Enzymatic Esterification of Functional Lipids for Specialty Fats: 1,3-Dipalmitoylglycerol and 1,3-Distearoylglycerol
Abstract
:1. Introduction
2. Results and Discussion
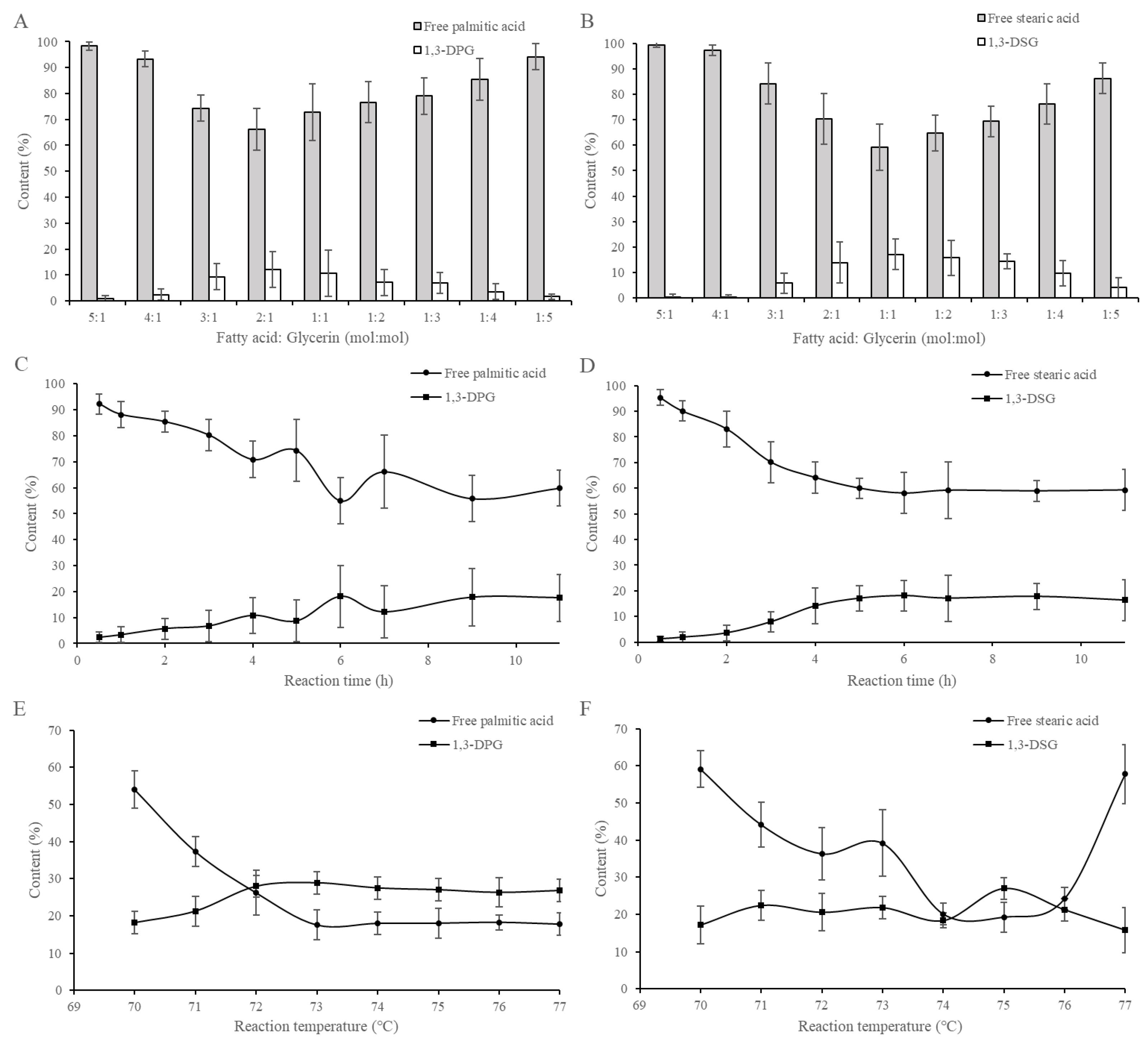
2.1. Gram-Scale Enzymatic Esterification of 1,3-DAGs
2.2. Pilot-Scale Enzymatic Esterification of 1,3-DAGs
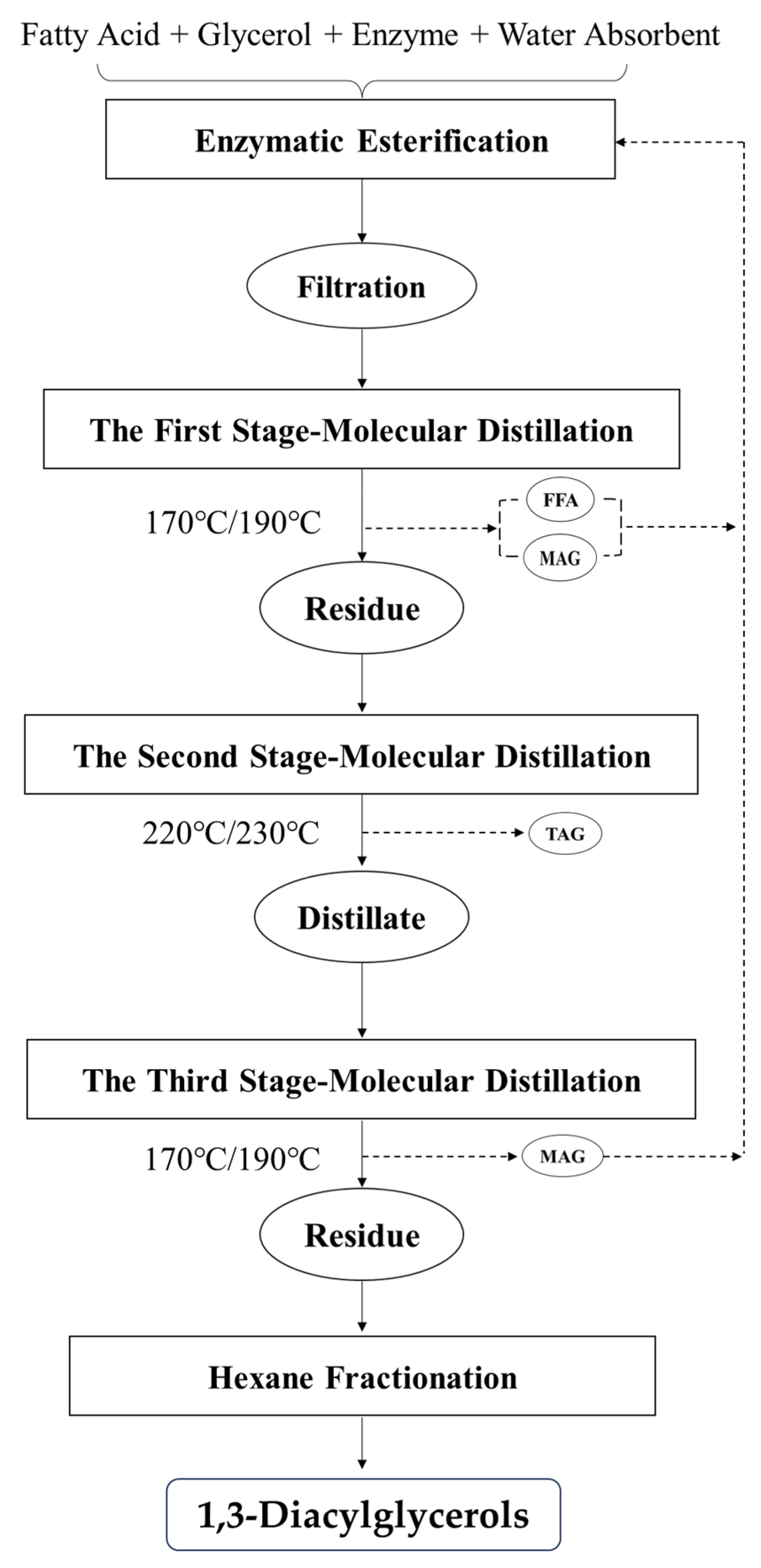
2.3. Purification of Esterified DAGs Using Multi-Stage Molecular Distillation
2.4. Solvent Fractionation for Concentrated 1,3-DAGs
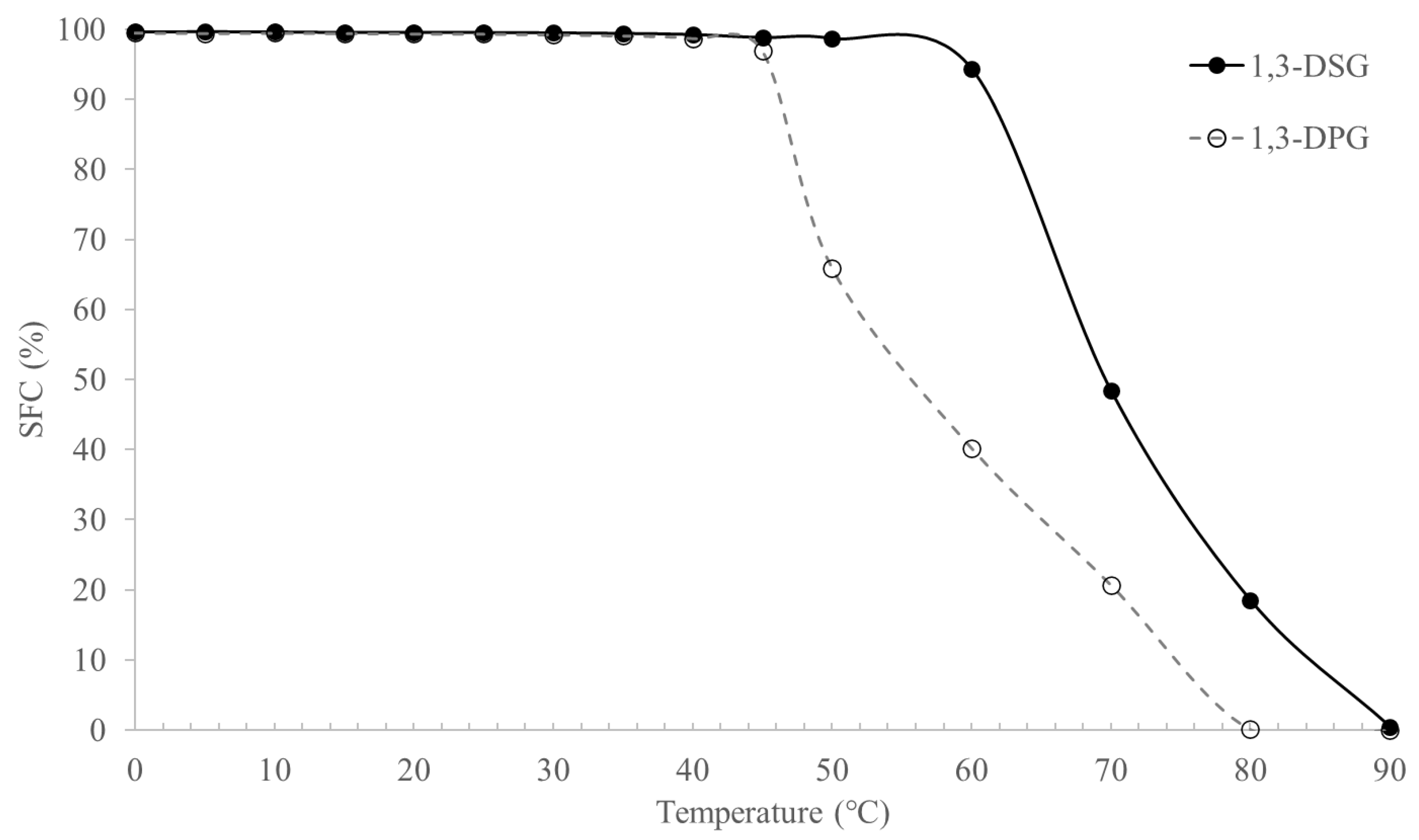
2.5. Solid Fat Contents of 1,3-DAGs
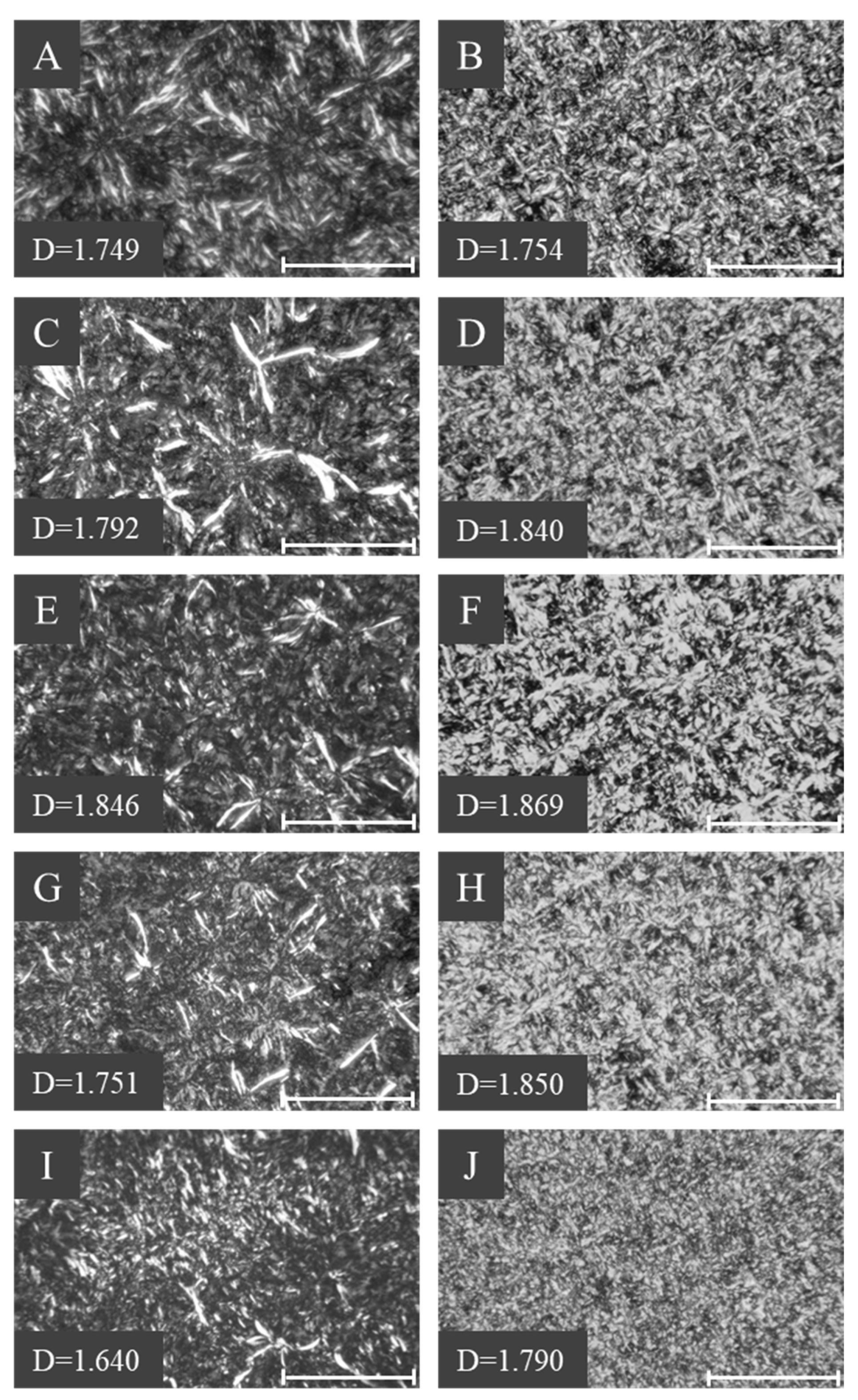
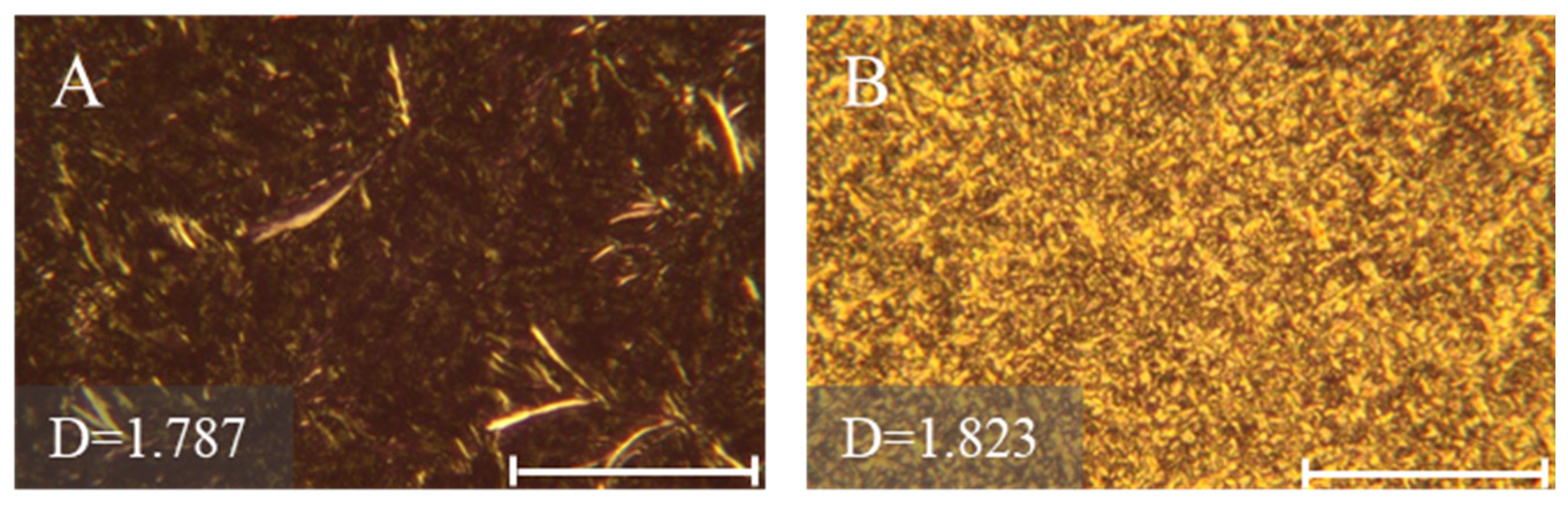
2.6. Fat Crystal Morphologies of 1,3-DAGs
2.7. Fat Crystal Types of 1,3-DAGs
3. Materials and Methods
3.1. Materials
3.2. Enzymatic Esterification of 1,3-DPG and 1,3-DSG
3.3. Purification of Esterified 1,3-DAGs Using Multi-Stage Molecular Distillation
3.4. Purification of 1,3-DAGs Using Solvent Fractionation
3.5. Determination of Lipid Components
3.6. Determination of Solid Fat Contents of 1,3-DAGs
3.7. Observation of Fat Crystal Microstructures of 1,3-DAGs
3.8. Determination of Polymorphism of 1,3-DAGs
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1,3-DPG | 1,3-dipalmitoylglycerol |
| 1,3-DSG | 1,3-distearoylglycerol |
| DAG | diacylglycerol |
| FFA | free fatty acid |
| MAG | monoglyceride |
| TAG | triglyceride |
References
- Koichi, Y.; Shinichiro, S.; Yuan-Li, Z.; Antonio, H.-O.; Ginsberg, H.N. Effects of triacylglycerol and diacylglycerol oils on blood clearance, tissue uptake, and hepatic apolipoprotein B secretion in mice. J. Lipid Res. 2007, 48, 1108–1121. [Google Scholar] [CrossRef]
- Teramoto, T.; Watanabe, H.; Ito, K.; Omata, Y.; Furukawa, T.; Shimoda, K.; Hoshino, M.; Nagao, T.; Naito, S. Significant effects of diacylglycerol on body fat and lipid metabolism in patients on hemodialysis. Clin. Nutr. 2004, 23, 1122–1126. [Google Scholar] [CrossRef]
- Maher, T.; Clegg, M.E. Dietary lipids with potential to affect satiety: Mechanisms and evidence. Crit. Rev. Food Sci. Nutr. 2018, 59, 1619–1644. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Onizawa, K.; Naito, S.; Taguchi, H.; Goto, N.; Nagao, T.; Matsuo, N.; Tokimitsu, I.; Yasukawa, T.; Tsushima, R.; et al. Fat-soluble vitamin status is not affected by diacylglycerol consumption. Ann. Nutr. Metab. 2001, 45, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Tokimitsu, I. Digestion and absorption of diacylglycerol. In Diacylglycerol Oil; AOCS Publishing: Champaign, IL, USA, 2019; pp. 30–45. [Google Scholar]
- Liu, X.; Xu, W.; Wang, W.; Luo, R.; Yang, B.; Lan, D.; Wang, Y. Physicochemical properties and feasibility of coconut oil-based diacylglycerol as an alternative fat for healthy non-dairy creamer. Food Chem. X 2023, 19, 100749. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.B.; Masuchi, M.H.; Miyasaki, E.K.; Domingues, M.A.F.; Stroppa, V.L.Z.; de Oliveira, G.M.; Kieckbusch, T.G. Crystallization modifiers in lipid systems. J. Food Sci. Technol. 2014, 52, 3925–3946. [Google Scholar] [CrossRef]
- Loisel, C.; Lecq, G.; Keller, G.; Ollivon, M. Dynamic crystallization of dark chocolate as affected by temperature and lipid additives. J. Food Sci. 1998, 63, 73–79. [Google Scholar] [CrossRef]
- Tietz, R.A.; Hartel, R.W. Effects of minor lipids on crystallization of milk fat-cocoa butter blends and bloom formation in chocolate. J. Am. Oil Chem. Soc. 2000, 77, 763–771. [Google Scholar] [CrossRef]
- De Clercq, N.; Depypere, F.; Delbaere, C.; Nopens, I.; Bernaert, H.; Dewettinck, K. Influence of cocoa butter diacylglycerols on migration induced fat bloom in filled chocolates. Eur. J. Lipid Sci. Technol. 2014, 116, 1388–1399. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Gu, X.-X.; Wang, Y.-X.; Liu, Z.-Y.; Zhu, Y.; Jin, J.; Jin, Q.-Z.; Wang, X.-G. Effects of diglycerides on crystallization of cocoa butter analogues and bloom of chocolates: A review. China Oils Fats 2023, 48, 46–50. [Google Scholar]
- Ray, J.; Smith, K.W.; Bhaggan, K.; Nagy, Z.K.; Stapley, A.G.F. Crystallization and polymorphic behavior of shea stearin and the effect of removal of polar components. Eur. J. Lipid Sci. Technol. 2013, 115, 1094–1106. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Y.; Huang, C.; Jian, L.; Lin, Z.; Lin, L.; Li, C.; Ye, Y. Insight into the influence of plant oils on the composition of diacylglycerol fabricated by glycerolysis and esterification. Ind. Crops Prod. 2023, 204, 117324. [Google Scholar] [CrossRef]
- Fregolente, P.B.L.; Pinto, G.M.F.; Wolf-Maciel, M.R.; Filho, R.M. Monoglyceride and diglyceride production through lipase-catalyzed glycerolysis and molecular distillation. Appl. Biochem. Biotechnol. 2010, 160, 1879–1887. [Google Scholar] [CrossRef]
- Siew, W.L.; Ng, W.L. Partition coefficient of diglycerides in crystallization of palm oil. J. Am. Oil Chem. Soc. 1995, 72, 591–595. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Song, K.; Wang, L.; Han, X.; Tang, S.; Wang, Y. Separation of diacylglycerols from enzymatically hydrolyzed soybean oil by molecular distillation. Sep. Purif. Technol. 2010, 75, 114–120. [Google Scholar] [CrossRef]
- Yeoh, C.M.; Phuah, E.T.; Tang, T.K.; Siew, W.L.; Abdullah, L.C.; Choong, T.S.Y. Molecular distillation and characterization of diacylglycerol-enriched palm olein. Eur. J. Lipid Sci. Technol. 2014, 116, 1654–1663. [Google Scholar] [CrossRef]
- Ando, Y.; Saito, S.; Yamanaka, N.; Suzuki, C.; Ono, T.; Osaki, N.; Katsuragi, Y. Alpha linolenic acid-enriched diacylglycerol consumption enhances dietary fat oxidation in healthy subjects: A randomized double-blind controlled trial. J. Oleo Sci. 2017, 66, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Yoo, H.J.; Kim, M.; Kim, M.; Choi, J.H.; Lee, C.; Lee, J.H. Effects of equivalent medium-chain diacylglycerol or long-chain triacylglycerol oil intake via muffins on postprandial triglycerides and plasma fatty acids levels. J. Funct. Foods 2019, 53, 299–305. [Google Scholar] [CrossRef]
- Lu, H.; Guo, T.; Fan, Y.; Deng, Z.; Luo, T.; Li, H. Effects of diacylglycerol and triacylglycerol from peanut oil and coconut oil on lipid metabolism in mice. J. Food Sci. 2020, 85, 1907–1914. [Google Scholar] [CrossRef]
- Basso, R.C.; Ribeiro, A.P.B.; Masuchi, M.H.; Gioielli, L.A.; Gonçalves, L.A.G.; dos Santos, A.O.; Cardoso, L.P.; Grimaldi, R. Tripalmitin and monoacylglycerols as modifiers in the crystallisation of palm oil. Food Chem. 2010, 122, 1185–1192. [Google Scholar] [CrossRef]
- Devi, A.; Khatkar, B.S. Physicochemical, rheological and functional properties of fats and oils in relation to cookie quality: A review. J. Food Sci. Technol. 2016, 53, 3633–3641. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Jin, Q.; Wang, X. Types and characteristic indexes of shortenings. China Oils Fats 2021, 46, 53–57. [Google Scholar]
- Gao, Z.; Xu, H.; Fan, Q.; Xie, P.; Jin, Q.; Wang, X.; Jin, J. Effects of fat unsaturation degree on whipping performance and foam stability of fat-reduced aerated emulsions. Int. J. Food Sci. Technol. 2024, 59, 3114–3125. [Google Scholar] [CrossRef]
- Xie, P.; Zheng, Y.; Lee, Y.-Y.; Zou, S.; Wu, Y.; Lai, J.; Wang, Y.; Zhang, Z. Effect of diacylglycerol on partial coalescence of aerated emulsions: Fat crystal-membrane interaction and air-liquid Interface interaction insights. Food Chem. 2024, 461, 140879. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Gao, Z.; Elbarbary, A.; Jin, J. Chemical compositions and crystallization characteristics of SOS-rich fats. J. Am. Oil Chem. Soc. 2024, 101, 1267–1276. [Google Scholar] [CrossRef]
- D’Souza, V.; Deman, J.M.; Deman, L. Short Spacings and Polymorphic Forms of Natural and Commercial Solid Fats: A Review. J. Am. Oil Chem. Soc. 1990, 67, 835–843. [Google Scholar] [CrossRef]
- Chen, J.; Ghazani, S.M.; Stobbs, J.A.; Marangoni, A.G. Tempering of cocoa butter and chocolate using minor lipidic components. Nat. Commun. 2021, 12, 5018. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, N.; Wang, D.; Luo, S.; Zhang, H.; Yu, D.; Wang, L.; Elfalleh, W.; Liao, C. Preparation of vacuum-assisted conjugated linoleic acid phospholipids under nitrogen: Mechanism of acyl migration of lysophospholipids. Food Chem. 2023, 436, 137680. [Google Scholar] [CrossRef]
- Laszlo, J.A.; Compton, D.L.; Vermillion, K.E. Acyl migration kinetics of vegetable oil 1,2-diacylglycerols. J. Am. Oil Chem. Soc. 2008, 85, 307–312. [Google Scholar] [CrossRef]
- Weber, N.; Mukherjee, K.D. Solvent-free lipase-catalyzed preparation of diacylglycerols. J. Agric. Food Chem. 2004, 52, 5347–5353. [Google Scholar] [CrossRef]
- Craven, R.J.; Lencki, R.W. Preparation of diacid 1,3-diacylglycerols. J. Am. Oil Chem. Soc. 2010, 87, 1281–1291. [Google Scholar] [CrossRef]
- Zhu, Y.; Jin, Q.; Wang, X.; Wang, X. Purification of 1,2-Diacylglycerols by a Two-Step Crystallization. Ind. Eng. Chem. Res. 2017, 56, 2197–2204. [Google Scholar] [CrossRef]
- Du, Y.; Zou, S.; Lee, Y.; Wang, Y.; Zhang, Z. Physical property improvement of fluid shortening using peanut oil-based diacylglycerols and their applications in sponge cake. Grain Oil Sci. Technol. 2024, 7, 150–158. [Google Scholar] [CrossRef]
- Xie, P.; Huang, M.; Liu, J.; Elbarbary, A.; Jin, Q.; Wang, X.; Jin, J. Effects of spans on whipping capabilities of aerated emulsions: Reinforcement of fat crystal-membrane interactions. J. Food Eng. 2024, 372, 112008. [Google Scholar] [CrossRef]
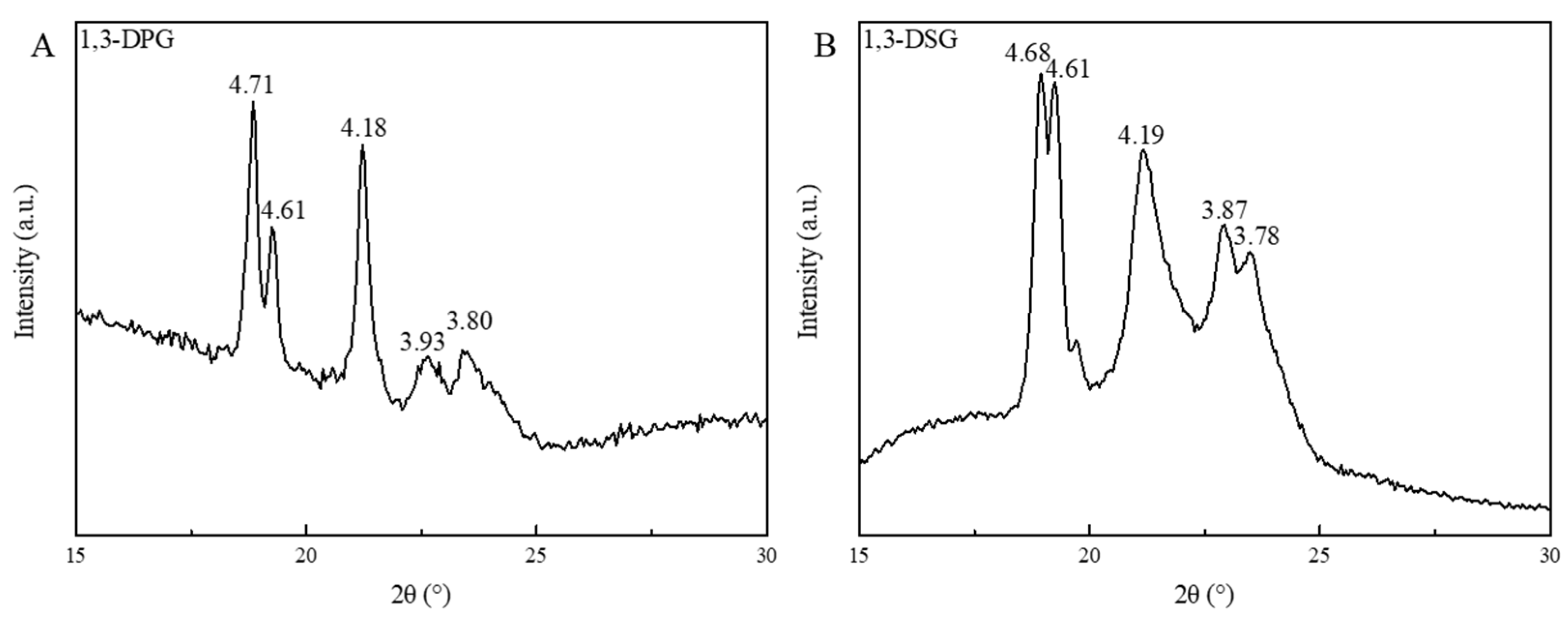
| Test Item | TAG | FFA | 1,3-DAG | 1,2-DAG | MAG | 1,3-DAG/(DAG + TAG) | 1,3-DAG/DAG | DAG/(DAG + TAG) | |
|---|---|---|---|---|---|---|---|---|---|
| Esterified 1,3-DPG | 22.64 ± 0.22 | 30.53 ± 0.31 | 26.27 ± 0.22 | 8.77 ± 0.02 | 11.79 ± 0.12 | 45.55 ± 1.03 | 74.98 ± 1.53 | 60.75 ± 1.32 | |
| Esterified 1,3-DSG | 25.64 ± 0.25 | 8.56 ± 0.03 | 20.75 ± 0.22 | 18.83 ± 0.12 | 26.22 ± 0.29 | 31.82 ± 0.83 | 52.43 ± 1.10 | 60.68 ± 1.78 | |
| The 1st molecular distillation | |||||||||
| 1,3-DPG | Residue | 60.57 ± 1.82 | 1.59 ± 0.01 | 25.28 ± 0.60 | 11.55 ± 0.13 | 1.00 ± 0.00 | 25.96 ± 0.41 | 68.63 ± 1.33 | 37.82 ± 0.89 |
| 1,3-DSG | Residue | 28.86 ± 0.50 | 1.85 ± 0.01 | 39.01 ± 0.67 | 18.06 ± 0.24 | 12.22 ± 0.11 | 45.40 ± 0.90 | 68.35 ± 1.45 | 66.42 ± 1.23 |
| The 2nd molecular distillation | |||||||||
| 1,3-DPG | Distillate | 5.65 ± 0.10 | 10.01 ± 0.17 | 23.48 ± 0.51 | 13.70 ± 0.33 | 47.16 ± 0.93 | 54.82 ± 0.99 | 63.15 ± 1.51 | 86.80 ± 1.88 |
| 1,3-DSG | Distillate | 6.47 ± 0.13 | 2.82 ± 0.07 | 14.12 ± 0.32 | 8.00 ± 0.17 | 68.60 ± 1.77 | 49.38 ± 0.71 | 63.82 ± 1.70 | 77.38 ± 1.88 |
| The 3rd molecular distillation | |||||||||
| 1,3-DPG | Residue | 10.10 ± 0.18 | 0.43 ± 0.00 | 58.42 ± 1.11 | 27.63 ± 0.49 | 3.42 ± 0.07 | 60.76 ± 1.11 | 67.89 ± 1.57 | 89.50 ± 2.57 |
| 1,3-DSG | Residue | 33.02 ± 0.64 | 1.30 ± 0.01 | 43.26 ± 0.88 | 19.85 ± 0.43 | 2.57 ± 0.05 | 45.00 ± 0.83 | 68.55 ± 1.47 | 65.65 ± 1.66 |
| Hexane fractionation | |||||||||
| 1,3-DPG | Stearin | 6.66 ± 0.12 | 0.19 ± 0.00 | 70.29 ± 1.44 | 18.63 ± 0.37 | 4.24 ± 0.03 | 73.54 ± 1.45 | 79.05 ± 1.50 | 93.03 ± 2.17 |
| 1,3-DSG | Stearin | 15.12 ± 0.37 | 0.18 ± 0.00 | 52.17 ± 1.16 | 31.60 ± 0.65 | 0.92 ± 0.01 | 52.76 ± 1.20 | 62.28 ± 1.22 | 84.71 ± 1.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Chi, J.; Wang, S.; Elbarbary, A.; Zhang, Y.; Jin, J. Enzymatic Esterification of Functional Lipids for Specialty Fats: 1,3-Dipalmitoylglycerol and 1,3-Distearoylglycerol. Molecules 2025, 30, 1328. https://doi.org/10.3390/molecules30061328
Yang Y, Chi J, Wang S, Elbarbary A, Zhang Y, Jin J. Enzymatic Esterification of Functional Lipids for Specialty Fats: 1,3-Dipalmitoylglycerol and 1,3-Distearoylglycerol. Molecules. 2025; 30(6):1328. https://doi.org/10.3390/molecules30061328
Chicago/Turabian StyleYang, Yuhuang, Juanjuan Chi, Shengyuan Wang, Abdelaziz Elbarbary, Yafei Zhang, and Jun Jin. 2025. "Enzymatic Esterification of Functional Lipids for Specialty Fats: 1,3-Dipalmitoylglycerol and 1,3-Distearoylglycerol" Molecules 30, no. 6: 1328. https://doi.org/10.3390/molecules30061328
APA StyleYang, Y., Chi, J., Wang, S., Elbarbary, A., Zhang, Y., & Jin, J. (2025). Enzymatic Esterification of Functional Lipids for Specialty Fats: 1,3-Dipalmitoylglycerol and 1,3-Distearoylglycerol. Molecules, 30(6), 1328. https://doi.org/10.3390/molecules30061328







