Google Scholar] [CrossRef]
- Gu, Y.; Huang, M.; Zhang, W.; Pearson, M.A.; Johnson, J.A. PolyMOF Nanoparticles: Dual Roles of a Multivalent polyMOF Ligand in Size Control and Surface Functionalization. Angew. Chem. Int. Ed. 2019, 58, 16676–16681. [Google Scholar] [CrossRef]
- Escorihuela, J.; Narducci, R.; Compañ, V.; Costantino, F. Proton Conductivity of Composite Polyelectrolyte Membranes with Metal-Organic Frameworks for Fuel Cell Applications. Adv. Mater. Interfaces 2019, 6, 1801146. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Wang, A.; Liu, X.; Chen, J.; Zeng, Q.; Zhang, L.; Liu, W.; Zhang, L. Covalently linked metal–organic framework (MOF)-polymer all-solid-state electrolyte membranes for room temperature high performance lithium batteries. J. Mater. Chem. A 2018, 6, 17227–17234. [Google Scholar] [CrossRef]
- Lin, R.; Ge, L.; Hou, L.; Strounina, E.; Rudolph, V.; Zhu, Z. Mixed Matrix Membranes with Strengthened MOFs/Polymer Interfacial Interaction and Improved Membrane Performance. ACS Appl. Mater. Interfaces 2014, 6, 5609–5618. [Google Scholar] [CrossRef]
- Tien-Binh, N.; Rodrigue, D.; Kaliaguine, S. In-situ cross interface linking of PIM-1 polymer and UiO-66-NH2 for outstanding gas separation and physical aging control. J. Membr. Sci. 2018, 548, 429–438. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Y.; Fang, W.; Shrestha, B.B.; Huang, M.; Jin, J. Constructing Strong Interfacial Interactions under Mild Conditions in MOF-Incorporated Mixed Matrix Membranes for Gas Separation. ACS Appl. Mater. Interfaces 2021, 13, 3166–3174. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Liu, T.; Li, Z.; Wang, S.; Li, Y.; Li, Z.; Ouyang, J.; Jiang, Z.; Wu, H. Mixed matrix membranes composed of sulfonated poly(ether ether ketone) and a sulfonated metal–organic framework for gas separation. J. Membr. Sci. 2015, 488, 67–78. [Google Scholar] [CrossRef]
- Li, Z.; He, G.; Zhao, Y.; Cao, Y.; Wu, H.; Li, Y.; Jiang, Z. Enhanced proton conductivity of proton exchange membranes by incorporating sulfonated metal-organic frameworks. J. Power Sources 2014, 262, 372–379. [Google Scholar] [CrossRef]
- Zhang, B.; Cao, Y.; Li, Z.; Wu, H.; Yin, Y.; Cao, L.; He, X.; Jiang, Z. Proton exchange nanohybrid membranes with high phosphotungstic acid loading within metal-organic frameworks for PEMFC applications. Electrochim. Acta 2017, 240, 186–194. [Google Scholar] [CrossRef]
- Narducci, R.; Sgreccia, E.; Knauth, P.; Di Vona, M.L. Anion Exchange Membranes with 1D, 2D and 3D Fillers: A Review. Polymers 2021, 13, 3887. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, C.-Y.V.; Chan, K.-Y.; Chen, Z.-N. Metal–Organic Framework Threaded with Aminated Polymer Formed in Situ for Fast and Reversible Ion Exchange. J. Am. Chem. Soc. 2014, 136, 7209–7212. [Google Scholar] [CrossRef]
- He, X.; Gang, M.; Li, Z.; He, G.; Yin, Y.; Cao, L.; Zhang, B.; Wu, H.; Jiang, Z. Highly conductive and robust composite anion exchange membranes by incorporating quaternized MIL-101(Cr). Sci. Bull. 2017, 62, 266–276. [Google Scholar] [CrossRef]
- Donnadio, A.; Narducci, R.; Casciola, M.; Marmottini, F.; D’Amato, R.; Jazestani, M.; Chiniforoshan, H.; Costantino, F. Mixed Membrane Matrices Based on Nafion/UiO-66/SO3H-UiO-66 Nano-MOFs: Revealing the Effect of Crystal Size, Sulfonation, and Filler Loading on the Mechanical and Conductivity Properties. ACS Appl. Mater. Interfaces 2017, 9, 42239–42246. [Google Scholar] [CrossRef]
- Ajpi, C.; Leiva, N.; Lundblad, A.; Lindbergh, G.; Cabrera, S. Synthesis and spectroscopic characterization of Fe3+-BDC metal organic framework as material for lithium ion batteries. J. Mol. Struct. 2023, 1272, 134127. [Google Scholar] [CrossRef]
- Scherb, C.; Schödel, A.; Bein, T. Directing the structure of metal-organic frameworks by oriented surface growth on an organic monolayer. Angew. Chem.-Int. Ed. 2008, 47, 5777–5779. [Google Scholar] [CrossRef]
- Di Vona, M.L.; Narducci, R.; Pasquini, L.; Pelzer, K.; Knauth, P. Anion-conducting ionomers: Study of type of functionalizing amine and macromolecular cross-linking. Int. J. Hydrogen Energy 2014, 39, 14039–14049. [Google Scholar] [CrossRef]
- Manning, G.S. Counterion binding in polyelectrolyte theory. Acc. Chem. Res. 1979, 12, 443–449. [Google Scholar] [CrossRef]
- Smith, B.C. The Carbonyl Group, Part V: Carboxylates—Coming Clean. Spectroscopy 2018, 33, 20–23. [Google Scholar]
- Pascual-Colino, J.; Artetxe, B.; Beobide, G.; Castillo, O.; Fidalgo-Mayo, M.L.; Isla-López, A.; Luque, A.; Mena-Gutiérrez, S.; Pérez-Yáñez, S. The Chemistry of Zirconium/Carboxylate Clustering Process: Acidic Conditions to Promote Carboxylate-Unsaturated Octahedral Hexamers and Pentanuclear Species. Inorg. Chem. 2022, 61, 4842–4851. [Google Scholar] [CrossRef]
- Namduri, H.; Nasrazadani, S. Quantitative analysis of iron oxides using Fourier transform infrared spectrophotometry. Corros. Sci. 2008, 50, 2493–2497. [Google Scholar] [CrossRef]
- Parak, S.; Nikseresht, A.; Alikarami, M.; Ghasemi, S. RSM optimization of biodiesel production by a novel composite of Fe(ΙΙΙ)-based MOF and phosphomolybdic acid. Res. Chem. Intermed. 2022, 48, 3773–3793. [Google Scholar] [CrossRef]
- Zhou, J.; Unlu, M.; Vega, J.A.; Kohl, P.A. Anionic polysulfone ionomers and membranes containing fluorenyl groups for anionic fuel cells. J. Power Sources 2009, 190, 285–292. [Google Scholar] [CrossRef]
- Solís, R.R.; Peñas-Garzón, M.; Belver, C.; Rodriguez, J.J.; Bedia, J. Highly stable UiO-66-NH2 by the microwave-assisted synthesis for solar photocatalytic water treatment. J. Environ. Chem. Eng. 2022, 10, 107122. [Google Scholar] [CrossRef]
- Conradie, J.; Erasmus, E. XPS Fe 2p peaks from iron tris(β-diketonates): Electronic effect of the β-diketonato ligand. Polyhedron 2016, 119, 142–150. [Google Scholar] [CrossRef]
- Derbali, Z.; Fahs, A.; Chailan, J.F.; Ferrari, I.V.; Di Vona, M.L.; Knauth, P. Composite anion exchange membranes with functionalized hydrophilic or hydrophobic titanium dioxide. Int. J. Hydrogen Energy 2017, 42, 19178–19189. [Google Scholar] [CrossRef]
- Navarathna, C.M.; Dewage, N.B.; Karunanayake, A.G.; Farmer, E.L.; Perez, F.; Hassan, E.; Mlsna, T.E.; Pittman, C.U. Rhodamine B Adsorptive Removal and Photocatalytic Degradation on MIL-53-Fe MOF/Magnetic Magnetite/Biochar Composites. J. Inorg. Organomet. Polym. Mater. 2020, 30, 214–229. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Virtanen, T.; Rudolph, G.; Lopatina, A.; Al-Rudainy, B.; Schagerlöf, H.; Puro, L.; Kallioinen, M.; Lipnizki, F. Analysis of membrane fouling by Brunauer-Emmet-Teller nitrogen adsorption/desorption technique. Sci. Rep. 2020, 10, 3427. [Google Scholar] [CrossRef]
- Zeng, S.; Lyu, F.; Sun, L.; Zhan, Y.; Ma, F.-X.; Lu, J.; Li, Y.Y. UiO-66-NO2 as an Oxygen “Pump” for Enhancing Oxygen Reduction Reaction Performance. Chem. Mater. 2019, 31, 1646–1654. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Z.; Lin, Y.; Zhang, Y.; Bi, P.; Jing, Q.; Luo, Y.; Sun, Z.; Liao, J.; Gao, Z. Molecular engineering of Fe-MIL-53 electrocatalyst for effective oxygen evolution reaction. Chem. Eng. J. 2023, 462, 142179. [Google Scholar] [CrossRef]
- Treimer, S.; Tang, A.; Johnson, D.C. A consideration of the application of Koutecky-Levich plots in the diagnoses of charge-transfer mechanisms at rotated disk electrodes. Electroanalysis 2002, 14, 165–171. [Google Scholar] [CrossRef]
- Wiberg, G.K.H.; Zana, A. Levich Analysis and the Apparent Potential Dependency of the Levich B Factor. Anal. Lett. 2016, 49, 2397–2404. [Google Scholar] [CrossRef]
- Davis, R.E.; Horvath, G.L.; Tobias, C.W. The solubility and diffusion coefficient of oxygen in potassium hydroxide solutions. Electrochim. Acta 1967, 12, 287–297. [Google Scholar] [CrossRef]
- Yan, W.Y.; Zheng, S.L.; Jin, W.; Peng, Z.; Wang, S.N.; Du, H.; Zhang, Y. The influence of KOH concentration, oxygen partial pressure and temperature on the oxygen reduction reaction at Pt electrodes. J. Electroanal. Chem. 2015, 741, 100–108. [Google Scholar] [CrossRef]
- Campos-Roldan, C.A.; Gonzalez-Huerta, R.G.; Alonso-Vante, N. Experimental Protocol for HOR and ORR in Alkaline Electrochemical Measurements. J. Electrochem. Soc. 2018, 165, J3001–J3007. [Google Scholar] [CrossRef]
- Sipos, P.M.; Hefter, G.; May, P.M. Viscosities and densities of highly concentrated aqueous MOH solutions (M+ = Na+, K+, Li+, Cs+, (CH3)(4)N+) at 25.0 degrees C. J. Chem. Eng. Data 2000, 45, 613–617. [Google Scholar] [CrossRef]
- Roy, S.; Bandyopadhyay, A.; Das, M.; Ray, P.P.; Pati, S.K.; Maji, T.K. Redox-active and semi-conducting donor-acceptor conjugated microporous polymers as metal-free ORR catalysts. J. Mater. Chem. A 2018, 6, 5587–5591. [Google Scholar] [CrossRef]
- Singh, A.; Verma, P.; Samanta, D.; Singh, T.; Maji, T.K. Bimodal Heterogeneous Functionality in Redox-Active Conjugated Microporous Polymer toward Electrocatalytic Oxygen Reduction and Photocatalytic Hydrogen Evolution. Chem.—A Eur. J. 2020, 26, 3810–3817. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.B.; Wang, K.; Wang, C.M.; Liu, W.P.; Pan, H.H.; Xiang, Y.J.; Qi, D.D.; Jiang, J.Z. Mixed phthalocyanine-porphyrin-based conjugated microporous polymers towards unveiling the activity origin of Fe-N4 catalysts for the oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 22851–22857. [Google Scholar] [CrossRef]
- Bai, J.; Li, R.C.; Huang, J.C.; Shang, X.F.; Wang, G.; Chao, S.J. Metal-free corrole-based donor-acceptor porous organic polymers as efficient bifunctional catalysts for hydrogen evolution and oxygen reduction reactions. Inorg. Chem. Front. 2024, 11, 5091–5102. [Google Scholar] [CrossRef]
- Mojet, B.L.; Ebbesen, S.D.; Lefferts, L. Light at the interface: The potential of attenuated total reflection infrared spectroscopy for understanding heterogeneous catalysis in water. Chem. Soc. Rev. 2010, 39, 4643–4655. [Google Scholar] [CrossRef]
- Di Vona, M.L.; Casciola, M.; Donnadio, A.; Nocchetti, M.; Pasquini, L.; Narducci, R.; Knauth, P. Anionic conducting composite membranes based on aromatic polymer and layered double hydroxides. Int. J. Hydrogen Energy 2017, 42, 3197–3205. [Google Scholar] [CrossRef]
- Narducci, R.; Chailan, J.F.; Fahs, A.; Pasquini, L.; Di Vona, M.L.; Knauth, P. Mechanical Properties of Anion Exchange Membranes by Combination of Tensile Stress-Strain Tests and Dynamic Mechanical Analysis. J. Polym. Sci. Part B-Polym. Phys. 2016, 54, 1180–1187. [Google Scholar] [CrossRef]



| Sample | C /µF | R1 /Ω | R2 /Ω | Q2 /µFs n−1 | n2 | Q3 /µFs n−1 | n3 |
|---|---|---|---|---|---|---|---|
| Fe-POP-QA | 728 | 44.6 | 2.7 | 42.4 | 0.82 | 1240 | 0.72 |
| Zr-POP-QA | 1170 | 53.6 | 3.8 | 54.0 | 0.74 | 1990 | 0.69 |
| Sample | Eon /V | E1/2 /V | n | b /mV | Ref. |
|---|---|---|---|---|---|
| Fe-POP-QA | 0.90 | 0.67 | 4.0 | 68 | This work |
| Zr-POP-QA | 0.90 | 0.68 | 3.2 | 60 | This work |
| Conjugated microporous polymers | 0.82 | - | 4.0 | - | [62] |
| Bola-amphiphilic conjugated microporous polymers | 0.81 | - | 3.8–3.9 | - | [63] |
| Phthalocyanine-porphyrin-based conjugated microporous polymers | 0.93 | 0.86 | 4.0 | 33 | [64] |
| Corrole-based POP | 0.81 | 0.66 | 3.0 | 90 | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narducci, R.; Sgreccia, E.; Montella, A.V.; Ercolani, G.; Kaciulis, S.; Syahputra, S.; Bloch, E.; Pasquini, L.; Knauth, P.; Di Vona, M.L. One-Component Catalytic Electrodes from Metal–Organic Frameworks Covalently Linked to an Anion Exchange Ionomer. Molecules 2025, 30, 1230. https://doi.org/10.3390/molecules30061230
Narducci R, Sgreccia E, Montella AV, Ercolani G, Kaciulis S, Syahputra S, Bloch E, Pasquini L, Knauth P, Di Vona ML. One-Component Catalytic Electrodes from Metal–Organic Frameworks Covalently Linked to an Anion Exchange Ionomer. Molecules. 2025; 30(6):1230. https://doi.org/10.3390/molecules30061230
Chicago/Turabian StyleNarducci, Riccardo, Emanuela Sgreccia, Alessio Vincenzo Montella, Gianfranco Ercolani, Saulius Kaciulis, Suanto Syahputra, Emily Bloch, Luca Pasquini, Philippe Knauth, and Maria Luisa Di Vona. 2025. "One-Component Catalytic Electrodes from Metal–Organic Frameworks Covalently Linked to an Anion Exchange Ionomer" Molecules 30, no. 6: 1230. https://doi.org/10.3390/molecules30061230
APA StyleNarducci, R., Sgreccia, E., Montella, A. V., Ercolani, G., Kaciulis, S., Syahputra, S., Bloch, E., Pasquini, L., Knauth, P., & Di Vona, M. L. (2025). One-Component Catalytic Electrodes from Metal–Organic Frameworks Covalently Linked to an Anion Exchange Ionomer. Molecules, 30(6), 1230. https://doi.org/10.3390/molecules30061230













