Enhancing the Magnetic Behaviors of Dy2 Complexes by Modulating the Crystal Field Environment with Different μ-O Bridging Ligands
Abstract
1. Introduction
2. Results and Discussion
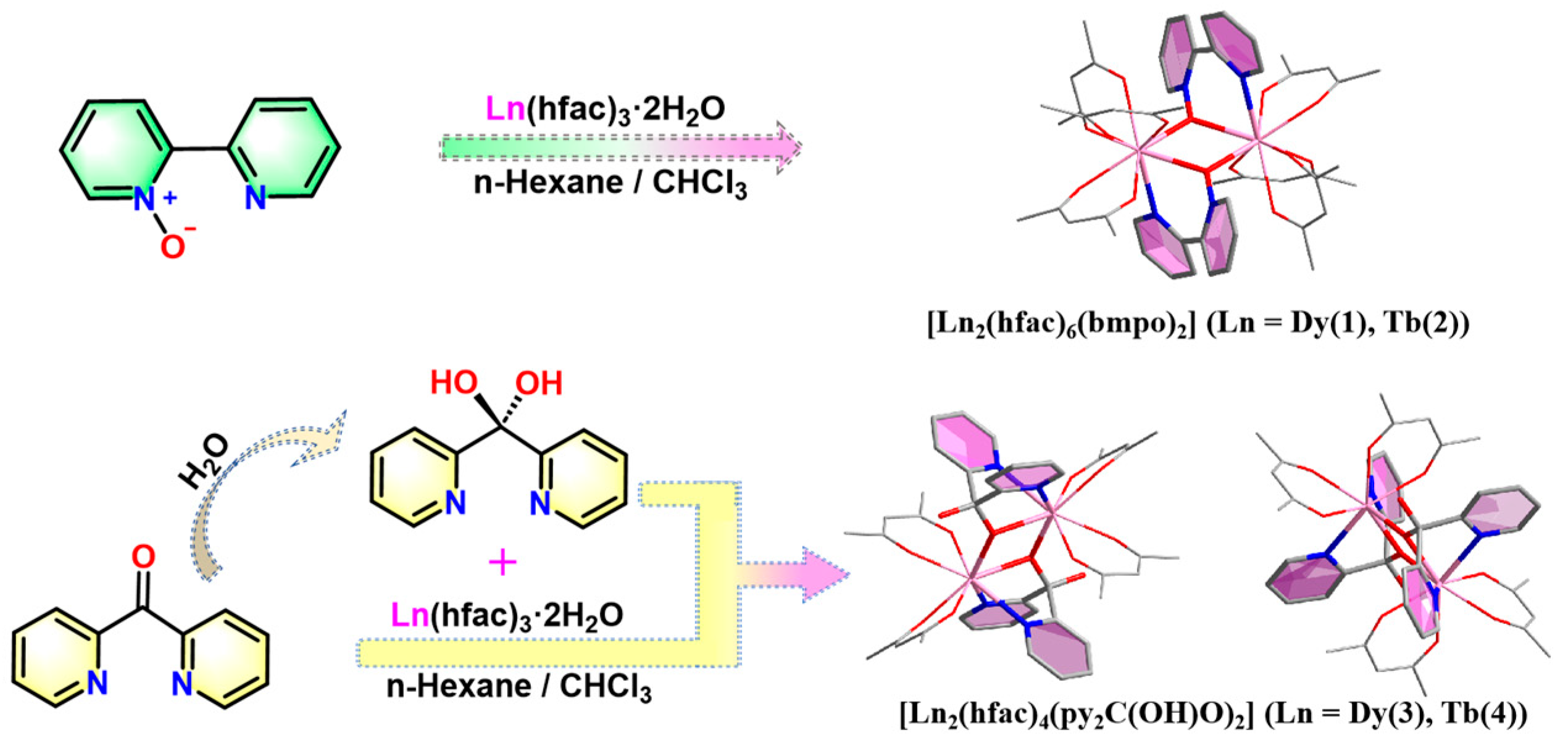
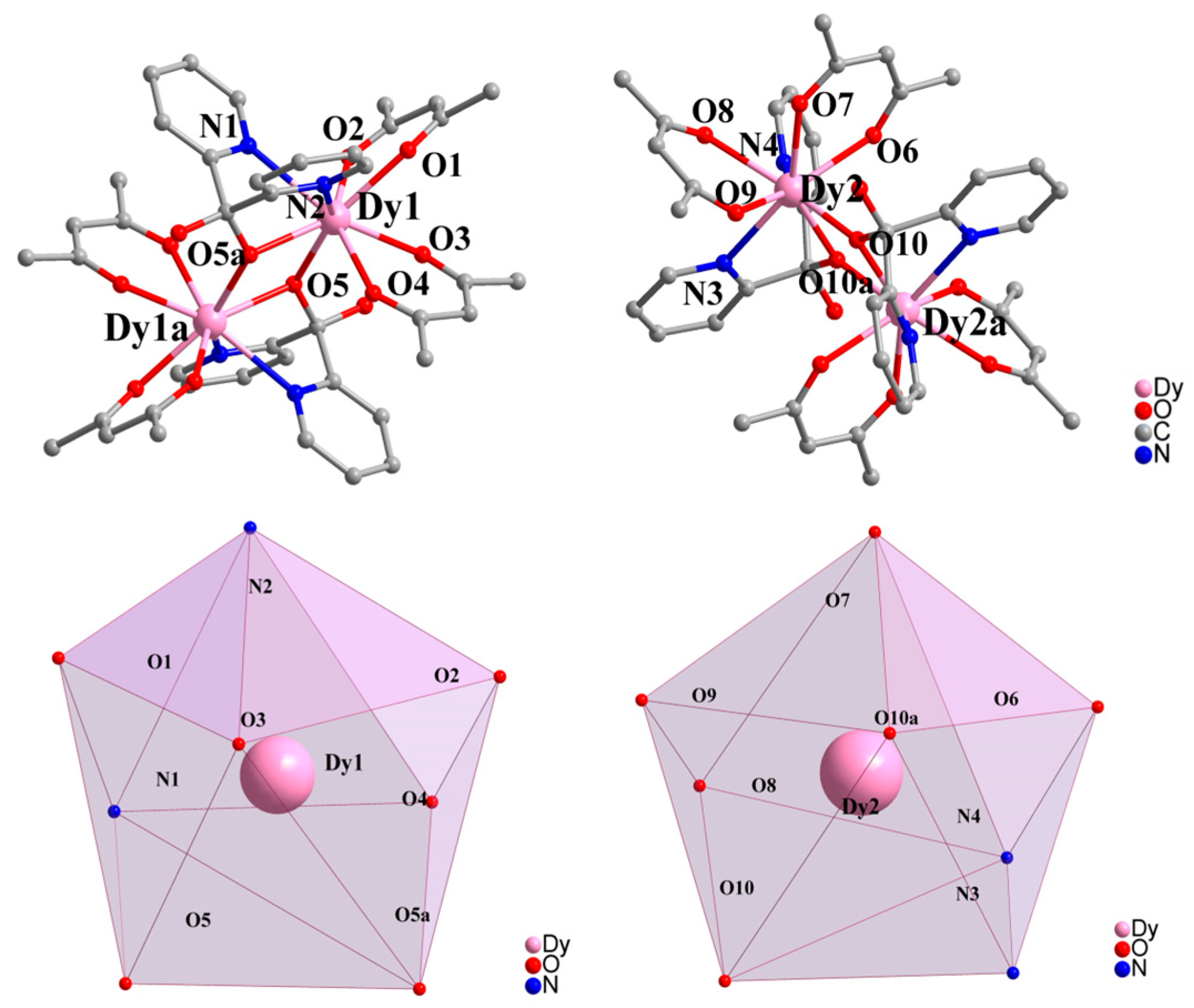
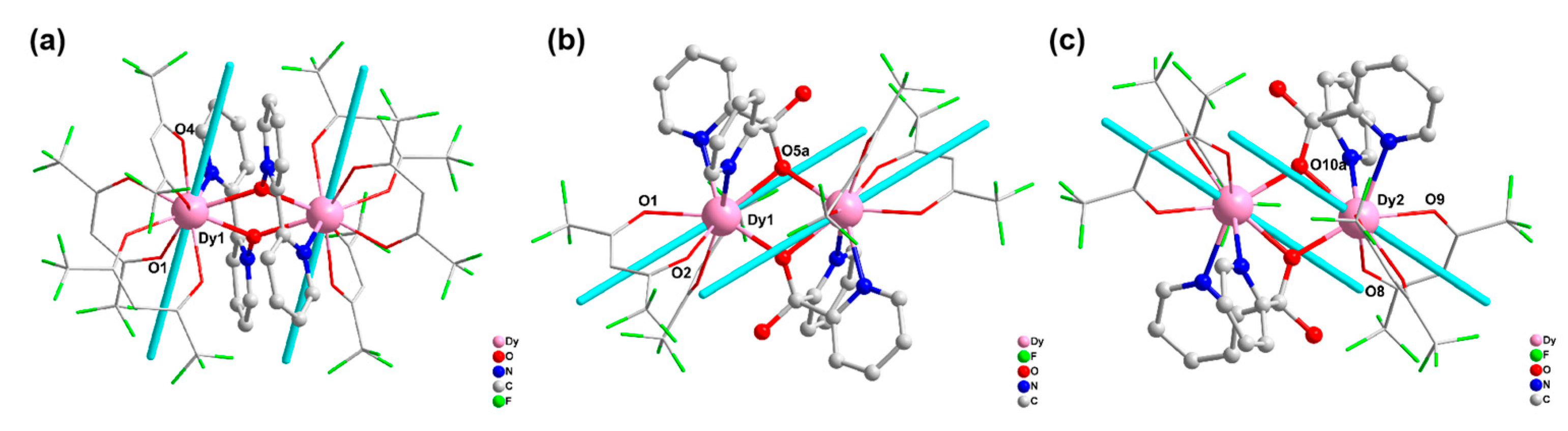
2.1. Crystal Structure Description
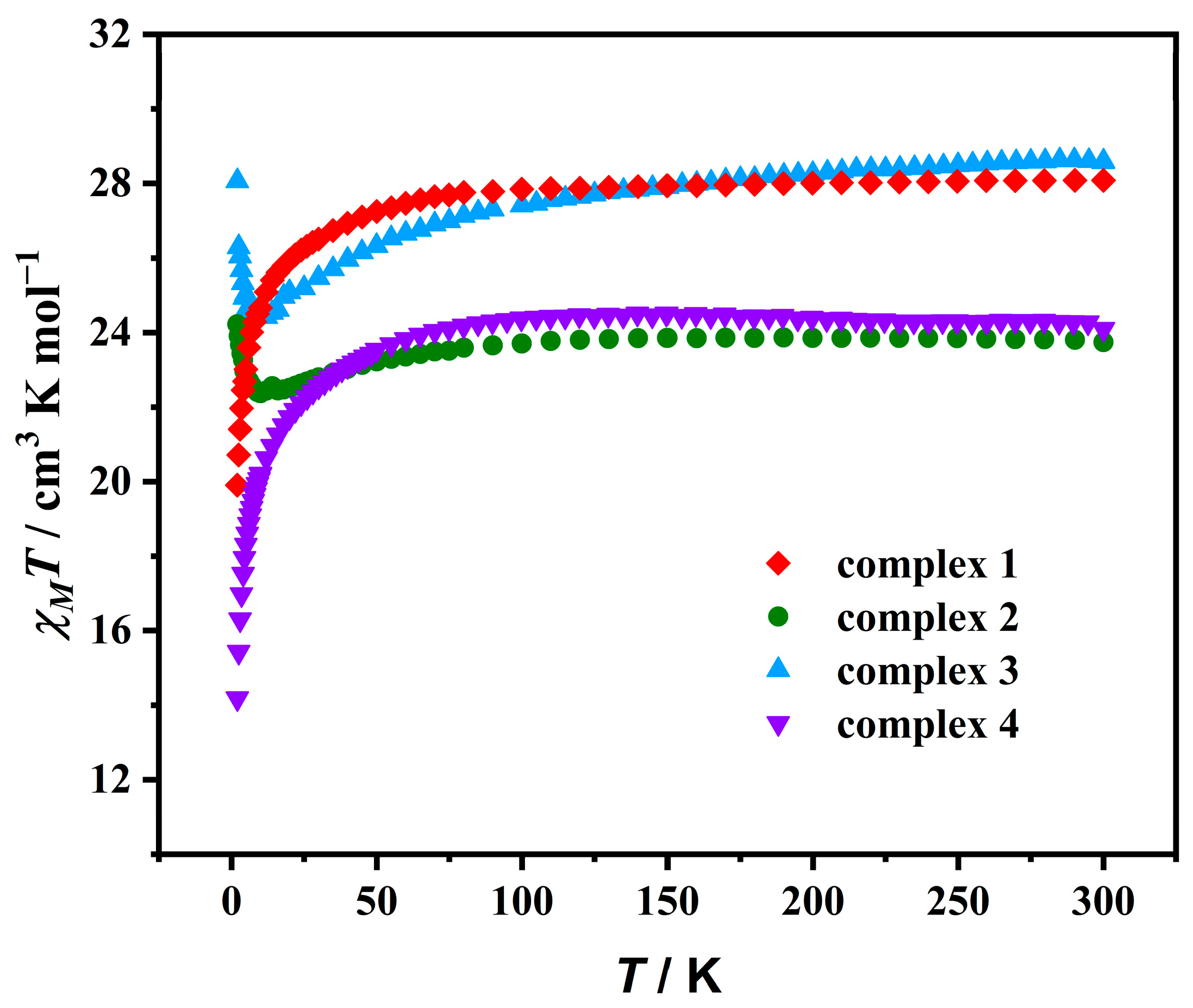
2.2. Static Magnetic Characterizations
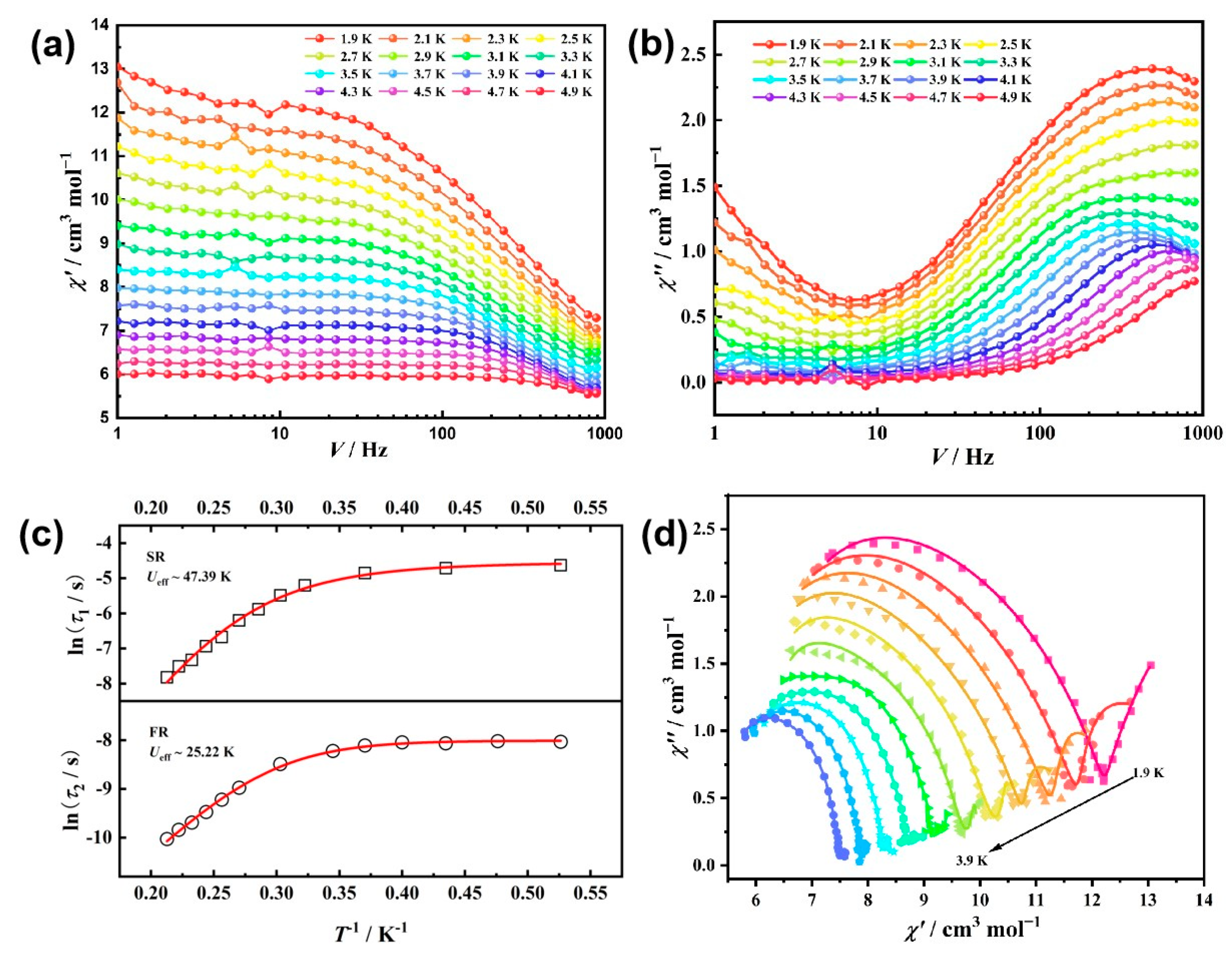
2.3. Dynamic Magnetic Measurements
2.4. Structure–Property Relationship
3. Experimental Section
3.1. Materials and Physical Techniques
3.2. X-Ray Crystallography
3.3. Synthesis of [Ln2(hfac)6(bmpo)2] (Ln = Dy(1), Tb(2))
3.4. Synthesis of [Ln2(hfac)4(py2C(OH)O)2] (Ln = Dy(3), Tb(4))
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronado, E. Molecular magnetism: From chemical design to spin control in molecules, materials and devices. Nat. Rev. Mater. 2020, 5, 87–104. [Google Scholar] [CrossRef]
- Mannini, M.; Pineider, F.; Sainctavit, P.; Danieli, C.; Otero, E.; Sciancalepore, C.; Talarico, A.M.; Arrio, M.-A.; Cornia, A.; Gatteschi, D.; et al. Magnetic memory of a single-molecule quantum magnet wired to a gold surface. Nat. Mater. 2009, 8, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Troiani, F.; Affronte, M. Molecular spins for quantum information technologies. Chem. Soc. Rev. 2011, 40, 3119–3129. [Google Scholar] [CrossRef] [PubMed]
- Aromí, G.; Aguilà, D.; Gamez, P.; Luis, F.; Roubeau, O. Design of magnetic coordination complexes for quantum computing. Chem. Soc. Rev. 2012, 41, 537–546. [Google Scholar] [CrossRef]
- Timco, G.A.; Faust, T.B.; Tuna, F.; Winpenny, R.E.P. Linking heterometallic rings for quantum information processing and amusement. Chem. Soc. Rev. 2011, 40, 3067–3075. [Google Scholar] [CrossRef]
- Leuenberger, M.N.; Loss, D. Quantum computing in molecular magnets. Nature 2001, 410, 789–793. [Google Scholar] [CrossRef]
- Habib, F.; Murugesu, M. Lessons learned from dinuclear lanthanide nano-magnets. Chem. Soc. Rev. 2013, 42, 3278–3288. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.-y.; Kaizu, Y. Lanthanide Double-Decker Complexes Functioning as Magnets at the Single-Molecular Level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef]
- Powell, A.K. Lanthanides and Actinides in Molecular Magnetism; Layfield, R.A., Murugesu, M., Eds.; Angewandte Chemie International Edition; Wiley: Hoboken, NJ, USA, 2015; Volume 54. [Google Scholar] [CrossRef]
- Liu, J.-L.; Chen, Y.-C.; Tong, M.-L. Symmetry strategies for high performance lanthanide-based single-molecule magnets. Chem. Soc. Rev. 2018, 47, 2431–2453. [Google Scholar] [CrossRef]
- Canaj, A.B.; Dey, S.; Martí, E.R.; Wilson, C.; Rajaraman, G.; Murrie, M. Insight into D6 Symmetry: Targeting Strong Axiality in Stable Dysprosium(III) Hexagonal Bipyramidal Single-Ion Magnets. Angew. Chem. Int. Ed. 2019, 58, 14146–14151. [Google Scholar] [CrossRef] [PubMed]
- Swain, A.; Sharma, T.; Rajaraman, G. Strategies to quench quantum tunneling of magnetization in lanthanide single molecule magnets. Chem. Commun. 2023, 59, 3206–3228. [Google Scholar] [CrossRef] [PubMed]
- Mannini, M.; Pineider, F.; Danieli, C.; Totti, F.; Sorace, L.; Sainctavit, P.; Arrio, M.A.; Otero, E.; Joly, L.; Cezar, J.C.; et al. Quantum tunnelling of the magnetization in a monolayer of oriented single-molecule magnets. Nature 2010, 468, 417–421. [Google Scholar] [CrossRef]
- Dey, A.; Bag, P.; Kalita, P.; Chandrasekhar, V. Heterometallic CuII–LnIII complexes: Single molecule magnets and magnetic refrigerants. Coord. Chem. Rev. 2021, 432, 213707. [Google Scholar] [CrossRef]
- Benelli, C.; Gatteschi, D. Magnetism of Lanthanides in Molecular Materials with Transition-Metal Ions and Organic Radicals. Chem. Rev. 2002, 102, 2369–2388. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhao, C.; Feng, T.; Liu, X.; Ying, X.; Li, X.-L.; Zhang, Y.-Q.; Tang, J. Air-Stable Chiral Single-Molecule Magnets with Record Anisotropy Barrier Exceeding 1800 K. J. Am. Chem. Soc. 2021, 143, 10077–10082. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.-C.; Liu, J.-L.; Vieru, V.; Ungur, L.; Jia, J.-H.; Chibotaru, L.F.; Lan, Y.; Wernsdorfer, W.; Gao, S.; et al. A Stable Pentagonal Bipyramidal Dy(III) Single-Ion Magnet with a Record Magnetization Reversal Barrier over 1000 K. J. Am. Chem. Soc. 2016, 138, 5441–5450. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef]
- Jiang, S.-D.; Wang, B.-W.; Su, G.; Wang, Z.-M.; Gao, S. A Mononuclear Dysprosium Complex Featuring Single-Molecule-Magnet Behavior. Angew. Chem. Int. Ed. 2010, 49, 7448–7451. [Google Scholar] [CrossRef]
- Zanella, S.; Aragon-Alberti, M.; Brite, C.D.S.; Salles, F.; Carlos, L.D.; Long, J. Luminescent Single-Molecule Magnets as Dual Magneto-Optical Molecular Thermometers. Angew. Chem. Int. Ed. 2023, 62, e202306970. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, N.; Mavragani, N.; Kitos, A.A.; Chartrand, D.; Maris, T.; Mansikkamäki, A.; Murugesu, M. Hard single-molecule magnet behavior and strong magnetic coupling in pyrazinyl radical-bridged lanthanide metallocenes. Chem 2024, 10, 2484–2499. [Google Scholar] [CrossRef]
- Deng, W.; Wu, S.-G.; Ruan, Z.-Y.; Gong, Y.-P.; Du, S.-N.; Wang, H.-L.; Chen, Y.-C.; Zhang, W.-X.; Liu, J.-L.; Tong, M.-L. Spin-State Control in Dysprosium(III) Metallacrown Magnets via Thioacetal Modification. Angew. Chem. Int. Ed. 2024, 63, e202404271. [Google Scholar] [CrossRef]
- Zhang, P.; Luo, Q.-C.; Zhu, Z.; He, W.; Song, N.; Lv, J.; Wang, X.; Zhai, Q.-G.; Zheng, Y.-Z.; Tang, J. Radical-Bridged Heterometallic Single-Molecule Magnets Incorporating Four Lanthanoceniums. Angew. Chem. Int. Ed. 2023, 62, e202218540. [Google Scholar] [CrossRef]
- Fernandez Garcia, G.; Guettas, D.; Montigaud, V.; Larini, P.; Sessoli, R.; Totti, F.; Cador, O.; Pilet, G.; Le Guennic, B. A Dy4 Cubane: A New Member in the Single-Molecule Toroics Family. Angew. Chem. Int. Ed. 2018, 57, 17089–17093. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wang, Y.; Wu, J.; Zhao, L.; Tang, J. Structures and magnetic properties of dysprosium complexes: The effect of crystallization temperature. Dalton Trans. 2017, 46, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Du, J.; Li, B.; Zhang, H.; Zhang, Y.; Sun, L.; Ma, P. Recent advances of dinuclear dysprosium-based single-molecule magnets: From mechanisms to application. J. Mater. Chem. C 2024, 12, 14754–14773. [Google Scholar] [CrossRef]
- Matheson, B.E.; Dais, T.N.; Donaldson, M.E.; Rowlands, G.J.; Plieger, P.G. The importance of second sphere interactions on single molecule magnet performance. Inorg. Chem. Front. 2023, 10, 6427–6439. [Google Scholar] [CrossRef]
- Long, J.; Habib, F.; Lin, P.-H.; Korobkov, I.; Enright, G.; Ungur, L.; Wernsdorfer, W.; Chibotaru, L.F.; Murugesu, M. Single-Molecule Magnet Behavior for an Antiferromagnetically Superexchange-Coupled Dinuclear Dysprosium(III) Complex. J. Am. Chem. Soc. 2011, 133, 5319–5328. [Google Scholar] [CrossRef]
- Guo, Y.-N.; Xu, G.-F.; Wernsdorfer, W.; Ungur, L.; Guo, Y.; Tang, J.; Zhang, H.-J.; Chibotaru, L.F.; Powell, A.K. Strong Axiality and Ising Exchange Interaction Suppress Zero-Field Tunneling of Magnetization of an Asymmetric Dy2 Single-Molecule Magnet. J. Am. Chem. Soc. 2011, 133, 11948–11951. [Google Scholar] [CrossRef]
- Wang, M.; Guo, Y.; Han, Z.; Cheng, X.; Zhang, Y.-Q.; Shi, W.; Cheng, P. Impact of Ligand Substituents on the Magnetization Dynamics of Mononuclear DyIII Single-Molecule Magnets. Inorg. Chem. 2022, 61, 9785–9791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-M.; Duan, Y.-Y.; Gao, H.-L.; Cui, J.-Z. Solvent-induced single-molecule magnet behavior and near-infrared luminescence properties of rare earth complexes. New J. Chem. 2020, 44, 19135–19143. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.-X.; Ge, Y.; Zhang, X.-M.; Xu, Y.; Li, Y.; Zhang, Y.-Q.; Yao, J.-L. Designing asymmetric Dy2 single-molecule magnets with two-step relaxation processes by the modification of the coordination environments of Dy(iii) ions. Dalton Trans. 2020, 49, 8976–8984. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-2014, Program for Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Sessoli, R.; Powell, A.K. Strategies towards single molecule magnets based on lanthanide ions. Coord. Chem. Rev. 2009, 253, 2328–2341. [Google Scholar] [CrossRef]
- Chilton, N.F.; Collison, D.; McInnes, E.J.L.; Winpenny, R.E.P.; Soncini, A. An electrostatic model for the determination of magnetic anisotropy in dysprosium complexes. Nat. Commun. 2013, 4, 2551. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Leng, J.-D.; Liu, J.-L.; Lin, W.-Q.; Gómez-Coca, S.; Aravena, D.; Ruiz, E.; Tong, M.-L. Unprecedented ferromagnetic dipolar interaction in a dinuclear holmium(iii) complex: A combined experimental and theoretical study. Chem. Commun. 2013, 49, 9341–9343. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, Y.-Q.; Zhu, Z.; Tang, J. Dysprosium Compounds with Hula-Hoop-like Geometries: The Influence of Magnetic Anisotropy and Magnetic Interactions on Magnetic Relaxation. Inorg. Chem. 2018, 57, 12213–12221. [Google Scholar] [CrossRef]
- Karbakhsh Ravari, A.; Pineda-Galvan, Y.; Huynh, A.; Ezhov, R.; Pushkar, Y. Facile Light-Induced Transformation of [RuII(bpy)2(bpyNO)]2+ to [RuII(bpy)3]2+. Inorg. Chem. 2020, 59, 13880–13887. [Google Scholar] [CrossRef]
- Richardson, M.F.; Wagner, W.F.; Sands, D.E. Rare-earth trishexafluoroacetylacetonates and related compounds. J. Inorg. Nucl. Chem. 1968, 30, 1275–1289. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, W.-B.; OuYang, Z.-J.; Yang, M.; Zhang, Y.-Q.; Gao, S.; Schulze, M.; Wernsdorfer, W.; Dong, W. Unprecedented one-dimensional chain and two-dimensional network dysprosium(iii) single-molecule toroics with white-light emission. Chem. Commun. 2020, 56, 2590–2593. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-S.; Chen, Y.; Hu, Z.-B.; Yin, C.-L.; Zhang, Z.; Pan, Z.-Q. Modulation of the directions of the anisotropic axes of DyIII ions through utilizing two kinds of organic ligands or replacing DyIII ions by FeIII ions. CrystEngComm 2019, 21, 5429–5439. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-2014, Program for Solution of Crystal Structures; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE 2.1; University of Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Wang, H.-S.; Zhang, K.; Song, Y.; Pan, Z.-Q. Recent advances in 3d-4f magnetic complexes with several types of non-carboxylate organic ligands. Inorganica Chim. Acta 2021, 521, 120318. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Rees, B.; Jenner, L.; Yusupov, M. Bulk-solvent correction in large macromolecular structures. Acta Crystallogr. Sect. D 2005, 61, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Hine, J.; Green, L.R.; Meng, P.C., Jr.; Thiagarajan, V. Structural effects on rates and equilibriums. XX. Rates and equilibriums in hydration and bisulfate addition by 1,3-dimethoxyacetone. J. Org. Chem. 1976, 41, 3343–3349. [Google Scholar] [CrossRef]
- Kokesh, F.C. The determination by proton nuclear magnetic resonance of the enol, hydrate and keto forms of oxaloacetic acid and its anions. J. Org. Chem. 1976, 41, 3593–3599. [Google Scholar] [CrossRef]
- Sayer, J.M. Hydration of p-nitrobenzaldehyde. J. Org. Chem. 1975, 40, 2545–2547. [Google Scholar] [CrossRef]
- Deveson, A.C.; Heath, S.L.; Harding, C.J.; Powell, A.K. Synthesis and structures of copper(II) anion-bridged aggregates and chains: Control over molecular shape. J. Chem. Soc. Dalton Trans. 1996, 15, 3173–3178. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Tangoulis, V.; Raptopoulou, C.P.; Terzis, A.; Kessissoglou, D.P. First Example of a CuII Polymeric Complex Having a Tetranuclear Repeating Unit with a S = 2 Ground State. Crystal Structure of [Cu4(dpk·CH3O)2Cl6]n (dpk·CH3OH = Unimethylated Diol of Di-2-pyridyl Ketone). Inorg. Chem. 1996, 35, 559–565. [Google Scholar] [CrossRef]
- Parker, O.J.; Aubol, S.L.; Breneman, G.L. Crystal structure of a copper stabilized hydrate of di-2-pyridyl ketone. Polyhedron 2000, 19, 623–626. [Google Scholar] [CrossRef]
- Moreno Pineda, E.; Chilton, N.F.; Marx, R.; Dörfel, M.; Sells, D.O.; Neugebauer, P.; Jiang, S.-D.; Collison, D.; van Slageren, J.; McInnes, E.J.L.; et al. Direct measurement of dysprosium(III)˙˙˙dysprosium(III) interactions in a single-molecule magnet. Nat. Commun. 2014, 5, 5243. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wang, L.; Zhu, G.-Z.; Liu, Y.-H.; Yang, H.; Li, X.; Yang, K. Controllable syntheses and magnetic properties of novel homoleptic triple-decker lanthanide complexes. Dalton Trans. 2019, 48, 13360–13368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-Y.; Tian, Y.-M.; Li, H.-F.; Chen, P.; Sun, W.-B.; Zhang, Y.-Q.; Yan, P.-F. A series of dinuclear Dy(iii) complexes bridged by 2-methyl-8-hydroxylquinoline: Replacement on the periphery coordinated β-diketonate terminal leads to different single-molecule magnetic properties. Dalton Trans. 2016, 45, 3863–3873. [Google Scholar] [CrossRef]
- Wang, W.-M.; Zhang, H.-X.; Wang, S.-Y.; Shen, H.-Y.; Gao, H.-L.; Cui, J.-Z.; Zhao, B. Ligand Field Affected Single-Molecule Magnet Behavior of Lanthanide(III) Dinuclear Complexes with an 8-Hydroxyquinoline Schiff Base Derivative as Bridging Ligand. Inorg. Chem. 2015, 54, 10610–10622. [Google Scholar] [CrossRef]
- Xiao, Z.-X.; Miao, H.; Shao, D.; Wei, H.-Y.; Zhang, Y.-Q.; Wang, X.-Y. Single-molecule magnet behaviour in a dysprosium-triradical complex. Chem. Commun. 2018, 54, 9726–9729. [Google Scholar] [CrossRef]
- Shen, F.-X.; Pramanik, K.; Brandão, P.; Zhang, Y.-Q.; Jana, N.C.; Wang, X.-Y.; Panja, A. Macrocycle supported dimetallic lanthanide complexes with slow magnetic relaxation in Dy2 analogues. Dalton Trans. 2020, 49, 14169–14179. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Sahu, P.P.; Tang, W.-J.; Zhang, Y.-L.; Zhou, Y.; Xu, F.-X.; Wei, X.-Q.; Tian, Z.; Singh, S.K.; Wang, X.-Y. A single-ion magnet building block strategy toward Dy2 single-molecule magnets with enhanced magnetic performance. Dalton Trans. 2022, 51, 18610–18621. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadis, N.C.; Kalofolias, D.A.; Philippidis, A.; Tzani, S.; Raptopoulou, C.P.; Psycharis, V.; Milios, C.J.; Escuer, A.; Perlepes, S.P. A family of dinuclear lanthanide(iii) complexes from the use of a tridentate Schiff base. Dalton Trans. 2015, 44, 10200–10209. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, Z.; Li, X.-L.; Tang, J. Air-stable chiral mono- and dinuclear dysprosium singlemolecule magnets: Steric hindrance of hexaazamacrocycles. Inorg. Chem. Front. 2022, 9, 4049–4055. [Google Scholar] [CrossRef]



| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Empirical formula | C50H22Dy2F36N4O14 | C50H22Tb2F36N4O14 | C42H22Dy2F24N4O12 | C42H22Tb2F24N4O12 |
| Formula weight | 1911.71 | 1904.56 | 1555.63 | 1548.47 |
| Temperature/K | 300.01(10) | 120.00(10) | 137.00 | 113.00 |
| Crystal system | monoclinic | monoclinic | triclinic | triclinic |
| Space group | C2/c | C2/c | Pī | Pī |
| a [Å] | 22.3250(4) | 22.2755(3) | 11.8996(9) | 11.966(2) |
| b [Å] | 15.5402(3) | 15.2108(3) | 12.0756(10) | 12.126(2) |
| c [Å] | 19.1924(3) | 18.9123(3) | 20.9612(16) | 21.023(4) |
| a [deg] | 90 | 90 | 76.529(7) | 76.41(3) |
| b [deg] | 99.915(2) | 99.5091(16) | 78.452(6) | 78.68(3) |
| g [deg] | 90 | 90 | 61.314(8) | 61.32(3) |
| Volume [Å3] | 6559.1(2) | 6319.95(19) | 2555.8(4) | 2588.7(12) |
| Z | 4 | 4 | 2 | 2 |
| ρcalc [g/cm−3] | 1.936 | 2.002 | 2.021 | 1.987 |
| μ [mm−1] | 13.586 | 12.455 | 3.052 | 2.859 |
| F(000) | 3672 | 3664 | 1492 | 1488 |
| Reflections collected | 19,975 | 24,225 | 17,394 | 23,369 |
| Unique/parameters | 6536/478 | 6351/478 | 8984/758 | 9102/936 |
| R(int) | 0.0882 | 0.0430 | 0.0497 | 0.1232 |
| Goodness-of-fit on F2 | 1.028 | 1.073 | 1.085 | 0.981 |
| R1, wR2 [I > 2σ(I)] | 0.0772, 0.2127 | 0.0477, 0.1211 | 0.0512, 0.0960 | 0.0783, 0.1740 |
| R1, wR2 (all data) | 0.0873, 0.2205 | 0.0554, 0.1250 | 0.0736, 0.1151 | 0.0988, 0.1908 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhou, M.; Wang, W.; Zhu, F.; Qin, S.; Li, X.; Bai, F.; Wang, Q.; Li, L.; Ma, Y.; et al. Enhancing the Magnetic Behaviors of Dy2 Complexes by Modulating the Crystal Field Environment with Different μ-O Bridging Ligands. Molecules 2025, 30, 1260. https://doi.org/10.3390/molecules30061260
Wang X, Zhou M, Wang W, Zhu F, Qin S, Li X, Bai F, Wang Q, Li L, Ma Y, et al. Enhancing the Magnetic Behaviors of Dy2 Complexes by Modulating the Crystal Field Environment with Different μ-O Bridging Ligands. Molecules. 2025; 30(6):1260. https://doi.org/10.3390/molecules30061260
Chicago/Turabian StyleWang, Xirong, Min Zhou, Wen Wang, Fangting Zhu, Shijia Qin, Xiulan Li, Feifei Bai, Qinglun Wang, Licun Li, Yue Ma, and et al. 2025. "Enhancing the Magnetic Behaviors of Dy2 Complexes by Modulating the Crystal Field Environment with Different μ-O Bridging Ligands" Molecules 30, no. 6: 1260. https://doi.org/10.3390/molecules30061260
APA StyleWang, X., Zhou, M., Wang, W., Zhu, F., Qin, S., Li, X., Bai, F., Wang, Q., Li, L., Ma, Y., & Zhao, B. (2025). Enhancing the Magnetic Behaviors of Dy2 Complexes by Modulating the Crystal Field Environment with Different μ-O Bridging Ligands. Molecules, 30(6), 1260. https://doi.org/10.3390/molecules30061260







