Boosting Visible-Light-Driven Hydrogen Evolution Enabled by Iodine-Linked Magnetically Curved Graphene with Mobius-like Electronic Paths
Abstract
:1. Introduction
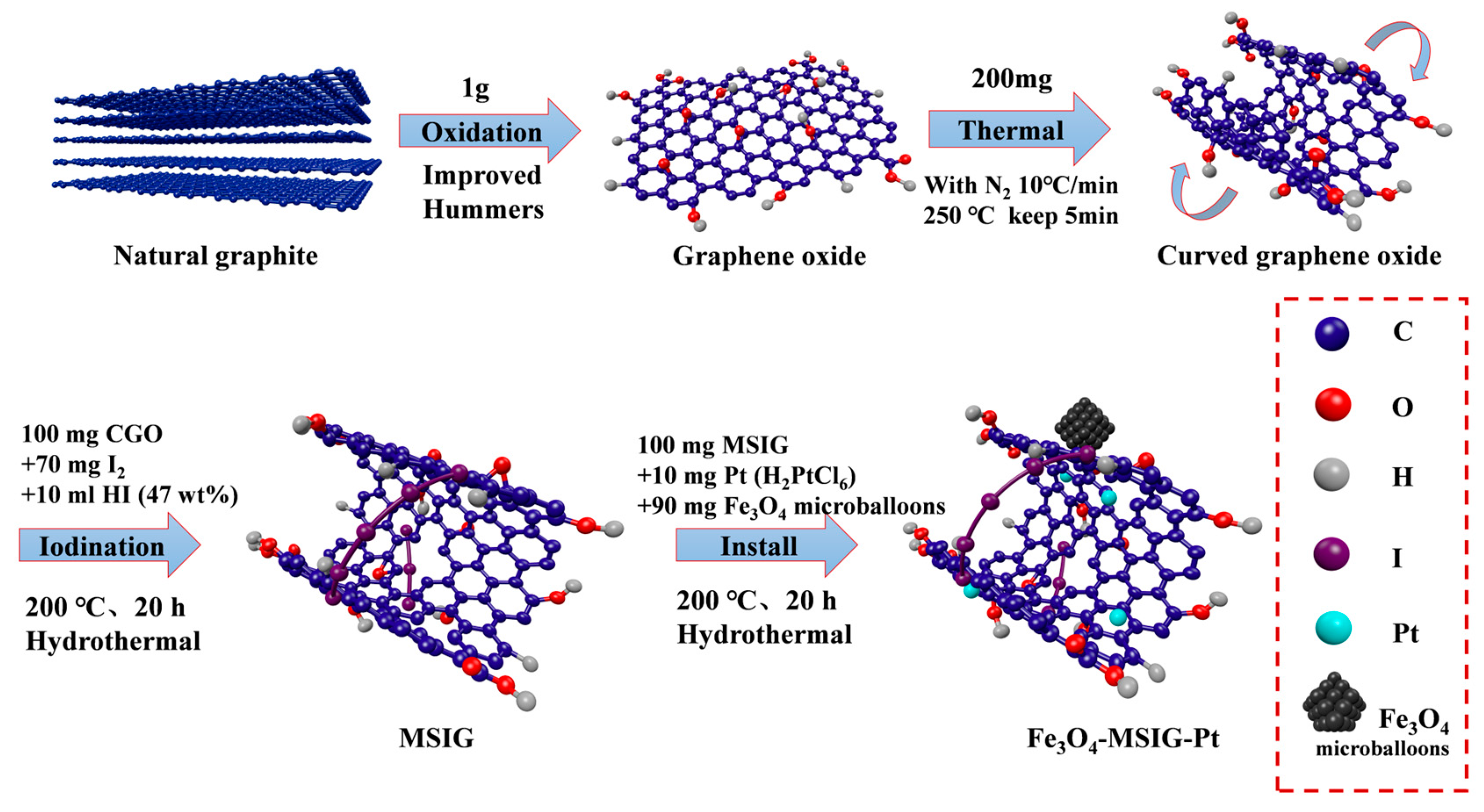
2. Results and Discussion
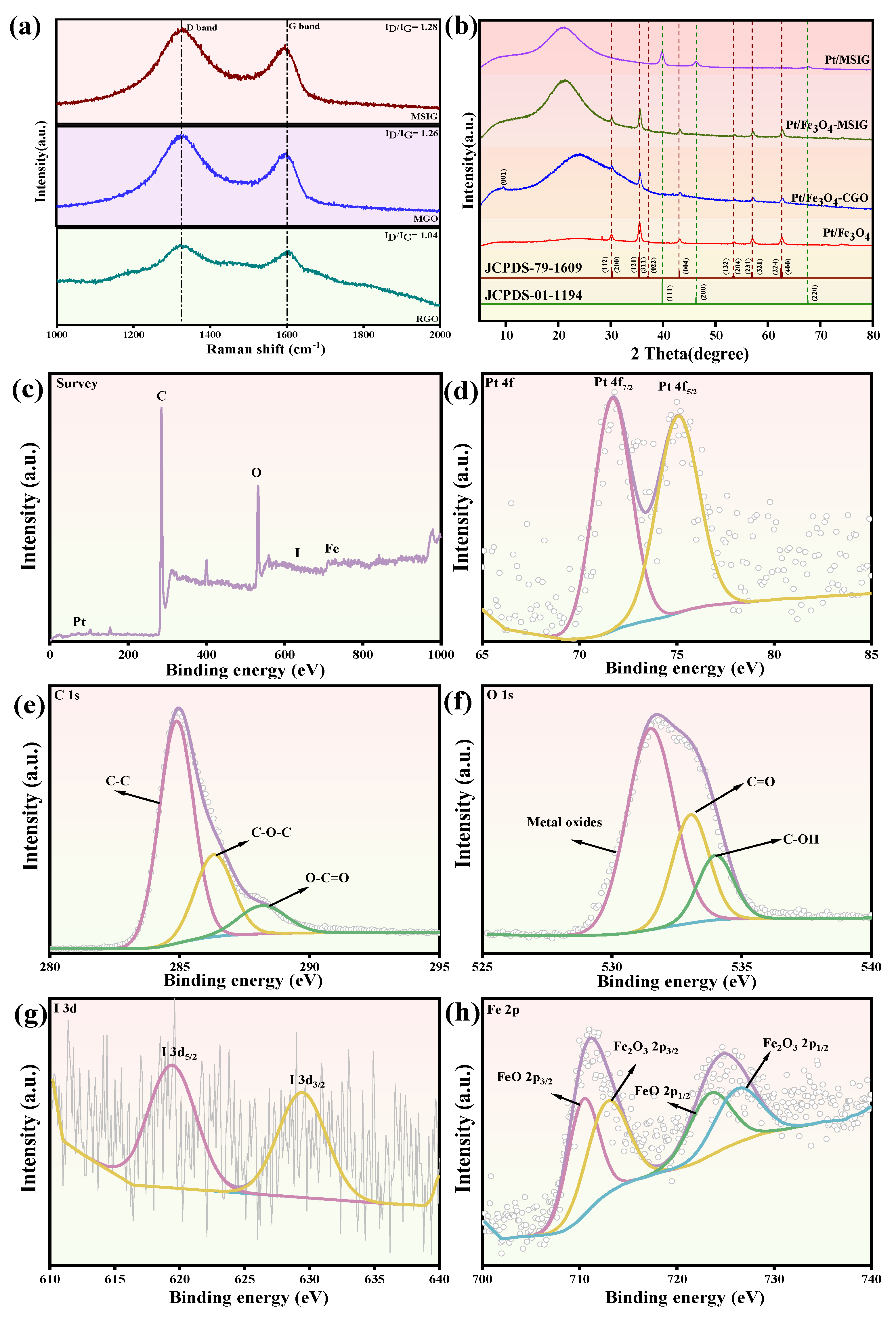
2.1. The XRD and Raman Analysis
2.2. XPS Analysis
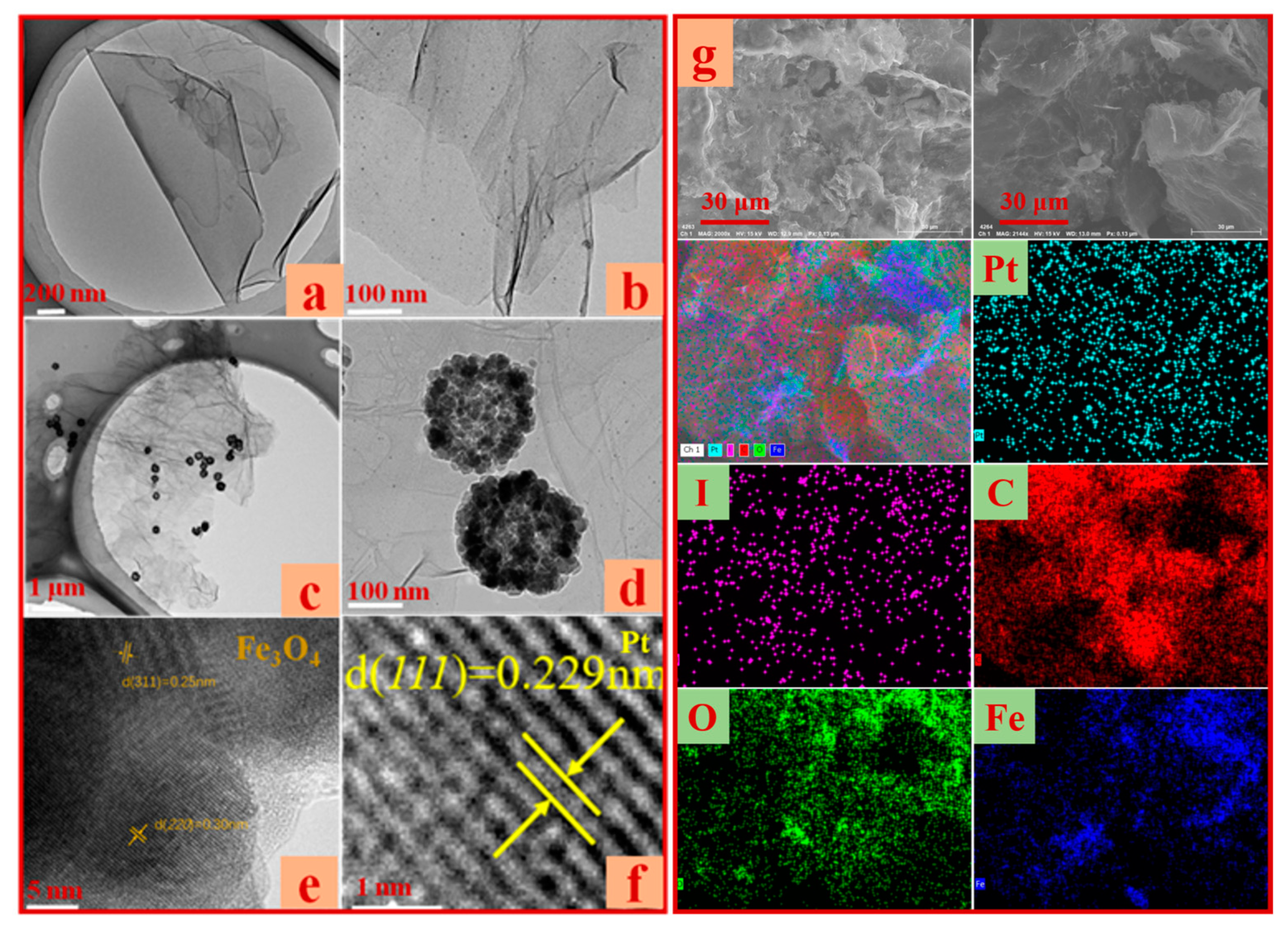
2.3. TEM Analysis
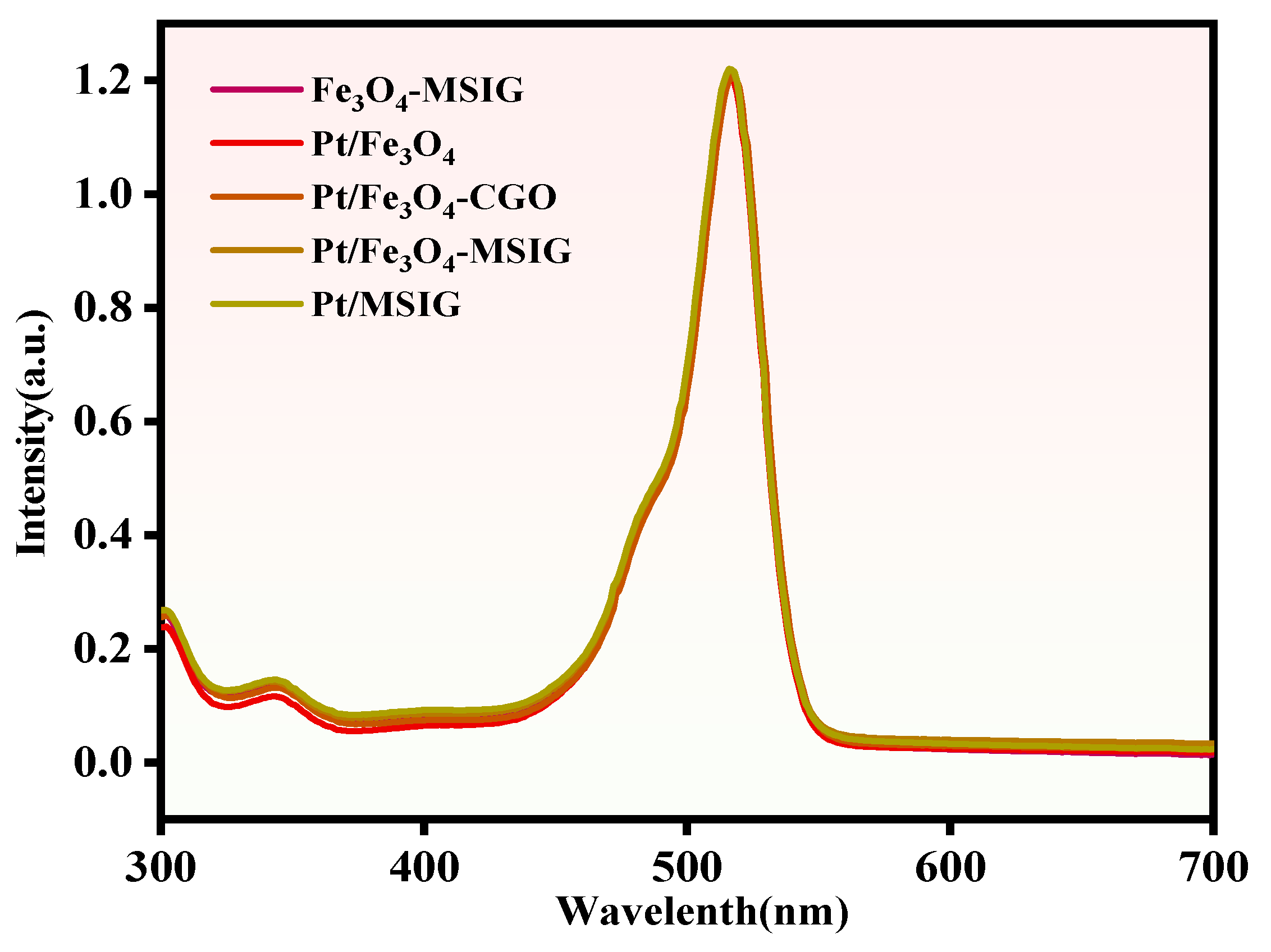
2.4. UV-Vis Analysis
2.5. Catalytic Activity Performance
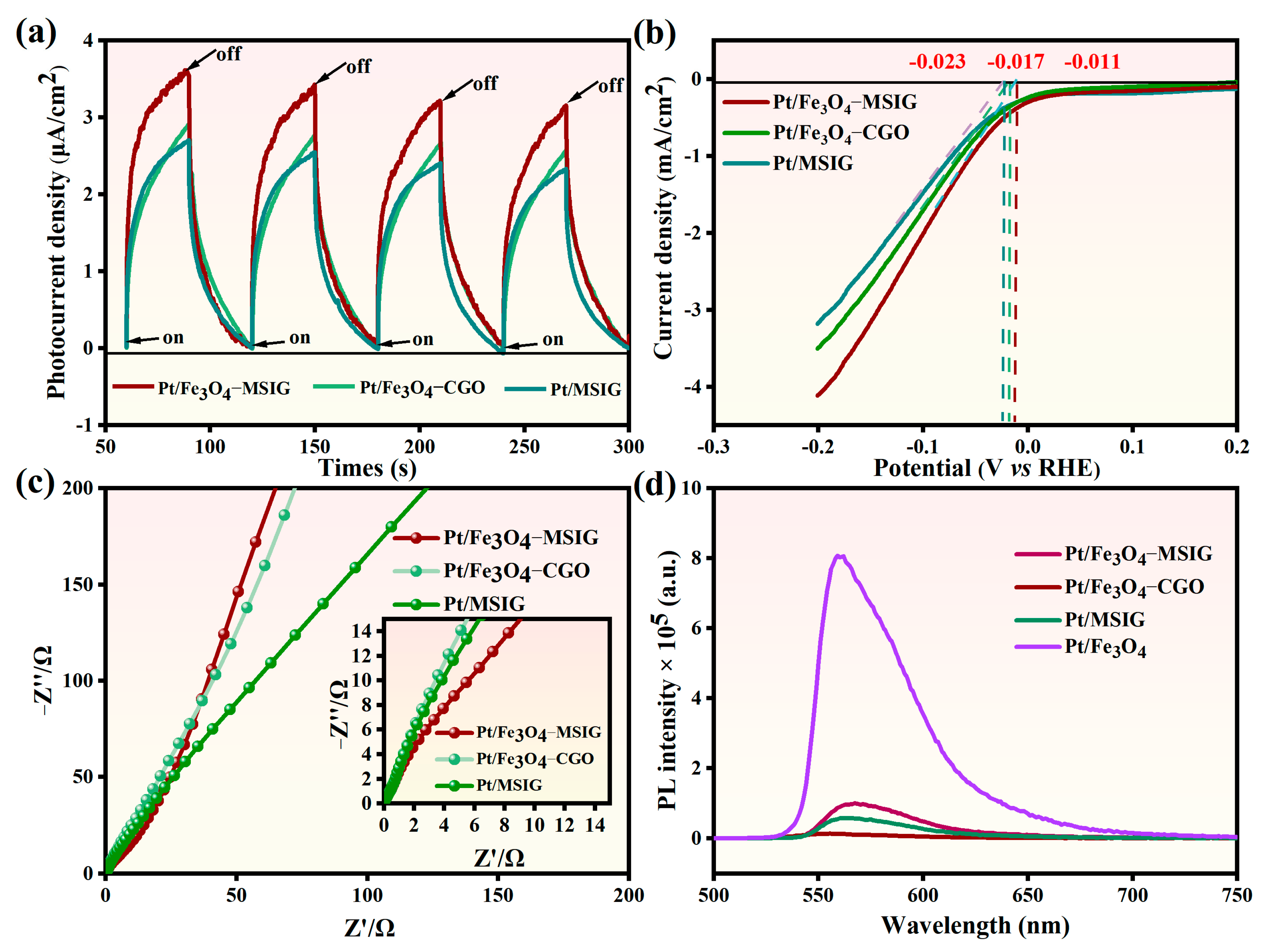
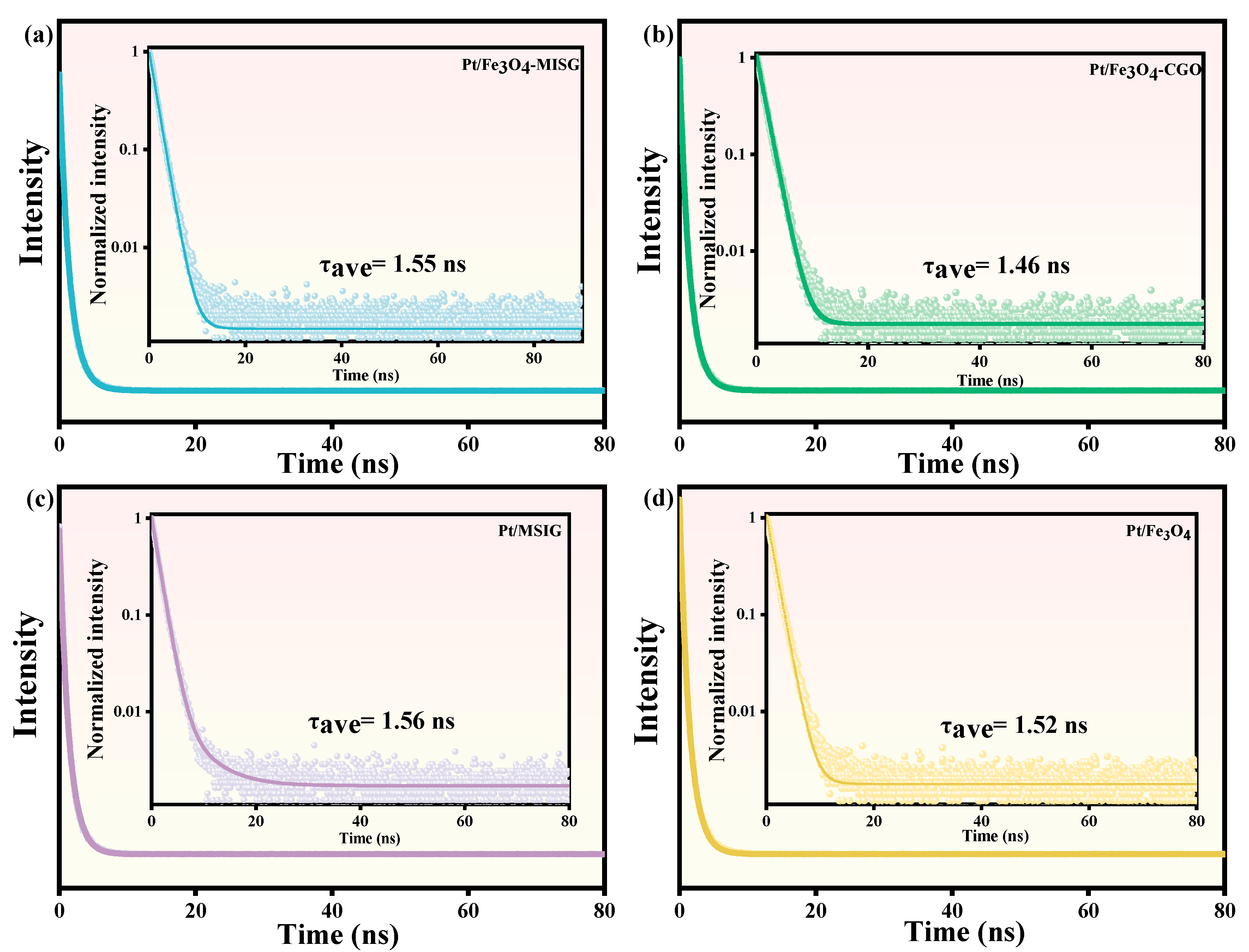
2.6. Photo-Electrochemical Analysis
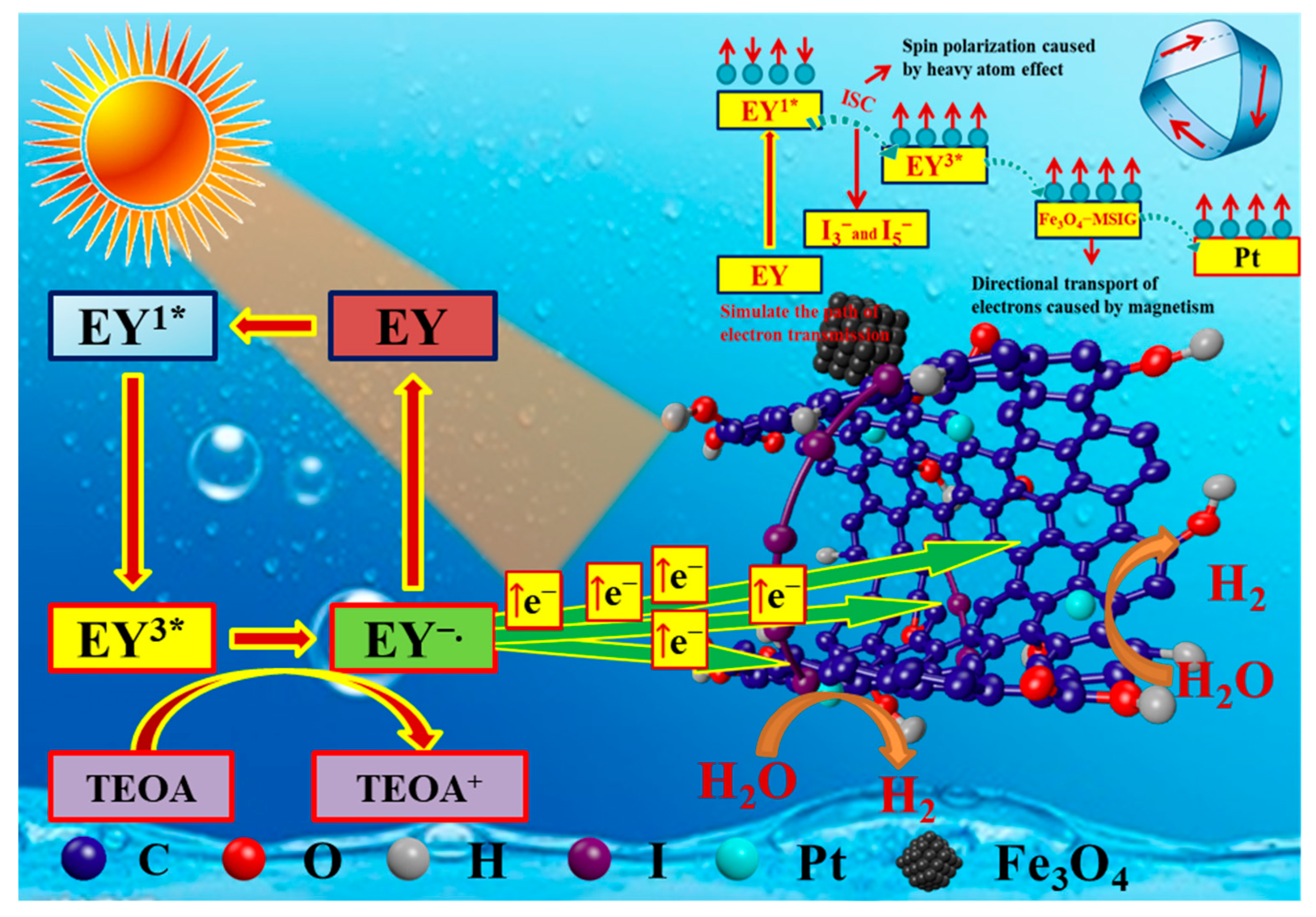
2.7. Catalytic Mechanism
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, X.; Li, X.; Dong, L.; Zhang, B.; Liu, D.; Hou, S.; Zhang, Y.; Zhang, F.-M.; Song, B. Enhancement and inhibition of photocatalytic hydrogen production by fine piezoelectric potential tuning over piezo-photocatalyst. Nano Energy 2024, 123, 109341. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, R.; Huang, J.; Jiang, Y.; Zhao, C.; Yang, X. In situ construction of protonated g-C3N4/Ti3C2 MXene Schottky heterojunctions for efficient photocatalytic hydrogen production. Chin. J. Catal. 2021, 42, 107–114. [Google Scholar] [CrossRef]
- Falcão, B.P.; Leitão, J.P.; Ricardo, L.; Águas, H.; Martins, R.; Pereira, R.N. Recombination of photo-generated charge carriers in H-terminated and (photo-)oxidized silicon nanoparticles. Appl. Mater. Today 2021, 23, 101071. [Google Scholar] [CrossRef]
- Li, F.; Cheng, L.; Fan, J.; Xiang, Q. Steering the behavior of photogenerated carriers in semiconductor photocatalysts: A new insight and perspective. J. Mater. Chem. A 2021, 9, 23765–23782. [Google Scholar] [CrossRef]
- Choi, Y.M.; Lee, B.W.; Jung, M.S.; Han, H.S.; Kim, S.H.; Chen, K.; Kim, D.H.; Heinz, T.F.; Fan, S.; Lee, J.; et al. Retarded Charge–Carrier Recombination in Photoelectrochemical Cells from Plasmon-Induced Resonance Energy Transfer. Adv. Energy Mater. 2020, 10, 2000570. [Google Scholar] [CrossRef]
- Chu, S.; Wang, Y.; Guo, Y.; Feng, J.; Wang, C.; Luo, W.; Fan, X.; Zou, Z. Band structure engineering of carbon nitride: In search of a polymer photocatalyst with high photooxidation property. ACS Catal. 2013, 3, 912–919. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Y.; Yu, H.; Wang, Q.; Wang, L.; Wu, J.; Wang, Y.; Shen, H.; Zhang, J.; Chen, N.; et al. Design of filamentous conductive catalyst as separator coating for high-efficiency lithium-sulfur batteries. Chem. Eng. J. 2024, 479, 147870. [Google Scholar] [CrossRef]
- Liang, S.; Wang, Z.; Zhou, L.; You, S.; Zhang, R.; Liu, F.; Niu, P.; Wang, X. Coupling Reliable Interfacial Carrier Migration Channels with Visible-Light Response Antennas in ZnO-Based Heterostructure for Ameliorated Photocatalytic Hydrogen Generation. ACS Appl. Mater. Interfaces 2024, 16, 17442–17452. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, S.; Zhang, H.; Yang, W.; Yi, Z.; Yi, Y.; Wang, J.; Ahmad, S.; Raza, R. Ultrathin broadband terahertz metamaterial based on single-layer nested patterned graphene. Phys. Lett. A 2025, 534, 130262. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, S.; Zhang, H.; Yi, Z.; Tang, B.; Chen, J.; Zhang, J.; Tang, C. Ultra wideband absorption absorber based on Dirac semimetallic and graphene metamaterials. Phys. Lett. A 2024, 517, 129675. [Google Scholar] [CrossRef]
- Li, W.; Yi, Y.; Yang, H.; Cheng, S.; Yang, W.; Zhang, H.; Yi, Z.; Yi, Y.; Li, H. Active tunable terahertz bandwidth absorber based on single layer graphene. Commun. Theor. Phys. 2023, 75, 045503. [Google Scholar] [CrossRef]
- Min, S.; Lu, G. Dye-Sensitized Reduced Graphene Oxide Photocatalysts for Highly Efficient Visible-Light-Driven Water Reduction. J. Phys. Chem. C 2011, 115, 13938–13945. [Google Scholar] [CrossRef]
- Yeh, T.F.; Syu, J.M.; Cheng, C.H.; Chang, T.-H.; Teng, H. Graphite Oxide as a Photocatalyst for Hydrogen Production from Water. Adv. Funct. Mater. 2010, 20, 2255–2262. [Google Scholar] [CrossRef]
- Zhang, S.; Du, P.; Xiao, H.; Wang, Z.; Zhang, R.; Luo, W.; An, J.; Gao, Y.; Lu, B. Fast Interfacial Carrier Dynamics Modulated by Bidirectional Charge Transport Channels in ZnIn2S4-based Composite Photoanodes Probed by Scanning Photoelectrochemical Microscopy. Angew. Chem. 2024, 136, e202315763. [Google Scholar] [CrossRef]
- Wang, D.; Hasegawa, S.; Shimizu, M.; Tanaka, J. Synthesis, doping and polarized Raman spectra of highly conducting polyacetylene. Synth. Met. 1992, 46, 85–91. [Google Scholar] [CrossRef]
- Yao, Z.; Nie, H.; Yang, Z.; Zhou, X.; Liu, Z.; Huang, S. Catalyst-free synthesis of iodine-doped graphenevia a facile thermal annealing process and its use for electrocatalytic oxygen reduction in an alkaline medium. Chem. Commun. 2012, 48, 1027–1029. [Google Scholar] [CrossRef]
- Kalita, G.; Wakita, K.; Takahashi, M.; Umeno, M. Iodine doping in solid precursor-based CVD growth graphene film. J. Mater. Chem. 2011, 21, 15209–15213. [Google Scholar] [CrossRef]
- Shu, A.; Qin, C.; Li, M.; Zhao, L.; Shangguan, Z.; Shu, Z.; Yuan, X.; Zhu, M.; Wu, Y.; Wang, H. Electric effects reinforce charge carrier behaviour for photocatalysis. Energy Environ. Sci. 2024, 17, 4907–4928. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, B.; Tang, C.Y.; Zhou, Q.; Zhu, Y. Photogenerated outer electric field induced electrophoresis of organic nanocrystals for effective solid-solid photocatalysis. Nat. Commun. 2024, 15, 428. [Google Scholar] [CrossRef]
- Houchins, G.; Crook, C.B.; Zhu, J.-X.; Balatsky, A.V.; Haraldsen, J.T. Voltage-dependent spin flip in magnetically substituted graphene nanoribbons: Towards the realization of graphene-based spintronic devices. Phys. Rev. B 2017, 95, 155450. [Google Scholar] [CrossRef]
- Kalinowski, J.; Cocchi, M.; Virgili, D.; Di Marco, P.; Fattori, V. Magnetic field effects on emission and current in Alq3-based electroluminescent diodes. Chem. Phys. Lett. 2003, 380, 710–715. [Google Scholar] [CrossRef]
- Zhai, X.; Blanter, Y.M. Proximity-induced diversified magnetic states and electrically controllable spin polarization in bilayer graphene: Towards layered spintronics. Phys. Rev. B 2022, 106, 075425. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, Y.; Shi, X.; Sun, W.; Felser, C.; Li, W.; Li, G. From Charge to Spin: An In-Depth Exploration of Electron Transfer in Energy Electrocatalysis. Adv. Mater. 2024, 36, e2312524. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Magnetic composite microspheres with exposed {001} faceted TiO2 shells: A highly active and selective visible-light photocatalyst. J. Mater. Chem. 2012, 22, 13341–13347. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Zhou, K.; Yang, X.; Li, C. Tailored anisotropic magnetic conductive film assembled from graphene-encapsulated multifunctional magnetic composite microspheres. J. Mater. Chem. 2012, 22, 545–550. [Google Scholar] [CrossRef]
- Su, W.; Zhang, T.; Li, L.; Xing, J.; He, M.; Zhong, Y.; Li, Z. Synthesis of small yolk–shell Fe3O4@TiO2 nanoparticles with controllable thickness as recyclable photocatalysts. RSC Adv. 2014, 4, 8901–8906. [Google Scholar] [CrossRef]
- Dodo, K.; Fujita, K.; Sodeoka, M. Raman Spectroscopy for Chemical Biology Research. J. Am. Chem. Soc. 2022, 144, 19651–19667. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Viswanath, P.; Rao, V.K.; Suzuki, S.; Yoshimura, M. New Insight into the Characterization of Graphene Oxide and Reduced Graphene Oxide Monolayer Flakes on Si-Based Substrates by Optical Microscopy and Raman Spectroscopy. J. Phys. Chem. C 2021, 125, 7791–7798. [Google Scholar] [CrossRef]
- Ikram, M.; Raza, A.; Imran, M.; Ul-Hamid, A.; Shahbaz, A.; Ali, S. Hydrothermal Synthesis of Silver Decorated Reduced Graphene Oxide (rGO) Nanoflakes with Effective Photocatalytic Activity for Wastewater Treatment. Nanoscale Res. Lett. 2020, 15, 95. [Google Scholar] [CrossRef]
- Bharat, B.S.; Babu, A.R. Iodine-doped reduced graphene oxide and titanium dioxide nanocomposite as effective amperometric glucose biosensor. Mater. Chem. Phys. 2024, 320, 129409. [Google Scholar] [CrossRef]
- Taniguchi, T.; Nurdiwijayanto, L.; Sakai, N.; Tsukagoshi, K.; Sasaki, T.; Tsugawa, T.; Koinuma, M.; Hatakeyama, K.; Ida, S. Revisiting the two-dimensional structure and reduction process of graphene oxide with in-plane X-ray diffraction. Carbon 2023, 202, 26–35. [Google Scholar] [CrossRef]
- Ma, J.; Ankit, G.; Zhong, F.; Li, C.; Liu, N.; Niu, W.; Cao, H. Coupling behavior and enhancement mechanism of porous structure, graphite microcrystals, and oxygen-containing groups of activated biochar for the adsorption of phenol. Environ. Sci. Water Res. Technol. 2023, 9, 1944–1957. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Mohd Nawawi, M.G. Well-Designed 3D/2D/2D WO3/Bt/g-C3N4 Z-Scheme Heterojunction for Tailoring Photocatalytic CO2 Methanation with 2D-Layered Bentonite-Clay as the Electron Moderator under Visible Light. Energy Fuels 2020, 34, 14400–14418. [Google Scholar] [CrossRef]
- Wu, N.; Ji, X.; Li, L.; Zhu, J.; Lu, X. Mesoscience in supported nano-metal catalysts based on molecular thermodynamic modeling: A mini review and perspective. Chem. Eng. Sci. 2021, 229, 116164. [Google Scholar] [CrossRef]
- Yao, Y.-H.; Yang, Y.; Wang, Y.; Zhang, H.; Tang, H.-L.; Zhang, H.-Y.; Zhang, G.; Wang, Y.; Zhang, F.-M.; Yan, H. Photo-induced synthesis of ternary Pt/rGO/COF photocatalyst with Pt nanoparticles precisely anchored on rGO for efficient visible-light-driven H2 evolution. J. Colloid. Interface Sci. 2022, 608, 2613–2622. [Google Scholar] [CrossRef]
- Liu, H.; Wang, M.; Zhang, X.; Ma, J.; Lu, G. High efficient catalytic hydrogen evolution from formaldehyde over sensitized Ag@Ag-Pd alloy catalyst under photo-visible light irradiation. Appl. Catal. B Environ. 2018, 237, 563–573. [Google Scholar] [CrossRef]
- Guo, S.-J.; Zhang, G.-H.; Zhu, Q.-H.; Yu, C.; Liu, J.-Y.; Yang, X.-L.; Qin, S.; Zhao, N.-R.; He, L.; Tao, G.-H. Efficient capture of iodine by charge-induced effect of nitrogen-rich ionic liquids. Chem. Eng. J. 2023, 475, 146221. [Google Scholar] [CrossRef]
- Wu, J.; Ke, K.; Qin, N.; Lin, E.; Kang, Z.; Bao, D. Magnetically retrievable Fe3O4@SiO2@ZnO piezo-photocatalyst: Synthesis and multiple catalytic properties. J. Colloid. Interface Sci. 2023, 636, 167–175. [Google Scholar] [CrossRef]
- Bayat, R.; Akin, M.; Yilmaz, B.; Bekmezci, M.; Bayrakci, M.; Sen, F. Biogenic platinum based nanoparticles: Synthesis, characterization and their applications for cell cytotoxic, antibacterial effect, and direct alcohol fuel cells. Chem. Eng. J. Adv. 2023, 14, 100471. [Google Scholar] [CrossRef]
- Liu, H.; Yan, X.; Luo, W.; Liu, J.; Ren, S. Effect of Pd crystal facet on the reaction of oxygen-promoted hydrogen evolution from formaldehyde driven by visible light. Colloids Surf. A Physicochem. Eng. Asp. 2023, 673, 131820. [Google Scholar] [CrossRef]
- Zhang, W.; Kong, C.; Lu, G. Super-paramagnetic nano-Fe3O4/graphene for visible-light-driven hydrogen evolution. Chem. Commun. 2015, 51, 10158–10161. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, B.; Zhen, W.; Li, Z.; Wu, Y.; Lu, G. Construction of Möbius-strip-like graphene for highly efficient charge transfer and high active hydrogen evolution. J. Catal. 2017, 354, 258–269. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, H.; Li, X.; Zhang, S. Enhanced photocatalytic activity of TiO2 nano-structured thin film with a silver hierarchical configuration. Appl. Surf. Sci. 2008, 254, 1630–1635. [Google Scholar] [CrossRef]
- Cui, L.; Ding, X.; Wang, Y.; Shi, H.; Huang, L.; Zuo, Y.; Kang, S. Facile preparation of Z-scheme WO3/g-C3N4 composite photocatalyst with ehanced photocatalytic performance under visible light. Appl. Surf. Sci. 2017, 391, 202–210. [Google Scholar] [CrossRef]
- Hu, S.; Tian, R.; Dong, Y.; Yang, J.; Liu, J.; Chang, Q. Modulation and effects of surface groups on photoluminescence and photocatalytic activity of carbon dots. Nanoscale 2013, 5, 11665–11671. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, A. Photophysics and Photocatalysis of Carbon Nitride Synthesized at Different Temperatures. J. Phys. Chem. C 2014, 118, 11628–11635. [Google Scholar] [CrossRef]
- Zhao, X.; Hou, Y.; Liu, L.; Zhao, J. Triplet Photosensitizers Showing Strong Absorption of Visible Light and Long-Lived Triplet Excited States and Application in Photocatalysis: A Mini Review. Energy Fuels 2021, 35, 18942–18956. [Google Scholar] [CrossRef]
- Li, Z.; Tian, B.; Zhen, W.; Zhang, W.; Zhang, X.; Wu, Y.; Lu, G. Enhancing photoactivity for hydrogen generation by electron tunneling via flip-flop hopping over iodinated graphitic carbon nitride. Appl. Catal. B Environ. 2017, 204, 33–42. [Google Scholar] [CrossRef]
- Zhang, W.; Kong, C.; Gao, W.; Lu, G. Intrinsic magnetic characteristics-dependent charge transfer and visible photo-catalytic H2 evolution reaction (HER) properties of a Fe3O4@PPy@Pt catalyst. Chem. Commun. 2016, 52, 3038–3041. [Google Scholar] [CrossRef] [PubMed]
- Tristant, D.; Puech, P.; Gerber, I.C. Theoretical study of graphene doping mechanism by iodine molecules. J. Phys. Chem. C 2015, 119, 12071–12078. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Wang, M.; Meng, X.; Li, J. P-type reduced graphene oxide membranes induced by iodine doping. J. Mater. Sci. 2013, 48, 2284–2289. [Google Scholar] [CrossRef]
- Lee, H.; Paeng, K.; Kim, I.S. A review of doping modulation in graphene. Synth. Met. 2018, 244, 36–47. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Zhang, M.; Wang, L.; Han, Y.; Xu, S.; Han, T.; Lin, J.; An, L.; Wang, J.; et al. Fluctuation-induced tunneling conduction in iodine-doped bilayer graphene. J. Appl. Phys. 2018, 123, 244302. [Google Scholar] [CrossRef]
- Hoyt, R.A.; Remillard, E.M.; Cubuk, E.D.; Vecitis, C.D.; Kaxiras, E. Polyiodide-Doped Graphene. J. Phys. Chem. C 2016, 121, 609–615. [Google Scholar] [CrossRef]
- Liu, H.; Wang, M.; Ma, J.; Lu, G. Hydrogen generation from toxic formaldehyde catalyzed by low-cost Pd–Sn alloys driven by visible light. J. Mater. Chem. A 2020, 8, 9616–9628. [Google Scholar] [CrossRef]
- Liu, H.; Wang, M.; Ma, J.; Lu, G. Modulation of HCHO, H2O and H adsorption on AgPd cocatalyst by optimizing of selective exposed facet to enhancing the efficiency of conversion toxic formaldehyde into hydrogen driven by visible light. J. Catal. 2019, 375, 493–506. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Peng, S. Facile Synthesis of Graphene Sponge from Graphene Oxide for Efficient Dye-Sensitized H2 Evolution. ACS Appl. Mater. Interfaces 2016, 8, 15187–15195. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Clark, S.; Segall, M.; Pickard, C.; Hasnip, P.; Probert, M.; Refson, K.; Payne, M. First principles methods using CASTEP. Z. Kristallogr. 2005, 220. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Perdew, Burke, and Ernzerhof Reply. Phys. Rev. Lett. 1998, 80, 891. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, L.; Liu, H.; Yan, X. Boosting Visible-Light-Driven Hydrogen Evolution Enabled by Iodine-Linked Magnetically Curved Graphene with Mobius-like Electronic Paths. Molecules 2025, 30, 1302. https://doi.org/10.3390/molecules30061302
Cai L, Liu H, Yan X. Boosting Visible-Light-Driven Hydrogen Evolution Enabled by Iodine-Linked Magnetically Curved Graphene with Mobius-like Electronic Paths. Molecules. 2025; 30(6):1302. https://doi.org/10.3390/molecules30061302
Chicago/Turabian StyleCai, Liangjun, Hongxia Liu, and Xiaoxiao Yan. 2025. "Boosting Visible-Light-Driven Hydrogen Evolution Enabled by Iodine-Linked Magnetically Curved Graphene with Mobius-like Electronic Paths" Molecules 30, no. 6: 1302. https://doi.org/10.3390/molecules30061302
APA StyleCai, L., Liu, H., & Yan, X. (2025). Boosting Visible-Light-Driven Hydrogen Evolution Enabled by Iodine-Linked Magnetically Curved Graphene with Mobius-like Electronic Paths. Molecules, 30(6), 1302. https://doi.org/10.3390/molecules30061302





