Modern Approaches in Organic Chromofluorescent Sensor Synthesis for the Detection of Considered First-Row Transition Metal Ions
Abstract
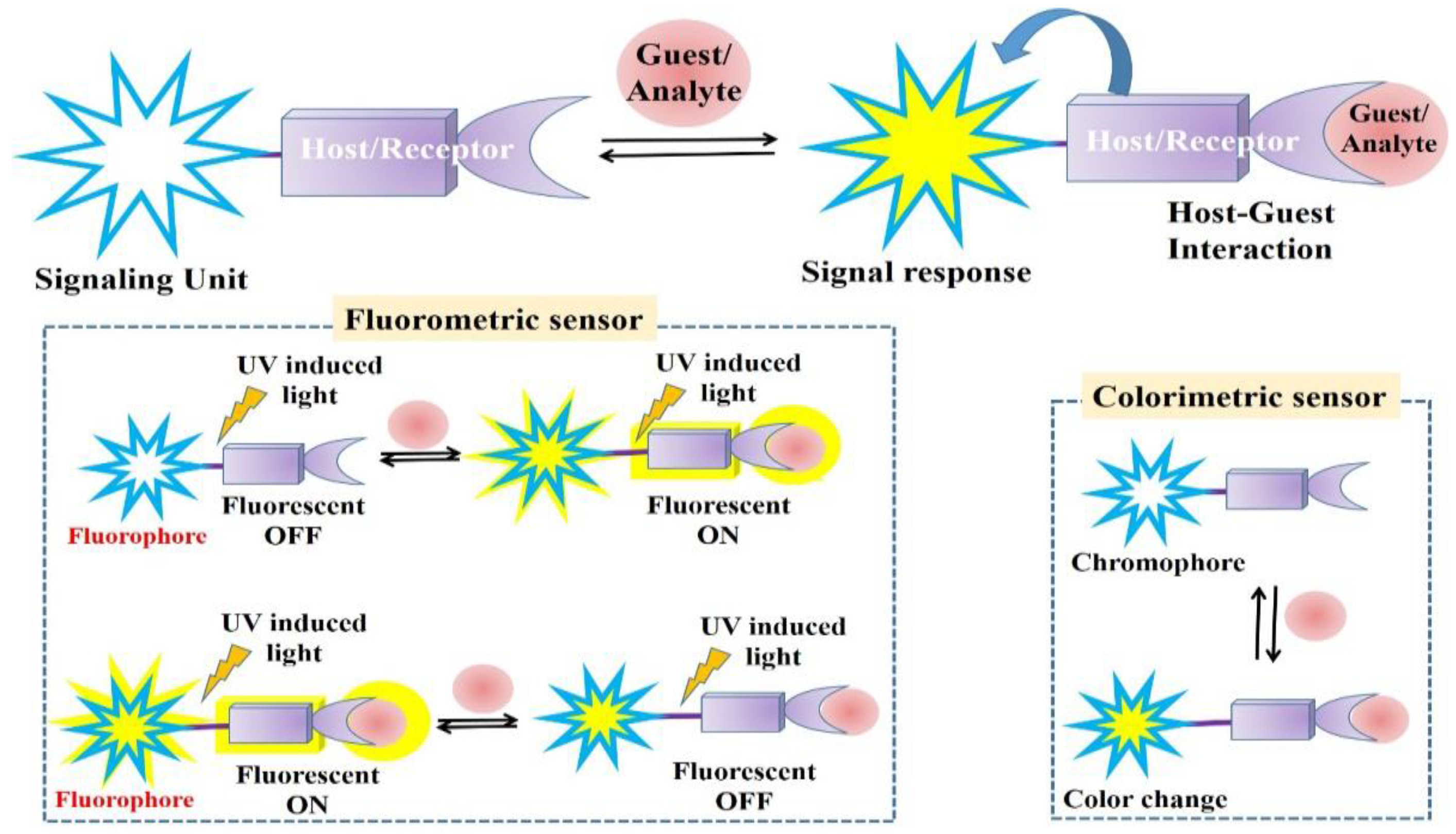
1. Introduction
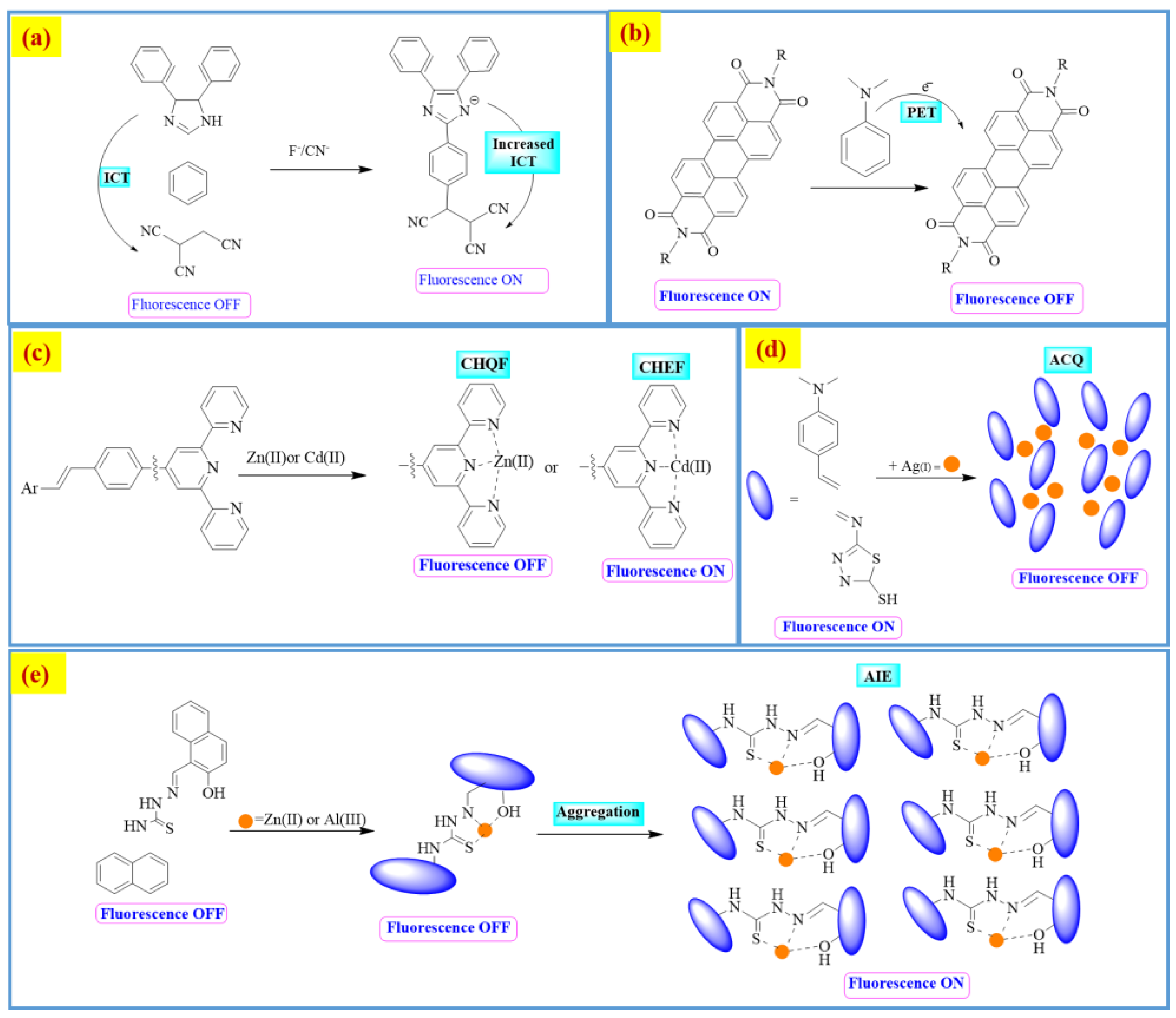
Colorimetric and Fluorometric Detections
2. Detection of Selected First-Row Transition Metal Ions
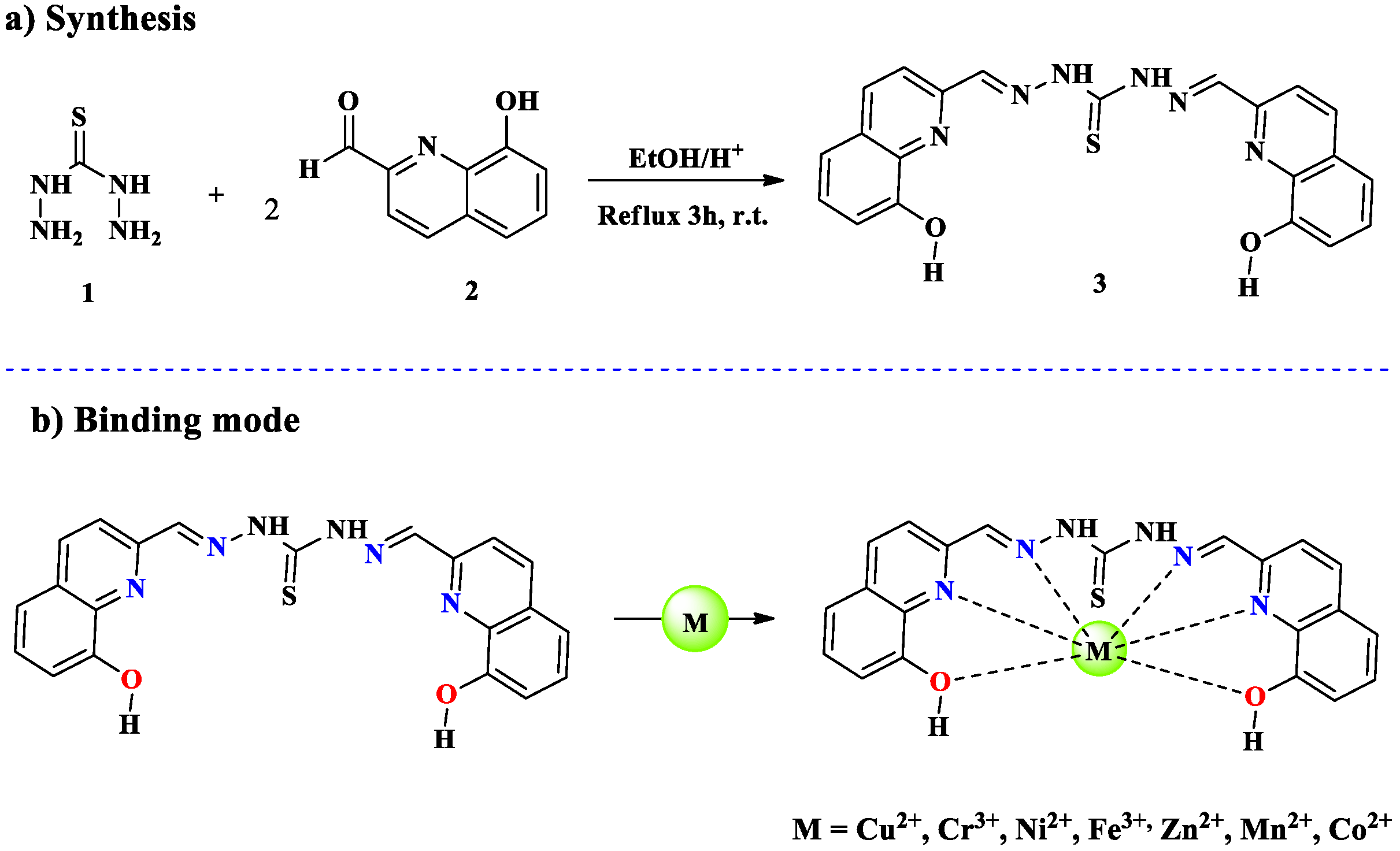
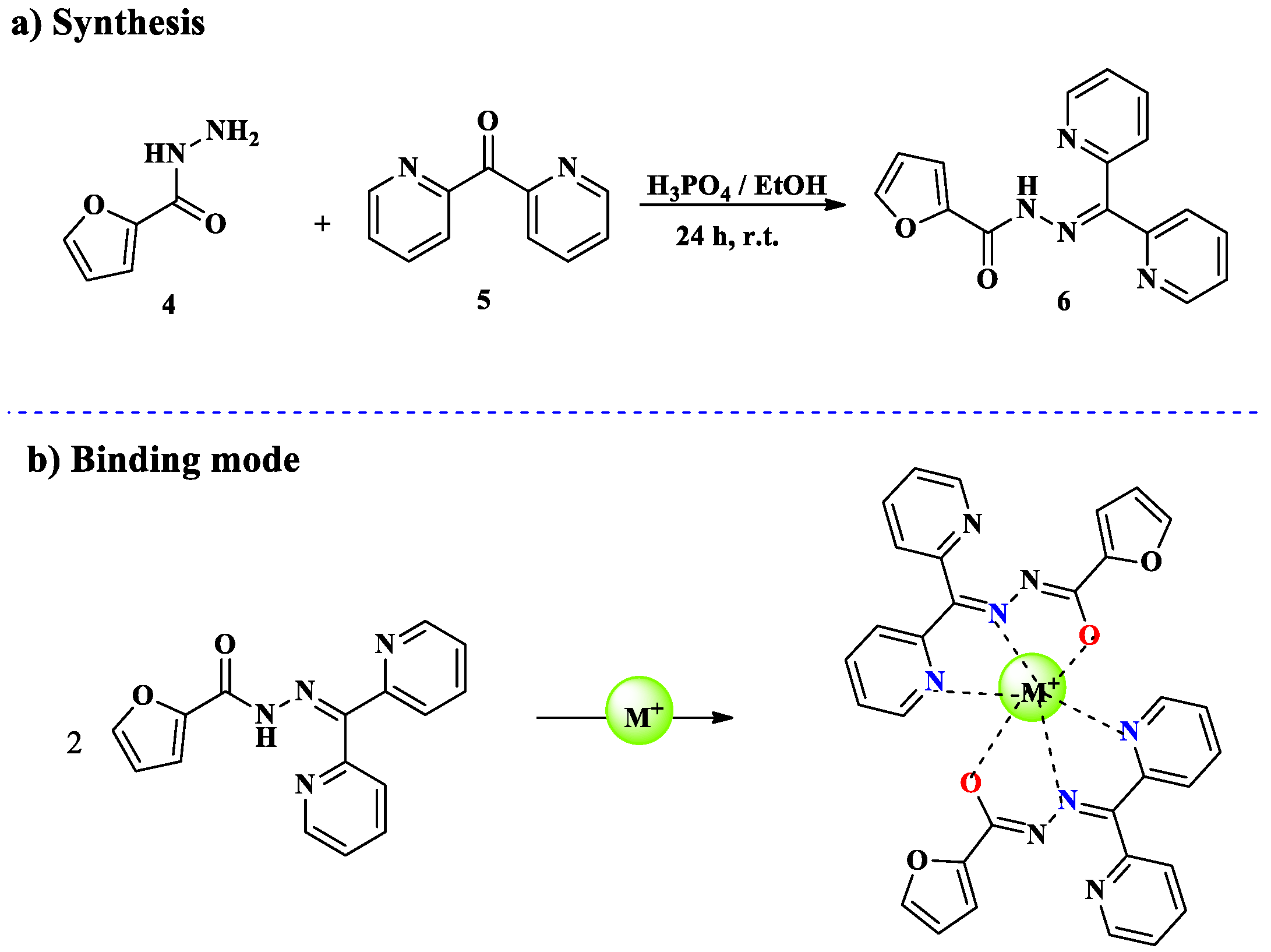
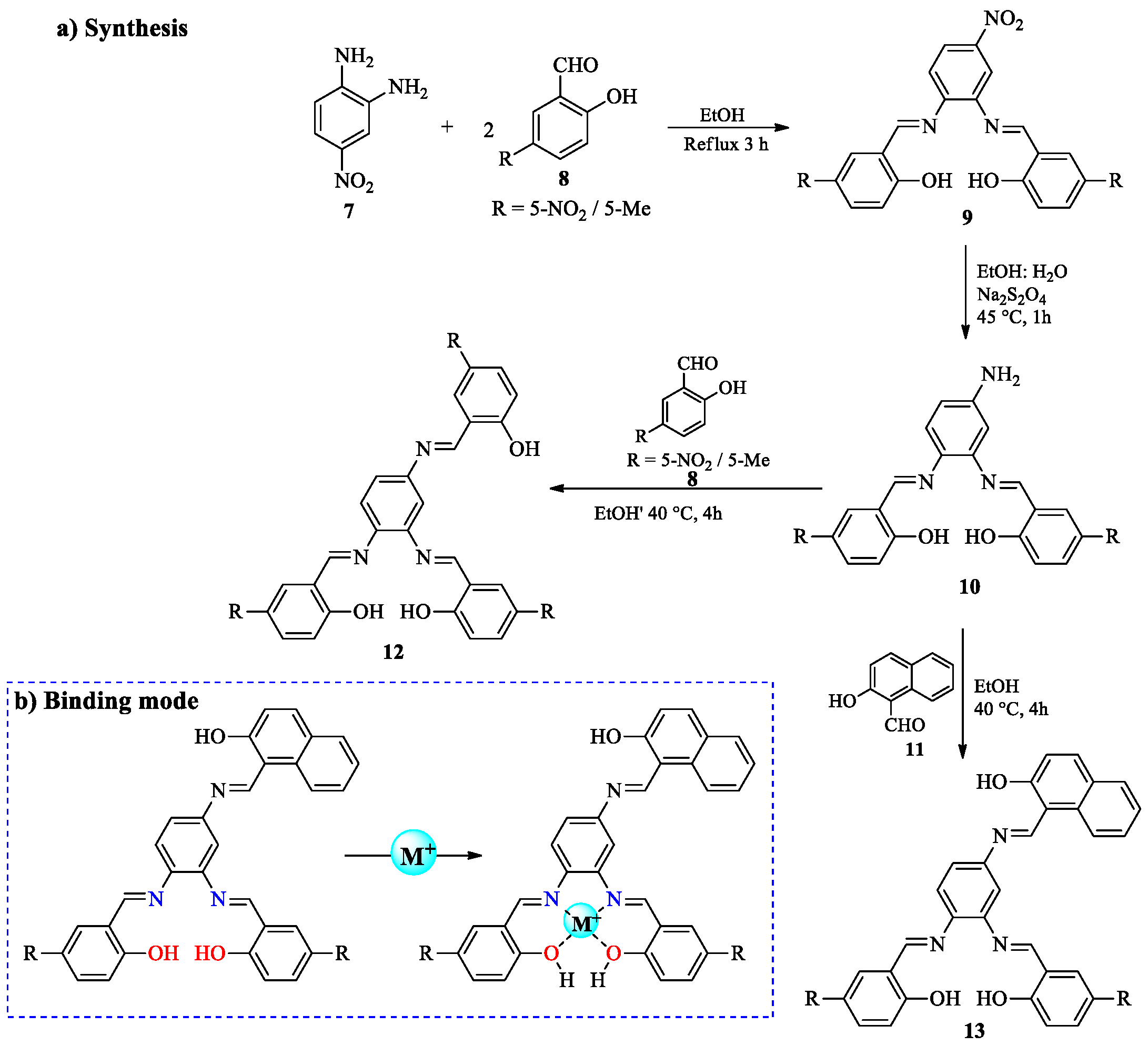
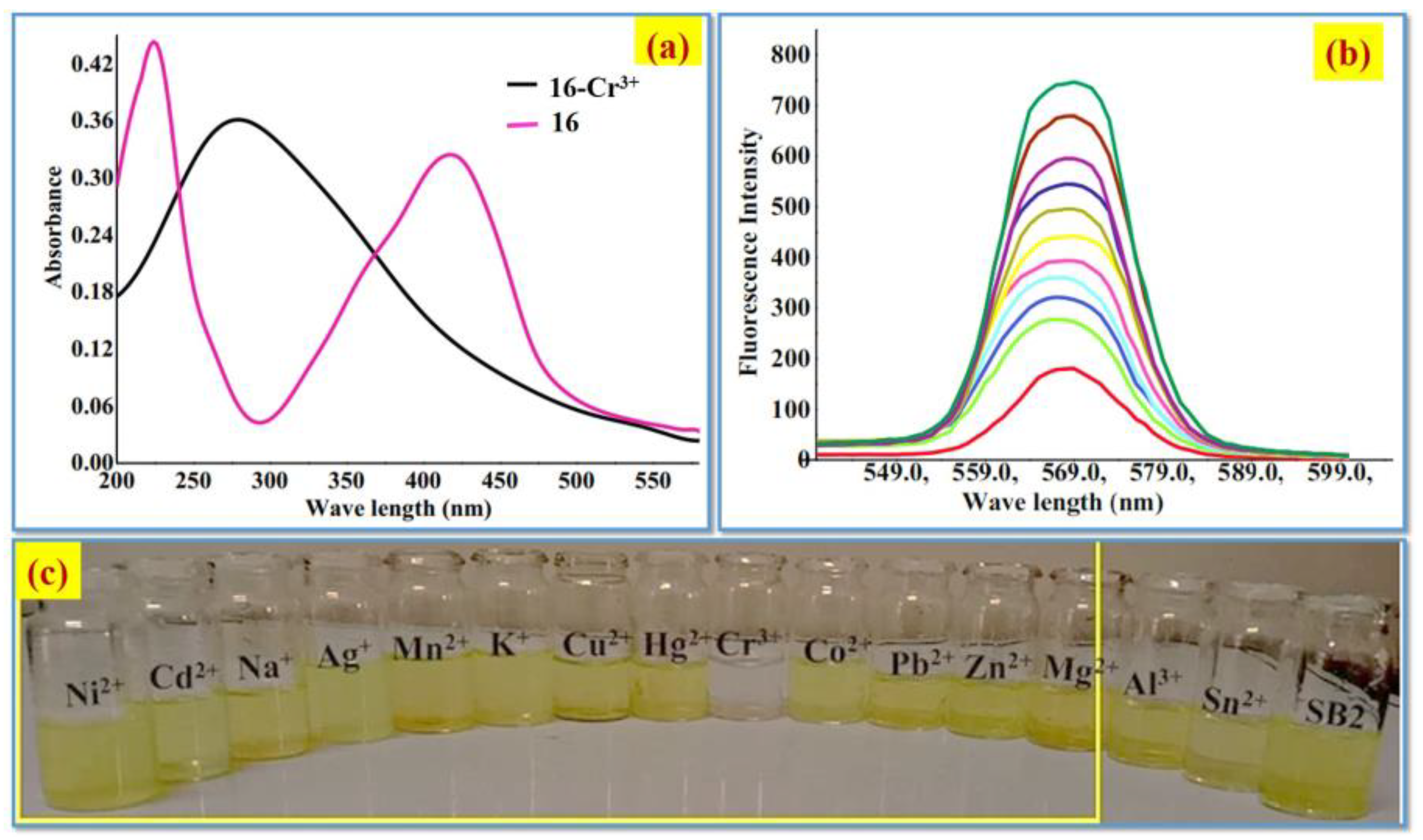
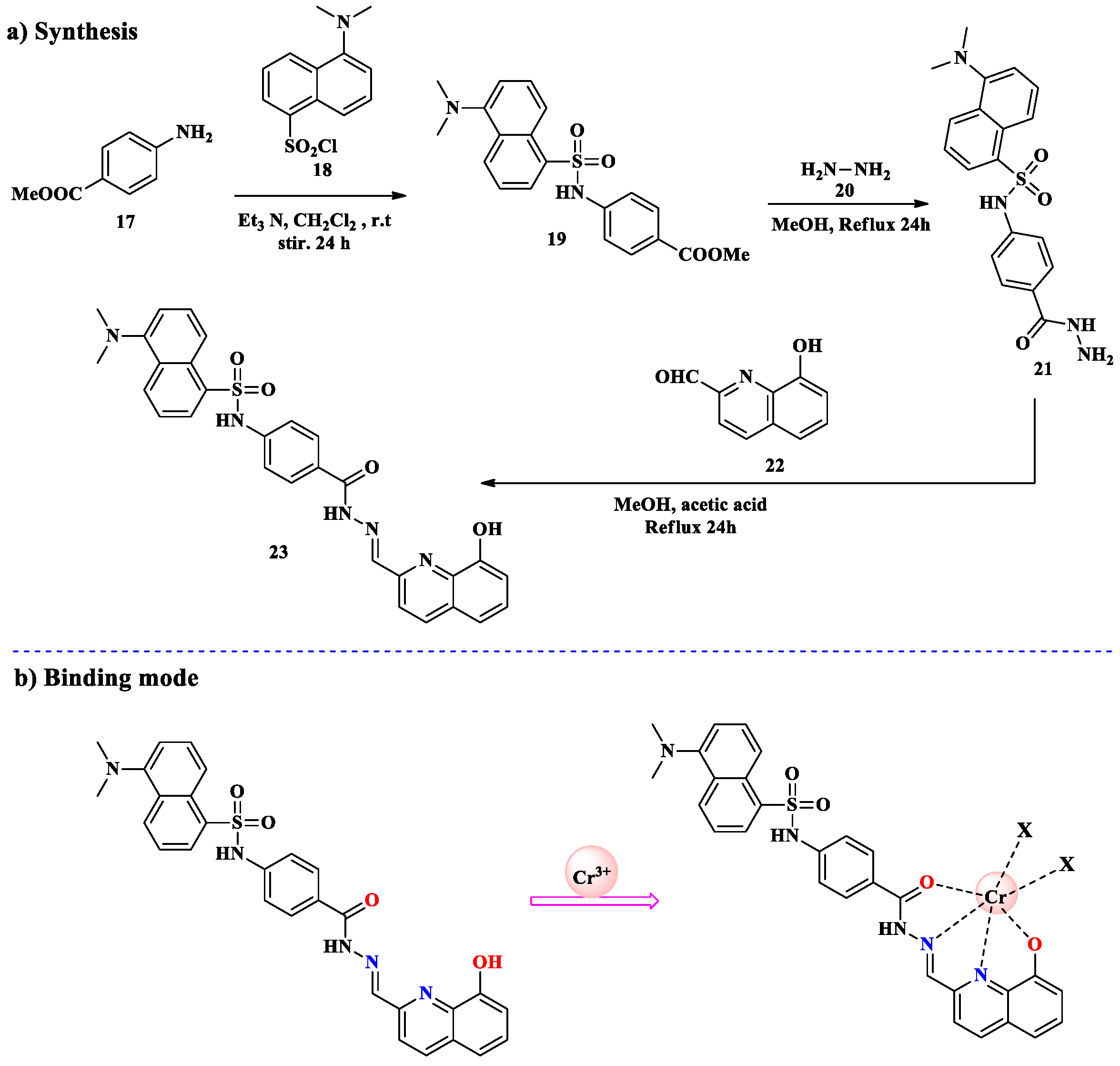
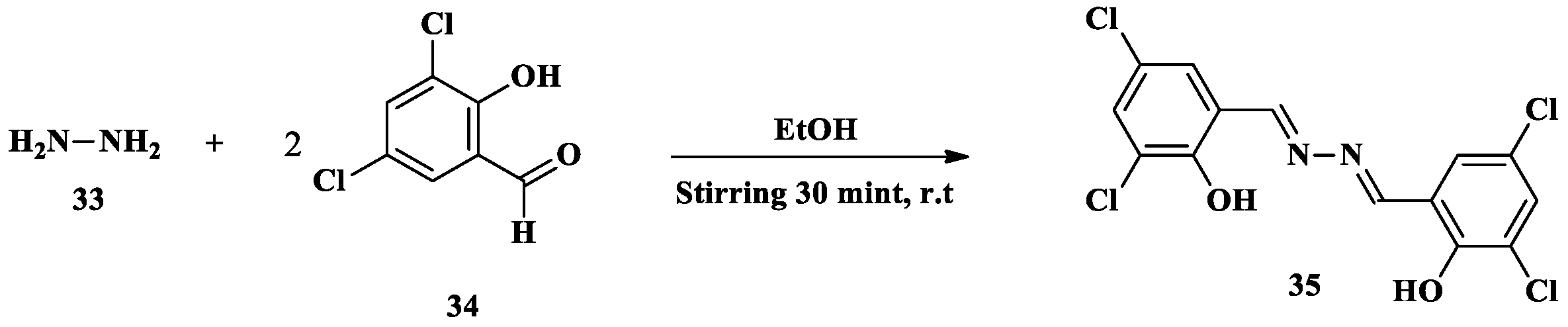
2.1. Cr3+ Ion Detection
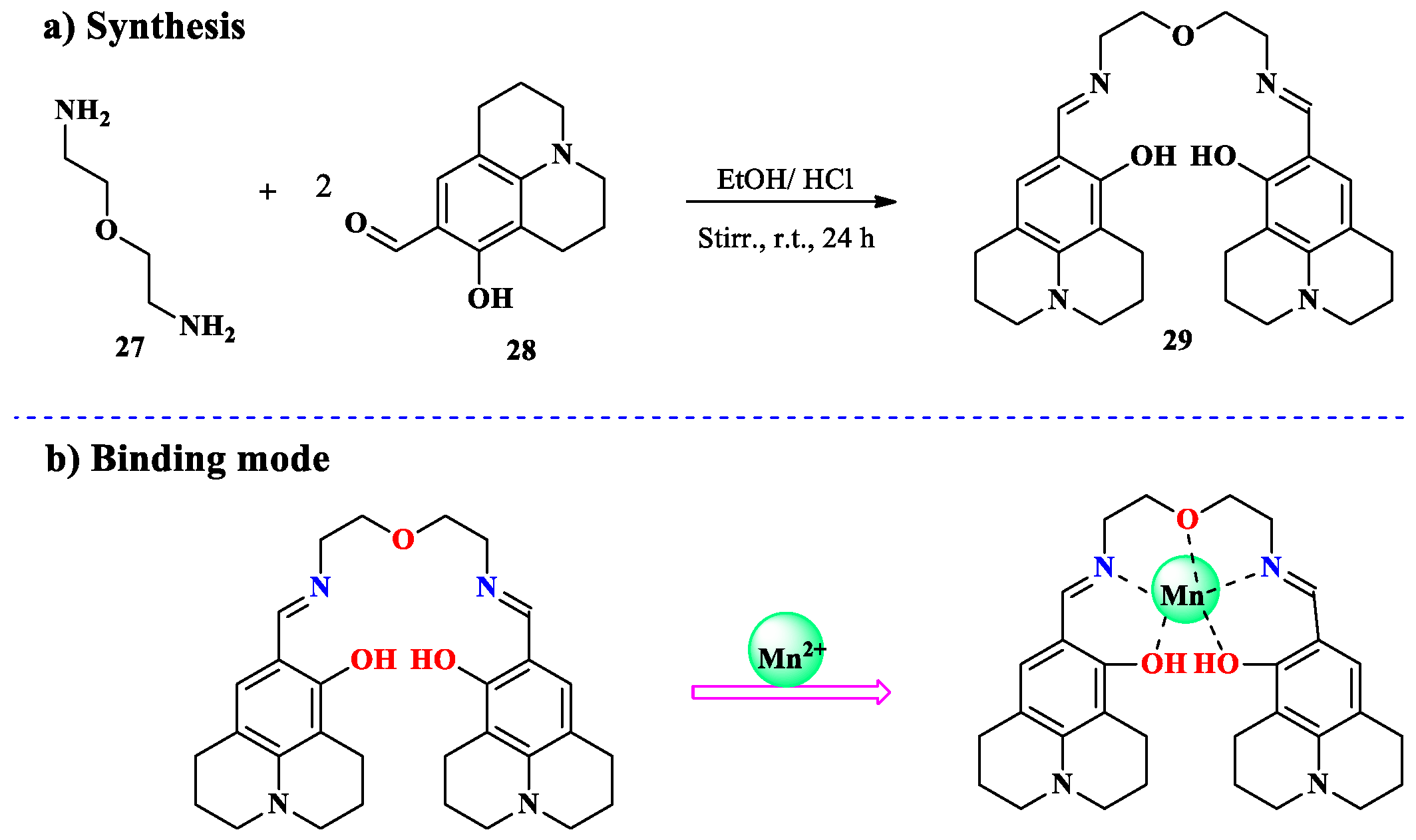
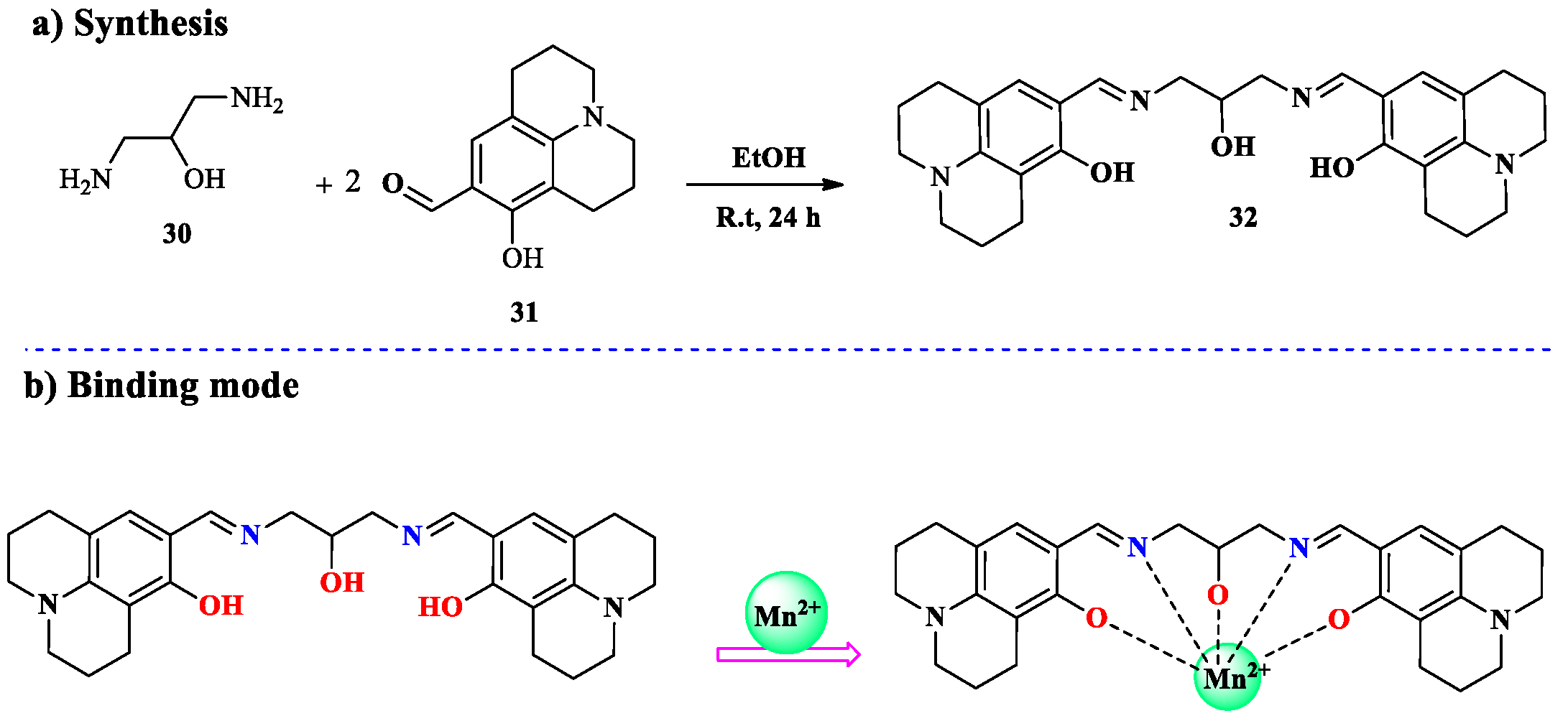
2.2. Mn2+ Ion Detection
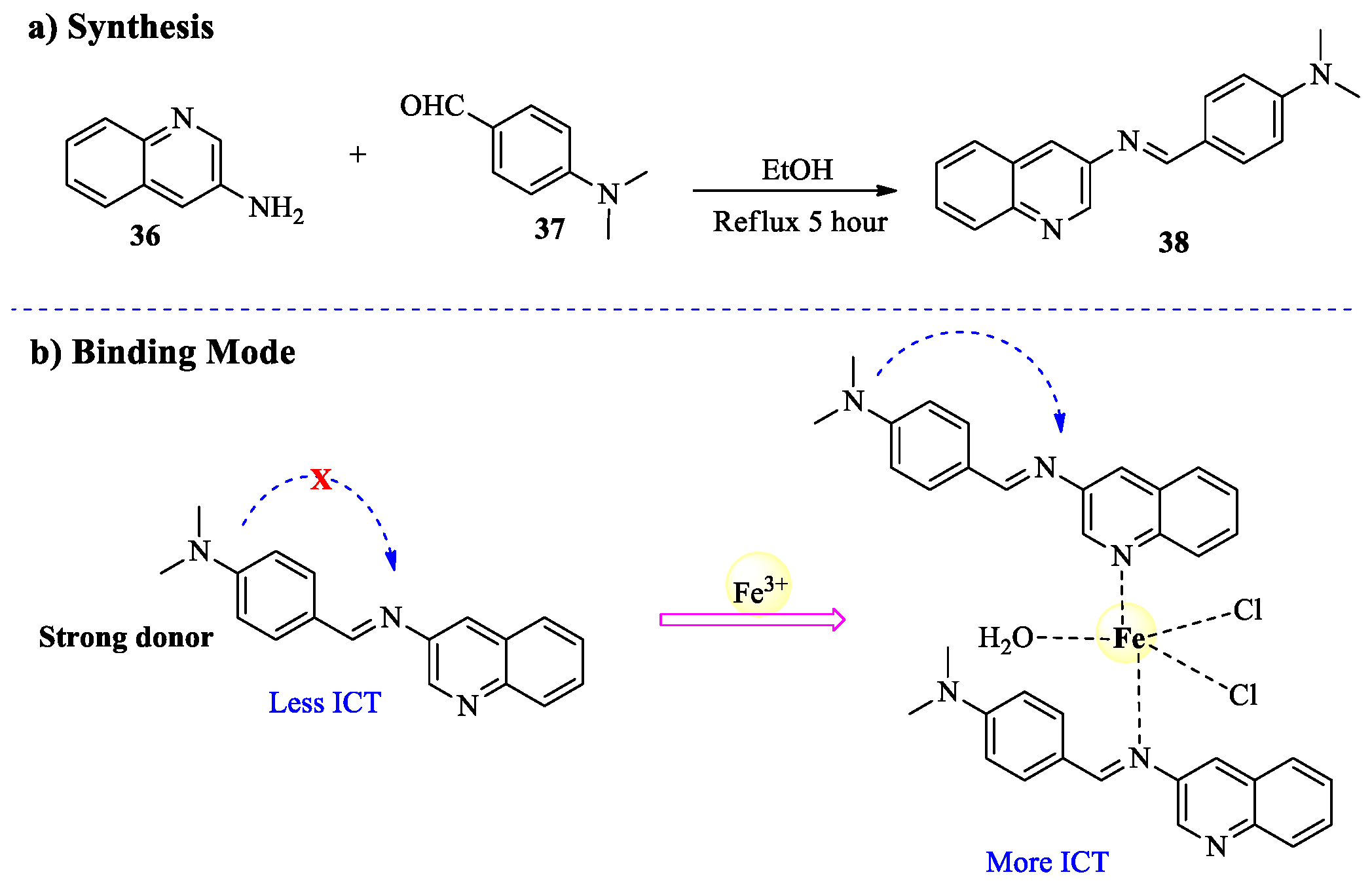
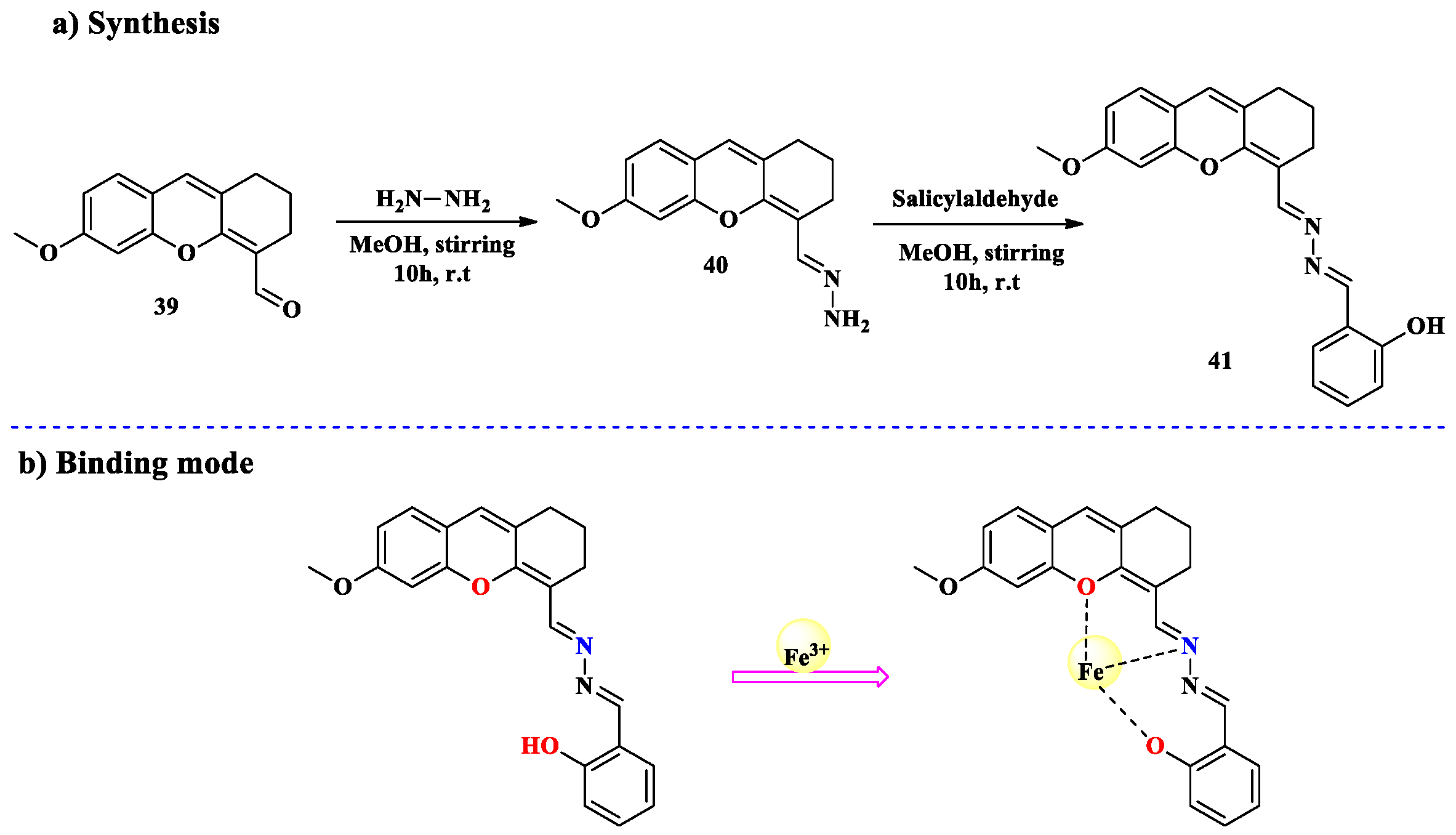
2.3. Fe3+ Ion Detection
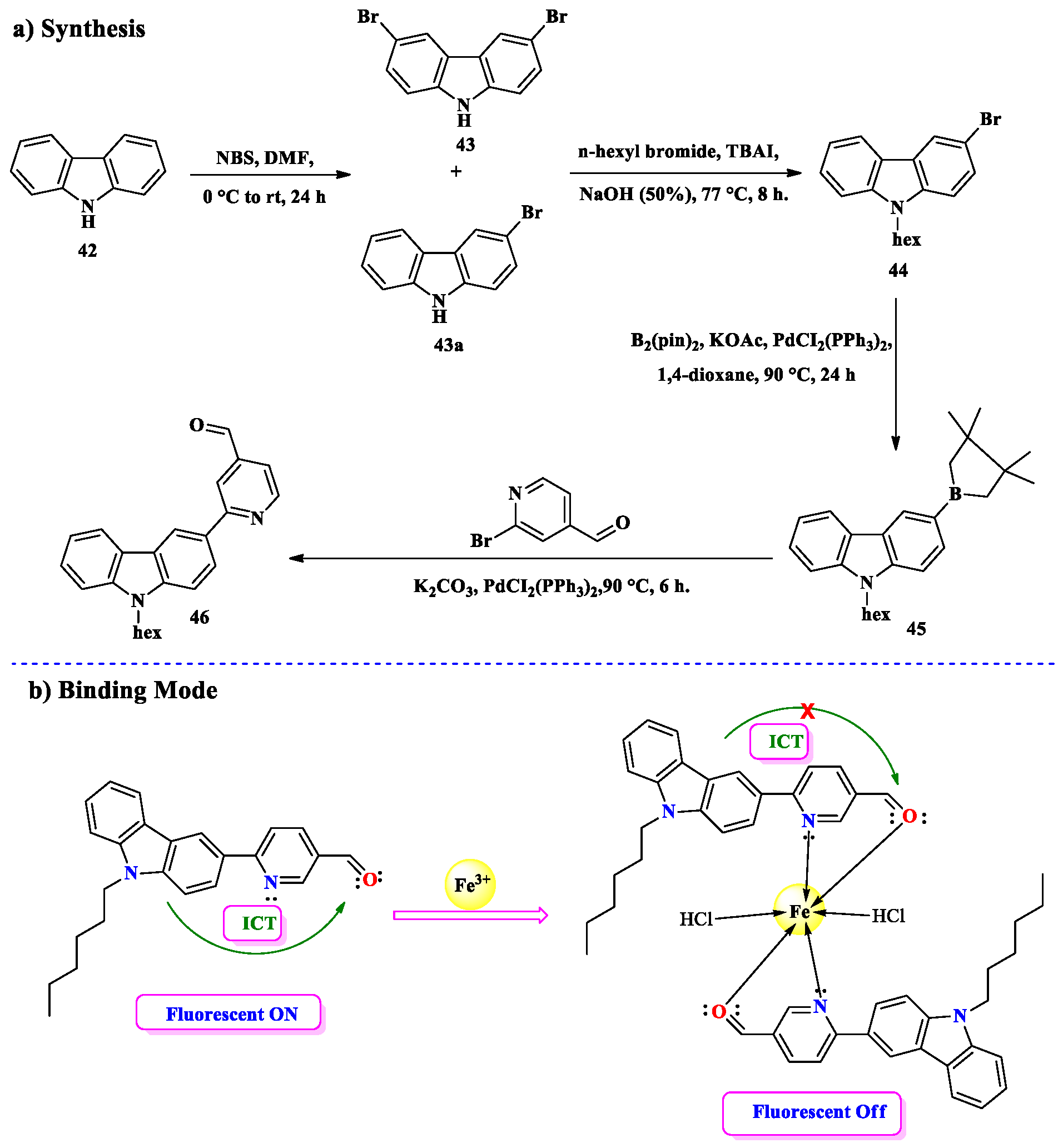
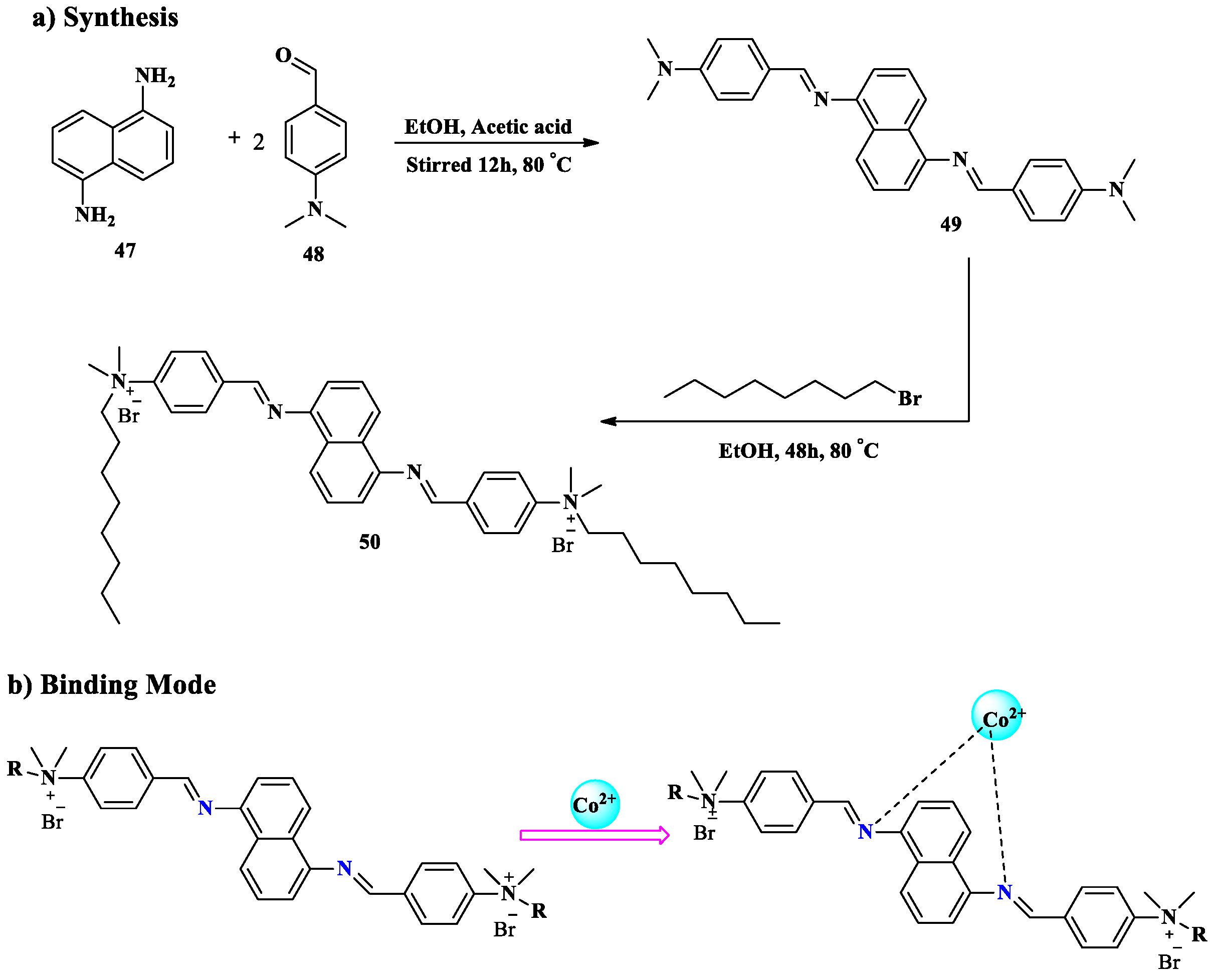
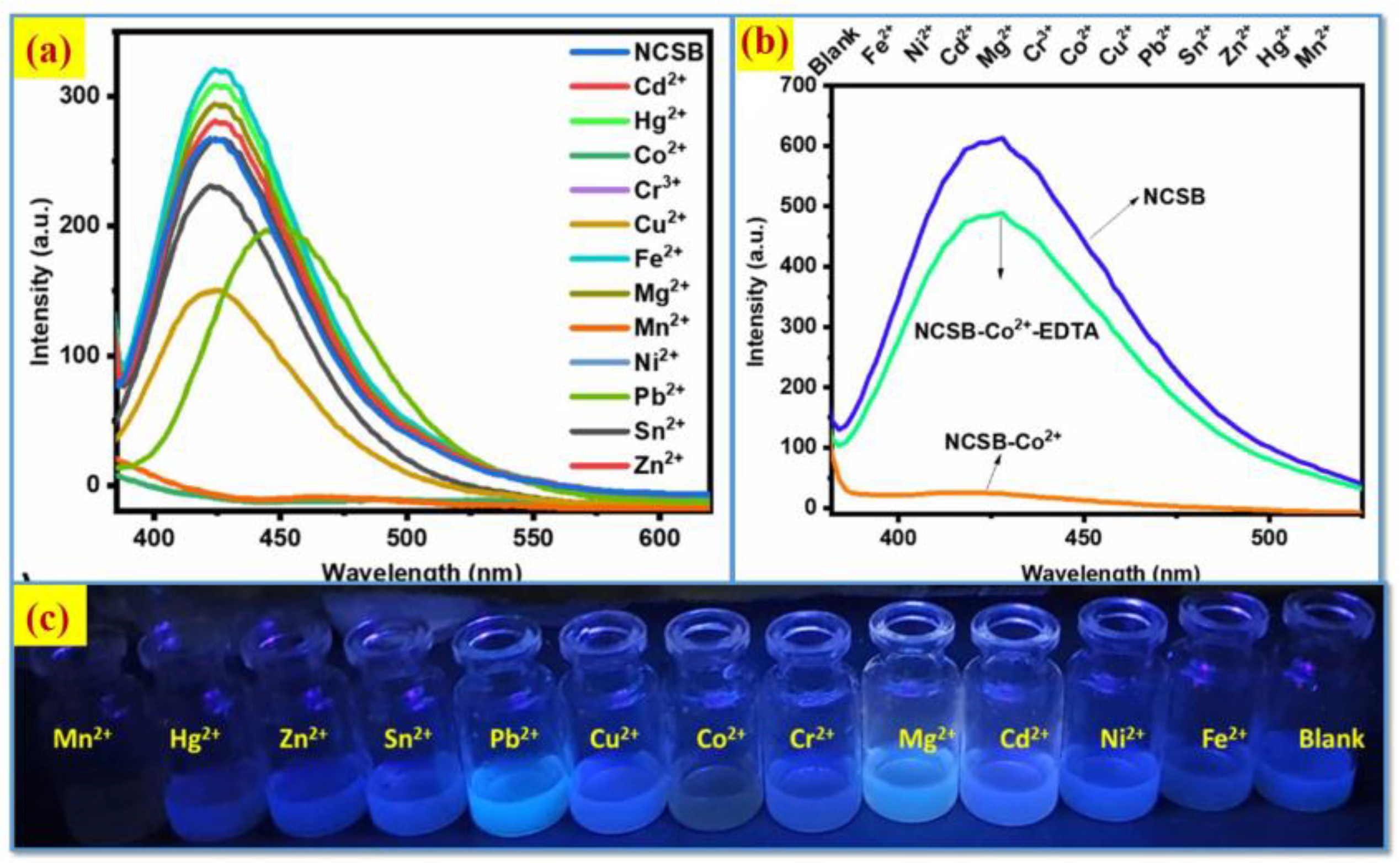
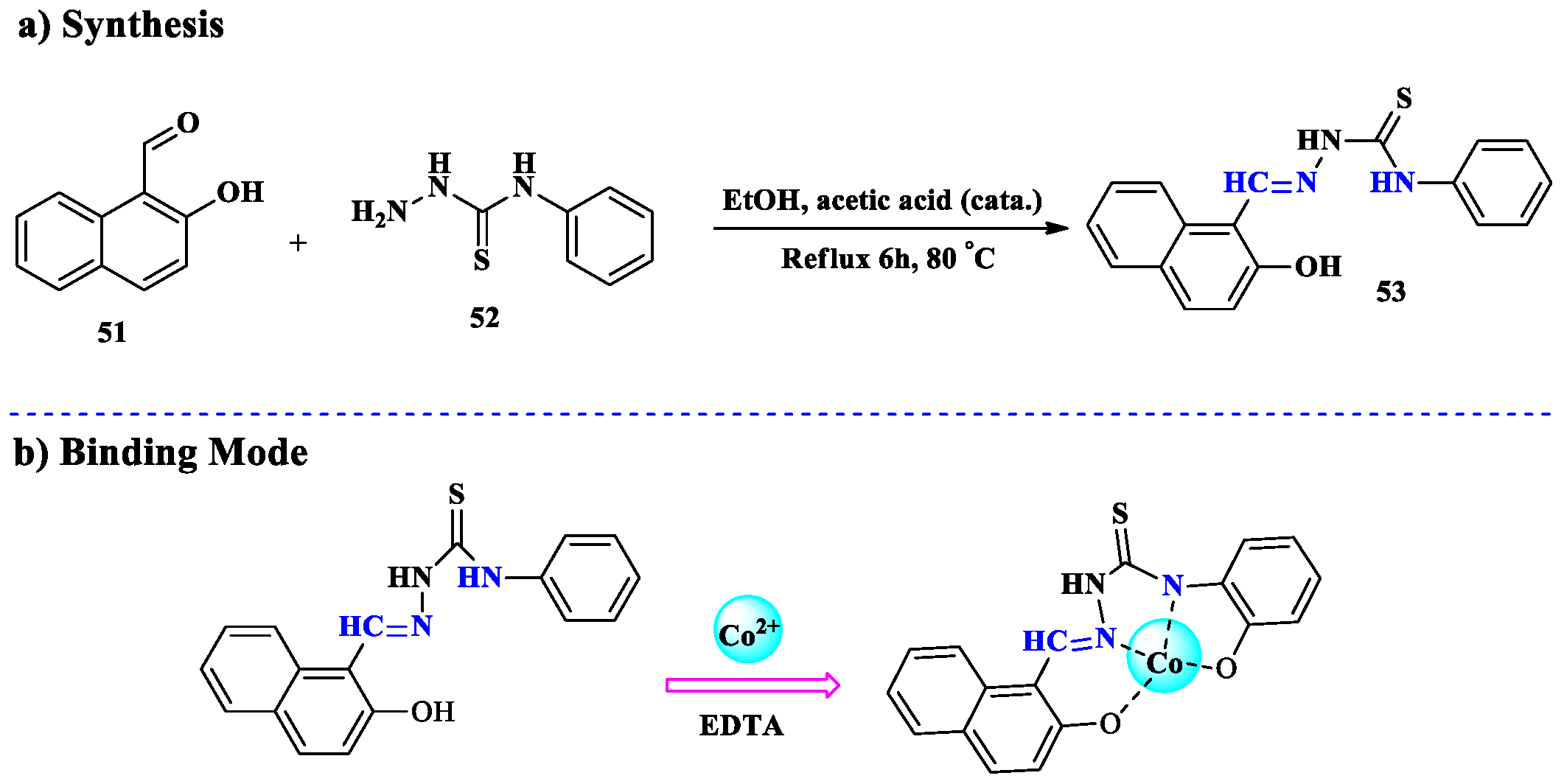
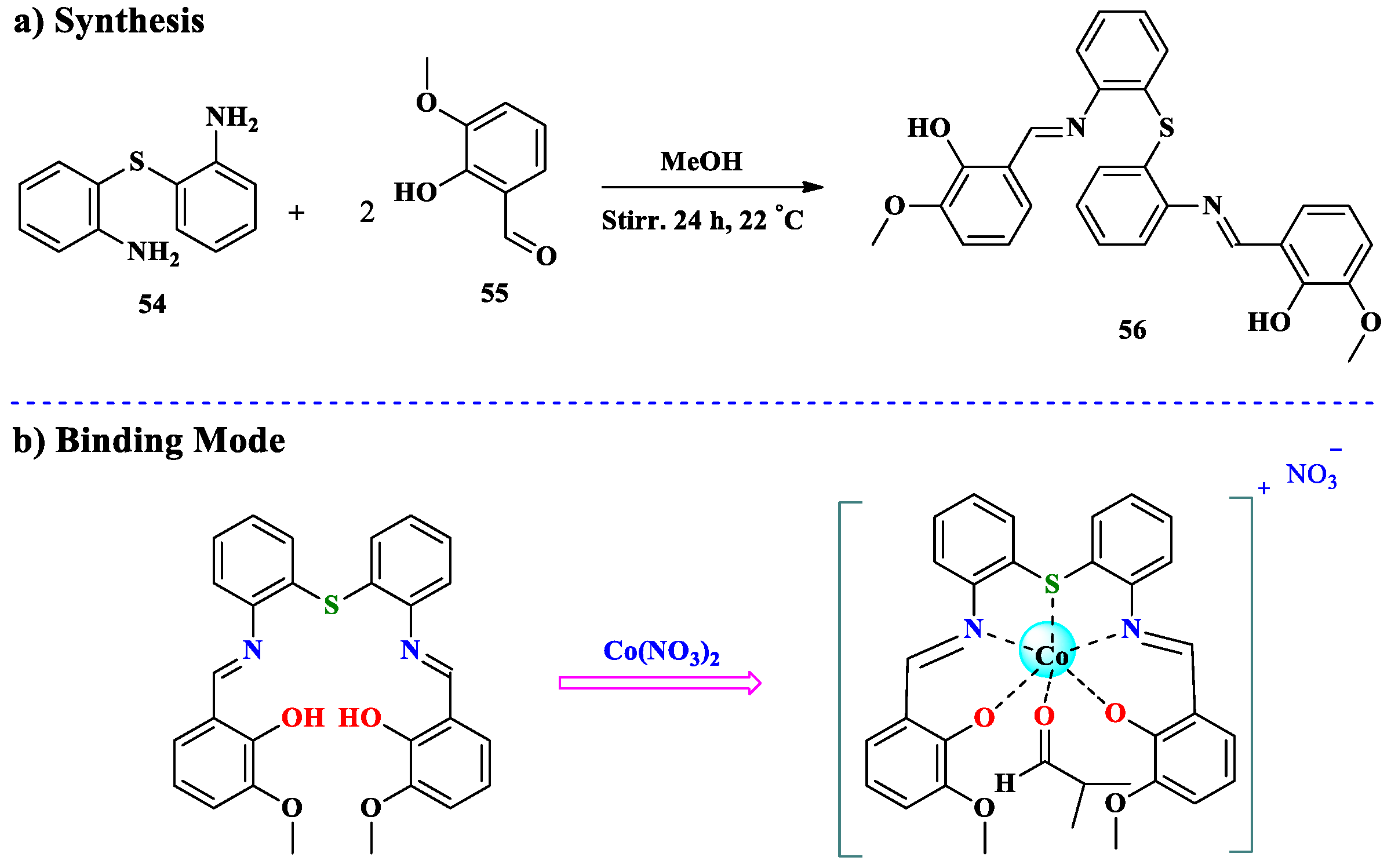
2.4. Co2+ Ion Detection
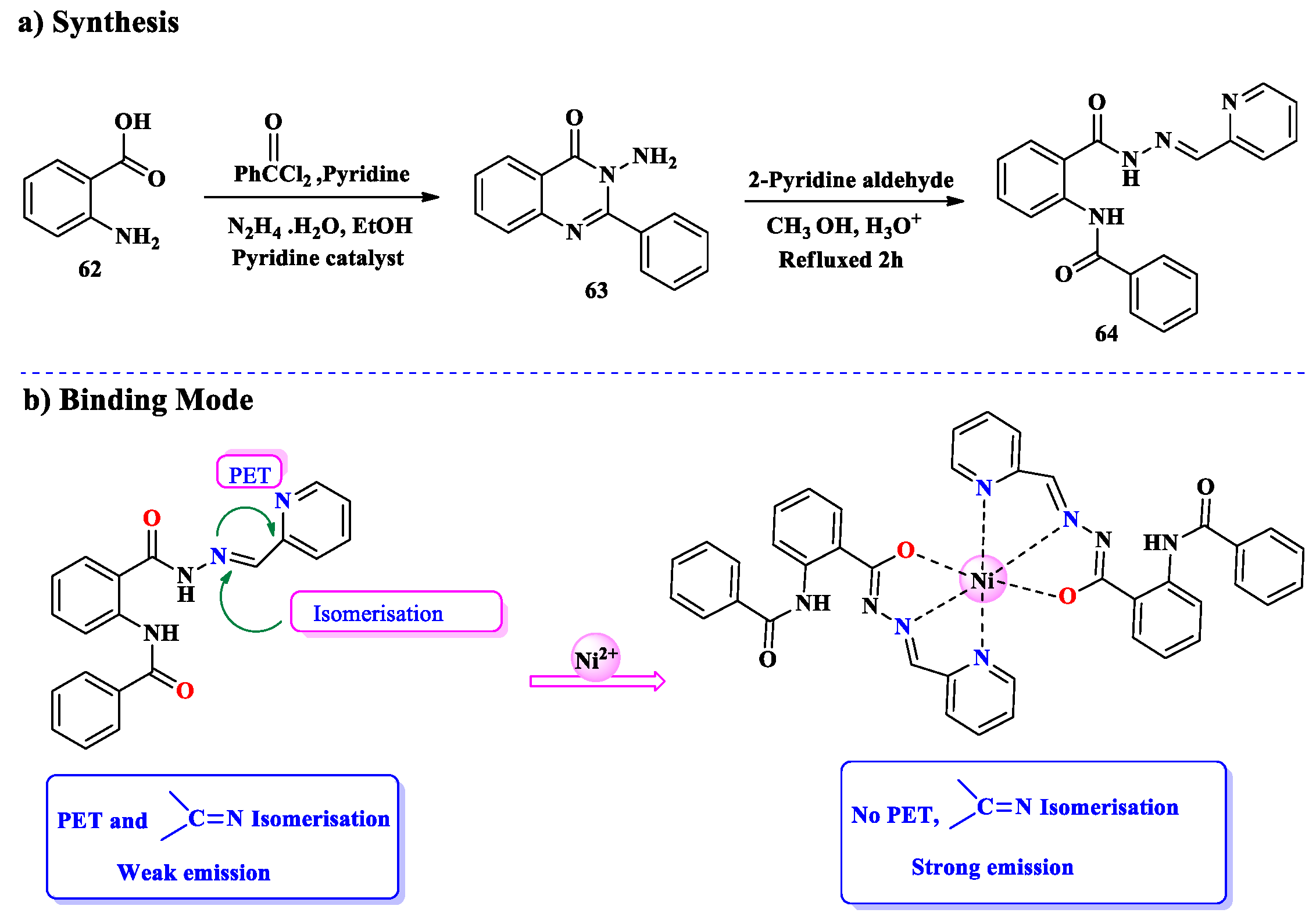
2.5. Ni2+ Ion Detection
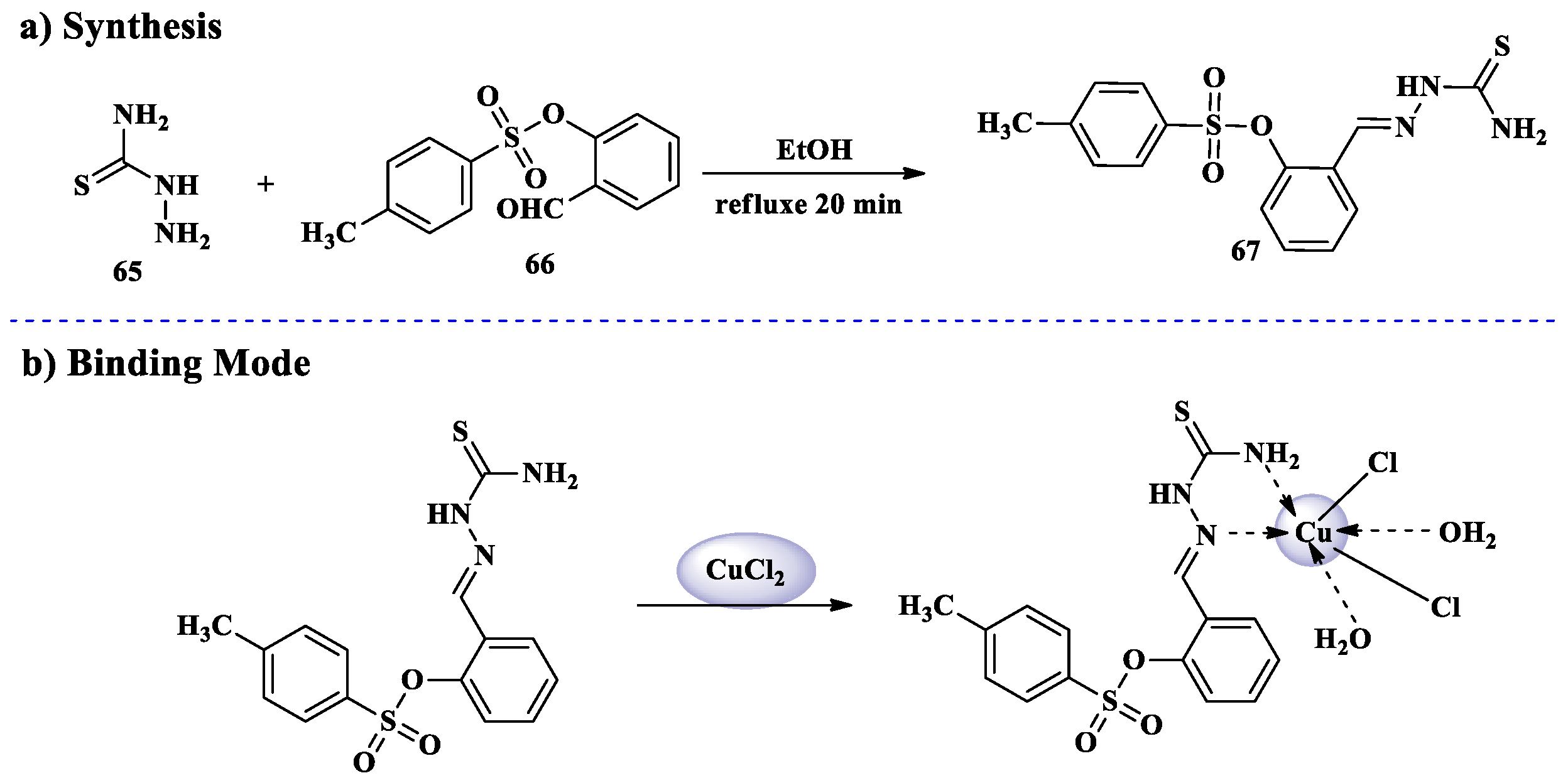
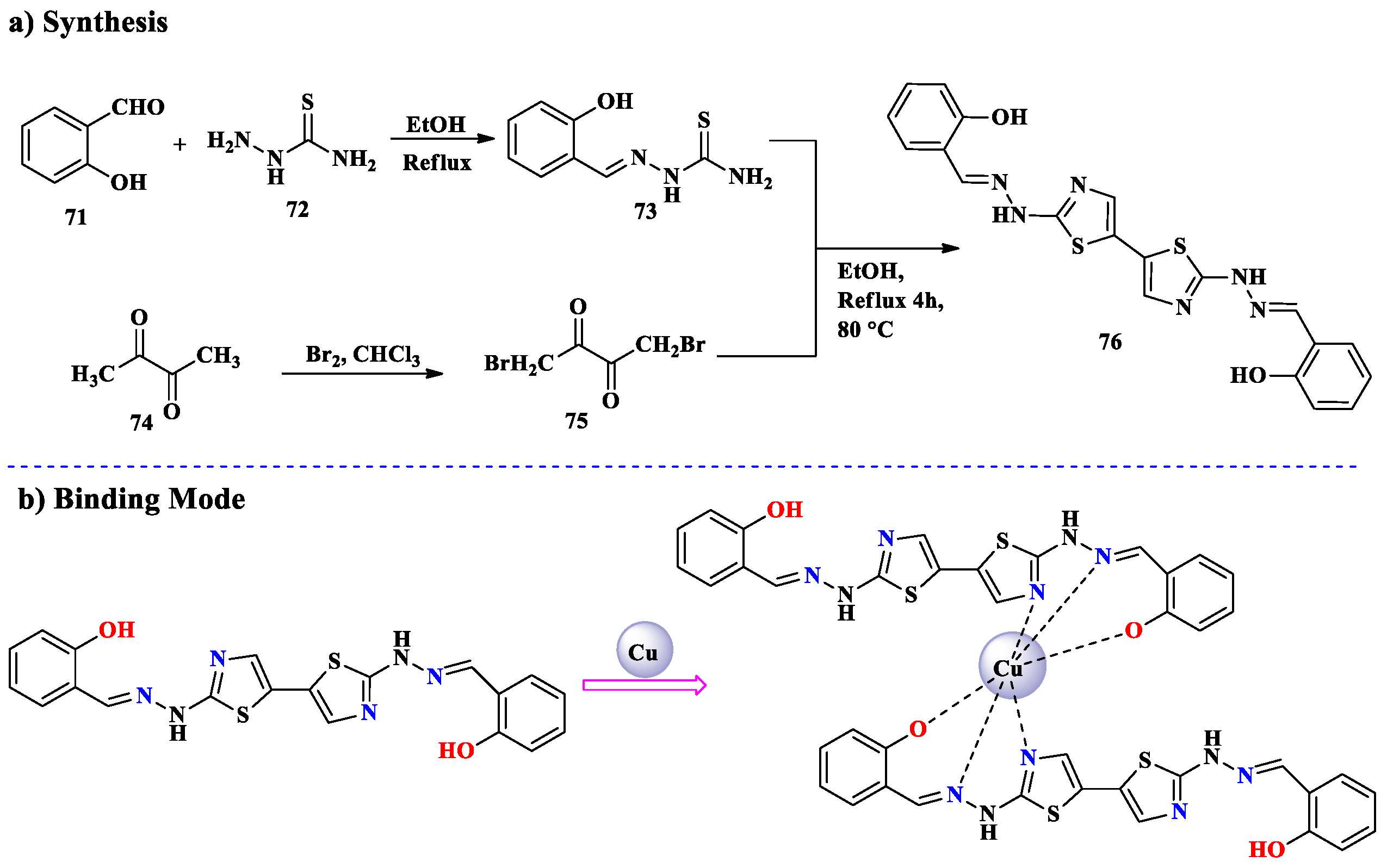
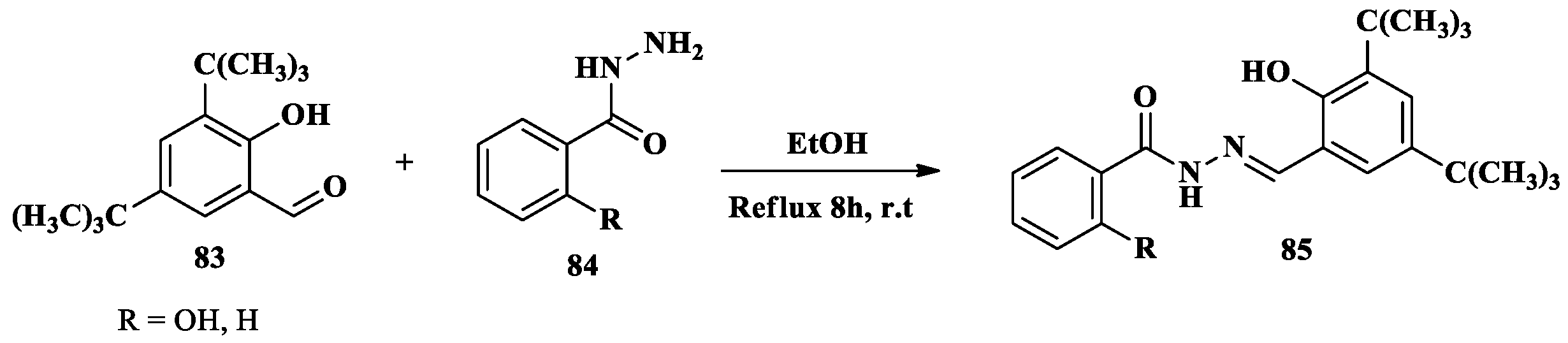
2.6. Cu2+ Ion Detection
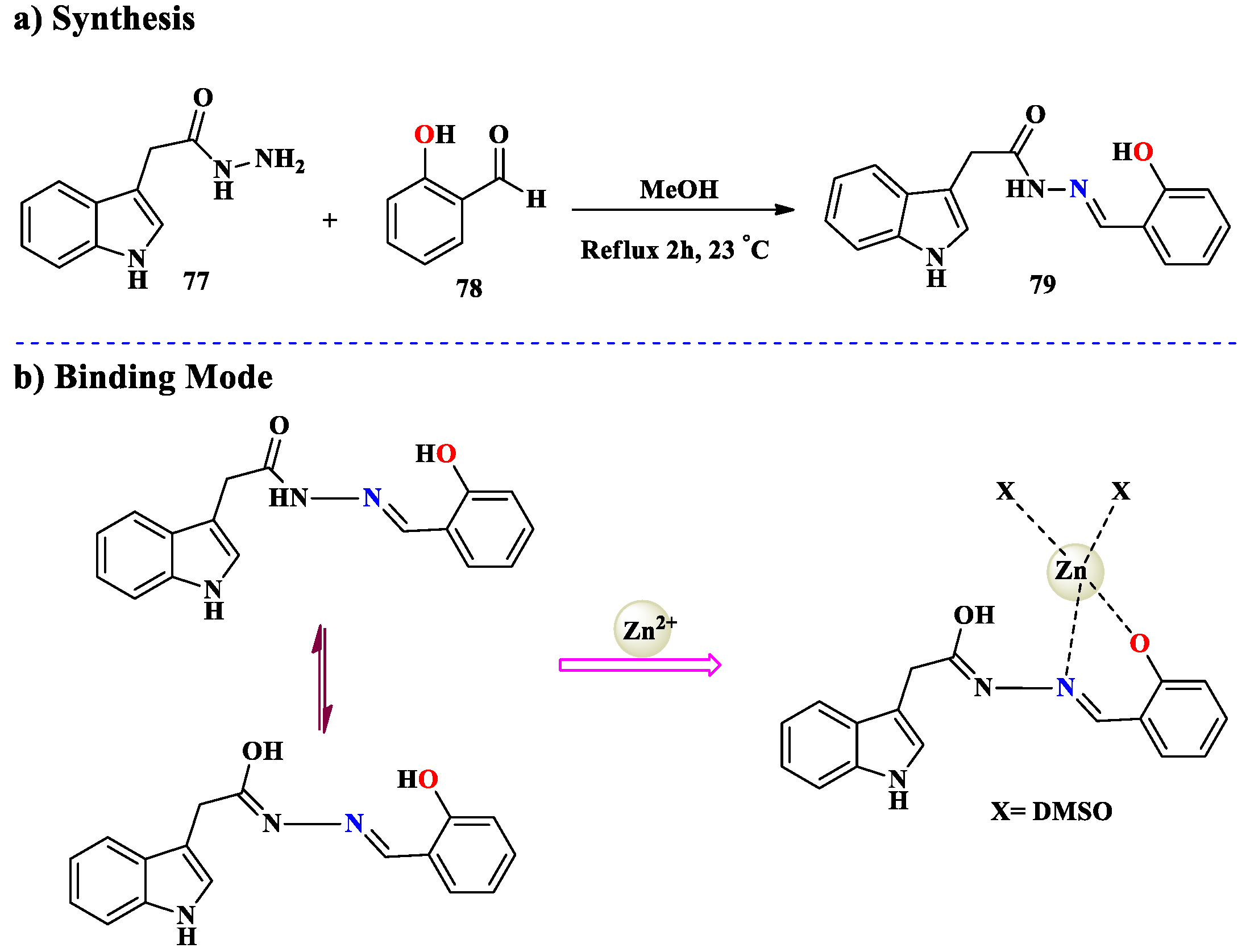
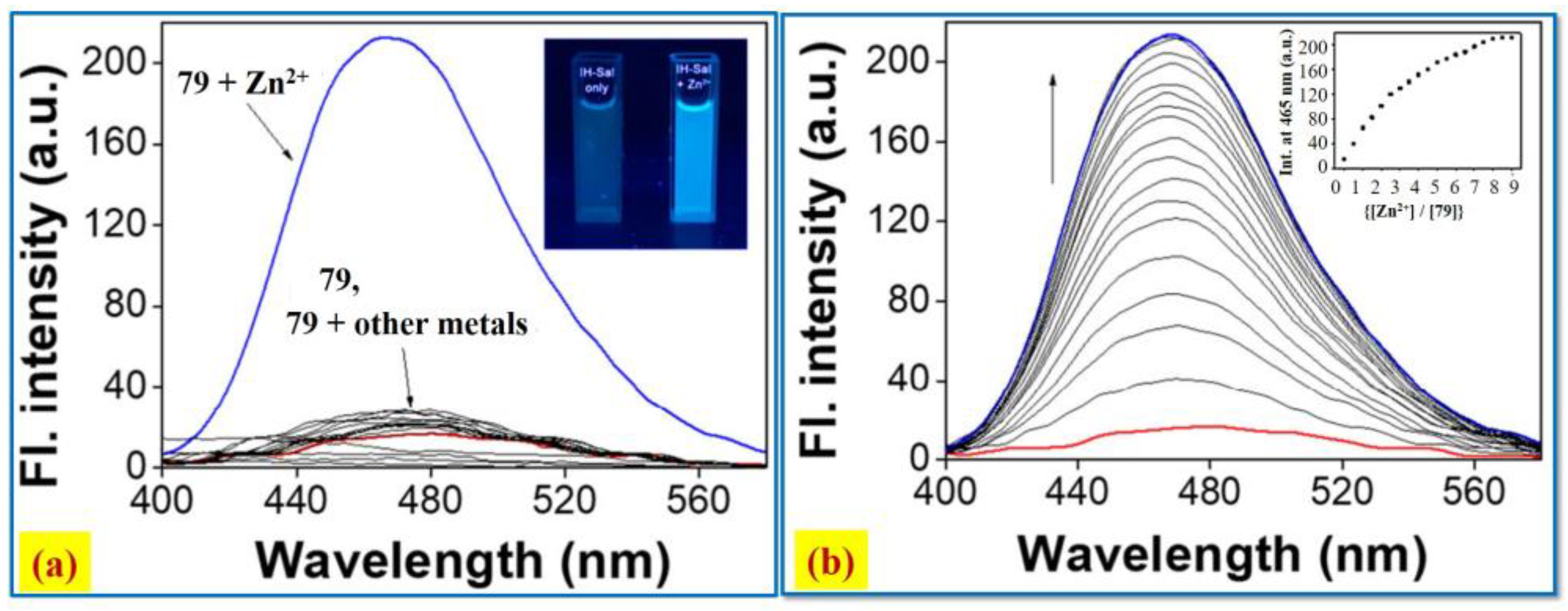
2.7. Zn2+ Ion Detection
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHMPMBS | 2-[(carbamothioylhydrazono)methyl-phenyl-4-methylbenzenesulfonate |
| WHO | World Health Organization |
| ESI-MS | Electrospray Ionization–Mass Spectrometry |
| µM | Micromolar |
| THF | Tetrahydrofuran |
| PBS | Phosphate-Buffered Saline |
| DMSO | Dimethyl Sulfoxide |
| DMFO | Dimethylformamide |
| EDTA | Ethylenediaminetetraacetic acid |
| LOD | Limit of detection |
| DBQA | N-(4-(dimethylamino) benzylidene) quinolone-3-amine |
| HEPES | N-(2-Hydroxyethyl)piperazine-N′-2-ethanesulfonic acid |
References
- Chen, S.-Y.; Li, Z.; Li, K.; Yu, X.-Q. Small molecular fluorescent probes for the detection of lead, cadmium and mercury ions. Coord. Chem. Rev. 2021, 429, 213691. [Google Scholar] [CrossRef]
- Gaur, V.K.; Sharma, P.; Gaur, P.; Varjani, S.; Ngo, H.H.; Guo, W.; Chaturvedi, P.; Singhania, R.R. Sustainable mitigation of heavy metals from effluents: Toxicity and fate with recent technological advancements. Bioengineered 2021, 12, 7297–7313. [Google Scholar] [CrossRef]
- Khan, S.; Chen, X.; Almahri, A.; Allehyani, E.S.; Alhumaydhi, F.A.; Ibrahim, M.M.; Ali, S. Recent developments in fluorescent and colorimetric chemosensors based on schiff bases for metallic cations detection: A review. J. Environ. Chem. Eng. 2021, 9, 106381. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bhattacharyya, S.; Ghosh, K.; Pal, S.; Halder, A.; Naseri, M.; Mohammadniaei, M.; Sarkar, S.; Ghosh, A.; Sun, Y. Sensory development for heavy metal detection: A review on translation from conventional analysis to field-portable sensor. Trends Food Sci. Technol. 2021, 109, 674–689. [Google Scholar] [CrossRef]
- Akpor, O.B.; Ohiobor, G.O.; Olaolu, D. Heavy metal pollutants in wastewater effluents: Sources, effects and remediation. Adv. Biosci. Bioeng. 2014, 2, 37–43. [Google Scholar] [CrossRef]
- Lopez Marzo, A.M.; Pons, J.; Blake, D.A.; Merkoçi, A. All-integrated and highly sensitive paper based device with sample treatment platform for Cd2+ immunodetection in drinking/tap waters. Anal. Chem. 2013, 85, 3532–3538. [Google Scholar] [CrossRef]
- Guerrero, M.; Garcia-Anton, J.; Tristany, M.; Pons, J.; Ros, J.; Philippot, K.; Lecante, P.; Chaudret, B. Design of new N, O hybrid pyrazole derived ligands and their use as stabilizers for the synthesis of Pd nanoparticles. Langmuir 2010, 26, 15532–15540. [Google Scholar] [CrossRef]
- Aragay, G.; Pons, J.; Merkoçi, A. Enhanced electrochemical detection of heavy metals at heated graphite nanoparticle-based screen-printed electrodes. J. Mater. Chem. 2011, 21, 4326–4331. [Google Scholar] [CrossRef]
- Sun, X.; Ji, J.; Jiang, D.; Li, X.; Zhang, Y.; Li, Z.; Wu, Y. Development of a novel electrochemical sensor using pheochromocytoma cells and its assessment of acrylamide cytotoxicity. Biosens. Bioelectron. 2013, 44, 122–126. [Google Scholar] [CrossRef]
- Khan, J. Synthesis and applications of fluorescent chemosensors: A review. J. Fluoresc. 2024, 34, 2485–2494. [Google Scholar] [CrossRef]
- Li, W.-T.; Wu, G.-Y.; Qu, W.-J.; Li, Q.; Lou, J.-C.; Lin, Q.; Yao, H.; Zhang, Y.-M.; Wei, T.-B. A colorimetric and reversible fluorescent chemosensor for Ag+ in aqueous solution and its application in IMPLICATION logic gate. Sens. Actuators B Chem. 2017, 239, 671–678. [Google Scholar] [CrossRef]
- Francisco, B.B.A.; Caldas, L.F.S.; Brum, D.M.; Cassella, R.J. Novel spectrophotometric method for the determination of aluminum in soda drinks packed in cans and plastic bottles. J. Hazard. Mater. 2010, 181, 485–490. [Google Scholar] [CrossRef]
- Tontrong, S.; Khonyoung, S.; Jakmunee, J. Flow injection spectrophotometry using natural reagent from Morinda citrifolia root for determination of aluminium in tea. Food Chem. 2012, 132, 624–629. [Google Scholar] [CrossRef]
- Sang, H.; Liang, P.; Du, D. Determination of trace aluminum in biological and water samples by cloud point extraction preconcentration and graphite furnace atomic absorption spectrometry detection. J. Hazard. Mater. 2008, 154, 1127–1132. [Google Scholar] [CrossRef]
- Herdan, J.; Feeney, R.; Kounaves, S.P.; Flannery, A.F.; Storment, C.W.; Kovacs, G.T.; Darling, R.B. Field evaluation of an electrochemical probe for in situ screening of heavy metals in groundwater. Environ. Sci. Technol. 1998, 32, 131–136. [Google Scholar] [CrossRef]
- Lian, H.; Kang, Y.; Bi, S.; Arkin, Y.; Shao, D.; Li, D.; Chen, Y.; Dai, L.; Gan, N.; Tian, L. Direct determination of trace aluminum with quercetin by reversed-phase high performance liquid chromatography. Talanta 2004, 62, 43–50. [Google Scholar] [CrossRef]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Yang, C.; Liu, H.; Gao, Z.; Zhu, J.; Bao, X.; Kan, C. Dual-binding pyridine and rhodamine B conjugate derivatives as fluorescent chemosensors for ferric ions in aqueous media and living cells. Analyst 2019, 144, 3094–3102. [Google Scholar] [CrossRef]
- Kumar, G.G.V.; Kesavan, M.P.; Sankarganesh, M.; Sakthipandi, K.; Rajesh, J.; Sivaraman, G. A Schiff base receptor as a fluorescence turn-on sensor for Ni2+ ions in living cells and logic gate application. New J. Chem. 2018, 42, 2865–2873. [Google Scholar] [CrossRef]
- Ghazali, S.; Fan, J.; Du, J.; Peng, X. Mito-targeted “turn-on” fluorescent probe for nickel (II) detection. Methods 2019, 168, 24–28. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Xu, S.; Li, C.; Dong, B. Recent progress in fluorescent probes for metal ion detection. Front. Chem. 2022, 10, 875241. [Google Scholar] [CrossRef]
- Hu, L.; Nie, L.; Xu, G.; Shi, H.; Xu, X.; Zhang, X.; Yan, Z. Spectral properties of 4-(4-hydroxy-1-naphthylazo) benzenesulfonic acid and its application for colorimetric determination of trace Fe3+. RSC Adv. 2014, 4, 19370–19374. [Google Scholar] [CrossRef]
- Zhao, G.; Guo, B.; Wei, G.; Guang, S.; Gu, Z.; Xu, H. A novel dual-channel Schiff base fluorescent chemo-sensor for Zn2+ and Ca2+ recognition: Synthesis, mechanism and application. Dye. Pigment. 2019, 170, 107614. [Google Scholar] [CrossRef]
- Komatsu, H.; Miki, T.; Citterio, D.; Kubota, T.; Shindo, Y.; Kitamura, Y.; Oka, K.; Suzuki, K. Single molecular multianalyte (Ca2+, Mg2+) fluorescent probe and applications to bioimaging. J. Am. Chem. Soc. 2005, 127, 10798–10799. [Google Scholar] [CrossRef] [PubMed]
- Kolanowski, J.L.; Liu, F.; New, E.J. Fluorescent probes for the simultaneous detection of multiple analytes in biology. Chem. Soc. Rev. 2018, 47, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Khatua, S.; Schmittel, M. A single molecular light-up sensor for quantification of Hg2+ and Ag+ in aqueous medium: High selectivity toward Hg2+ over Ag+ in a mixture. Org. Lett. 2013, 15, 4422–4425. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhou, Y.; Yoon, J.; Kim, J.S. Recent progress in fluorescent and colorimetric chemosensors for detection of precious metal ions (silver, gold and platinum ions). Chem. Soc. Rev. 2011, 40, 3416–3429. [Google Scholar] [CrossRef]
- Kaim, W.; Schwederski, B.; Klein, A. Bioinorganic Chemistry—Inorganic Elements in the Chemistry of Life: An Introduction and Guide; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Butler, A.; Carrano, C.J. Coordination chemistry of vanadium in biological systems. Coord. Chem. Rev. 1991, 109, 61–105. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Pal, S.; Chatterjee, N.; Bharadwaj, P.K. Selectively sensing first-row transition metal ions through fluorescence enhancement. RSC Adv. 2014, 4, 26585–26620. [Google Scholar] [CrossRef]
- Fabbrizzi, L.; Poggi, A. Sensors and switches from supramolecular chemistry. Chem. Soc. Rev. 1995, 24, 197–202. [Google Scholar] [CrossRef]
- Igiri, B.E.; Okoduwa, S.I.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: A review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Kaur, N.; Kumar, S. Colorimetric metal ion sensors—A comprehensive review of the years 2011–2016. Coord. Chem. Rev. 2018, 358, 13–69. [Google Scholar] [CrossRef]
- Bicker, K.L.; Wiskur, S.L.; Lavigne, J.J. Colorimetric sensor design. In Chemosensors: Principles, Strategies, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 275–295. [Google Scholar]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef]
- Priyangga, K.T.A.; Kurniawan, Y.S.; Ohto, K.; Jumina, J. A review on calixarene fluorescent chemosensor agents for various analytes. J. Multidiscip. Appl. Nat. Sci. 2022, 2, 23–40. [Google Scholar] [CrossRef]
- Ali, R.; Razi, S.S.; Shahid, M.; Srivastava, P.; Misra, A. Off–On–Off fluorescence behavior of an intramolecular charge transfer probe toward anions and CO2. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 168, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, C.; Zhang, Z.; Luo, Y.; Sun, S.; Zhang, G. Cd (II) enhanced fluorescence and Zn (II) quenched fluorescence with phenylenevinylene terpyridine: A theoretical investigation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 209, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Manna, U.; Ray, T.; Das, G. An aggregation-induced emission (AIE) active probe for multiple targets: A fluorescent sensor for Zn2+ and Al3+ & a colorimetric sensor for Cu2+ and F−. Dalton Trans. 2015, 44, 18902–18910. [Google Scholar]
- Fukuhara, G. Analytical supramolecular chemistry: Colorimetric and fluorimetric chemosensors. J. Photochem. Photobiol. C Photochem. Rev. 2020, 42, 100340. [Google Scholar] [CrossRef]
- Aragay, G.; Pons, J.; Merkoçi, A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef]
- Darwish, N.B.; Kurdi, A.; Alshihri, S.; Tabbakh, T. Organic heterocyclic-based colorimetric and fluorimetric chemosensors for the detection of different analytes: A review (from 2015 to 2022). Mater. Today Chem. 2023, 27, 101347. [Google Scholar] [CrossRef]
- Jo, T.G.; Na, Y.J.; Lee, J.J.; Lee, M.M.; Lee, S.Y.; Kim, C. A multifunctional colorimetric chemosensor for cyanide and copper (II) ions. Sens. Actuators B Chem. 2015, 211, 498–506. [Google Scholar] [CrossRef]
- Hu, Y.; Yin, J.; Yoon, J. A multi-responsive cyanine-based colorimetric chemosensor containing dipicolylamine moieties for the detection of Zn (II) and Cu (II) ions. Sens. Actuators B Chem. 2016, 230, 40–45. [Google Scholar] [CrossRef]
- Mohammadi, A.; Yaghoubi, S. A new dual colorimetric chemosensor based on quinazolinone for CNˉ, AcOˉ and Cu2+ ions. Sens. Actuators B Chem. 2017, 241, 1069–1075. [Google Scholar] [CrossRef]
- Yang, M.; Chae, J.B.; Kim, C.; Harrison, R.G. A visible chemosensor based on carbohydrazide for Fe (II), Co (II) and Cu (II) in aqueous solution. Photochem. Photobiol. Sci. 2019, 18, 1249–1258. [Google Scholar] [CrossRef]
- Hu, J.-H.; Li, J.-B.; Qi, J.; Sun, Y. Acylhydrazone based fluorescent chemosensor for zinc in aqueous solution with high selectivity and sensitivity. Sens. Actuators B Chem. 2015, 208, 581–587. [Google Scholar] [CrossRef]
- Han, F.; Bao, Y.; Yang, Z.; Fyles, T.M.; Zhao, J.; Peng, X.; Fan, J.; Wu, Y.; Sun, S. Simple bisthiocarbonohydrazones as sensitive, selective, colorimetric, and switch-on fluorescent chemosensors for fluoride anions. Chem.-A Eur. J. 2007, 13, 2880–2892. [Google Scholar] [CrossRef]
- Zhou, Y.; You, X.-Y.; Fang, Y.; Li, J.-Y.; Liu, K.; Yao, C. A thiophen-thiooxorhodamine conjugate fluorescent probe for detecting mercury in aqueous media and living cells. Org. Biomol. Chem. 2010, 8, 4819–4822. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Chen, Y.; Bi, N.; Zheng, X.; Qi, H.; Qin, M.; Liao, X.; Zhang, H.; Tian, Y. Determination of mercury (II) by surface-enhanced Raman scattering spectroscopy based on thiol-functionalized silver nanoparticles. Microchim. Acta 2012, 177, 341–348. [Google Scholar] [CrossRef]
- Matesanz, A.I.; Cuadrado, I.; Pastor, C.; Souza, P. A Novel Sulfur-bridged Dimeric Zinc (II) Complex with 2, 6-Diacetylpyridine Bis (thiosemicarbazone). Z. Anorg. Allg. Chem. 2005, 631, 780–784. [Google Scholar] [CrossRef]
- Casas, J.S.; Castellano, E.E.; Ellena, J.; García-Tasende, M.S.; Sánchez, A.; Sordo, J.; Vázquez-López, E.M.; Vidarte, M.J. Synthesis, Spectroscopic and Structural Aspects of Mono-and Diorganothallium (III) Complexes of 2, 6-Diacetylpyridine Bis (thiosemicarbazone)(H2DAPTSC)—A New Coordination Mode of the HDAPTSC—Anion. Z. Anorg. Allg. Chem. 2003, 629, 261–267. [Google Scholar] [CrossRef]
- Paswan, S.; Bharty, M.; Bharati, P.; Sonkar, P.K.; Ganesan, V.; Butcher, R. Square planar Ni (ii) complexes of acetone N 4-phenyl-thiosemicarbazone and in situ generated benzoyl thiosemicarbazide ligands: Synthesis, spectral and structural characterizations, thermal behaviour and electrochemical studies. New J. Chem. 2017, 41, 15466–15474. [Google Scholar] [CrossRef]
- Ren, W.; Zhu, C.; Wang, E. Enhanced sensitivity of a direct SERS technique for Hg2+ detection based on the investigation of the interaction between silver nanoparticles and mercury ions. Nanoscale 2012, 4, 5902–5909. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Li, X.; Li, J.-Y.; Wang, G.-Y.; Zhou, Y.; Xu, N.-Z.; Hu, Y.; Yao, C. Thiooxo-Rhodamine B hydrazone derivatives bearing bithiophene group as fluorescent chemosensors for detecting mercury (II) in aqueous media and living HeLa cells. Sens. Actuators B Chem. 2018, 255, 1182–1190. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, B.; Zhao, Y.; Du, J.; Xiao, D. A Hg2+ selective fluorescent chemosensor based on rhodamine B thiohydrazide and its application in bioimaging. Anal. Methods 2012, 4, 2369–2374. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Li, L.; Liang, X.; Li, S.; Fu, Y. A dual thiourea-appended perylenebisimide “turn-on” fluorescent chemosensor with high selectivity and sensitivity for Hg2+ in living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118678. [Google Scholar] [CrossRef]
- Anbazhagan, R.; Sankaran, K. Design, synthesis, computational calculation and biological evaluation of some novel 2-thiazolyl hydrazones. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 984–993. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Biernasiuk, A. Synthesis and investigation of antimicrobial activities of nitrofurazone analogues containing hydrazide-hydrazone moiety. Saudi Pharm. J. 2017, 25, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Dubey, G.; Sran, B.S.; Bharatam, P.V.; Hundal, G. Fabrication of a Hydrazone-based Al (III)-selective “turn-on” fluorescent chemosensor and ensuing potential recognition of picric acid. ACS Omega 2019, 4, 18520–18529. [Google Scholar] [CrossRef]
- Pothulapadu, C.A.S.; Jayaraj, A.; Swathi, N.; Priyanka, R.N.; Sivaraman, G. Novel benzothiazole-based highly selective ratiometric fluorescent turn-on sensors for Zn2+ and colorimetric chemosensors for Zn2+, Cu2+, and Ni2+ ions. ACS Omega 2021, 6, 24473–24483. [Google Scholar] [CrossRef]
- Moussa, Z.; Al-Mamary, M.; Al-Juhani, S.; Ahmed, S.A. Preparation and biological assessment of some aromatic hydrazones derived from hydrazides of phenolic acids and aromatic aldehydes. Heliyon 2020, 6, e05019. [Google Scholar] [CrossRef]
- Popiołek, Ł. Hydrazide–hydrazones as potential antimicrobial agents: Overview of the literature since 2010. Med. Chem. Res. 2017, 26, 287–301. [Google Scholar] [CrossRef]
- Duval, C.; Loc, T.B. Applications variees du thiocarbohydrazide en chimie analytique. Comptes Rendus Hebd. Séances L’académie Sci. 1955, 240, 1097–1099. [Google Scholar]
- Alvarez, F.; de Pablos, F.; Ariza, J.L.G. Evaluation of two new asymmetric derivatives of thiocarbohydrazide as spectrophotometric analytical reagents. Talanta 1988, 35, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Duval, C.; Xuong, N.D. Emploi des thiocarbohydrazones en analyse organique fonctionelle. Microchim. Acta 1956, 44, 747–749. [Google Scholar] [CrossRef]
- Duval, C.; Xuong, N.D. Analyse organique fonctionnelle: Analyse thermogravimétrique des dinitro-2,4 phénylhydrazones. Anal. Chim. Acta 1954, 10, 520–522. [Google Scholar] [CrossRef]
- Esteban-Gómez, D.; Fabbrizzi, L.; Licchelli, M. Why, on interaction of urea-based receptors with fluoride, beautiful colors develop. J. Org. Chem. 2005, 70, 5717–5720. [Google Scholar] [CrossRef]
- de La Rosa, F.B.; Ariza, J.G.; Pino, F. Derivatives of carbohydrazide, thiocarbohydrazide and diaminoguanidine as photometric analytical reagents—I: 1,3-bis [(2-pyridyl) methyleneamino] thiourea and 1,3-bis [(2-pyridyl) methyleneamino] guanidine. Talanta 1983, 30, 555–564. [Google Scholar] [CrossRef]
- Momidi, B.K.; Tekuri, V.; Trivedi, D.R. Multi-signaling thiocarbohydrazide based colorimetric sensors for the selective recognition of heavy metal ions in an aqueous medium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 180, 175–182. [Google Scholar] [CrossRef]
- Udhayakumari, D. Mechanistic Innovations in Fluorescent Chemosensors for Detecting Toxic Ions: PET, ICT, ESIPT, FRET and AIE Approaches. J. Fluoresc. 2024, 1–30. [Google Scholar] [CrossRef]
- Özdemir, Ö. Novel symmetric diimine-Schiff bases and asymmetric triimine-Schiff bases as chemosensors for the detection of various metal ions. J. Mol. Struct. 2016, 1125, 260–271. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Xie, Y.; Bi, J.; Li, Y.; Song, Y.; Cheng, S.; Li, D.; Tan, M. Facile one-step synthesis of highly luminescent N-doped carbon dots as an efficient fluorescent probe for chromium (vi) detection based on the inner filter effect. New J. Chem. 2018, 42, 3729–3735. [Google Scholar] [CrossRef]
- Gong, X.; Liu, Y.; Yang, Z.; Shuang, S.; Zhang, Z.; Dong, C. An “on-off-on” fluorescent nanoprobe for recognition of chromium (VI) and ascorbic acid based on phosphorus/nitrogen dual-doped carbon quantum dot. Anal. Chim. Acta 2017, 968, 85–96. [Google Scholar] [CrossRef]
- Li, P.; Hong, Y.; Feng, H.; Li, S.F. An efficient “off–on” carbon nanoparticle-based fluorescent sensor for recognition of chromium (VI) and ascorbic acid based on the inner filter effect. J. Mater. Chem. B 2017, 5, 2979–2988. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Peng, J.; Peng, H.; Liu, S.; Xiao, H.; Liu, D.; Pan, Z.; Chen, Y.; Chen, F.; He, Y. Fluorescent carbon dots for the sensitive detection of Cr (VI) in aqueous media and their application in test papers. RSC Adv. 2016, 6, 95469–95475. [Google Scholar] [CrossRef]
- Zheng, M.; Xie, Z.; Qu, D.; Li, D.; Du, P.; Jing, X.; Sun, Z. On–off–on fluorescent carbon dot nanosensor for recognition of chromium (VI) and ascorbic acid based on the inner filter effect. ACS Appl. Mater. Interfaces 2013, 5, 13242–13247. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, P.; Wang, J.; Zhao, L.; Duan, C. Dansyl-based fluorescent chemosensors for selective responses of Cr (III). New J. Chem. 2009, 33, 653–658. [Google Scholar] [CrossRef]
- Yang, M.; Sun, M.; Zhang, Z.; Wang, S. A novel dansyl-based fluorescent probe for highly selective detection of ferric ions. Talanta 2013, 105, 34–39. [Google Scholar] [CrossRef]
- Yu, M.; Du, W.; Zhou, W.; Li, H.; Liu, C.; Wei, L.; Li, Z.; Zhang, H. A 1,8-naphthalimide-based chemosensor with an off-on fluorescence and lifetime imaging response for intracellular Cr3+ and further for S2−. Dye. Pigment. 2016, 126, 279–285. [Google Scholar] [CrossRef]
- Khan, S.; Muhammad, M.; Algethami, J.S.; Al-Saidi, H.M.; Almahri, A.; Hassanian, A.A. Synthesis, characterization and applications of schiff base chemosensor for determination of Cr (III) ions. J. Fluoresc. 2022, 32, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Shakir, M. An inner filter effect based Schiff base chemosensor for recognition of Cr (VI) and ascorbic acid in water matrices. New J. Chem. 2018, 42, 293–300. [Google Scholar] [CrossRef]
- Emsley, J. Nature’s Building Blocks: An AZ Guide to the Elements; Oxford University Press: New York, USA, 2011. [Google Scholar]
- Gray, T. Elements: A Visual Exploration of Every Known Atom in the Universe; Hachette: London, UK, 2012. [Google Scholar]
- Charles Dismukes, G.; van Willigen, R.T. Manganese: The Oxygen-Evolving Complex & Models. In Encyclopedia of Inorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Raymond, J.; Blankenship, R.E. The origin of the oxygen-evolving complex. Coord. Chem. Rev. 2008, 252, 377–383. [Google Scholar] [CrossRef]
- Liang, J.; Canary, J.W. Discrimination between hard metals with soft ligand donor atoms: An on-fluorescence probe for manganese (II). Angew. Chem. (Int. Ed. Engl.) 2010, 49, 7710. [Google Scholar] [CrossRef] [PubMed]
- McCreary, J.K. Applications of Manganese-Enhanced Magnetic Resonance Imaging in Neuroscience; University of Lethbridge, Department of Neuroscience: Lethbridge, AB, Canada, 2012. [Google Scholar]
- Drapeau, P.; Nachshen, D. Manganese fluxes and manganese-dependent neurotransmitter release in presynaptic nerve endings isolated from rat brain. J. Physiol. 1984, 348, 493–510. [Google Scholar] [CrossRef]
- Hunter, D.R.; Haworth, R.A.; Berkoff, H.A. Cellular manganese uptake by the isolated perfused rat heart: A probe for the sarcolemma calcium channel. J. Mol. Cell. Cardiol. 1981, 13, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Sotogaku, N.; Oku, N. Influence of manganese on the release of neurotransmitters in rat striatum. Brain Res. 2003, 965, 279–282. [Google Scholar] [CrossRef]
- Abe, S.; Fujii, K.; Sono, T. Liquid-liquid extraction of manganese (II), copper (II) and zinc (II) with acyclic and macrocyclic Schiff bases containing bisphenol A subunits. Anal. Chim. Acta 1994, 293, 325–330. [Google Scholar] [CrossRef]
- Epstein, D.M.; Choudhary, S.; Churchill, M.R.; Keil, K.M.; Eliseev, A.V.; Morrow, J.R. Chloroform-soluble Schiff-base Zn (II) or Cd (II) complexes from a dynamic combinatorial library. Inorg. Chem. 2001, 40, 1591–1596. [Google Scholar] [CrossRef]
- Oshima, S.; Hirayama, N.; Kubono, K.; Kokusen, H.; Honjo, T. Structural control of Schiff base ligands for selective extraction of copper (II). Anal. Sci. 2002, 18, 1351–1355. [Google Scholar] [CrossRef]
- Zhou, X.-X.; Fang, H.-C.; Ge, Y.-Y.; Zhou, Z.-Y.; Gu, Z.-G.; Gong, X.; Zhao, G.; Zhan, Q.-G.; Zeng, R.-H.; Cai, Y.-P. Assembly of a series of trinuclear zinc (II) compounds with N2O2 donor tetradentate symmetrical Schiff base ligand. Cryst. Growth Des. 2010, 10, 4014–4022. [Google Scholar] [CrossRef]
- Hirayama, N.; Taga, J.; Oshima, S.; Honjo, T. Sulfonamide-type di-Schiff base ligands as chelate extraction reagents for divalent metal cations. Anal. Chim. Acta 2002, 466, 295–301. [Google Scholar] [CrossRef]
- Hariharan, P.; Anthony, S.P. Substitutional group dependent colori/fluorimetric sensing of Mn2+, Fe3+ and Zn2+ ions by simple Schiff base chemosensor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.H. A review on organic colorimetric and fluorescent chemosensors for the detection of Zn (II) ions. Crit. Rev. Anal. Chem. 2023, 53, 1472–1488. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lim, C.; Suh, H.; Song, E.J.; Kim, C. A multifunctional sensor: Chromogenic sensing for Mn2+ and fluorescent sensing for Zn2+ and Al3+. Sens. Actuators B Chem. 2014, 201, 535–544. [Google Scholar] [CrossRef]
- Kim, K.B.; Park, G.J.; Kim, H.; Song, E.J.; Bae, J.M.; Kim, C. A novel colorimetric chemosensor for multiple target ions in aqueous solution: Simultaneous detection of Mn (II) and Fe (II). Inorg. Chem. Commun. 2014, 46, 237–240. [Google Scholar] [CrossRef]
- Raju, V.; Kumar, R.S.; Tharakeswar, Y.; Kumar, S.A. A multifunctional Schiff-base as chromogenic chemosensor for Mn2+ and fluorescent chemosensor for Zn2+ in semi-aqueous environment. Inorganica Chim. Acta 2019, 493, 49–56. [Google Scholar] [CrossRef]
- Qin, J.-c.; Yang, Z.-y.; Wang, G.-q. A novel ratiometric fluorescent probe for detection of Fe3+ by rhodamine–quinoline conjugate. J. Photochem. Photobiol. A Chem. 2015, 310, 122–127. [Google Scholar] [CrossRef]
- Liu, S.-R.; Wu, S.-P. New water-soluble highly selective fluorescent chemosensor for Fe (III) ions and its application to living cell imaging. Sens. Actuators B Chem. 2012, 171, 1110–1116. [Google Scholar] [CrossRef]
- Sen, S.; Sarkar, S.; Chattopadhyay, B.; Moirangthem, A.; Basu, A.; Dhara, K.; Chattopadhyay, P. A ratiometric fluorescent chemosensor for iron: Discrimination of Fe2+ and Fe3+ and living cell application. Analyst 2012, 137, 3335–3342. [Google Scholar] [CrossRef]
- Li, S.; Zhang, D.; Xie, X.; Ma, S.; Liu, Y.; Xu, Z.; Gao, Y.; Ye, Y. A novel solvent-dependently bifunctional NIR absorptive and fluorescent ratiometric probe for detecting Fe3+/Cu2+ and its application in bioimaging. Sens. Actuators B Chem. 2016, 224, 661–667. [Google Scholar] [CrossRef]
- Tamil Selvan, G.; Varadaraju, C.; Tamil Selvan, R.; Enoch, I.V.; Mosae Selvakumar, P. On/off fluorescent chemosensor for selective detection of divalent iron and copper ions: Molecular logic operation and protein binding. ACS Omega 2018, 3, 7985–7992. [Google Scholar] [CrossRef]
- Nagarajan, R.; Vanjare, B.D.; Lee, K.H. The first tryptophan based turn-off chemosensor for Fe2+ ion detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120103. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Wang, H.; Wang, Y.; Huang, Q.; Zhang, Q. A novel colorimetric and turn-on fluorescent chemosensor for iron (III) ion detection and its application to cellular imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 168, 37–44. [Google Scholar] [CrossRef]
- Huang, L.; Hou, F.; Cheng, J.; Xi, P.; Chen, F.; Bai, D.; Zeng, Z. Selective off–on fluorescent chemosensor for detection of Fe3+ ions in aqueous media. Org. Biomol. Chem. 2012, 10, 9634–9638. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qian, Y. A novel naphthalimide-rhodamine dye: Intramolecular fluorescence resonance energy transfer and ratiometric chemodosimeter for Hg2+ and Fe3+. Dye. Pigment. 2017, 136, 782–790. [Google Scholar] [CrossRef]
- Guo, X.; Pan, Q.; Song, X.; Guo, Q.; Zhou, S.; Qiu, J.; Dong, G. Embedding carbon dots in Eu3+-doped metal-organic framework for label-free ratiometric fluorescence detection of Fe3+ ions. J. Am. Ceram. Soc. 2021, 104, 886–895. [Google Scholar] [CrossRef]
- Ganesan, J.S.; Sepperumal, M.; Balasubramaniem, A.; Ayyanar, S. A novel pyrazole bearing imidazole frame as ratiometric fluorescent chemosensor for Al3+/Fe3+ ions and its application in HeLa cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 117993. [Google Scholar] [CrossRef]
- Wei, T.-B.; Zhang, P.; Shi, B.-B.; Chen, P.; Lin, Q.; Liu, J.; Zhang, Y.-M. A highly selective chemosensor for colorimetric detection of Fe3+ and fluorescence turn-on response of Zn2+. Dye. Pigment. 2013, 97, 297–302. [Google Scholar] [CrossRef]
- Chen, Z.-E.; Zang, X.-F.; Zhang, H. An ethyl thioglycolate-based chemosensor: Spectrophotometric detection of Fe3+ and fluorometric detection of Hg2+ with high selectivity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 260, 119955. [Google Scholar] [CrossRef] [PubMed]
- Mustufa, M.; Abbasi, A.; Hanif, S.; Bhat, Z.U.H.; Alam, M.J.; Ahmad, M.; Shakir, M. A combined theoretical and experimental study of ratiometric fluorescent Schiff base chemosensor for detection of Fe3+ ion and its anticancer activity. Chem. Inorg. Mater. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Singh, G.; Singh, J.; Singh, J.; Mangat, S.S. Design of selective 8-methylquinolinol based ratiometric Fe2+ and Fe3+/H2PO4− fluorescent chemosensor mimicking NOR and IMPLICATION logic gates. J. Lumin. 2015, 165, 123–129. [Google Scholar] [CrossRef]
- Li, B.; Gu, X.; Wang, M.; Liu, X.; Xu, K. A novel “off-on-off” fluorescent probe for sensing of Fe3+ and F− successively in aqueous solution and its application in cells. Dye. Pigment. 2021, 194, 109637. [Google Scholar] [CrossRef]
- Battal, A.; Kassa, S.B.; Gultekin, N.A.; Tavasli, M.; Onganer, Y. A Carbazole-based Fluorescent Turn-off Chemosensor for Iron (II/III) Detection in a Dimethyl Sulfoxide. J. Fluoresc. 2023, 33, 1421–1429. [Google Scholar] [CrossRef]
- Maity, D.; Raj, A.; Karthigeyan, D.; Kundu, T.K.; Govindaraju, T. Reaction-based probes for Co (II) and Cu (I) with dual output modes: Fluorescence live cell imaging. RSC Adv. 2013, 3, 16788–16794. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Wang, Y.; Ding, X.-J.; Sun, Y.-X.; Liu, H.-B.; Xie, C.-Z.; Qian, J.; Li, Q.-Z.; Xu, J.-Y. A highly selective colorimetric and fluorescent probe for quantitative detection of Cu2+/Co2+: The unique ON-OFF-ON fluorimetric detection strategy and applications in living cells/zebrafish. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117763. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Choi, Y.W.; You, G.R.; Lee, S.Y.; Kim, C. A phthalazine-based two-in-one chromogenic receptor for detecting Co2+ and Cu2+ in an aqueous environment. Dalton Trans. 2015, 44, 13305–13314. [Google Scholar] [CrossRef] [PubMed]
- Maity, D.; Govindaraju, T. Highly selective colorimetric chemosensor for Co2+. Inorg. Chem. 2011, 50, 11282–11284. [Google Scholar] [CrossRef]
- Jang, H.J.; Chae, J.B.; Jung, J.M.; So, H.; Kim, C. Colorimetric detection of Co2+, Cu2+, and Zn2+ by a multifunctional chemosensor in aqueous solution. Bull. Korean Chem. Soc. 2019, 40, 650–657. [Google Scholar] [CrossRef]
- Min, C.H.; Na, S.; Shin, J.E.; Kim, J.K.; Jo, T.G.; Kim, C. A new Schiff-based chemosensor for chromogenic sensing of Cu2+, Co2+ and S2− in aqueous solution: Experimental and theoretical studies. New J. Chem. 2017, 41, 3991–3999. [Google Scholar] [CrossRef]
- Tawfik, S.M.; Farag, A.A.; Abd-Elaal, A.A. Fluorescence Naphthalene Cationic Schiff Base Reusable Paper as a Sensitive and Selective for Heavy Metals Cations Sensor: RSM, Optimization, and DFT Modelling. J. Fluoresc. 2024, 34, 2139–2155. [Google Scholar] [CrossRef]
- Azizi Khereshki, N.; Mohammadi, A.; Zavvar Mousavi, H.; Alizadeh, N. A novel thiosemicarbazide based chemosensor for colorimetric detection of Co2+ in commercial B12 vitamin and Co2+, Ni2+ simultaneously in aqueous media. Supramol. Chem. 2021, 33, 513–526. [Google Scholar] [CrossRef]
- Kim, P.A.; Lee, H.; So, H.; Kim, C. A chelated-type colorimetric chemosensor for sensing Co2+ and Cu2+. Inorganica Chim. Acta 2020, 505, 119502. [Google Scholar] [CrossRef]
- Liu, X.; Lin, Q.; Wei, T.-B.; Zhang, Y.-M. A highly selective colorimetric chemosensor for detection of nickel ions in aqueous solution. New J. Chem. 2014, 38, 1418–1423. [Google Scholar] [CrossRef]
- Kang, J.H.; Lee, S.Y.; Ahn, H.M.; Kim, C. A novel colorimetric chemosensor for the sequential detection of Ni2+ and CN− in aqueous solution. Sens. Actuators B Chem. 2017, 242, 25–34. [Google Scholar] [CrossRef]
- Goswami, S.; Chakraborty, S.; Das, A.K.; Manna, A.; Bhattacharyya, A.; Quah, C.K.; Fun, H.-K. Selective colorimetric and ratiometric probe for Ni (II) in quinoxaline matrix with the single crystal X-ray structure. RSC Adv. 2014, 4, 20922–20926. [Google Scholar] [CrossRef]
- Peralta-Domínguez, D.; Rodríguez, M.; Ramos-Ortíz, G.; Maldonado, J.L.; Meneses-Nava, M.A.; Barbosa-García, O.; Santillan, R.; Farfán, N. A Schiff base derivative from cinnamaldehyde for colorimetric detection of Ni2+ in water. Sens. Actuators B Chem. 2015, 207, 511–517. [Google Scholar] [CrossRef]
- Alqasaimeh, M.M.; Abu-Yamin, A.-A.M.; Matar, S.A.; Sarairah, I.A.; Salman, M.M.; Al-As’ad, R.M. Synthesis and characterization of a new Schiff-base derivative as an optical nickel (II) chemosensor and its antimicrobial activity. J. Photochem. Photobiol. A Chem. 2024, 447, 115277. [Google Scholar] [CrossRef]
- Manna, A.K.; Chowdhury, S.; Patra, G.K. A novel hydrazide-based selective and sensitive optical chemosensor for the detection of Ni2+ ions: Applications in live cell imaging, molecular logic gates and smart phone-based analysis. Dalton Trans. 2019, 48, 12336–12348. [Google Scholar] [CrossRef]
- Korzec, M.; Senkała, S.; Rzycka-Korzec, R.; Kotowicz, S.; Schab-Balcerzak, E.; Polański, J. A highly selective and sensitive sensor with imine and phenyl-ethynyl-phenyl units for the visual and fluorescent detection of copper in water. J. Photochem. Photobiol. A Chem. 2019, 382, 111893. [Google Scholar] [CrossRef]
- Jiang, J.; Gou, C.; Luo, J.; Yi, C.; Liu, X. A novel highly selective colorimetric sensor for Ni (II) ion using coumarin derivatives. Inorg. Chem. Commun. 2012, 15, 12–15. [Google Scholar] [CrossRef]
- Lu, W.; Chen, J.; Shi, J.; Li, Z.; Xu, L.; Jiang, W.; Yang, S.; Gao, B. An acylhydrazone coumarin as chemosensor for the detection of Ni2+ with excellent sensitivity and low LOD: Synthesis, DFT calculations and application in real water and living cells. Inorganica Chim. Acta 2021, 516, 120144. [Google Scholar] [CrossRef]
- Patil, N.; Dhake, R.; Phalak, R.; Fegade, U.; Naushad, M.; Bathula, C.; Kanchi, S.; Govender, K. A colorimetric chemosensor for distinct color change with (E)-2-(1-(3-aminophenyl) ethylideneamino) benzenethiol to detect Cu2+ in real water samples. Anal. Sci. 2023, 39, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Leung, K.-H.; Lin, S.; Chan, D.S.-H.; Kwong, D.W.; Leung, C.-H.; Ma, D.-L. A colorimetric chemosensor for Cu2+ ion detection based on an iridium (III) complex. Sci. Rep. 2014, 4, 6794. [Google Scholar] [CrossRef]
- Li, G.; Tao, F.; Wang, H.; Li, Y.; Wang, L. A novel reversible colorimetric chemosensor for rapid naked-eye detection of Cu2+ in pure aqueous solution. Sens. Actuators B Chem. 2015, 211, 325–331. [Google Scholar] [CrossRef]
- Kowser, Z.; Rayhan, U.; Akther, T.; Redshaw, C.; Yamato, T. A brief review on novel pyrene based fluorometric and colorimetric chemosensors for the detection of Cu2+. Mater. Chem. Front. 2021, 5, 2173–2200. [Google Scholar] [CrossRef]
- Hrichi, H.; Elkanzi, N.A.; Ali, A.M.; Abdou, A. A novel colorimetric chemosensor based on 2-[(carbamothioylhydrazono) methyl] phenyl 4-methylbenzenesulfonate (CHMPMBS) for the detection of Cu (II) in aqueous medium. Res. Chem. Intermed. 2023, 49, 2257–2276. [Google Scholar] [CrossRef]
- Abdou, A.; Mostafa, H.M.; Abdel-Mawgoud, A.-M.M. Seven metal-based bi-dentate NO azocoumarine complexes: Synthesis, physicochemical properties, DFT calculations, drug-likeness, in vitro antimicrobial screening and molecular docking analysis. Inorganica Chim. Acta 2022, 539, 121043. [Google Scholar] [CrossRef]
- Rupa, S.A.; Patwary, M.A.M.; Ghann, W.E.; Abdullahi, A.; Uddin, A.R.; Mahmud, M.M.; Haque, M.A.; Uddin, J.; Kazi, M. Synthesis of a novel hydrazone-based compound applied as a fluorescence turn-on chemosensor for iron (iii) and a colorimetric sensor for copper (ii) with antimicrobial, DFT and molecular docking studies. RSC Adv. 2023, 13, 23819–23828. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Wei, J.; Ren, Y.; Zhang, N.; Hao, X.-Q.; Zhu, X.; Xu, Y. A new colorimetric and fluorescent chemosensor based on thiazolyl-hydrazone for sensitive detection of copper ions. J. Iran. Chem. Soc. 2020, 17, 609–614. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, J.J.; Lee, S.Y.; Jo, T.G.; Kim, C. A highly sensitive benzimidazole-based chemosensor for the colorimetric detection of Fe (II) and Fe (III) and the fluorometric detection of Zn (II) in aqueous media. RSC Adv. 2016, 6, 61505–61515. [Google Scholar] [CrossRef]
- Liu, D.; Deng, X.; Yin, X.; Wang, Y.; Guo, J.; Chen, J.; Yang, G.; He, H. 1, 8-Naphthalimide-based fluorescent sensor with high selectivity and sensitivity for Zn2+ and its imaging in living cells. Inorg. Chem. Commun. 2019, 101, 117–120. [Google Scholar] [CrossRef]
- Burdette, S.C.; Walkup, G.K.; Spingler, B.; Tsien, R.Y.; Lippard, S.J. Fluorescent sensors for Zn2+ based on a fluorescein platform: Synthesis, properties and intracellular distribution. J. Am. Chem. Soc. 2001, 123, 7831–7841. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Y.; Qi, J.; Liu, X.-Y.; He, H.-R.; Chen, J.-T.; Yang, G.-M. 4-Amino-1,8-naphthalimide-based fluorescent sensor with high selectivity and sensitivity for Zn2+ imaging in living cells. Inorg. Chem. Commun. 2014, 43, 173–178. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Z.; Li, Y.; He, W.; Gao, X.; Guo, Z. In vitro and in vivo imaging application of a 1, 8-naphthalimide-derived Zn2+ fluorescent sensor with nuclear envelope penetrability. Chem. Commun. 2013, 49, 11430–11432. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhao, Y.; Shi, J.; Zhu, H.; Zhang, T.; Qi, P.; Chen, J.; Yang, G.; He, H. A highly selective and sensitive 1, 8-naphthalimide-based fluorescent sensor for Zn2+ imaging in living cells. Bioorganic Med. Chem. Lett. 2019, 29, 2646–2649. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.E.; Lee, S.; You, Y.; Baek, K.-H.; Ohkubo, K.; Cho, J.; Fukuzumi, S.; Shin, I.; Park, S.Y.; Nam, W. Fluorescent zinc sensor with minimized proton-induced interferences: Photophysical mechanism for fluorescence turn-on response and detection of endogenous free zinc ions. Inorg. Chem. 2012, 51, 8760–8774. [Google Scholar] [CrossRef]
- Wu, Y.; Peng, X.; Guo, B.; Fan, J.; Zhang, Z.; Wang, J.; Cui, A.; Gao, Y. Boron dipyrromethene fluorophore based fluorescence sensor for the selective imaging of Zn (II) in living cells. Org. Biomol. Chem. 2005, 3, 1387–1392. [Google Scholar] [CrossRef]
- Fan, J.; Peng, X.; Wu, Y.; Lu, E.; Hou, J.; Zhang, H.; Zhang, R.; Fu, X. A new PET fluorescent sensor for Zn2+. J. Lumin. 2005, 114, 125–130. [Google Scholar] [CrossRef]
- Lee, H.G.; Lee, J.H.; Jang, S.P.; Park, H.M.; Kim, S.-J.; Kim, Y.; Kim, C.; Harrison, R.G. Zinc selective chemosensor based on pyridyl-amide fluorescence. Tetrahedron 2011, 67, 8073–8078. [Google Scholar] [CrossRef]
- Choe, D.; So, H.; Park, S.; Lee, H.; Chae, J.B.; Kim, J.; Kim, K.-T.; Kim, C. An indole-based fluorescent chemosensor for detecting Zn2+ in aqueous media and zebrafish. Sensors 2021, 21, 5591. [Google Scholar] [CrossRef]
- Mathew, M.M.; Sreekanth, A. Zn2+ ion responsive fluorescent chemosensor probe of Thiophene-diocarbohydrazide derivatives. Inorganica Chim. Acta 2021, 516, 120149. [Google Scholar] [CrossRef]
- Peng, X.; Tang, X.; Qin, W.; Dou, W.; Guo, Y.; Zheng, J.; Liu, W.; Wang, D. Aroylhydrazone derivative as fluorescent sensor for highly selective recognition of Zn2+ ions: Syntheses, characterization, crystal structures and spectroscopic properties. Dalton Trans. 2011, 40, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Singh, K. Analyte detection: A decade of progress in the development of optical/fluorescent sensing probes. Chem. Rec. 2023, 23, e202200184. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.S.; Shaw, I.; Liu, Y.; Chen, C. Perspective Chapter: Advanced Nanotechnology Approach for Heavy Metal Toxicity–Analysis. In Heavy Metals in the Environment—Contamination, Risk, and Remediation; IntechOpen Limited: London, UK, 2025. [Google Scholar] [CrossRef]
- Khalajiolyaie, A.; Jian, C. Advances in Graphene-Based Materials for Metal Ion Sensing and Wastewater Treatment: A Review. Environments 2025, 12, 43. [Google Scholar] [CrossRef]


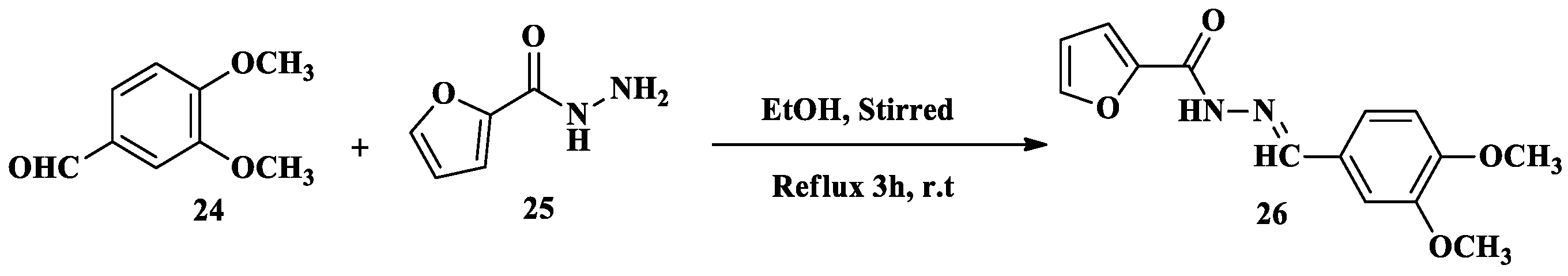







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslam, S.; Kousar, I.; Rani, S.; Zainab, I.; Bristy, S.; Skouta, R. Modern Approaches in Organic Chromofluorescent Sensor Synthesis for the Detection of Considered First-Row Transition Metal Ions. Molecules 2025, 30, 1263. https://doi.org/10.3390/molecules30061263
Aslam S, Kousar I, Rani S, Zainab I, Bristy S, Skouta R. Modern Approaches in Organic Chromofluorescent Sensor Synthesis for the Detection of Considered First-Row Transition Metal Ions. Molecules. 2025; 30(6):1263. https://doi.org/10.3390/molecules30061263
Chicago/Turabian StyleAslam, Samina, Iram Kousar, Sadia Rani, Isra Zainab, Sadia Bristy, and Rachid Skouta. 2025. "Modern Approaches in Organic Chromofluorescent Sensor Synthesis for the Detection of Considered First-Row Transition Metal Ions" Molecules 30, no. 6: 1263. https://doi.org/10.3390/molecules30061263
APA StyleAslam, S., Kousar, I., Rani, S., Zainab, I., Bristy, S., & Skouta, R. (2025). Modern Approaches in Organic Chromofluorescent Sensor Synthesis for the Detection of Considered First-Row Transition Metal Ions. Molecules, 30(6), 1263. https://doi.org/10.3390/molecules30061263







