Advancing Cancer Treatment and Diagnosis: A Review on Photodynamic Therapy Using OLED Technology
Abstract
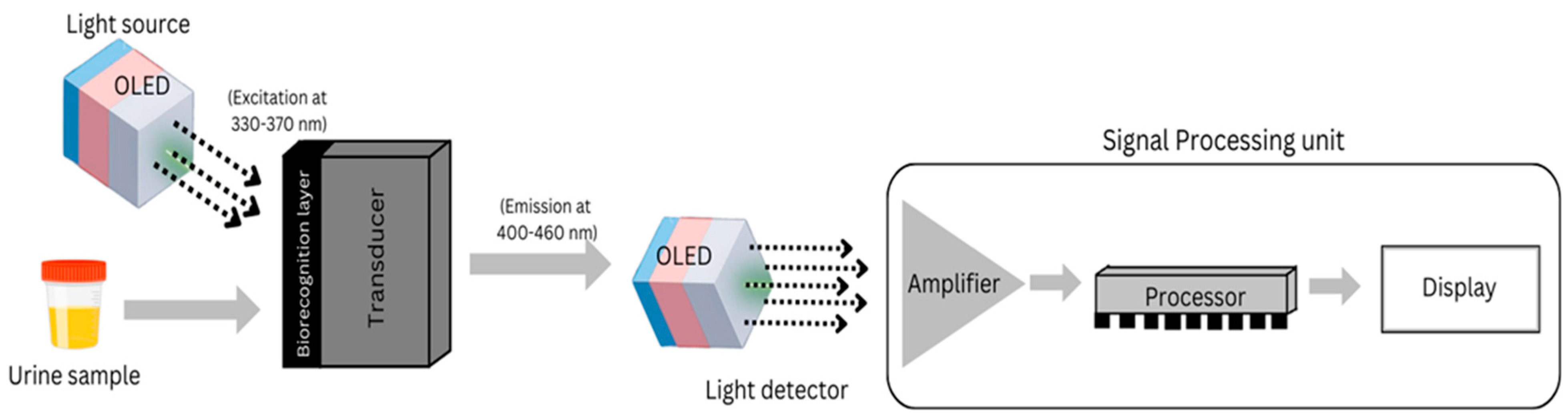
:1. Introduction
2. OLEDs in Healthcare
3. Photodynamic Diagnosis and Treatment
3.1. Ovarian and Prostate Cancer
3.2. Cutaneous Tumors and Wound Healing
3.3. Glioma/Brain Injury
3.4. Breast Cancer
3.5. Detection of Biomarkers
3.6. Neonatal Jaundice
4. Near-IR OLED
5. Comprehensive Analysis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sim, J.H.; Kwon, J.; Chae, H.; Kim, S.-B.; Cho, H.; Lee, W.; Kim, S.H.; Byun, C.-W.; Hahn, S.; Park, D.H.; et al. Oled catheters for inner-body phototherapy: A case of type 2 diabetes mellitus improved via duodenal photobiomodulation. Sci. Adv. 2023, 9, eadh8619. [Google Scholar] [CrossRef] [PubMed]
- Piksa, M.; Fortuna, W.; Lian, C.; Gacka, M.; Samuel, I.D.W.; Matczyszyn, K.; Pawlik, K.J. Treatment of antibiotic-resistant bacteria colonizing diabetic foot ulcers by oled induced antimicrobial photodynamic therapy. Sci. Rep. 2023, 13, 14087. [Google Scholar] [CrossRef]
- Choi, S.; Jeon, Y.; Kwon, J.H.; Ihm, C.; Kim, S.Y.; Choi, K.C. Wearable photomedicine for neonatal jaundice treatment using blue organic light-emitting diodes (oleds): Toward textile-based wearable pho-totherapeutics. Adv. Sci. 2022, 9, 2204622. [Google Scholar] [CrossRef]
- Smith, J.T.; O’Brien, B.; Lee, Y.-K.; Bawolek, E.J.; Christen, J.B. Application of flexible oled display technology for electro-optical stimulation and/or silencing of neural activity. J. Disp. Technol. 2014, 10, 514–520. [Google Scholar] [CrossRef]
- Smith, J.; Shah, A.; Lee, Y.; O’Brien, B.; Kullman, D.; Sridharan, A.; Muthuswamy, J.; Christen, J.B. Optogenetic neurostimulation of auricular vagus using flexible oled display technology to treat chronic inflammatory disease and mental health disorders. Electron. Lett. 2016, 52, 900–902. [Google Scholar] [CrossRef]
- Negi, S.; Mitta, P.; Kumar, B.; Bhatia, A. Detection of ovarian cancer using organic light emitting diodes. In Proceedings of the 2018 5th IEEE Uttar Pradesh Section International Conference on Electrical, Electronics and Computer Engineering (UPCON), Gorakhpur, India, 2–4 November 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–4. [Google Scholar]
- American Cancer Society. Cancer Facts and Figures. 2024. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2024/2024-cancer-facts-and-figures-acs.pdf (accessed on 30 December 2024).
- Clancy, E. Acs report shows prostate cancer on the rise, cervical cancer on the decline. In Renal & Urology News; Haymarket Media, Inc.: New York, NY, USA, 2023. [Google Scholar]
- Indian Cancer Society. Cervical Cancer: White Paper. 2024. Available online: https://www.indiancancersociety.org/pdf/ics-whitepaper-cervical-cancer-jan-24.pdf (accessed on 25 June 2024).
- Dobson, J.; de Queiroz, G.F.; Golding, J.P. Photodynamic therapy and diagnosis: Principles and comparative aspects. Vet. J. 2018, 233, 8–18. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic therapy for the treatment and diagnosis of cancer–a review of the current clinical status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef] [PubMed]
- Vasudeo, B.S.; Gill, S.S.; Raj, B. Applications of oleds for flexible electronics, biophotonic, chronic, optogenetic applications & different sensors. In Proceedings of the International Conference on Emerging Technologies: AI, IoT, and CPS for Science & Technology Applications, Chandigarh, India, 6–7 September 2021. [Google Scholar]
- Islam, A.; Shah, S.H.U.; Haider, Z.; Imran, M.; Amin, A.; Haider, S.K.; Li, M.-D. Biological interfacial materials for organic light-emitting diodes. Micromachines 2023, 14, 1171. [Google Scholar] [CrossRef]
- Cabral, F.V.; Lian, C.; Persheyev, S.; Smith, T.K.; Ribeiro, M.S.; Samuel, I.D. Organic light-emitting diodes as an innovative approach for treating cutaneous leishmaniasis. Adv. Mater. Technol. 2021, 6, 2100395. [Google Scholar] [CrossRef]
- Guo, H.-W.; Lin, L.-T.; Chen, P.-H.; Ho, M.-H.; Huang, W.-T.; Lee, Y.-J.; Chiou, S.-H.; Hsieh, Y.-S.; Dong, C.-Y.; Wang, H.-W. Low-fluence rate, long duration photodynamic therapy in glioma mouse model using organic light emitting diode (oled). Photodiagn. Photodyn. Ther. 2015, 12, 504–510. [Google Scholar] [CrossRef]
- Genaro, J.F.A.; Higuera, J.M.L.; Cobo, L.R.; García, A.C. Advanced light source technologies for photodynamic therapy of skin cancer lesions. Pharmaceutics 2023, 15, 2075. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Chung, P.-S.; Ahn, J.C. 630 nm-oled accelerates wound healing in mice via regulation of cytokine release and genes expression of growth factors. Curr. Opt. Photonics 2019, 3, 485–495. [Google Scholar]
- Park, Y.; Choi, H.-R.; Shin, J.W.; Huh, C.-H.; Choi, K.C. A wearable oled medical device for enhanced cutaneous wound healing and patient comfort: Revolutionizing dermatology. J. Inf. Disp. 2014, 25, 151–156. [Google Scholar] [CrossRef]
- Negi, S.; Mittal, P.; Kumar, B. Modeling and analysis of high-performance triple hole block layer organic led based light sensor for detection of ovarian cancer. IEEE Trans. Circuits Syst. I Regul. Pap. 2021, 68, 3254–3264. [Google Scholar] [CrossRef]
- Nath, S.; Saad, M.A.; Pigula, M.; Swain, J.W.; Hasan, T. Photoimmunotherapy of ovarian cancer: A unique niche in the management of advanced disease. Cancers 2019, 11, 1887. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gao, Y.; Liu, N.; Suo, Y. Nanoparticles loading porphyrin sensitizers in improvement of photo-dynamic therapy for ovarian cancer. Photodiagn. Photodyn. Ther. 2021, 33, 102156. [Google Scholar] [CrossRef]
- Ahmad, M.; Hasan, M.; Tarannum, N.; Ahmed, S. Recent advances in optical and photoelectrochemical nanobiosensor technology for cancer biomarker detection. Biosens. Bioelectron. X 2023, 14, 100375. [Google Scholar] [CrossRef]
- Lin, T.; Zou, P.; Lin, R.; Guan, H.; Fang, Z.; Chen, J.; Long, Z.; Zhang, Y.; Xing, L.; Qi, F.; et al. A self-powered wireless detachable drug/light injector for metronomic photodynamic therapy in cancer treatment. Nano Energy 2023, 116, 108826. [Google Scholar] [CrossRef]
- Samuel, I.D.; Lian, C.; Piksa, M.; Matczyszyn, K.; Yoshida, K.; Persheyev, S.; Pawlik, K.; Cabral, F.; Ribeiro, M.; Lindoso, J. Oleds: Wearable light sources for medicine. In Organic and Hybrid Sensors and Bioelectronics XIII; SPIE: Bellingham, WA, USA, 2020; Volume 11475, p. 114750C. [Google Scholar]
- Erkiert-Polguj, A.; Halbina, A.; Polak-Pacholczyk, I.; Rotsztejn, H. Light-emitting diodes in photody-namic therapy in non-melanoma skin cancers–own observations and literature review. J. Cosmet. Laser Ther. 2016, 18, 105–110. [Google Scholar] [CrossRef]
- Olszowy, M.; Nowak-Perlak, M.; Woźniak, M. Current strategies in photodynamic therapy (pdt) and photodynamic diagnostics (pdd) and the future potential of nanotechnology in cancer treatment. Pharmaceutics 2023, 15, 1712. [Google Scholar] [CrossRef]
- Lian, C.; Piksa, M.; Yoshida, K.; Persheyev, S.; Pawlik, K.J.; Matczyszyn, K.; Samuel, I.D. Flexible organic light-emitting diodes for antimicrobial photodynamic therapy. Npj Flex. Electron. 2019, 3, 18. [Google Scholar] [CrossRef]
- Marcello, A.; Sblattero, D.; Cioarec, C.; Maiuri, P.; Melpignano, P. A deep-blue oled-based biochip for protein microarray fluorescence detection. Biosens. Bioelectron. 2013, 46, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Han, D.; Ting, J.; Ahmed, M.; Nagisetty, R.; Arias, A.C. Organic multi-channel optoelectronic sensors for wearable health monitoring. IEEE Access 2019, 7, 128114–128124. [Google Scholar] [CrossRef]
- Saczko, J.; Choromańska, A.; Rembiałkowska, N.; Dubińska-Magiera, M.; Bednarz-Misa, I.; Bar, J.; Marcinkowska, A.; Kulbacka, J. Oxidative modification induced by photodynamic therapy with photofrin® ii and 2-methoxyestradiol in human ovarian clear carcinoma (ovbh-1) and human breast ade-nocarcinoma (mcf-7) cells. Biomed. Pharmacother. 2015, 71, 30–36. [Google Scholar] [CrossRef]
- Negi, S.; Mittal, P.; Kumar, B.; Juneja, P.K. Organic led based light sensor for detection of ovarian cancer. Microelectron. Eng. 2019, 218, 111154. [Google Scholar] [CrossRef]
- Takagi, D.; Hirai, Y.; Tsuchiya, T.; Tabata, O.; Anai, S.; Chihara, Y.; Fujimoto, K.; Hirao, Y. Microfluidic cell sorter for photodynamic urine diagnosis. In Proceedings of the 7th IEEE International Conference on Nano/Molecular Medicine and Engineering, Phuket, Thailand, 10–13 November 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 22–26. [Google Scholar]
- Sánchez-Ramírez, D.R.; Domínguez-Ríos, R.; Juárez, J.; Valdés, M.; Hassan, N.; Quintero-Ramos, A.; Del Toro-Arreola, A.; Barbosa, S.; Taboada, P.; Topete, A.; et al. Biodegradable photoresponsive nanoparticles for chemo-, photothermal-and photodynamic therapy of ovarian cancer. Mater. Sci. Eng. C 2020, 116, 111196. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Ji, S.-Y.; Xu, H.-Y.; Zhao, W.; Xu, J.-J.; Chen, H.-Y. Bidirectional electrochemiluminescence color switch: An application in detecting multimarkers of prostate cancer. Anal. Chem. 2018, 90, 3570–3575. [Google Scholar] [CrossRef]
- Jeon, Y.; Choi, H.-R.; Park, K.-C.; Choi, K.C. Flexible organic light-emitting-diode-based photonic skin for attachable phototherapeutics. J. Soc. Inf. Disp. 2020, 28, 324–332. [Google Scholar] [CrossRef]
- Smith, T.; Persheyev, S.; Samuel, I. Organic and inorganic light emitting diodes for photodynamic therapy of cutaneous leishmaniasis. Glob. J. Infect. Dis. Clin. Res. 2016, 9, 025–030. [Google Scholar] [CrossRef]
- Jeon, Y.; Choi, H.-R.; Lim, M.; Choi, S.; Kim, H.; Kwon, J.H.; Park, K.-C.; Choi, K.C. A wearable photobiomodulation patch using a flexible red-wavelength oled and its in vitro differential cell proliferation effects. Adv. Mater. Technol. 2018, 3, 1700391. [Google Scholar] [CrossRef]
- Sari, M.; Bintanjoyo, L.; Kusumaputra, B.; Citrashanty, I.; Hidayati, A.N.; Murtiastutik, D.; Listi-awan, M.; Prakoeswa, C. A retrospective study of demographic, clinical, and histopathological profiles of cutaneous tumors. Berk. Ilmu Kesehat. Kulit Dan Kelamin 2022, 34, 149–155. [Google Scholar] [CrossRef]
- Querfeld, C.; Leung, S.; Myskowski, P.L.; Curran, S.A.; Goldman, D.A.; Heller, G.; Wu, X.; Kil, S.H.; Sharma, S.; Finn, K.J.; et al. Primary t cells from cutaneous t-cell lymphoma skin explants display an exhausted immune checkpoint profile. Cancer Immunol. Res. 2018, 6, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.; Castel, H. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef] [PubMed]
- Avanaki, K.; Andersen, P.E. Optical coherence tomography for melanoma detection. In New Technologies in Dermatological Science and Practice; CRC Press: Boca Raton, FL, USA, 2021; pp. 47–58. [Google Scholar]
- Madheswaran, S.; Mungra, N.; Biteghe, F.A.; De la Croix Ndong, J.; Arowolo, A.T.; Adeola, H.A.; Rama-murthy, D.; Naran, K.; Khumalo, N.P.; Barth, S.; et al. Antibody-based targeted interventions for the diagnosis and treatment of skin cancers. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2021, 21, 162–186. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Löhr, F.; Raap, U. Photodynamic therapy and skin cancer. In Dermatologic Surgery and Procedures; IntechOpen: London, UK, 2017. [Google Scholar]
- Luo, O.D.; Bose, R.; Bawazir, M.A.; Thuraisingam, T.; Ghazawi, F.M. A review of the dermatologic clinical applications of topical photodynamic therapy. J. Cutan. Med. Surg. 2024, 28, NP1–NP18. [Google Scholar] [CrossRef]
- Kibbi, N.; Zhang, Y.; Leffell, D.J.; Christensen, S.R. Photodynamic therapy for cutaneous squamous cell carcinoma in situ: Impact of anatomic location, tumor diameter, and incubation time on effectiveness. J. Am. Acad. Dermatol. 2020, 82, 1124–1130. [Google Scholar] [CrossRef]
- Keyal, U.; Bhatta, A.K.; Zhang, G.; Wang, X.L. Present and future perspectives of photodynamic therapy for cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2019, 80, 765–773. [Google Scholar] [CrossRef]
- Pierre, M.B. Nanocarriers for photodynamic therapy intended to cutaneous tumors. Curr. Drug Targets 2021, 22, 1090–1107. [Google Scholar] [CrossRef]
- Al-Niaimi, F.; Sheth, N.; Kurwa, H.A.; Mallipeddi, R. Photodynamic therapy followed by mohs micro-graphic surgery compared to mohs micrographic surgery alone for the treatment of basal cell carcinoma: Results of a pilot single-blinded randomised controlled trial. J. Cutan. Aesthetic Surg. 2015, 8, 88–91. [Google Scholar] [CrossRef]
- Yu, N.; Wu, L.; Su, J.; Chen, M.; Lu, L.; Huang, K.; Li, Y.; Jiang, Z.; Liu, S.; Peng, L.; et al. Photodynamic therapy combined with surgery versus mohs micrographic surgery for the treatment of difficult-to-treat basal cell carcinoma: A retrospective clinical study. J. Dermatol. Treat. 2023, 34, 2200871. [Google Scholar] [CrossRef]
- Cerrati, E.W.; Nguyen, S.A.; Farrar, J.D.; Lentsch, E.J. The efficacy of photodynamic therapy in the treatment of oral squamous cell carcinoma: A meta-analysis. Ear Nose Throat J. 2015, 94, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, Y.; Zhang, X.; Yang, Y.; Kou, H.; Wang, Y. Clinical efficacy of mohs surgery combined with topical photodynamic therapy for facial basal cell carcinoma. J. Cancer Res. Ther. 2020, 16, 1051–1055. [Google Scholar]
- Williams, G.; Backhouse, C.; Aziz, H. Integration of organic light emitting diodes and organic photode-tectors for lab-on-a-chip bio-detection systems. Electronics 2014, 3, 43–75. [Google Scholar] [CrossRef]
- Wu, X.; Alberico, S.; Saidu, E.; Khan, S.R.; Zheng, S.; Romero, R.; Chae, H.S.; Li, S.; Mochizuki, A.; Anders, J. Organic light emitting diode improves diabetic cutaneous wound healing in rats. Wound Repair Regen. 2015, 23, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, T. Photodynamic diagnosis and photodynamic therapy for the brain tumors. Prog. Neuro-Oncol. 2014, 21, 14–21. [Google Scholar]
- Sroka, R.; Stepp, H.; Beyer, W.; Markwardt, N.; Rühm, A. Photodynamic diagnosis and related optical techniques for the management of malignant glioma. In Proceedings of the International Conference on Biophotonics V, Perth, Australia, 30 April–1 May 2017; SPIE: Bellingham, WA, USA, 2017; pp. 55–57. [Google Scholar]
- Mahmoudi, K.; Garvey, K.; Bouras, A.; Cramer, G.; Stepp, H.; Raj, J.J.; Bozec, D.; Busch, T.; Hadjipanayis, C. 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. J. Neuro-Oncol. 2019, 141, 595–607. [Google Scholar] [CrossRef]
- Bhanja, D.; Wilding, H.; Baroz, A.; Trifoi, M.; Shenoy, G.; Slagle-Webb, B.; Hayes, D.; Soudagar, Y.; Connor, J.; Mansouri, A. Photodynamic therapy for glioblastoma: Illuminating the path toward clinical applica-bility. Cancers 2023, 15, 3427. [Google Scholar] [CrossRef]
- Hsia, T.; Small, J.L.; Yekula, A.; Batool, S.M.; Escobedo, A.K.; Ekanayake, E.; You, D.G.; Lee, H.; Carter, B.S.; Balaj, L. Systematic review of photodynamic therapy in gliomas. Cancers 2023, 15, 3918. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Serafin, I.; Dynarowicz, K.; Aebisher, D. Photodynamic therapy and associated targeting methods for treatment of brain cancer. Front. Pharmacol. 2023, 14, 1250699. [Google Scholar] [CrossRef]
- Abdurashitov, A.; Tuchin, V.; Semyachkina-Glushkovskaya, O. Photodynamic therapy of brain tumors and novel optical coherence tomography strategies for in vivo monitoring of cerebral fluid dynamics. J. Innov. Opt. Health Sci. 2020, 13, 2030004. [Google Scholar] [CrossRef]
- Hempstead, J.; Jones, D.P.; Ziouche, A.; Cramer, G.M.; Rizvi, I.; Arnason, S.; Hasan, T.; Celli, J.P. Low-cost photodynamic therapy devices for global health settings: Characterization of battery-powered led performance and smartphone imaging in 3d tumor models. Sci. Rep. 2015, 5, 10093. [Google Scholar] [CrossRef]
- Jamali, Z.; Hejazi, S.M.; Ebrahimi, S.M.; Moradi-Sardareh, H.; Paknejad, M. Effects of led-based photodynamic therapy using red and blue lights, with natural hydrophobic photosensitizers on human glioma cell line. Photodiagn. Photodyn. Ther. 2018, 21, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Borisova, E.; Kyurkchiev, D.; Tumangelova-Yuzeir, K.; Angelov, I.; Genova-Hristova, T.; Semyachkina-Glushkovskaya, O.; Minkin, K. Evaluation of photodynamic treatment efficiency on glioblastoma cells received from malignant lesions: Initial studies. In Proceedings of the Saratov Fall Meeting 2017: Optical Technologies in Biophysics and Medicine XIX, Saratov, Russia, 26–30 September 2017; SPIE: Bellingham, WA, USA, 2018; p. 1071602. [Google Scholar]
- Kim, D.; Yokota, T.; Suzuki, T.; Lee, S.; Woo, T.; Yukita, W.; Koizumi, M.; Tachibana, Y.; Yawo, H.; Onodera, H.; et al. Ultraflexible organic light-emitting diodes for optogenetic nerve stimulation. Proc. Natl. Acad. Sci. USA 2020, 117, 21138–21146. [Google Scholar] [CrossRef] [PubMed]
- Aebisher, D.; Przygórzewska, A.; Myśliwiec, A.; Dynarowicz, K.; Krupka-Olek, M.; Bożek, A.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. Current photodynamic therapy for glioma treatment: An update. Biomedicines 2024, 12, 375. [Google Scholar] [CrossRef]
- Mani, M.S.A.; Thivakaran, T. A survey on various combination of breast cancer biomarkers. In Proceedings of the 2022 IEEE International Conference on Signal Processing, Informatics, Communication and Energy Systems (SPICES), Trivandrum, India, 10–12 March 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 146–151. [Google Scholar]
- Ranjan, P.; Parihar, A.; Jain, S.; Kumar, N.; Dhand, C.; Murali, S.; Mishra, D.; Sanghi, S.K.; Chaurasia, J.; Srivastava, A.K.; et al. Biosensor-based diagnostic approaches for various cellular biomarkers of breast cancer: A comprehensive review. Anal. Biochem. 2020, 610, 113996. [Google Scholar] [CrossRef] [PubMed]
- Ostańska, E.; Aebisher, D.; Bartusik-Aebisher, D. The potential of photodynamic therapy in current breast cancer treatment methodologies. Biomed. Pharmacother. 2021, 137, 111302. [Google Scholar] [CrossRef]
- Gustalik, J.; Aebisher, D.; Bartusik-Aebisher, D. Photodynamic therapy in breast cancer treatment. J. Appl. Biomed. 2022, 20, 98–105. [Google Scholar] [CrossRef]
- Gustalik-Nowicka, J.; Aebisher, D.; Adamczyk, M.; Cieślar, G.; Kawczyk-Krupka, A. Therapeutic use of singlet oxygen for breast cancer ex vivo. Acta Pol. Pharm. 2021, 78, 447–456. [Google Scholar] [CrossRef]
- Shang, L.; Zhou, X.; Zhang, J.; Shi, Y.; Zhong, L. Metal nanoparticles for photodynamic therapy: A potential treatment for breast cancer. Molecules 2021, 26, 6532. [Google Scholar] [CrossRef]
- Kim, E.H.; Park, S.; Kim, Y.K.; Moon, M.; Park, J.; Lee, K.J.; Lee, S.; Kim, Y.-P. Self-luminescent photodynamic therapy using breast cancer targeted proteins. Sci. Adv. 2020, 6, eaba3009. [Google Scholar] [CrossRef]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. Organic nanoparticle based active targeting for photody-namic therapy treatment of breast cancer cells. Oncotarget 2020, 11, 2120. [Google Scholar] [CrossRef]
- Czarnecka-Czapczyńska, M.; Aebisher, D.; Oleś, P.; Sosna, B.; Krupka-Olek, M.; Dynarowicz, K.; Latos, W.; Cieślar, G.; Kawczyk-Krupka, A. The role of photodynamic therapy in breast cancer—A review of in vitro research. Biomed. Pharmacother. 2021, 144, 112342. [Google Scholar] [CrossRef]
- Jayanthi, V.S.A.; Das, A.B.; Saxena, U. Recent advances in biosensor development for the detection of cancer biomarkers. Biosens. Bioelectron. 2017, 91, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, D.; Basilion, J.P. Photodynamic therapy: Targeting cancer biomarkers for the treatment of cancers. Cancers 2021, 13, 2992. [Google Scholar] [CrossRef] [PubMed]
- Sinibaldi, A. Cancer biomarker detection with photonic crystals-based biosensors: An overview. J. Light. Technol. 2021, 39, 3871–3881. [Google Scholar] [CrossRef]
- Smith, J.T.; Katchman, B.A.; Kullman, D.E.; Obahiagbon, U.; Lee, Y.-K.; O’Brien, B.P.; Raupp, G.B.; Anderson, S.; Christen, J.B. Application of flexible oled display technology to point-of-care medical diagnostic testing. J. Disp. Technol. 2016, 12, 273–280. [Google Scholar] [CrossRef]
- Park, S.; Kang, Y.J.; Majd, S. A review of patterned organic bioelectronic materials and their biomedical applications. Adv. Mater. 2015, 27, 7583–7619. [Google Scholar] [CrossRef]
- Smith, J.T.; Katchman, B.A.; Lee, Y.-K.; O’Brien, B.P.; Bawolek, E.J.; Shah, S.S.; Christen, J.B. Dis-posable point-of-use optical biosensor for multiple biomarker detection. In Proceedings of the 2014 IEEE Biomedical Circuits and Systems Conference (BioCAS) Proceedings, Lausanne, Switzerland, 22–24 October 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 268–271. [Google Scholar]
- Zauk, A. Phototherapy: A simple and safe treatment for neonatal jaundice. J. Pediatr. Neonatal. Care 2015, 2, 00070. [Google Scholar] [CrossRef]
- Ebbesen, F.; Hansen, T.W.; Maisels, M.J. Update on phototherapy in jaundiced neonates. Curr. Pediatr. Rev. 2017, 13, 176–180. [Google Scholar] [CrossRef]
- Yurdakok, M. Phototherapy in the newborn: What’s new? J. Pediatr. Neonatal Individ. Med. (JPNIM) 2015, 4, e040255. [Google Scholar]
- Woodgate, P.; Jardine, L.A. Neonatal jaundice: Phototherapy. BMJ Clin. Evid. 2015, 2015, 0319. [Google Scholar] [PubMed]
- Savedra, R.M.L.; Fonseca, A.M.T.; Silva, M.D.M.; Bianchi, R.F.; Siqueira, M. White led phototherapy as an improved treatment for neonatal jaundice. Rev. Sci. Instrum. 2021, 92, 064101. [Google Scholar] [CrossRef]
- Sherbiny, H.S.; Youssef, D.M.; Sherbini, A.S.; El-Behedy, R.; Sherief, L.M. High-intensity light-emitting diode vs fluorescent tubes for intensive phototherapy in neonates. Paediatr. Int. Child Health 2016, 36, 127–133. [Google Scholar] [CrossRef]
- Inamori, G.; Kamoto, U.; Nakamura, F.; Isoda, Y.; Uozumi, A.; Matsuda, R.; Shimamura, M.; Okubo, Y.; Ito, S.; Ota, H. Neonatal wearable device for colorimetry-based real-time detection of jaundice with simultaneous sensing of vitals. Sci. Adv. 2021, 7, eabe3793. [Google Scholar] [CrossRef]
- Smith, J.; Bawolek, E.; Lee, Y.; O’Brien, B.; Marrs, M.; Howard, E.; Strnad, M.; Christen, J.B.; Goryll, M. Application of flexible flat panel display technology to wearable biomedical devices. Electron. Lett. 2015, 51, 1312–1314. [Google Scholar] [CrossRef]
- Zhu, H.; Cheng, P.; Chen, P.; Pu, K. Recent progress in the development of near-infrared organic photothermal and photodynamic nanotherapeutics. Biomater. Sci. 2018, 6, 746–765. [Google Scholar] [CrossRef] [PubMed]
- Teh, D.B.L.; Bansal, A.; Chai, C.; Toh, T.B.; Tucker, R.A.J.; Gammad, G.G.L.; Yeo, Y.; Lei, Z.; Zheng, X.; Yang, F.; et al. A flexi-pegda upconversion implant for wireless brain photodynamic therapy. Adv. Mater. 2020, 32, 2001459. [Google Scholar] [CrossRef]
- Huang, L.; Li, Z.; Zhao, Y.; Zhang, Y.; Wu, S.; Zhao, J.; Han, G. Ultralow-power near infrared lamp light operable targeted organic nanoparticle photodynamic therapy. J. Am. Chem. Soc. 2016, 138, 14586–14591. [Google Scholar] [CrossRef]
- Huang, L.; Li, Z.; Zhao, Y.; Yang, J.; Yang, Y.; Pendharkar, A.I.; Zhang, Y.; Kelmar, S.; Chen, L.; Wu, W.; et al. Enhancing photodynamic therapy through resonance energy transfer constructed near-infrared photosen-sitized nanoparticles. Adv. Mater. 2017, 29, 1604789. [Google Scholar] [CrossRef]
- Ruan, Z.; Miao, W.; Yuan, P.; Le, L.; Jiao, L.; Hao, E.; Yan, L. High singlet oxygen yield photosensitizer based polypeptide nanoparticles for low-power near-infrared light imaging-guided photodynamic therapy. Bioconjug. Chem. 2018, 29, 3441–3451. [Google Scholar] [CrossRef]
- Sharma, N.; Ankalgi, A.D.; Thakur, U.; Ashawat, M.S.; Sharma, N. Advancement of near infrared techniques in diagnosis and treatment of cancer. J. Drug Deliv. Ther. 2022, 12, 192–198. [Google Scholar] [CrossRef]
- Mudeng, V.; Ayana, G.; Zhang, S.-U.; Choe, S.-W. Progress of near-infrared-based medical imaging and cancer cell suppressors. Chemosensors 2022, 10, 471. [Google Scholar] [CrossRef]
- Cho, E.H.; Choi, H.-R.; Park, Y.; Jeong, S.Y.; Song, Y.J.; Hwang, Y.H.; Lee, J.; Chi, Y.; Wang, S.-F.; Jeon, Y.; et al. Wearable and wavelength-tunable near-infrared organic light-emitting diodes for biomedical applications. ACS Appl. Mater. Interfaces 2023, 15, 57415–57426. [Google Scholar] [CrossRef]
- Cai, Q.; Li, Z.; Li, B.; Jiang, J.; Li, X.; Meng, W.; Zhu, S. Precise diagnosis and therapy of bone cancer using near-infrared lights. Front. Bioeng. Biotechnol. 2021, 9, 771153. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Choyke, P.L. Near-infrared photoimmunotherapy of cancer. Acc. Chem. Res. 2019, 52, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lu, Y.; Sun, Y. Precise diagnosis and therapy using near-infrared light. Front. Bioeng. Biotechnol. 2022, 10, 864759. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Choi, H.-R.; Jeon, Y.; Kim, H.; Shin, J.W.; Huh, C.-H.; Park, K.-C.; Choi, K.-C. Cell proliferation effect of deep-penetrating microcavity tandem nir oleds with therapeutic trend analysis. Sci. Rep. 2022, 12, 10935. [Google Scholar] [CrossRef]
- Pal, M.K.; Rashid, M.; Bisht, M. Multiplexed Magnetic Nanoparticle-Antibody Conjugates(MNPs-ABS) based Prognostic Detection of Ovarian Cancer Biomarkers, CA-125, β-2M and ApoA1 Using Fluorescence Spectroscopy with comparison of Surface Plasmon Resonance (SPR) analysis. Biosens. Bioelectron. 2015, 73, 146–152. [Google Scholar] [CrossRef]
- Kaur, B.; Kumar, S.; Kaushik, B.K. Recent Advancements in Optical Biosensors for cancer Detection. Biosens. Bioelectron. 2022, 197, 113805. [Google Scholar] [CrossRef]

| S.No | Ref. | Source and Year of Publication | Advantages | Limitations |
|---|---|---|---|---|
| 1. | [6] | 2018 IEEE | - A home-useable gadget that is lightweight and unobtrusive for early detection. - User-friendly and early detection enhances the survival rate. | Not mentioned. |
| 2. | [31] | 2019 IEEE | - Robust, lightweight, and low power requirements. Superior light detection capabilities. - The ability of the dual-gate OTFT indual-gate mode to generate the 18 V required to operate the triple-hole block-layer OLED light source. | - An immobile, non-flexible ovarian cancer screening and diagnosis tool. - Limit its ability to detect a wider range of wavelengths. |
| 3. | [19] | 2021 IEEE | - An increase in luminous power efficiency of 74%. - Improved recombination rates. - Good response to varying wavelengths with a maximum photocurrent of 93 mA. - The capacity to distinguish between healthy people and those who have ovarian cancer using the cathode current and fluorescence that urine samples produce. | Not mentioned. |
| 4. | [20] | 2019 MDPI | - It can overcome challenges associated with ovarian cancer, including chemoresistance. It can resensitize ovarian tumours to chemotherapy, increasing treatment options. - Allows combination therapy and dose reduction of toxic drugs. - It has the potential to improve patient quality of life. | - Non-specific localization of photosen sitizers leads to dose-limiting toxicities. - Lower effective concentration of photo sensitizers delivered to target cells with PICs compared to free photosensitizers. - Limited light penetration depth. |
| 5. | [21] | 2020 Elsevier | - Increased stability and loading efficiency of the photosensitizers. - Improved delivery capacity and stronger quantum yield/phototoxicity. - Overcoming the lack of tumour specificity of photosensitizers. | - Tumour heterogeneity. - Lack of specificity of photosensitizers for tumours. - Variability in tissue optical properties. - Potential for additional biological barriers in vivo that may prevent effective drug delivery and discrepancies between in vitro and in vivo results due to additional factors. |
| 6 | [34] | 2018 | - Ability to selectively excite different electrochemical luminophores (ECL). - Ability to detect multiple biomarkers for prostate cancer (PSA, miRNA-141, sarcosine). - Separation of the organic solvent containing the ECL reagents from the bioanalytes using the closed BPE design, enabling biological applications. | - The low water-solubility of the iridium complexes used limits their biological application. - The potential for ECL emission quenching at high concentrations of the co-reactant TPrA. - The need to optimize the concentration range of the Ru(II) complex to avoid the classic ECL emission mechanism. |
| S.No | Ref. | Source and Year of Publication | Advantages | Limitations |
|---|---|---|---|---|
| 1. | [35] | 2020 Wiley | - It is extremely thin, at only 6 µm, skin-like platform, making it suitable for attachable phototherapeutics. - The photonic skin has a long operating lifetime of over 100 h. | - The optimal wavelength and irradiation interval for the OLED skin were not fully determined and could be further optimized in the context of surgical wounds, and its applicability to other treatment areas may need further investigation. |
| 2. | [36] | 2023 | - OLED light sources can be large-area (80 mm × 80 mm), thin, lightweight, and potentially flexible or conformable to the skin. - Inorganic LEDs have advantages like high power output, high efficiency, and low cost. | - The OLED light intensity of 2 mW/cm2 may not be sufficient for effectively killing and need major, and higher intensities around 10 mW/cm2 are needed. |
| - The current OLED light sources are not bright enough. | ||||
| 3. | [14] | 2021 Wiley | - They are intrinsically area light sources, which provide uniform illumination over the surface area of topical infections and lesions. - They have shown effectiveness in treating skin cancer, and their antimicrobial efficiency has been demonstrated for bacterial infections, suggesting their potential for treating cutaneous leishmaniosis. | - This study was only conducted in vitro, and further, in vivo studies are needed to evaluate the effectiveness of OLED-APDT for treating cutaneous leishmaniasis in actual patients. - The effectiveness of different photo-sensitizers may depend on the specific antioxidant defences of the Leishmania species, which could limit the generalizability of the findings to other photosensitizers |
| 4. | [17] | 2019 | - Improved wound-healing parameters such as wound size, collagen density, neo-epidermis thickness, and number of new blood vessels, fibroblasts, and neutrophils. - Modulation of cytokine levels, in-creasing anti-inflammatory IL-1β and IL-6, and decreasing proinflammatory TNF-α. | Not mentioned. |
| 5. | [37] | 2018 Wiley | -Lightweight and thin design (0.82 g, 676 µm). - Flexible with a 20 mm bending radius. - Long operation life (>300 h). - Low-temperature operation (<40 °C). - Wide and safe application irrespective of location and time. | - Further research is needed to deter-mine the optimal wavelength of OLED. - This study only looked at in vitro Effects on fibroblasts, and further research is needed to evaluate the in vivo wound healing effects of the OLED device. |
| S.No | Ref. | Source and Year of Publication | Advantages | Limitations |
|---|---|---|---|---|
| 1. | [15] | 2015 | OLED: lightweight, flexible, power-efficient, compact, suitable for mPDT in small animals. | - A small sample size hinders generalization. - Need for larger sample size and extended treatment days. |
| 2. | [65] | 2024 MDPI | - Photodynamic therapy offers improved survival rates for brain tumour patients. - Minimal side effects. - Selective accumulation in cancer cells | - Insufficient accumulation of PSs in the tumour hampers PDT success. - transport to the tumour postoperative resection area. |
| 3. | [58] | 2023 | - PDT induces cell death through oxida - Light dosimetry can be optimized by altering delivery geometry and timing. | - High costs and specialized equipment requirements - PS accumulation variability and reduced efficacy in hypoxic regions. - PDT efficacy is hindered by the hypoxic glioma microenvironment. |
| 4. | [4] | 2014 IEEE | - Selectively activates neurons. - Uses biocompatible plastic substrates. - Offers high-resolution emissive arrays. - Reduces power consumption significantly | - Glass substrates are rigid and not suitable for in vivo applications. - Autoclave sterilization damages OLED organic layers, affecting optical performance. |
| 5. | [5] | 2016 | - Non-invasive optogenetic therapy for chronic diseases and mental health disorders. - Precision in targeting specific afferent vagus nerve branches for treatment. - Red OLED technology provides bright light for therapeutic optical stimulation. | - Existing optogenetic therapies require invasive surgery for deep brain placement. - Electrical vagus nerve stimulation devices are large and invasive. - Transcutaneous and implanted transducers lack precision for specific nerve branches. |
| S.No | Ref. | Source Year of Publication | Advantages | Limitations |
|---|---|---|---|---|
| 1. | [68] | 2021 Elsevier | - Selective and targetable. - The use of near-infrared light in PDT allows for deeper penetration into tissue compared to other wavelengths, which could be beneficial for treating deep-seated breast tumours | - Limited light penetration depth. - The need to optimize the photosensitizer and its ability to generate cytotoxic reactive oxygen species upon light activation |
| 2. | [23] | 2023 Elsevier | - Minimally invasive design with a detectable actuator that can be easily replaced or refilled, providing convenience and flexibility - Wireless control capability for on demand delivery of drugs and light. - Partial self-powering using body motion energy, reducing the need for external power sources and increasing the device’s portability and convenience | - The device still needs further development and testing before it can be used in clinical cancer treatment and testing before it can be used in clinical cancer treatment - The current device is not fully selfpowered |
| 3. | [74] | 2021 Elsevier | - It is minimally invasive. - In vitro research on PDT can help identify optimal methods for clinical treatment. - PDT has demonstrated cytotoxic potential against breast cancer cell lines, particularly MCF-7, and the potential for enhancement through nanotechnology. | Not mentioned. |
| S.No | Ref. | Source and Year of Publication | Advantages | Limitations |
|---|---|---|---|---|
| 1. | [78] | 2021 IEEE | - Ability to leverage existing OLED display technology to produce low-cost biosensor substrates at scale. - High diagnostic sensitivity. - Ability to combine OLED display technology with bio recognition microarray technology to create a new type of point-of-care diagnostic device. | - Cost and scalability of manufacturing the biosensor substrate. |
| 2. | [79] | 2016 | - It enables efficient andcost-effective point-of-care molecular diagnostics health. - High-density, programmable, and multiplexed bio recognition in a compact and disposable configuration with clinical-level sensitivity. - Significantly reduced the cost of the biosensor substrate to just pennies persquare centimetre. | - The current approach has limitations in terms of density, programmability, and multiplexing capabilities that the authors are trying to address with their new technology. - The current approach is limited to detecting certain biomarkers. |
| 3. | [80] | 2014 IEEE | - Miniaturized design - Uses OLED and photodiode technology. - Achieves good optical performance with a bright OLED emitter and optical filters. | - Lower sensitivity compared to laboratory fluorescence-based instruments - Evaluation limited to a specific test structure with certain optical components, which may not be representative of real-world applications. |
| 4. | [22] | 2023 Elsevier | - High sensitivity and specificity for Detecting cancer biomarkers, making them desirable over traditional tech. - Low cost, high sensitivity, and high specificity of photo electrochemical techniques. - Ability to detect very low concentrations of biomarkers, which is crucial for early cancer diagnosis. | - Performance limitations of optical and photo electrochemical nanobiosensors. |
| S.No | Ref. | Source and Year of Publication | Advantages | Limitations |
|---|---|---|---|---|
| 1. | [3] | 2022 Wiley | - It can move flexibly and conform to the curvature of the human body, addressing the issue of separation from parents in traditional LED-based phototherapy. - Low voltages, addressing the disadvantages of water loss and retinal damage in traditional LED-based phototherapy. | - Limitations in terms of the wavelength and power output. - The operating reliability and temperature range of the OLED platform. - This study was limited to in vitro testing, and further research would be needed to evaluate the effectiveness of the OLED platform in actual clinical settings. |
| 2. | [85] | 2021 | - Promotes more efficient bilirubin degradation, which is a key factor in the treatment of neonatal jaundice. | Not mentioned. |
| 3. | [84] | 2015 | Not mentioned. | - The review considered potential harms or safety issues in addition to effectiveness. |
| 4. | [86] | 2016 | -Significantly higher bilirubin decline rates in both haemolytic and non-haemolytic neonates. - Comparable rates of rebound jaundice. - Lower rates of side effects such as hyperthermia, dehydration, and skin rash. | - Small sample size of 100 neonates per group. - Higher rates of side effects (hyperthermia, dehydration, skin rash) in the fluorescent tube group compared to the LED group. |
| 5. | [88] | 2015 Wiley | - Reduced manufacturing cost. - Potential improvement in diagnostic functionality. | Not mentioned. |
| S.No | Disease | Type of OLEDs | Wavelength | Remarks |
|---|---|---|---|---|
| 1. | Ovarian cancer | Violet (diagnosis) NIR (treatment) | 420–440 nm 700–800 nm | Triple-hole block-layer and TADF OLEDs are more efficient. |
| 2. | Prostate cancer | Red | 630–850 nm | Triple-hole block-layer and TADF OLEDs are more efficient. |
| 3. | Cutaneous cancer and wound healing | Red | 560–770 nm (wound healing) - Red wavelength (630–650 nm) for photobiomodulation - Near-infrared (NIR) wavelength (850 nm) for photodynamic therapy | Wavelength-tunable OLEDs are preferred for cancer detection and treatment. |
| 4. | Glioma/brain cancer | Red | Range: 590–750 nm | Flexible and semitransparent OLEDs are used; more research is required in this domain. |
| 615–635 nm murine glioma study | ||||
| 630 nm brain tumour treatment | ||||
| 5. | Mental health | Blue to red | 620 nm optogenetic stimulation | More research is required. |
| 450–460 optimum level of activation | ||||
| 6. | Breast cancer | Red to NIR | 600–1100 nm | Micro-LEDs are used, which can be implemented in OLEDs for enhancement. |
| 7. | Neonatal jaundice | Blue | 460–490 nm | Flexible blue OLEDs are preferred. |
| OLED-PDT Generations | Technology Used | Relative Cost | Relative Efficiency | Wavelength Purity |
|---|---|---|---|---|
| 1G | Fluorescence | Low | Low | High |
| 2G | Phosphorescence | High | High | Low |
| 3G | Thermally activated delayed fluorescence | Low | High | Low |
| 4G | Hyper fluorescence | Low | High | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, R.K.; Mishra, R.; Sharma, S.K.; Prabhu, N.; Nagar, M.R.; Grigalevicius, S. Advancing Cancer Treatment and Diagnosis: A Review on Photodynamic Therapy Using OLED Technology. Molecules 2025, 30, 1305. https://doi.org/10.3390/molecules30061305
Tiwari RK, Mishra R, Sharma SK, Prabhu N, Nagar MR, Grigalevicius S. Advancing Cancer Treatment and Diagnosis: A Review on Photodynamic Therapy Using OLED Technology. Molecules. 2025; 30(6):1305. https://doi.org/10.3390/molecules30061305
Chicago/Turabian StyleTiwari, Rajesh Kumar, Rajesh Mishra, Sanjay Kumar Sharma, Nakshathra Prabhu, Mangey Ram Nagar, and Saulius Grigalevicius. 2025. "Advancing Cancer Treatment and Diagnosis: A Review on Photodynamic Therapy Using OLED Technology" Molecules 30, no. 6: 1305. https://doi.org/10.3390/molecules30061305
APA StyleTiwari, R. K., Mishra, R., Sharma, S. K., Prabhu, N., Nagar, M. R., & Grigalevicius, S. (2025). Advancing Cancer Treatment and Diagnosis: A Review on Photodynamic Therapy Using OLED Technology. Molecules, 30(6), 1305. https://doi.org/10.3390/molecules30061305







