Phosphorylated Nitrones—Synthesis and Applications
Abstract
1. Introduction
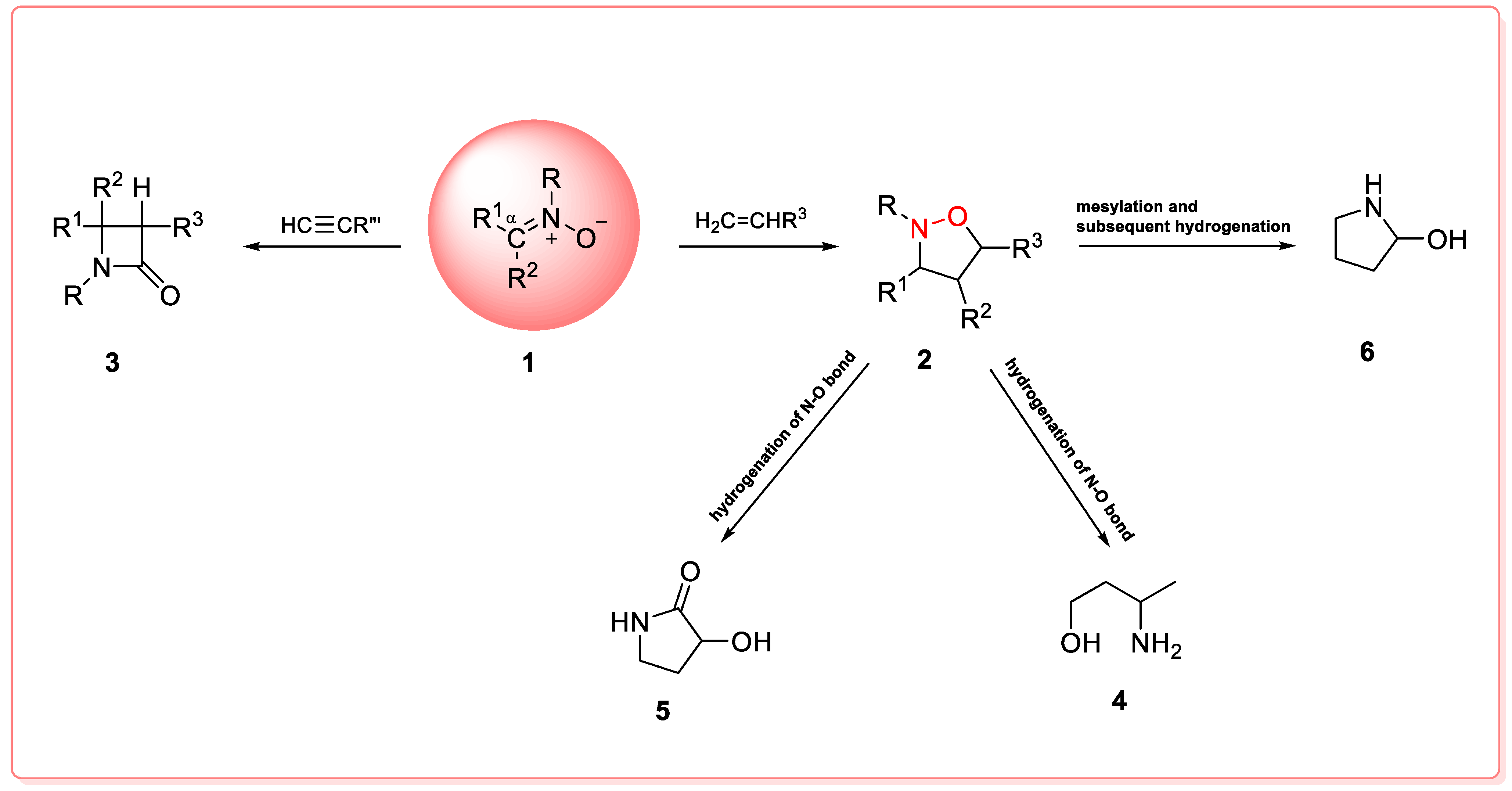
2. Synthesis of Nitrones
2.1. N-Substituted C-Phosphorylated Nitrones
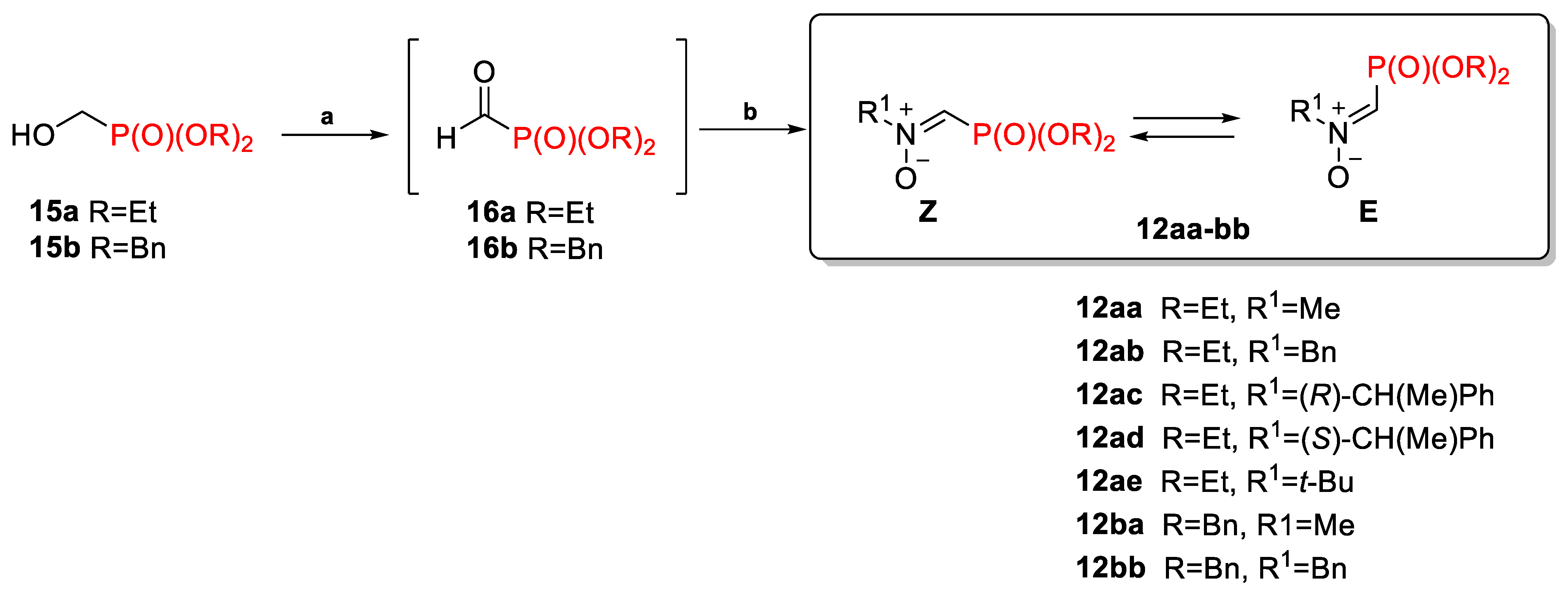
2.1.1. N-Substituted C-(Dialkoxyphosphoryl)Nitrones 12
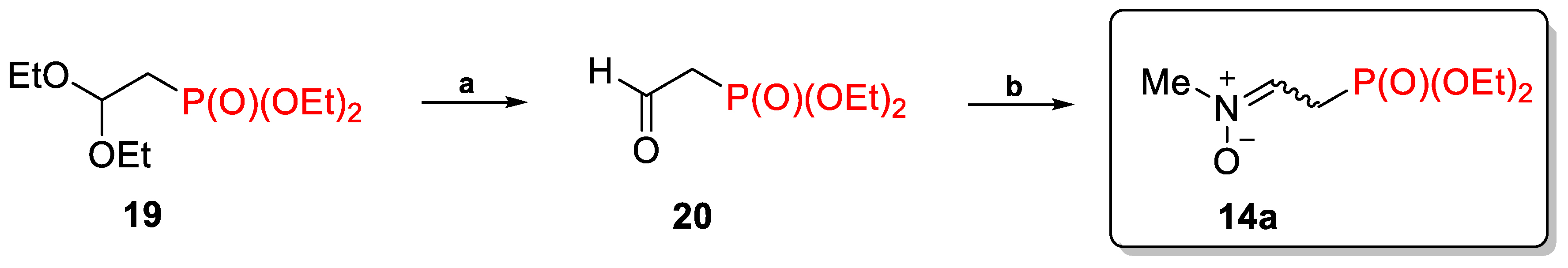
2.1.2. N-Substituted C-(Diethoxyphosphorylalkyl)Nitrones 14
2.2. “Non-Cyclic” Phosphorylated Nitrones
2.2.1. PPN-Type Nitrones 8
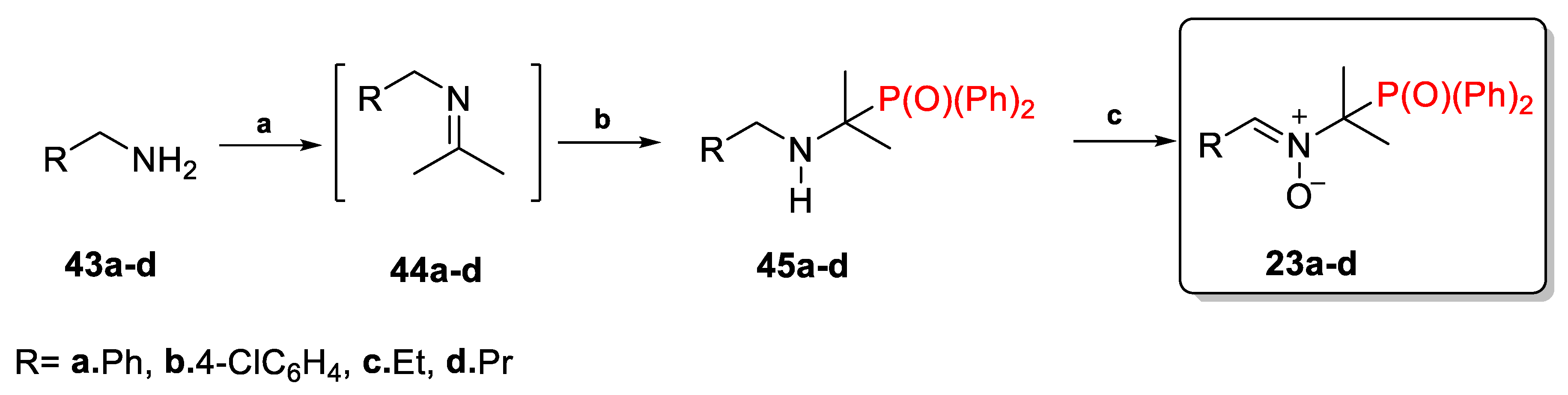
2.2.2. Nitrones with Diphenylphosphinyl Group 23
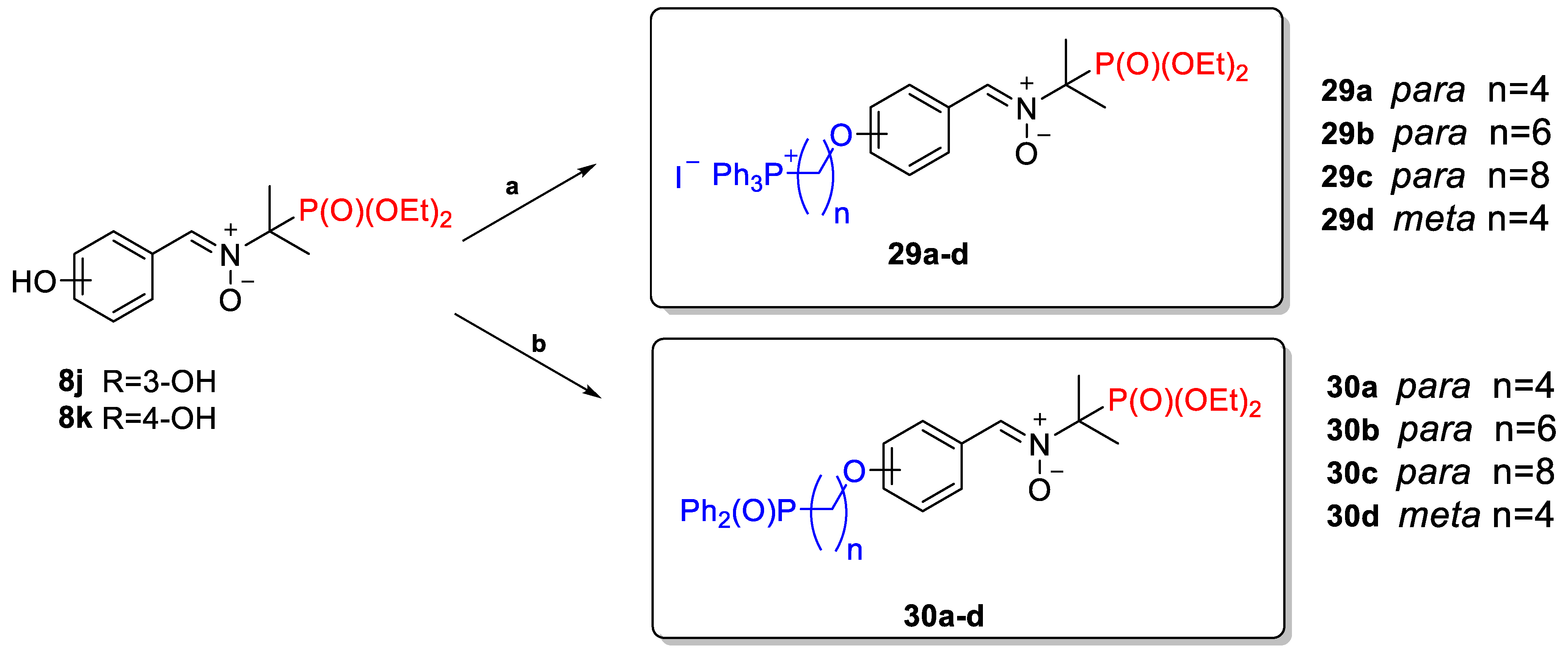
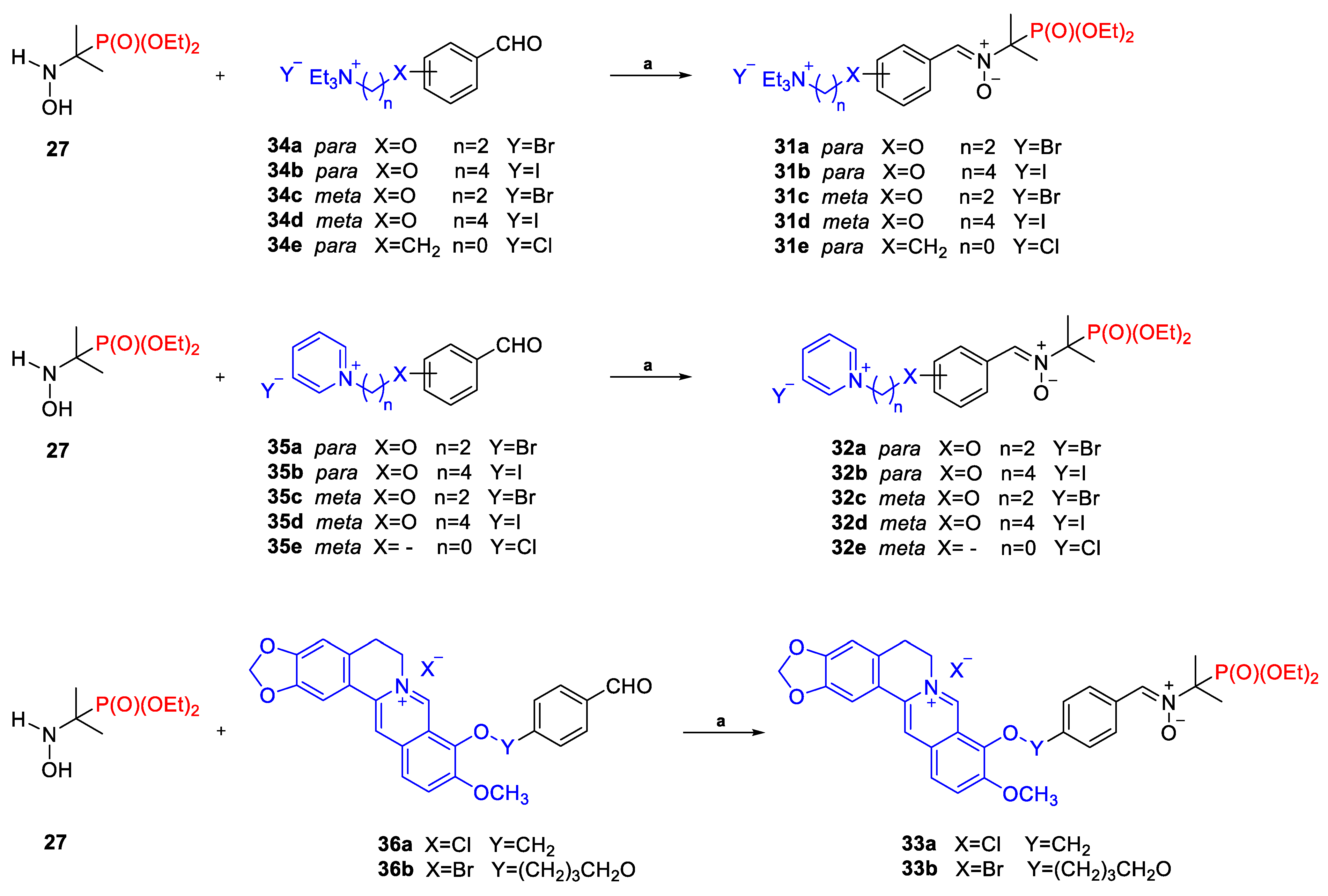
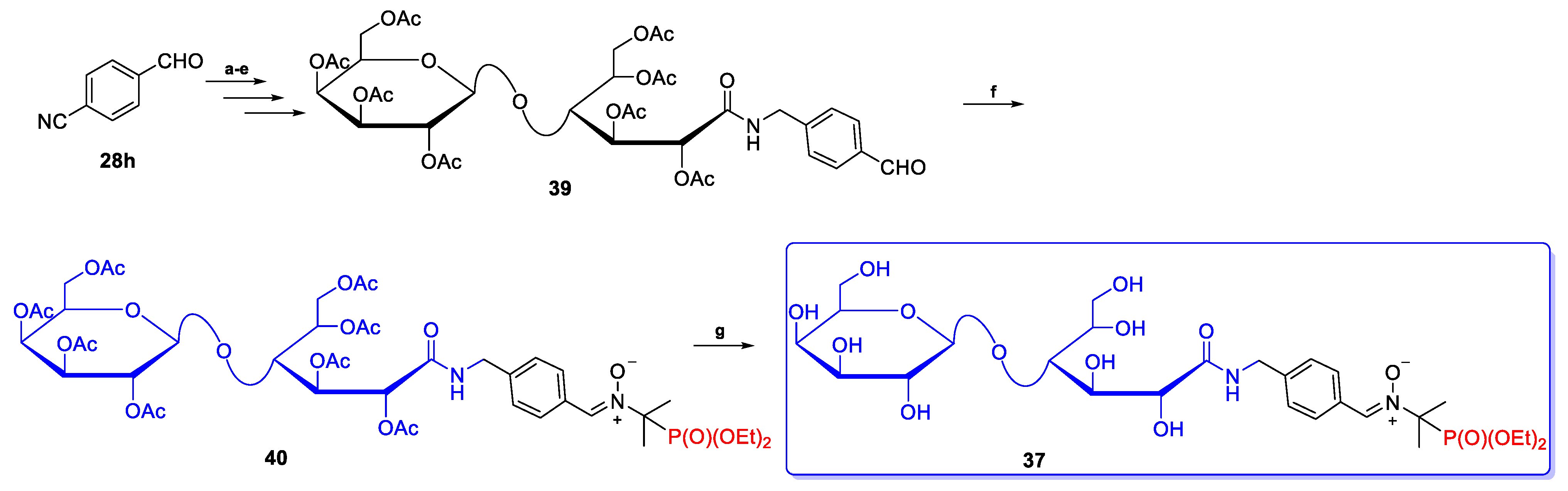
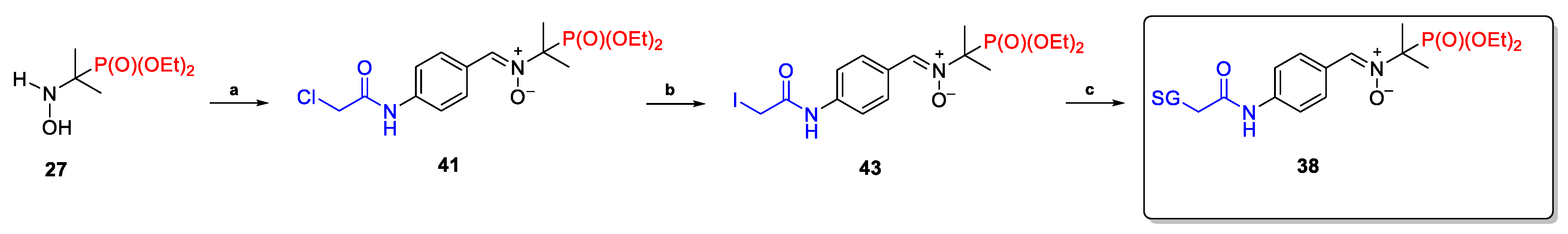
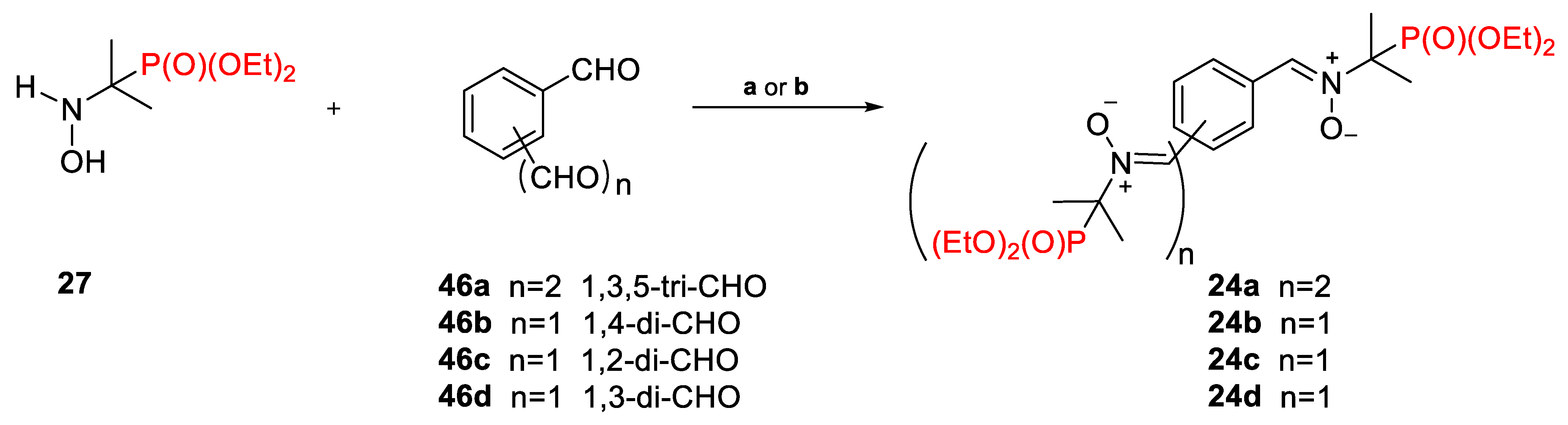
2.2.3. Poly(Phosphoryl)Nitrones 24
2.3. Cyclic Phosphorylated Nitrones
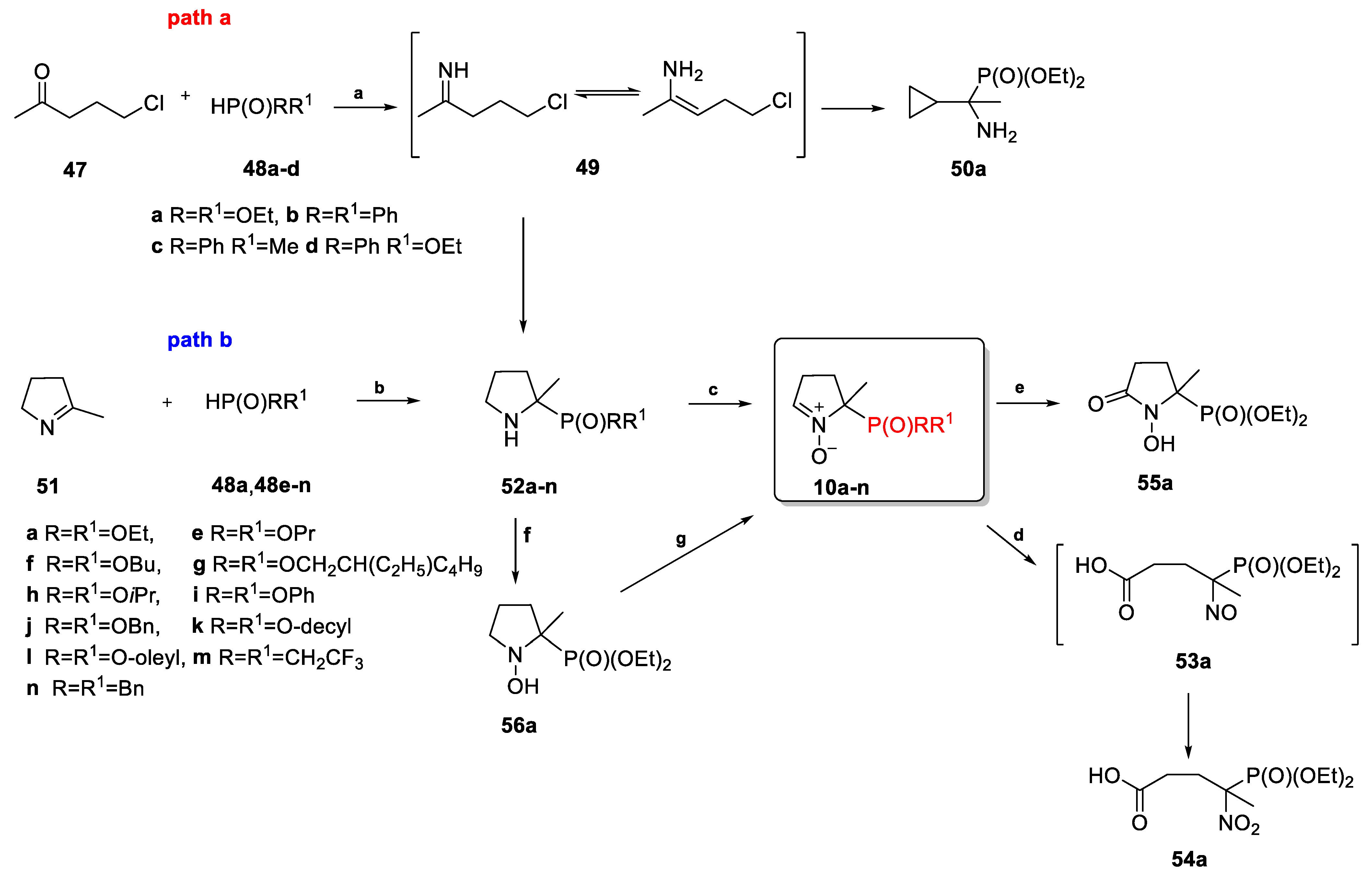
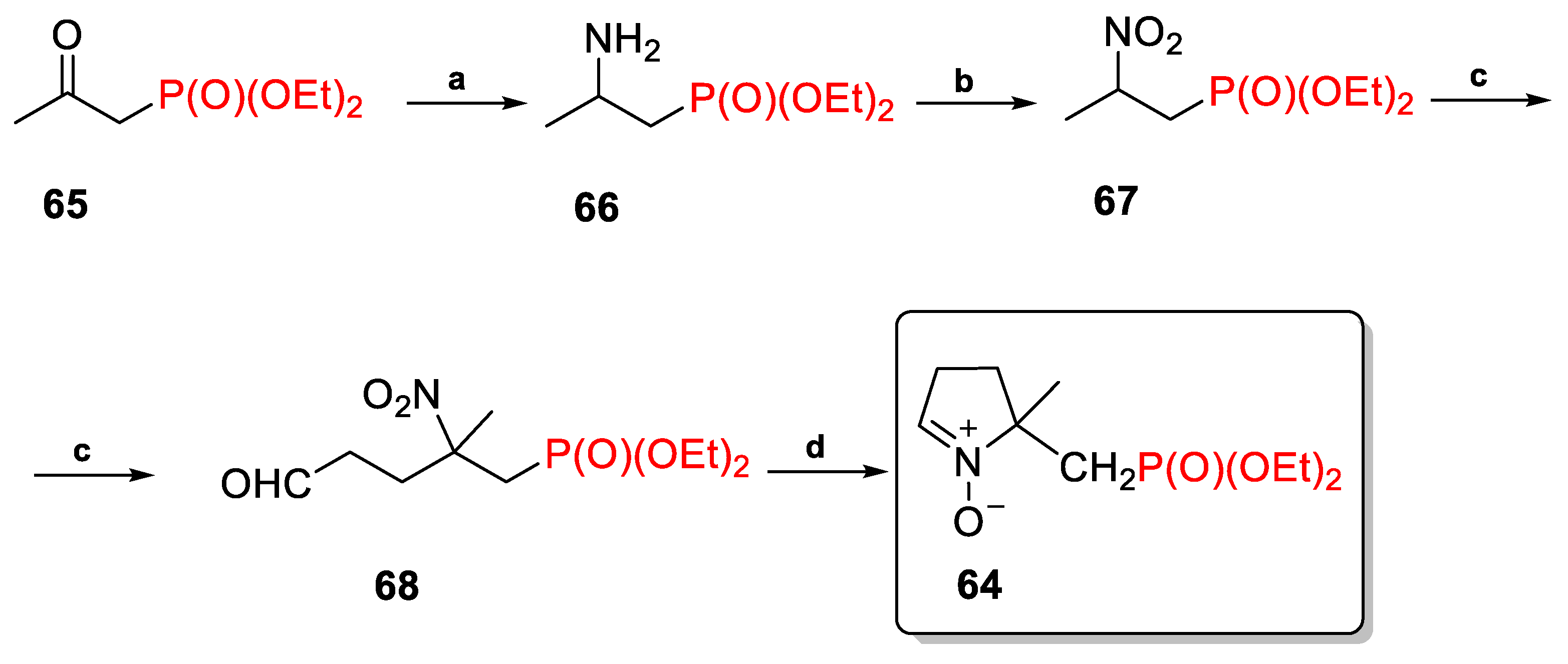
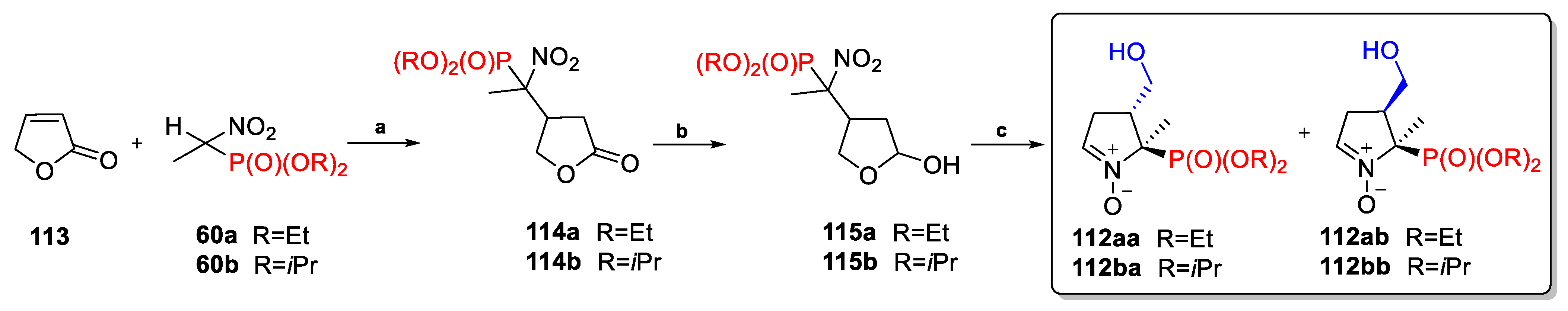
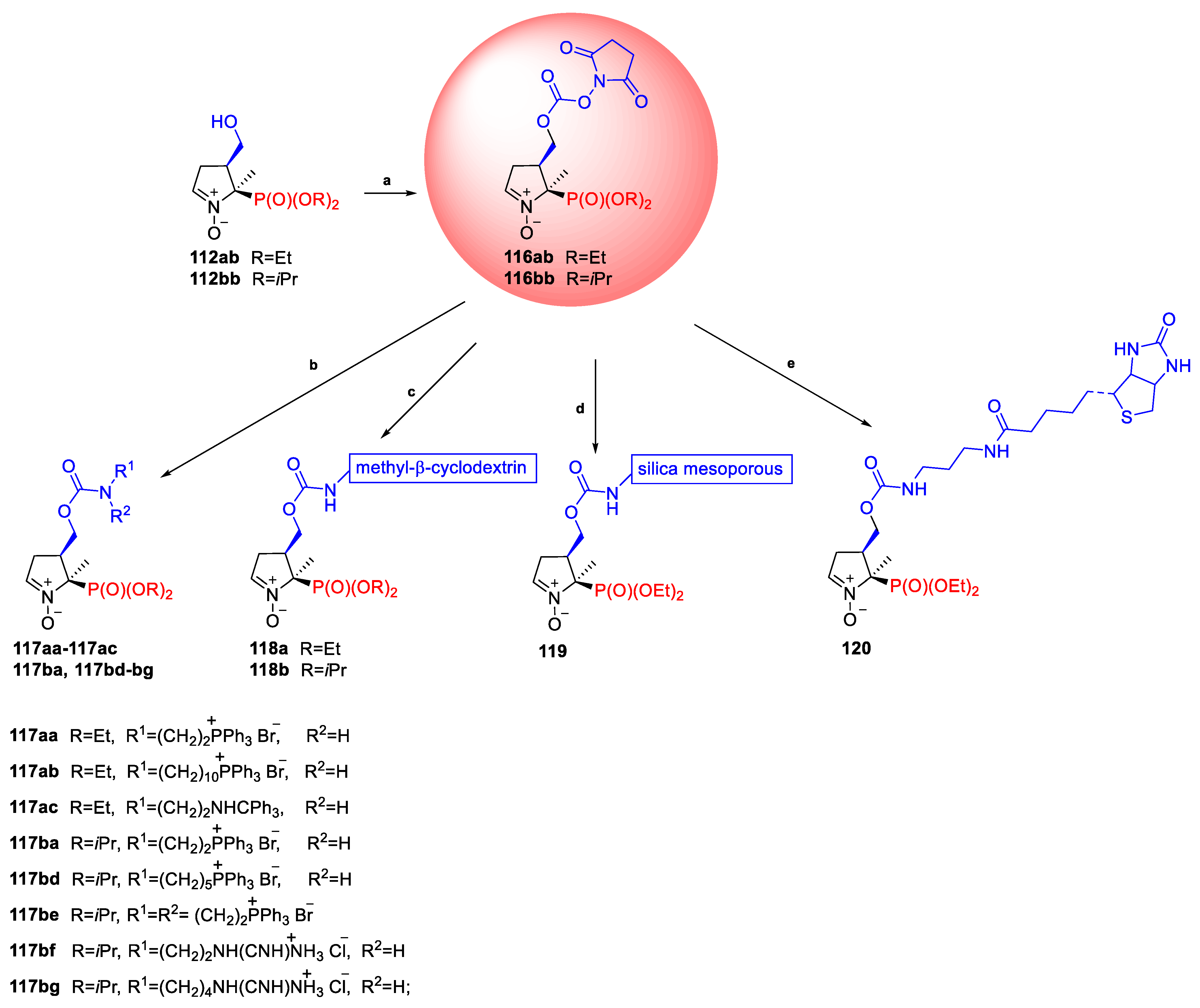
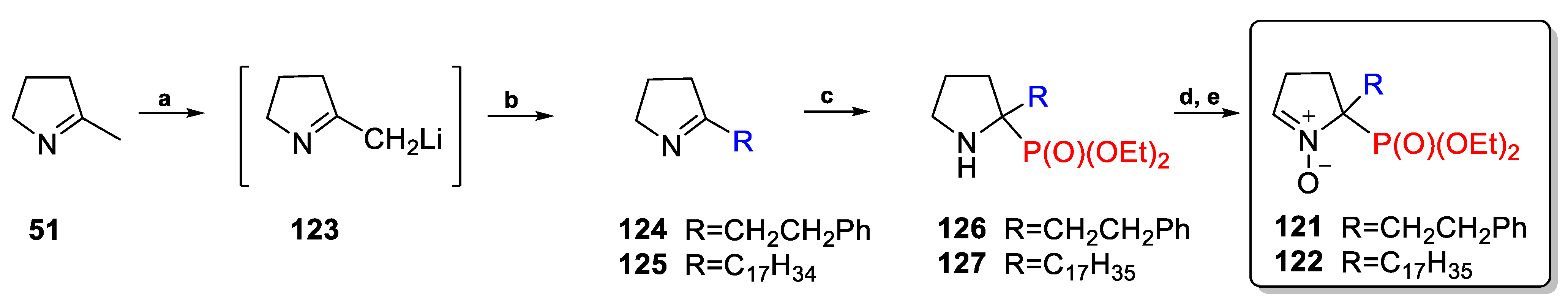
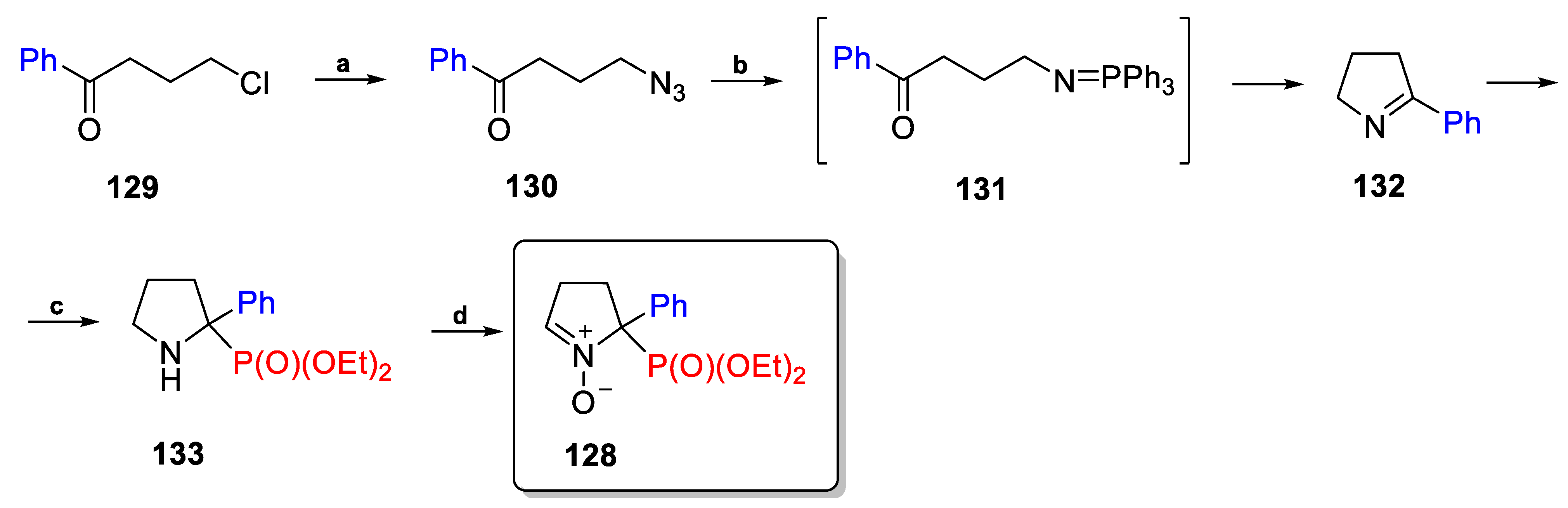
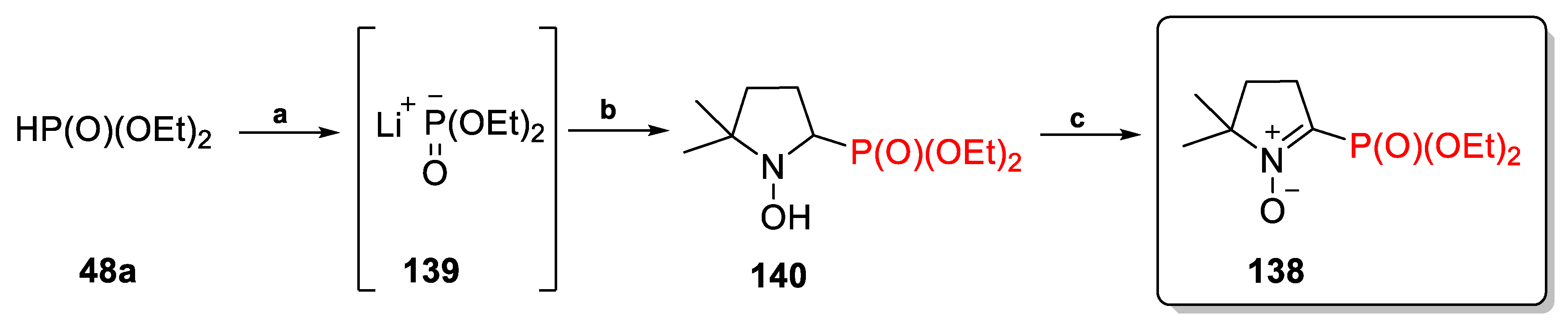
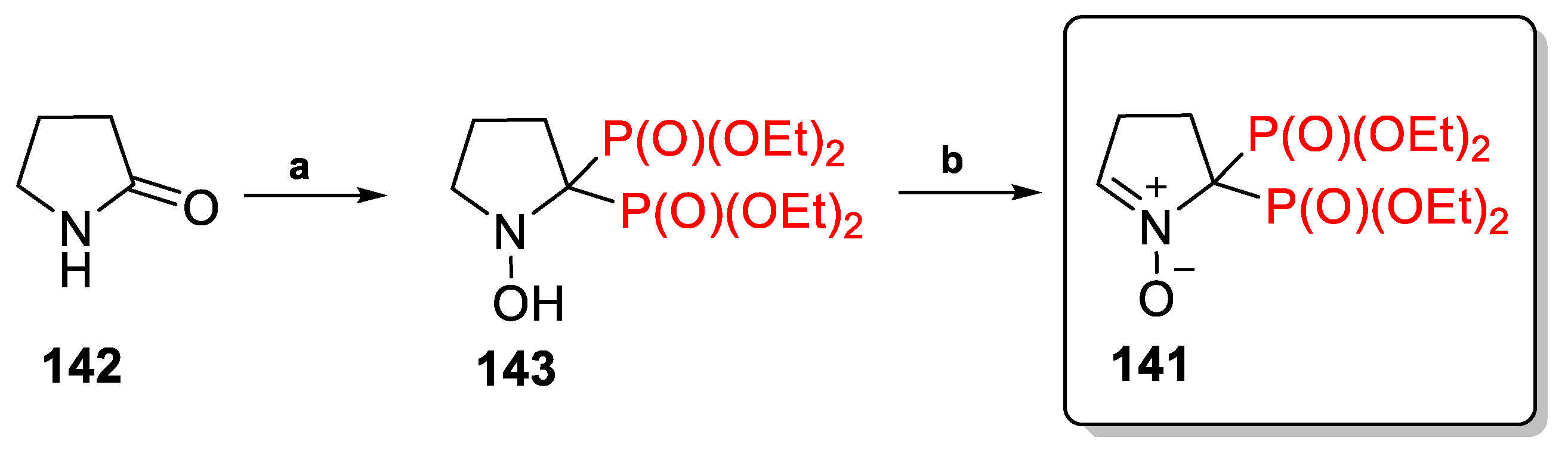
2.3.1. Depmpo and Its Alkoxy-, Alkyl-, and Aryl-Phosphorylated Derivatives
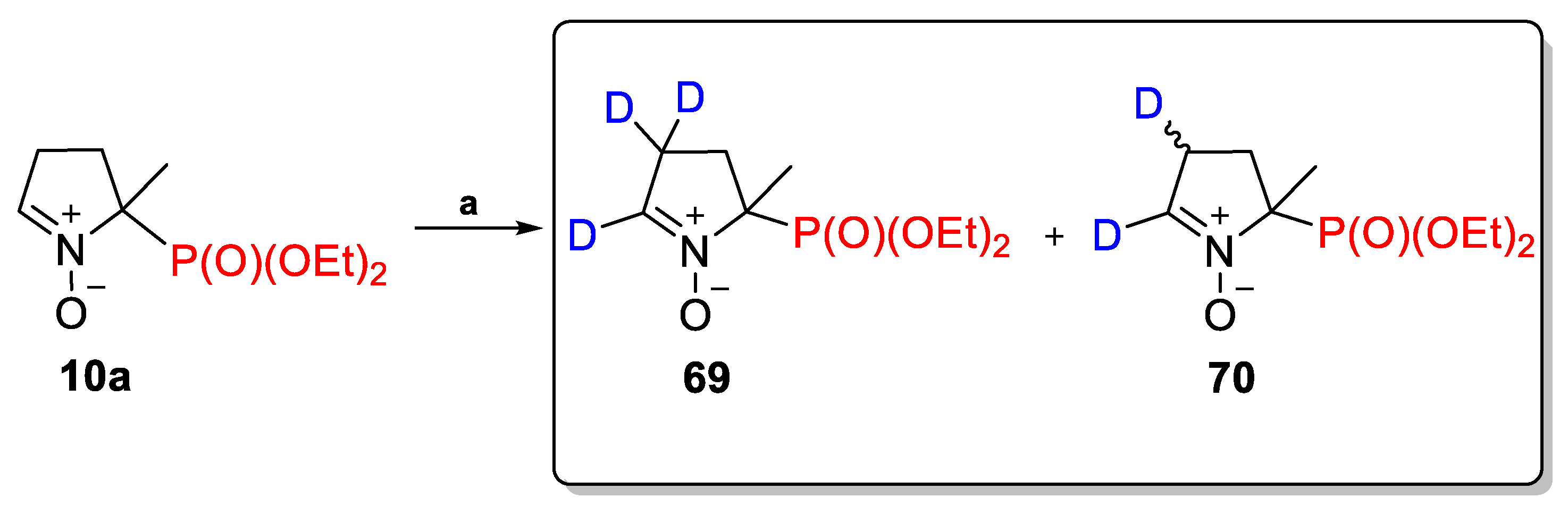
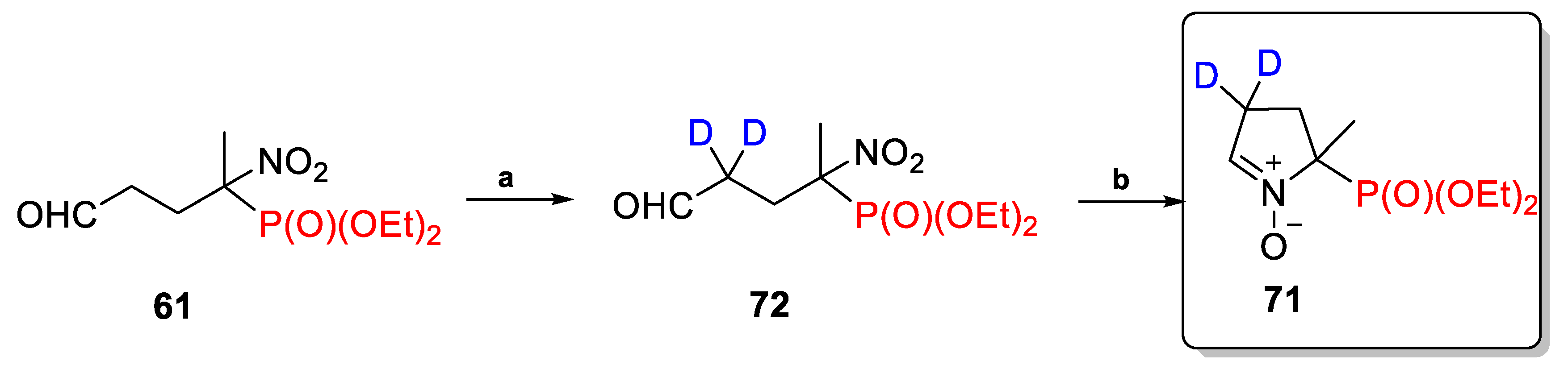
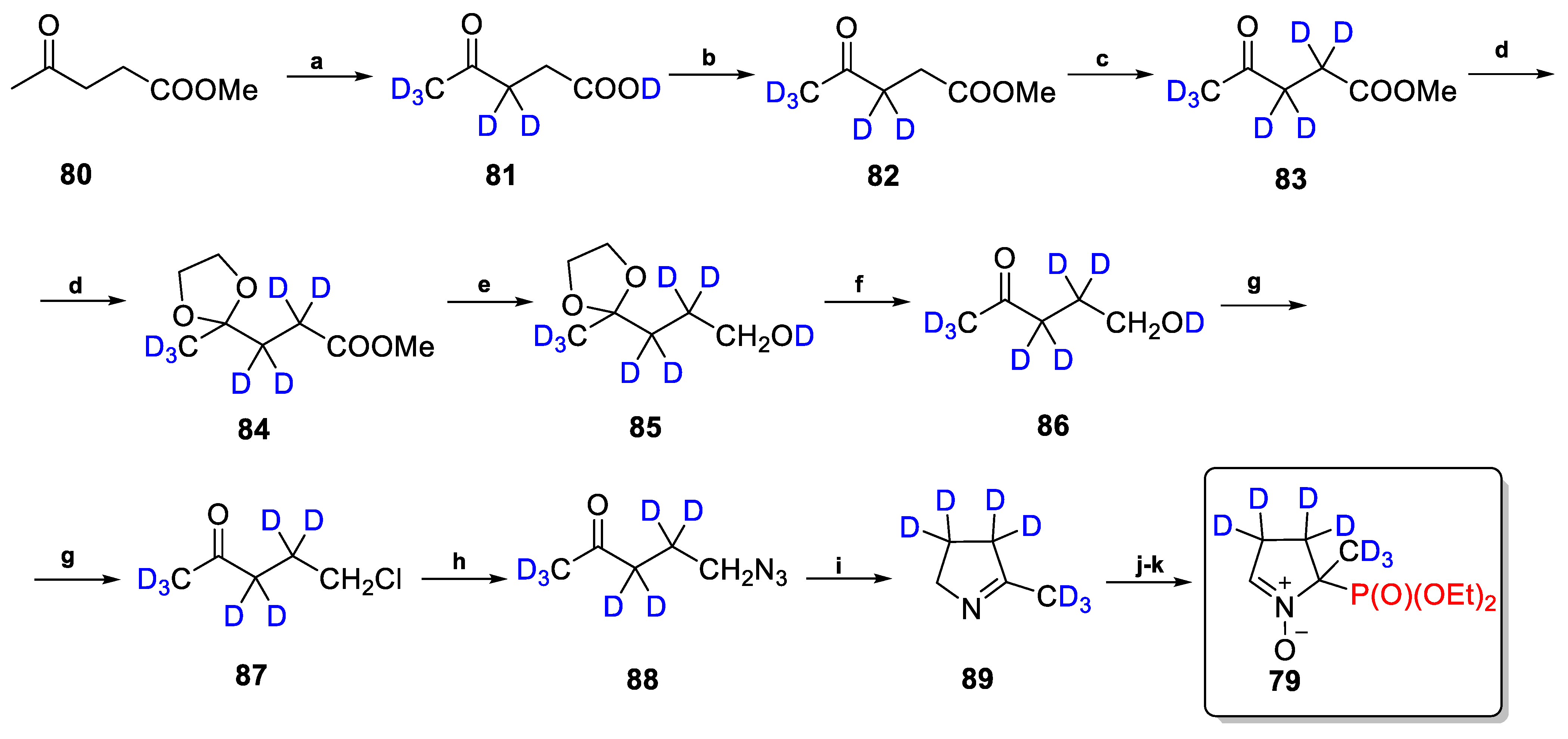
2.3.2. Deuterium-Labeled Analogs of DEPMPO
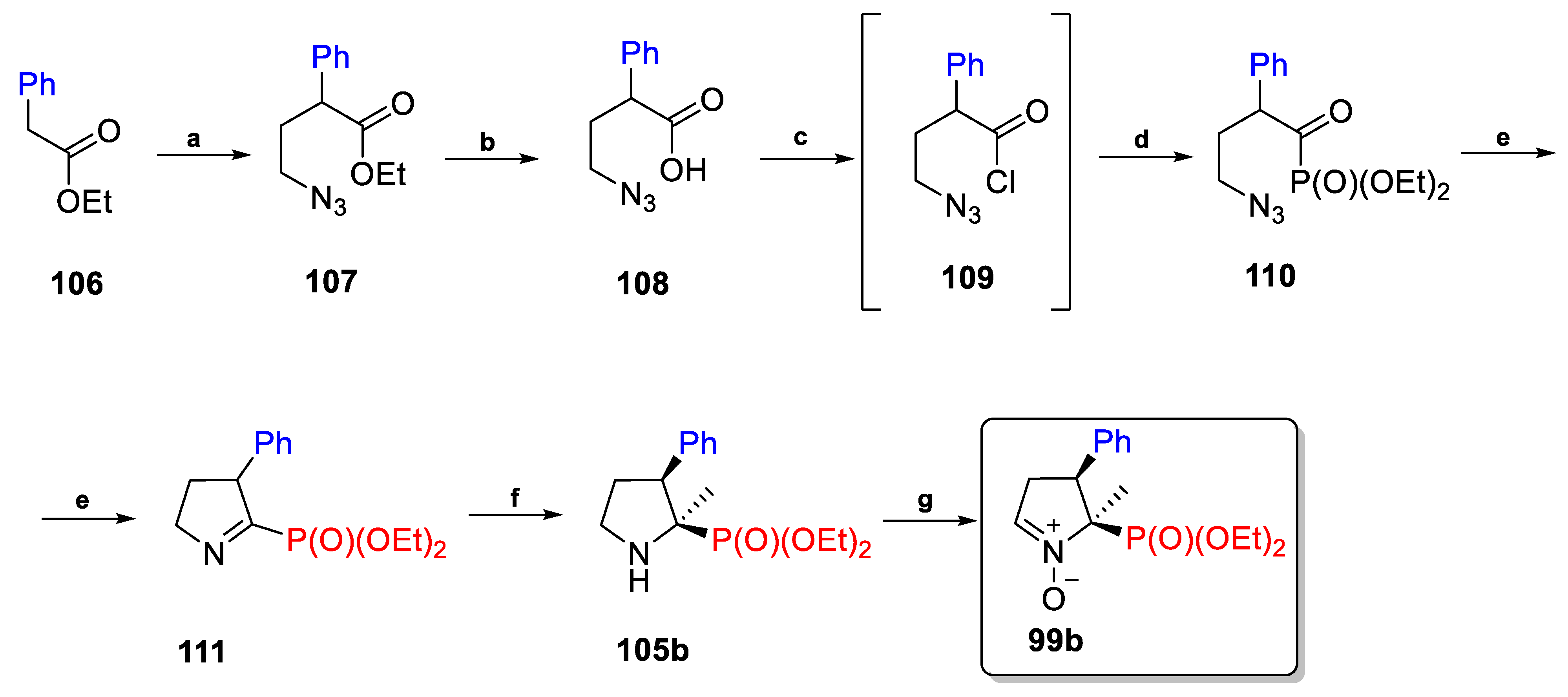
2.3.3. C3-Substituted Derivatives of DEPMPO
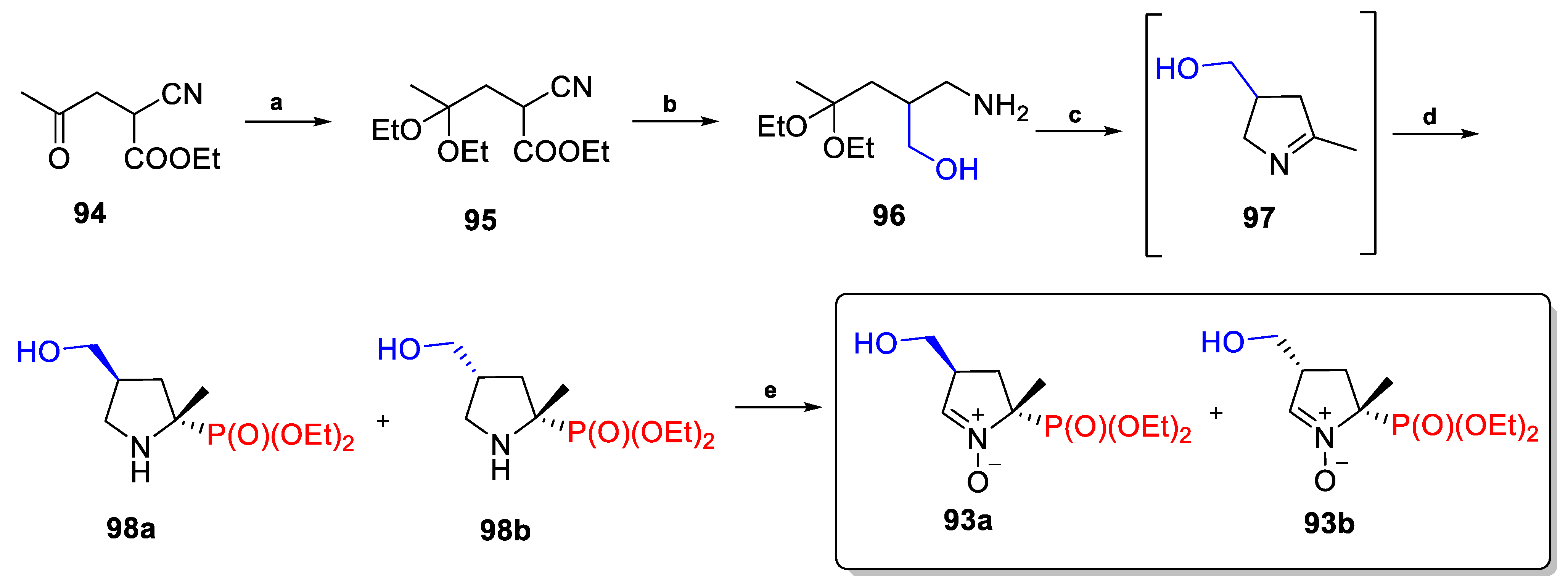
2.3.4. C4-Substituted Derivatives of DEPMPO
2.3.5. C5-Modified Derivatives of DEPMPO
2.3.6. Analog of DEPMPO with Phosphoryl Group at C2
2.3.7. Diphosphorylated Derivative of DEPMPO
3. Detection of Free Radicals
3.1. “Non-Cyclic” Phosphorylated Nitrones
3.2. Cyclic Phosphorylated Nitrones
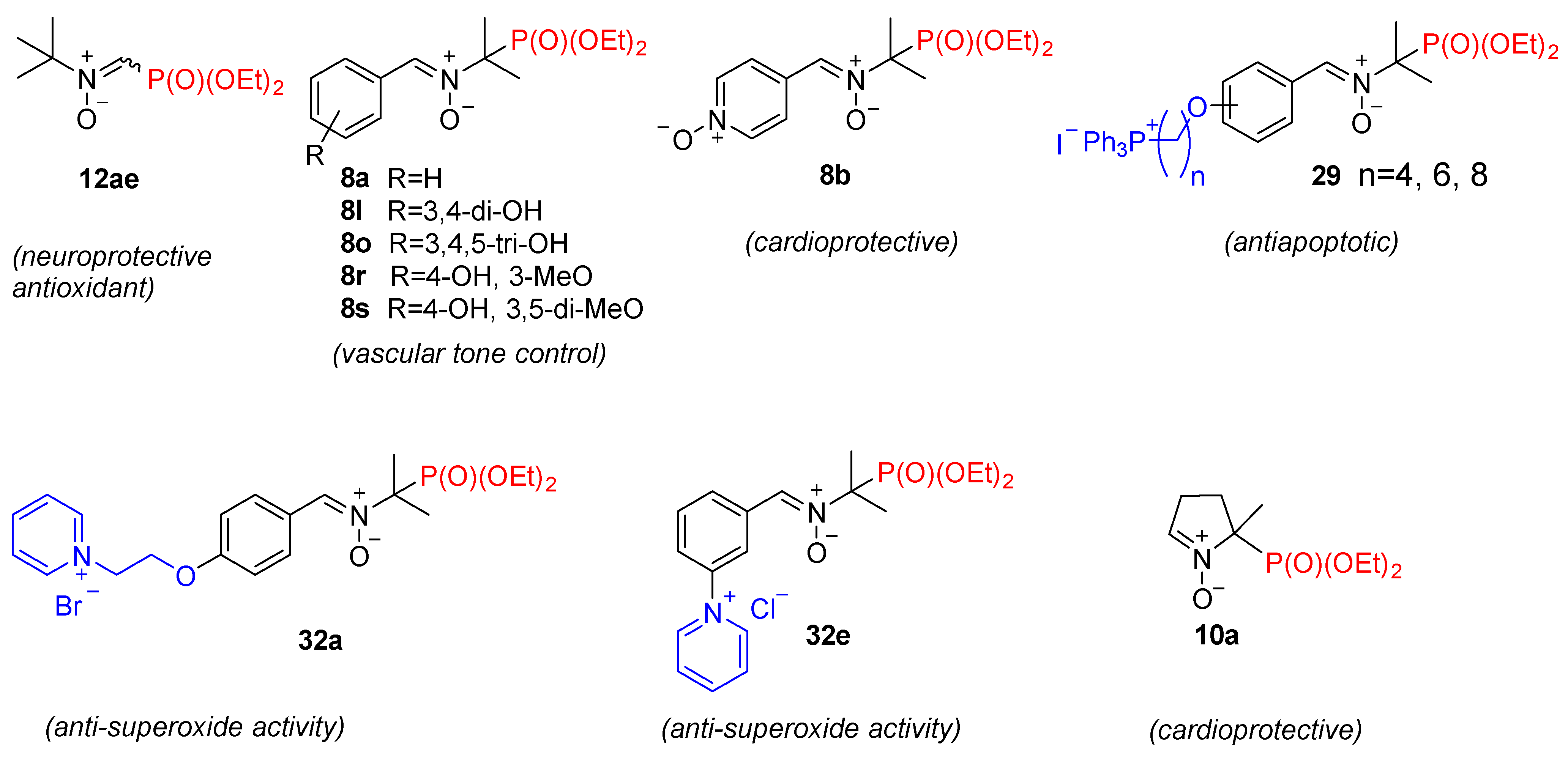
4. Pharmacological Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delpierre, G.R.; Lamchen, M. Nitrones. Q. Rev. Chem. Soc. 1965, 19, 329–348. [Google Scholar] [CrossRef]
- Gothelf, K.V.; Jorgensen, K.A. Asymmetric 1,3-dipolar cycloaddition reactions. Chem. Rev. 1998, 98, 863–909. [Google Scholar] [CrossRef] [PubMed]
- Padwa, A.; Fisera, L.; Koehler, K.F.; Rodriguez, A.; Wong, G.S.K. Regioselectivity associated with the 1,3-dipolar cycloaddition of nitrones with electron-deficient dipolarophiles. J. Org. Chem. 1984, 49, 276–281. [Google Scholar] [CrossRef]
- Novelli, G.P.; Angiolini, P.; Consales, G.; Lippi, R.; Tani, R. Anti-Shock Action of phenyl-tert-butyl-nitrone, a spin trapper. In Oxygen Free Radicals in Shock; Novelli, G.P., Ursini, F., Eds.; Karger: Basel, Switzerland, 1986; pp. 119–124. [Google Scholar]
- Novelli, G.P.; Angiolini, P.; Tani, R.; Consales, G.; Bordi, L. Phenyl t-butyl nitrone is active against traumatic shock in rats. Free Radic. Res. Commun. 1985, 1, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, S.; Tsuchidate, R.; Smith, M.L.; Maples, K.R.; Siesjo, B.K. Neuroprotective effects of a novel nitrone, NXY-059, after transient focal cerebral ischemia in the rat. J. Cereb. Blood Flow Metab. 1999, 19, 778–787. [Google Scholar] [CrossRef]
- Fong, J.J.; Rhoney, D.H. NXY-059: Review of neuroprotective potential for acute stroke. Ann. Pharmacother. 2006, 40, 461–471. [Google Scholar] [CrossRef]
- Chamorro, B.; Gtowacka, I.E.; Gotkowska, J.; Gulej, R.; Hadjipavlou-Litina, D.; Lopez-Munoz, F.; Marco-Contelles, J.; Piotrowska, D.G.; Oset-Gasque, M.J. Nucleobase-Derived Nitrones: Synthesis and Antioxidant and Neuroprotective Activities in an In Vitro Model of Ischemia-Reperfusion. Int. J. Mol. Sci. 2022, 23, 3411. [Google Scholar] [CrossRef]
- Perkins, M.J. Spin Trapping. Adv. Phys. Org. Chem. 1980, 17, 1–64. [Google Scholar]
- Villamena, F.A.; Zweier, J.L. Detection of reactive oxygen and nitrogen species by EPR spin trapping. Antioxid. Redox Signal. 2004, 6, 619–629. [Google Scholar] [CrossRef]
- Pfeiffer, P. Lichtchemische synthese von indolderivaten. Justus Liebigs Ann. Chem. 1916, 411, 72–159. [Google Scholar] [CrossRef]
- Villamena, F.A.; Das, A.; Nash, K.M. Potential implication of the chemical properties and bioactivity of nitrone spin traps for therapeutics. Future Med. Chem. 2012, 4, 1171–1207. [Google Scholar] [CrossRef]
- Marco-Contelles, J. β-Lactam synthesis by the Kinugasa reaction. Angew. Chem. Int. Ed. Engl. 2004, 43, 2198–2200. [Google Scholar] [CrossRef]
- Maciejko, M.; Stecko, S.; Staszewska-Krajewska, O.; Jurczak, M.; Furman, B.; Chmielewski, M. An Entry to the Carbapenem Antibiotic Scaffold via the Asymmetric Kinugasa Reaction. Synthesis 2012, 44, 2825–2839. [Google Scholar] [CrossRef]
- Pellissier, H. Asymmetric 1,3-dipolar cycloadditions. Tetrahedron 2007, 63, 3235–3285. [Google Scholar] [CrossRef]
- Evans, D.A.; Song, H.J.; Fandrick, K.R. Enantioselective nitrone cycloadditions of α,β-unsaturated 2-acyl imidazoles catalyzed by bis(oxazolinyl)pyridine-cerium(IV) triflate complexes. Org. Lett. 2006, 8, 3351–3354. [Google Scholar] [CrossRef] [PubMed]
- Heinz, L.J.; Lunn, W.H.W.; Murff, R.E.; Paschal, J.W.; Spangle, L.A. An efficient synthesis of cis- and trans-methyl-3-hydroxy-2-pyrrolidone-5-carboxylates, key intermediates for the synthesis of γ-substituted glutamic acid analogs. J. Org. Chem. 1996, 61, 4838–4841. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Głowacka, I.E. Enantiomerically pure phosphonate analogues of cis- and trans-4-hydroxyprolines. Tetrahedron Asymmetry 2007, 18, 1351–1363. [Google Scholar] [CrossRef]
- Damodiran, M.; Sivakumar, P.M.; SenthilKumar, R.; Muralidharan, D.; Kumar, B.; Doble, M.; Perumal, P.T. Antibacterial Activity, Quantitative Structure-Activity Relationship and Diastereoselective Synthesis of Isoxazolidine Derivatives Via 1,3-Dipolar Cycloaddition of d-glucose Derived Nitrone with Olefin. Chem. Biol. Drug Des. 2009, 74, 494–506. [Google Scholar] [CrossRef]
- Kumar, K.R.R.; Mallesha, H.; Rangappa, K.S. Synthesis of novel isoxazolidine derivatives and their antifungal and antibacterial properties. Arch. Pharm. 2003, 336, 159–164. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Andrei, G.; Schols, D.; Snoeck, R.; Grabkowska-Drużyc, M. New Isoxazolidine-Conjugates of Quinazolinones-Synthesis, Antiviral and Cytostatic Activity. Molecules 2016, 21, 959. [Google Scholar] [CrossRef]
- Łysakowska, M.; Balzarini, J.; Piotrowska, D.G. Design, Synthesis, Antiviral, and Cytostatic Evaluation of Novel Isoxazolidine Analogs of Homonucleotides. Arch. Pharm. 2014, 347, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Khazir, J.; Singh, P.P.; Reddy, D.M.; Hyder, I.; Shafi, S.; Sawant, S.D.; Chashoo, G.; Mahajan, A.; Alam, M.S.; Saxena, A.K.; et al. Synthesis and anticancer activity of novel spiro-isoxazoline and spiro-isoxazolidine derivatives of α-santonin. Eur. J. Med. Chem. 2013, 63, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, B. Solvent-free synthesis and 1,3-dipolar cycloaddition reactions of N-methyl-C-(2-furyl) nitrone in a ball mill and anticancer activities of the new cycloadducts. J. Heterocycl. Chem. 2020, 57, 477–485. [Google Scholar] [CrossRef]
- Piperno, A.; Giofrè, S.V.; Iannazzo, D.; Romeo, R.; Romeo, G.; Chiacchio, U.; Rescifina, A.; Piotrowska, D.G. Synthesis of C-4ʹTruncated Phosphonated Carbocyclic 2ʹ-Oxa-3ʹ-azanucleosides as Antiviral Agents. J. Org. Chem. 2010, 75, 2798–2805. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Cieślak, M.; Królewska, K.; Wróblewski, A.E. Design, synthesis and cytotoxicity of a new series of isoxazolidines derived from substituted chalcones. Eur. J. Med. Chem. 2011, 46, 1382–1389. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Balzarini, J.; Głowacka, I.E. Design, synthesis, antiviral and cytostatic evaluation of novel isoxazolidine nucleotide analogues with a 1,2,3-triazole linker. Eur. J. Med. Chem. 2012, 47, 501–509. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Andrei, G.; Schols, D.; Snoeck, R.; Łysakowska, M. Synthesis, anti-varicella-zoster virus and anti-cytomegalovirus activity of quinazoline-2,4-diones containing isoxazolidine and phosphonate substructures. Eur. J. Med. Chem. 2017, 126, 84–100. [Google Scholar] [CrossRef]
- Łysakowska, M.; Głowacka, I.E.; Honkisz-Orzechowska, E.; Handzlik, J.; Piotrowska, D.G. New 3-(Dibenzyloxyphosphoryl)isoxazolidine Conjugates of N1-Benzylated Quinazoline-2,4-diones as Potential Cytotoxic Agents against Cancer Cell Lines. Molecules 2024, 29, 3050. [Google Scholar] [CrossRef]
- Chiacchio, U.; Balestrieri, E.; Macchi, B.; Iannazzo, D.; Piperno, A.; Rescifina, A.; Romeo, R.; Saglimbeni, M.; Sciortino, M.T.; Valveri, V.; et al. Synthesis of phosphonated carbocyclic 2′-oxa-3′-aza-nucleosides: Novel inhibitors of reverse transcriptase. J. Med. Chem. 2005, 48, 1389–1394. [Google Scholar] [CrossRef]
- Chiacchio, U.; Rescifina, A.; Iannazzo, D.; Piperno, A.; Romeo, R.; Borrello, L.; Sciortino, M.T.; Balestrieri, E.; Macchi, B.; Mastino, A.; et al. Phosphonated carbocyclic 2′-Oxa-3′-azanucleosides as new antiretroviral agents. J. Med. Chem. 2007, 50, 3747–3750. [Google Scholar] [CrossRef]
- Giofre, S.V.; Romeo, R.; Garozzo, A.; Cicero, N.; Campisi, A.; Lanza, G.; Chiacchio, M.A. 5-(3-Phosphonated 1H-1,2,3-triazol-4-yl)isoxazolidines: Synthesis, DFT studies and biological properties. Arkivoc 2015, 7, 253–269. [Google Scholar] [CrossRef]
- Łysakowska, M.; Głowacka, I.E.; Andrei, G.; Schols, D.; Snoeck, R.; Lisiecki, P.; Szemraj, M.; Piotrowska, D.G. Design, synthesis, anti-varicella-zoster virus and antimicrobial activity of (isoxazolidin-3-yl)phosphonate conjugates of N1-functionalised quinazoline-2,4-diones. Molecules 2022, 27, 6526. [Google Scholar] [CrossRef] [PubMed]
- Romeo, R.; Carnovale, C.; Giofre, S.V.; Romeo, G.; Macchi, B.; Frezza, C.; Marino-Merlo, F.; Pistara, V.; Chiacchio, U. Truncated phosphonated C-1′-branched N,O-nucleosides: A new class of antiviral agents. Bioorg. Med. Chem. 2012, 20, 3652–3657. [Google Scholar] [CrossRef] [PubMed]
- Chiacchio, U.; Iannazzo, D.; Piperno, A.; Romeo, R.; Romeo, G.; Rescifina, A.; Saglimbeni, M. Synthesis and biological evaluation of phosphonated carbocyclic 2-oxa-3′-aza-nucleosides. Bioorg. Med. Chem. 2006, 14, 955–959. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Bujnowicz, A.; Wróblewski, A.E.; Głowacka, I.E. A New Approach to the Synthesis of 4-Phosphonylated β-lactams. Synlett 2015, 26, 375–379. [Google Scholar] [CrossRef]
- Głowacka, I.; Grabkowska-Drużyc, M.; Lebelt, L.; Andrei, G.; Schols, D.; Snoeck, R.; Piotrowska, D. β-lactam analogs of oxetanocins-synthesis and biological activity. Acta Pol. Pharm. 2022, 79, 167–196. [Google Scholar] [CrossRef]
- Glowacka, I.E.; Grabkowska-Drużyc, M.; Andrei, G.; Schols, D.; Snoeck, R.; Witek, K.; Podlewska, S.; Handzlik, J.; Piotrowska, D.G. Novel N-Substituted 3-Aryl-4-(diethoxyphosphoryl)azetidin-2-ones as Antibiotic Enhancers and Antiviral Agents in Search for a Successful Treatment of Complex Infections. Int. J. Mol. Sci. 2021, 22, 8032. [Google Scholar] [CrossRef]
- Głowacka, I.E.; Hartwich, A.; Rozpara, I.; Piotrowska, D.G. Synthesis of Functionalized Diethyl(pyrrolidin-2-yl)phosphonate and Diethyl(5-oxopyrrolidin-2-yl)phosphonate. Molecules 2021, 26, 3160. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Głowacka, I.E. Enantioselective synthesis of phosphonate analogues of (R)- and (S)-homoserine. Tetrahedron Asymmetry 2007, 18, 2787–2790. [Google Scholar] [CrossRef]
- Lebelt, L.; Głowacka, I.E.; Piotrowska, D.G. Synthesis of Four Enantiomers of (1-Amino-3-Hydroxypropane-1,3-Diyl)Diphosphonic Acid as Diphosphonate Analogues of 4-Hydroxyglutamic Acid. Molecules 2022, 27, 2699. [Google Scholar] [CrossRef]
- Piotrowska, D.G. Stereochemistry of cycloaddition of (S)-N-(1-phenylethyl)-C-phosphorylated nitrone with cyclopentene and 2,3-dihydrofuran. Tetrahedron Asymmetry 2008, 19, 2323–2329. [Google Scholar] [CrossRef]
- Lobo, F.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional food: Impact on human healths. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, K.H.; Slater, T.F. An introduction to free-radical biochemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Long, Y.C.; Barbosa, T.D.; Karlsson, H.K.R.; Glund, S.; Zavadoski, W.J.; Gibbs, E.M.; Koistinen, H.A.; Wallberg-Henriksson, H.; Zierath, J.R. Nitric oxide increases cyclic GMP levels, AMP-activated protein kinase (AMPK)α1-specific activity and glucose transport in human skeletal muscle. Diabetologia 2010, 53, 1142–1150. [Google Scholar] [CrossRef]
- Hool, L.C.; Corry, B. Redox control of calcium channels: From mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2007, 9, 409–435. [Google Scholar] [CrossRef]
- Adachi, T.; Weisbrod, R.M.; Pimentel, D.R.; Ying, J.; Sharov, V.S.; Schoneich, C.; Cohen, R.A. S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat. Med. 2004, 10, 1200–1207. [Google Scholar] [CrossRef]
- Babior, B.M. Oxidants from phagocytes: Agents of defense and destruction. Blood 1984, 64, 959–966. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Bahorun, T.; Soobrattee, M.A.; Luximon-Ramma, V.; Aruoma, O.I. Free Radicals and Antioxidants in Cardiovascular Health and Disease. Internet J. Med. Update 2006, 1, 25–41. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzi, G.; Tousoulis, D.; Stefanadis, C. The Role of Oxidative Stress in Atherosclerosis. Hellenic J. Cardiol. 2009, 50, 402–409. [Google Scholar]
- Perrelli, M.G.; Pagliaro, P.; Penna, C. Ischemia/reperfusion injury and cardioprotective mechanisms: Role of mitochondria and reactive oxygen species. World J. Cardiol. 2011, 3, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.G.; Gongora, M.C.; Guzik, T.J.; Widder, J. Oxidative stress and hypertension. J. Am. Soc. Hypertens. 2007, 1, 30–44. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Mitran, S.; Sivanesan, S.; Chang, E.; Buga, A.M. ROS and Brain Diseases: The Good, the Bad, and the Ugly. Oxid. Med. Cell. Longev. 2013, 2013, 1–14. [Google Scholar] [CrossRef]
- Zuo, L.; Motherwell, M.S. The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson’s disease. Gene 2013, 532, 18–23. [Google Scholar] [CrossRef]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef]
- Zeghdaoui, A.; Tuccio, B.; Finet, J.P.; Cerri, V.; Tordo, P. β-Phosphorylated α-phenyl-N-tert-butylnitrone (PBN) analogues: A new series of spin traps for oxyl radicals. J. Chem. Soc. Perkin Trans. 2 1995, 12, 2087–2089. [Google Scholar] [CrossRef]
- Frejaville, C.; Karoui, H.; Tuccio, B.; Lemoigne, F.; Culcasi, M.; Pietri, S.; Lauricella, R.; Tordo, P. 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide: A new efficient phosphorylated nitrone for the in vitro and in vivo spin trapping of oxygen-centered radicals. J. Med. Chem. 1995, 38, 258–265. [Google Scholar] [CrossRef]
- Liu, K.J.; Miyake, M.; Panz, T.; Swartz, H. Evaluation of DEPMPO as a spin trapping agent in biological systems. Free Radic. Biol. Med. 1999, 26, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.M.; Starkereed, P.E.; Oliver, C.N.; Landum, R.W.; Cheng, M.S.; Wu, J.F.; Floyd, R.A. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proc. Natl Acad. Sci. USA 1991, 88, 3633–3636. [Google Scholar] [CrossRef] [PubMed]
- Floyd, R.A. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990, 4, 2587–2597. [Google Scholar] [CrossRef] [PubMed]
- Shuaib, A.; Lees, K.R.; Lyden, P.; Grotta, J.; Davalos, A.; Davis, S.M.; Diener, H.; Ashwood, T.; Wasiewski, W.W.; Emeribe, U.; et al. NXY-059 for the treatment of acute ischemic stroke. N. Engl. J. Med. 2007, 357, 562–571. [Google Scholar] [CrossRef]
- Antonic, A.; Dottori, M.; Macleod, M.R.; Donnan, G.A.; Howells, D.W. NXY-059, a Failed Stroke Neuroprotectant, Offers No Protection to Stem Cell-Derived Human Neurons. J. Stroke Cerebrovasc. Dis. 2018, 27, 2158–2165. [Google Scholar] [CrossRef]
- Floyd, R.A.; Neto, H.; Zimmerman, G.A.; Hensley, K.; Towner, R.A. Nitrone-based therapeutics for neurodegenerative diseases: Their use alone or in combination with lanthionines. Free Radic. Biol. Med. 2013, 62, 145–156. [Google Scholar] [CrossRef]
- Garteiser, P.; Doblas, S.; Watanabe, Y.; Saunders, D.; Hoyle, J.; Lerner, M.; He, T.; Floyd, R.A.; Towner, R.A. Multiparametric Assessment of the Anti-glioma Properties of OKN007 by Magnetic Resonance Imaging. J. Magn. Reson. Imaging 2010, 31, 796–806. [Google Scholar] [CrossRef]
- He, T.; Doblas, S.; Saunders, D.; Casteel, R.; Floyd, R.A.; Towner, R.A. Effects of PBN and OKN007 in rodent glioma models assessed by 1H MR spectroscopy. Free Radic. Biol. Med. 2011, 51, 490–502. [Google Scholar] [CrossRef]
- Towner, R.A.; Smith, N.; Saunders, D.; Browns, C.A.; Cai, X.; Ziegler, J.; Mallory, S.; Dozmorov, M.G.; De Souza, P.C.; Wiley, G.; et al. OKN-007 Increases temozolomide (TMZ) Sensitivity and Suppresses TMZ-Resistant Glioblastoma (GBM) Tumor Growth. Transl. Oncol. 2019, 12, 320–335. [Google Scholar] [CrossRef]
- Towner, R.A.; Hocker, J.; Smith, N.; Saunders, D.; Battiste, J.; Hanas, J. OKN-007 Alters Protein Expression Profiles in High-Grade Gliomas: Mass Spectral Analysis of Blood Sera. Brain Sci. 2022, 12, 100. [Google Scholar] [CrossRef]
- Zalles, M.; Smith, N.; Saunders, D.; Lerner, M.; Fung, K.M.; Battiste, J.; Towner, R.A. A tale of two multi-focal therapies for glioblastoma: An antibody targeting ELTD1 and nitrone-based OKN-007. J. Cell. Mol. Med. 2022, 26, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Cassien, M.; Petrocchi, C.; Thetiot-Laurent, S.; Robin, M.; Ricquebourg, E.; Kandouli, C.; Asteian, A.; Rockenbauer, A.; Mercier, A.; Culcasi, M.; et al. On the vasoprotective mechanisms underlying novel β-phosphorylated nitrones: Focus on free radical characterization, scavenging and NO-donation in a biological model of oxidative stress. Eur. J. Med. Chem. 2016, 119, 197–217. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Mediavilla, L.; Cuarental, L.; Głowacka, I.E.; Marco-Contelles, J.; Hadjipavlou-Litina, D.; Lopez-Munoz, F.; Oset-Gasque, M.J. Synthesis and Neuroprotective Properties of N-Substituted C-Dialkoxyphosphorylated Nitrones. ACS Omega 2019, 4, 8581–8587. [Google Scholar] [CrossRef]
- Floyd, R.A.; Kopke, R.D.; Choi, C.H.; Foster, S.B.; Doblas, S.; Towner, R.A. Nitrones as therapeutics. Free Radic. Biol. Med. 2008, 45, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, S.I.; Imada, Y. Synthesis and Transformations of Nitrones for Organic Synthesis. Chem. Rev. 2019, 119, 4684–4716. [Google Scholar] [CrossRef]
- Piotrowska, D.G. N-substituted C-diethoxyphosphorylated nitrones as useful synthons for the synthesis of α-aminophosphonates. Tetrahedron Lett. 2006, 47, 5363–5366. [Google Scholar] [CrossRef]
- Piotrowska, D.G. Stereochemistry of substituted isoxazolidines derived from N-methyl C-diethoxyphosphorylated nitrone. Tetrahedron 2006, 62, 12306–12317. [Google Scholar] [CrossRef]
- Coppola, G. Amberlyst-15, A Superior Acid catalyst for the clevage of acetals. Synthesis 1984, 1984, 1021–1023. [Google Scholar] [CrossRef]
- Rizzi, C.; Marque, S.; Belin, F.; Bouteiller, J.C.; Lauricella, R.; Tuccio, B.; Cerri, V.; Tordo, P. PPN-type nitrones: Preparation and use of a new series of β-phosphorylated spin-trapping agents. J. Chem. Soc. Perkin Trans. 2 1997, 12, 2513–2518. [Google Scholar] [CrossRef]
- Rizzi, C.; Lauricella, R.; Bouteiller, J.C.; Roubaud, V.; Tuccio, A. Distribution of the methyl radical adduct of N-benzylidene-1-diethoxyphosphoryl-1-methylethylamine N-oxide-type nitrones in water-sodium dodecyl sulfate micelles: An electron paramagnetic resonance spin trapping study. Magn. Res. Chem. 2002, 40, 273–278. [Google Scholar] [CrossRef]
- Deletraz, A.; Zeamari, K.; Di Meo, F.; Fabre, P.L.; Reybier, K.; Trouillas, P.; Tuccio, B.; Durand, G. Reactivities of MeO-substituted PBN-type nitrones. New J. Chem. 2019, 43, 15754–15762. [Google Scholar] [CrossRef]
- Petrocchi, C.; Thétiot-Laurent, S.; Culcasi, M.; Pietri, S. Novel mitochondria-targeted triphenylphosphonium conjugates of linear β-phosphorylated nitrones: Preparation, 31PNMR mitochondrial distribution, EPRSpin trapping reporting, and Site-directed antiapoptotic properties. In Mitochondrial Medicine; Weissig, V., Edeas, M., Eds.; Springer: New York, NY, USA, 2021; Volume 1, pp. 65–85. [Google Scholar]
- Shioji, K.; Takao, M.; Okuma, K. Convenient synthesis of linear spin traps containing diphenylphosphoryl groups. Chem. Lett. 2006, 35, 1332–1333. [Google Scholar] [CrossRef]
- Roubaud, V.; Dozol, H.; Rizzi, C.; Lauricella, R.; Bouteiller, J.C.; Tuccio, B. Poly(β-phosphorylated nitrones): Preparation and characterisation of a new class of spin trap. J. Chem. Soc. Perkin Trans. 2 2002, 5, 958–964. [Google Scholar] [CrossRef]
- Roubaud, V.; Lauricella, R.; Tuccio, B.; Bouteiller, J.C.; Tordo, P. Decay of superoxide spin adducts of new PBN-type phosphorylated nitrones. Res. Chem. Intermed. 1996, 22, 405–416. [Google Scholar] [CrossRef]
- Ji, Y.Q.; Wang, Z.Y.; Wang, L.F.; Liu, K.J.; Liu, Y. Prediction and Evaluation of the Piperonylidene Analogue of PBN by DFT Calculations & NBT Reduction Mediated Spectral Assay. Chin. J. Chem. 2008, 26, 1780–1786. [Google Scholar] [CrossRef]
- Tuccio, B.; Zeghdaoui, A.; Finet, J.P.; Cerri, V.; Tordo, P. Use of new β-phosphorylated nitrones for the spin trapping of free radicals. Res. Chem. Intermed. 1996, 22, 393–404. [Google Scholar] [CrossRef]
- Culcasi, M.; Delehedde, C.; Esgulian, M.; Cassien, M.; Chocry, M.; Rockenbauer, A.; Pietri, S.; Thétiot-Laurent, S. New Biocompatible β-Phosphonylated Linera Nitrones Targeting Mitochondria: Ptorective Effect in Apoptotic Cells. ChemBioChem 2023, 24, e202200749. [Google Scholar] [CrossRef]
- Liu, Y.P.; Song, Y.G.; Du, L.B.; Villamena, F.A.; Ji, Y.Q.; Tian, Q.; Liu, K.J.; Liu, Y. Novel glutathione-linked nitrones as dual free radical probes. New J. Chem. 2011, 35, 1485–1490. [Google Scholar] [CrossRef]
- Ouari, O.; Chalier, F.; Bonaly, R.; Pucci, B.; Tordo, P. Synthesis and spin-trapping behaviour of glycosylated nitrones. J. Chem. Soc. Perkin Trans. 2 1998, 10, 2299–2307. [Google Scholar] [CrossRef]
- Arroyo, C.M. Spin Trapping and Spin Labelling Studied Using EPR Spectroscopy. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 4, pp. 209–217. [Google Scholar] [CrossRef]
- Chalier, F.; Tordo, P. 5-Diisopropoxyphosphoryl-5-methyl-1-pyrroline N-oxide, DIPPMPO, a crystalline analog of the nitrone DEPMPO: Synthesis and spin trapping properties. J. Chem. Soc. Perkin Trans. 2 2002, 12, 2110–2117. [Google Scholar] [CrossRef]
- Clement, J.L.; Frejaville, C.; Tordo, P. DEPMPO and lipophilic analogues: Synthesis and EPR studies. Res. Chem. Intermed. 2002, 28, 175–190. [Google Scholar] [CrossRef]
- Nishizawa, M.; Shioji, K.; Kurauchi, Y.; Okuma, K.; Kohno, M. Spin-trapping properties of 5-(diphenylphosphinoyl)-5-methyl-4,5-dihydro-3H-pyrrole N-oxide (DPPMDPO). Bull. Chem. Soc. Jpn. 2007, 80, 495–497. [Google Scholar] [CrossRef]
- Shioji, K.; Tsukimoto, S.; Tanaka, H.; Okuma, K. Synthesis of 5-(alkylphenylphosphoryl)-5-methyl-3,4-dihydro-2H-pyrroline N-oxide as a new spin trapping reagent. Chem. Lett. 2003, 32, 604–605. [Google Scholar] [CrossRef]
- Shioji, K.; Matsumoto, A.; Takao, M.; Kurauchi, Y.; Shigetomi, T.; Yokomori, Y.; Okuma, K. Cycloadditions of 3,4-dihydro-2H-pyrrole N-oxide with thioketones and a selenoketone. Bull. Chem. Soc. Jpn. 2007, 80, 743–746. [Google Scholar] [CrossRef]
- Shioji, K.; Iwashita, H.; Shimomura, T.; Yamaguchi, T.; Okuma, K. ESR measurement using 2-diphenylphosphinoyl-2-methyl-3,4-dihydro-2H-pyrrole N-oxide (DPhPMPO) in human erythrocyte ghosts. Bull. Chem. Soc. Jpn. 2007, 80, 758–762. [Google Scholar] [CrossRef]
- Stolze, K.; Udilova, N.; Nohl, H. Spin trapping of lipid radicals with DEPMPO-derived spin traps: Detection of superoxide, alkyl and alkoxyl radicals in aqueous and lipid phase. Free Radic. Biol. Med. 2000, 29, 1005–1014. [Google Scholar] [CrossRef]
- Huang, S.P.; Chen, Z.; Du, L.B.; Tian, Q.; Liu, Y.P.; Zheng, Y.S.; Liu, Y. Site-Specific Detection of Free Radicals in Membranes Using an Amphiphilic Spin Trap. Appl. Magn. Reson. 2015, 46, 489–504. [Google Scholar] [CrossRef]
- Karoui, H.; Nsanzumuhire, C.; Le Moigne, F.; Tordo, P. Synthesis of a new spin trap: 2-(diethoxyphosphoryl)-2-phenyl-3,4-dihydro-2H-pyrrole 1-oxide. J. Org. Chem. 1999, 64, 1471–1477. [Google Scholar] [CrossRef]
- Xu, Y.K.; Chen, Z.W.; Sun, J.; Liu, K.; Chen, W.; Shi, W.; Wang, H.M.; Liu, Y. Synthesis, crystal structure, and ESR study of a novel phosphorylated lipophilic spin trap. J. Org. Chem. 2002, 67, 7624–7630. [Google Scholar] [CrossRef]
- Chalier, F.; Clement, J.L.; Hardy, M.; Tordo, P.; Rockenbauer, A. ESR study of the spin adducts of three analogues of DEPMPO substituted at C-4 or C-3. RSC Adv. 2014, 4, 11610–11623. [Google Scholar] [CrossRef]
- Nsanzumuhire, C.; Clement, J.L.; Ouari, O.; Karoui, H.; Finet, J.P.; Tordo, P. Synthesis of the cis diastereoisomer of 5-diethoxyphosphoryl-5-methyl-3-phenyl-1-pyrroline N-oxide (DEPMPPOc) and ESR study of its superoxide spin adduct. Tetrahedron Lett. 2004, 45, 6385–6389. [Google Scholar] [CrossRef]
- Chalier, F.; Hardy, M.; Ouari, O.; Rockenbauer, A.; Tordo, P. Design of new derivatives of nitrone DEPMPO functionalized at C-4 for further specific applications in super xide radical detection. J. Org. Chem. 2007, 72, 7886–7892. [Google Scholar] [CrossRef] [PubMed]
- Hardy, M.; Chalier, F.; Finet, J.P.; Rockenbauer, A.; Tordo, P. Diastereoselective synthesis and ESR study of 4-PhenylDEPMPO spin traps. J. Org. Chem. 2005, 70, 2135–2142. [Google Scholar] [CrossRef] [PubMed]
- Hardy, M.; Poulhes, F.; Rizzato, E.; Rockenbauer, A.; Banaszak, K.; Karoui, H.; Lopez, M.; Zielonka, J.; Vasquez-Vivar, J.; Sethumadhavan, S.; et al. Mitochondria-Targeted Spin Traps: Synthesis, Superoxide Spin Trapping, and Mitochondrial Uptake. Chem. Res. Toxicol. 2014, 27, 1155–1165. [Google Scholar] [CrossRef]
- Barbati, S.; Clement, J.L.; Frejaville, C.; Bouteiller, J.C.; Tordo, P.; Michel, J.C.; Yadan, J.C. P-31-labeled pyrroline N-oxides: Synthesis of 5-diethylphosphono-5-methyl-1-pyrroline N-oxide (DEPMPO) by oxidation of diethyl (2-methylpyrrolidin-2-yl)phosphonate. Synthesis 1999, 1999, 2036–2040. [Google Scholar] [CrossRef]
- Clement, J.L.; Barbati, S.; Frejaville, C.; Rockenbauer, A.; Tordo, P. Synthesis and use as spin-trap of 5-methyl-5-phosphono-1-pyrroline N-oxide (DHPMPO). pH dependence of the EPR parameters of the spin adducts. J. Chem. Soc. Perkin Trans. 2 2001, 2, 1471–1475. [Google Scholar] [CrossRef]
- Roubaud, V.; Mercier, A.; Olive, G.; LeMoigne, F.; Tordo, P. 5-(Diethoxyphosphorylmethyl)-5-methyl-4,5-dihydro-3H-pyrrole N-oxide: Synthesis and evaluation of spin trapping properties. J. Chem. Soc., Perkin Trans. 2 1997, 2, 1827–1830. [Google Scholar] [CrossRef]
- Clement, J.L.; Finet, J.P.; Frejaville, C.; Tordo, P. Deuterated analogues of the free radical trap DEPMPO: Synthesis and, EPR studies. Org. Biomol. Chem. 2003, 1, 1591–1597. [Google Scholar] [CrossRef]
- Ohno, T.; Sakai, M.; Ishino, Y.; Shibata, T.; Maekawa, H.; Nishiguchi, I. Mg-promoted regio- and stereoselective C-acylation of aromatic α,β-unsaturated carbonyl compounds. Org. Lett. 2001, 3, 3439–3442. [Google Scholar] [CrossRef]
- Hardy, M.; Chalier, F.; Ouari, O.; Finet, J.P.; Rockenbauer, A.; Kalyanaraman, B.; Tordo, P. Mito-DEPMPO synthesized from a novel NH2-reactive DEPMPO spin trap: A new and improved trap for the detection of superoxide. Chem. Commun. 2007, 10, 1083–1085. [Google Scholar] [CrossRef]
- Abbas, K.; Hardy, M.; Poulhes, F.; Karoui, H.; Tordo, P.; Ouari, O.; Peyrot, F. Detection of superoxide production in stimulated and unstimulated living cells using new cyclic nitrone spin traps. Free Radic. Biol. Med. 2014, 71, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Beziere, N.; Hardy, M.; Poulhes, F.; Karoui, H.; Tordo, P.; Ouari, O.; Frapart, Y.M.; Rockenbauer, A.; Boucher, J.L.; Mansuy, D.; et al. Metabolic stability of superoxide adducts derived from newly developed cyclic nitrone spin traps. Free Radic. Biol. Med. 2014, 67, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Hardy, M.; Rockenbauer, A.; Vasquez-Vivar, J.; Felix, C.; Lopez, M.; Srinivasan, S.; Avadhani, N.; Tordo, P.; Kalyanaraman, B. Detection, characterization, and decay kinetics of ROS and thiyl adducts of Mito-DEPMPO spin trap. Chem. Res. Toxicol. 2007, 20, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Hardy, M.; Bardelang, D.; Karoui, H.; Rockenbauer, A.; Finet, J.P.; Jicsinszky, L.; Rosas, R.; Ouari, O.; Tordo, P. Improving the Trapping of Superoxide Radical with a β-Cyclodextrin-5-Diethoxyphosphoryl-5-methyl-1-pyrroline-N-oxide (DEPMPO) Conjugate. Chem. Eur. J. 2009, 15, 11114–11118. [Google Scholar] [CrossRef]
- Besson, E.; Gastaldi, S.; Bloch, E.; Zielonka, J.; Zielonka, M.; Kalyanaraman, B.; Aslan, S.; Karoui, H.; Rockenbauer, A.; Ouari, O.; et al. Embedding cyclic nitrone in mesoporous silica particles for EPR spin trapping of superoxide and other radicals. Analyst 2019, 144, 4194–4203. [Google Scholar] [CrossRef]
- Gosset, G.; Clement, J.L.; Culcasi, M.; Rockenbauer, A.; Pietri, S. CyDEPMPOs: A class of stable cyclic DEPMPO derivatives with improved properties as mechanistic markers of stereoselective hydroxyl radical adduct formation in biological systems. Bioorg. Med. Chem. 2011, 19, 2218–2230. [Google Scholar] [CrossRef]
- Kamibayashi, M.; Oowada, S.; Kameda, H.; Okada, T.; Inanami, O.; Ohta, S.; Ozawa, T.; Makino, K.; Kotake, Y. Synthesis and characterization of a practically better DEPMPO-type spin trap, 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO). Free Radic. Res. 2006, 40, 1166–1172. [Google Scholar] [CrossRef]
- Sueishi, Y.; Kamogawa, E.; Nakamura, H.; Ukai, M.; Kunieda, M.; Okada, T.; Shimmei, M.; Kotake, Y. Kinetic Evaluation of Spin Trapping Rate Constants of New CYPMPO-type Spin Traps for Superoxide and Other Free Radicals. Z. Phys. Chem. 2015, 229, 317–326. [Google Scholar] [CrossRef]
- Janzen, E.G.; Zhang, Y.K. Identification of reactive free radicals with a new 31P-labeled DMPO spin trap. J. Org. Chem. 1995, 60, 5441–5445. [Google Scholar] [CrossRef]
- Olive, G.; Le Moigne, F.; Mercier, A.; Rockenbauer, A.; Tordo, P. Synthesis of tetraalkyl(pyrrolidine-2,2-diyl)bisphosphonates and 2,2-bis(diethoxyphosphoryl)-3,4-dihydro-2H-pyrrole 1-oxide; ESR study of derived nitroxides. J. Org. Chem. 1998, 63, 9095–9099. [Google Scholar] [CrossRef]
- Sandstrom, M.E.; Zhang, S.J.; Bruton, J.; Silva, J.P.; Reid, M.B.; Westerblad, H.; Katz, A. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J. Physiol. 2006, 575, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R.; Jurkiewicz, B.A. Ascorbate free-radical as a marker of oxidative stress-an EPR study. Free Radic. Biol. Med. 1993, 14, 49–55. [Google Scholar] [CrossRef]
- Yu, J.H.; Chen, J.R.; Li, C.; Wang, X.S.; Zhang, B.W.; Ding, H.Y. ESR signal of superoxide radical anion adsorbed on TiO2 generated at room temperature. J. Phys. Chem. B 2004, 108, 2781–2783. [Google Scholar] [CrossRef]
- Bosnjakovic, A.; Schlick, S. Naflon perfluorinated membranes treated in Fenton media: Radical species detected by ESR spectroscopy. J. Phys. Chem. B 2004, 108, 4332–4337. [Google Scholar] [CrossRef]
- Villamena, F.A. EPR Spin Trapping. In Reactive Species Detection in Biology: From Fluorescence to Electron Paramagnetic Resonance Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 163–202. [Google Scholar] [CrossRef]
- Harbour, J.R.; Chow, V.; Bolton, J.R. Electron spin resonance study of spin adducts of OH and H2O radicals with nitrones in ultraviolet photolysis of aqueous hydrogen-peroxide solutions. Can. J. Chem. 1974, 52, 3549–3553. [Google Scholar] [CrossRef]
- Buettner, G.R.; Oberley, L.W. Considerations in spin trapping of superoxide and hydroxyl radical in aqueous systems using 5,5-dimethyl-1-pyrroline-1-oxide. Biochem. Biophys. Res. Commun. 1978, 83, 69–74. [Google Scholar] [CrossRef]
- Kotake, Y.; Janzen, E.G. Decay and fate of the hydroxyl radical adduct of alpha-phenyl-n-tert-butylnitrone in aqueous media. J. Am. Chem. Soc. 1991, 113, 9503–9506. [Google Scholar] [CrossRef]
- Yamazaki, I.; Piette, L.H. ESR spin trapping studies on the reaction of Fe2+ ions with H2O2 reactive species in oxygen toxicity in biology. J. Biol. Chem. 1990, 265, 13589–13594. [Google Scholar] [CrossRef]
- Chalier, F.; Ouari, O.; Tordo, P. ESR study of spin-trapping with two glycosylated analogues of PBN able to target cell membrane lectins. Org. Biomol. Chem. 2004, 2, 927–934. [Google Scholar] [CrossRef]
- Wilchek, M.; Bayer, E.A. The Avidin-biotin complex in bioanalytical applications. Anal. Biochem. 1988, 171, 1–32. [Google Scholar] [CrossRef]
- Vergely, C.; Renard, C.; Moreau, D.; Perrin-Sarrado, C.; Roubaud, V.; Tuccio, B.; Rochette, L. Effect of two new PBN-derived phosphorylated nitrones against postischaemic ventricular dysrhythmias. Fundam. Clin. Pharmacol. 2003, 17, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Willis, C.L.; Ray, D.E. Antioxidants attenuate MK-801-induced cortical neurotoxicity in the rat. Neurotoxicology 2007, 28, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Milatovic, D.; Radic, Z.; Zivin, M.; Dettbarn, W.D. Atypical effect of some spin trapping agents: Reversible inhibition of acetylcholinesterase. Free Radic. Biol. Med. 2000, 28, 597–603. [Google Scholar] [CrossRef] [PubMed]
- La Fontaine, M.A.; Geddes, J.W.; Banks, A.; Butterfield, D.A. Effect of exogenous and endogenous antioxidants on 3-nitropropionic acid-induced in vivo oxidative stress and striatal lesions: Insights into Huntington’s disease. J. Neurochem. 2000, 75, 1709–1715. [Google Scholar] [CrossRef]
- Pietri, S.; Liebgott, T.; Frejaville, C.; Tordo, P.; Culcasi, M. Nitrone spin traps and their pyrrolidine analogs in myocardial reperfusion injury: Hemodynamic and ESR implications—Evidence for a cardioprotective phosphonate effect for 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide in rat hearts. Eur. J. Biochem. 1998, 254, 256–265. [Google Scholar] [CrossRef]
- Maurelli, E.; Culcasi, M.; Delmas-Beauvieux, M.C.; Miollan, M.; Gallis, J.L.; Tron, T.; Pietri, S. New perspectives on the cardioprotective phosphonate effect of the spin trap 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide: An hemodynamic and P-31 NMR study in rat hearts. Free Radic. Biol. Med. 1999, 27, 34–41. [Google Scholar] [CrossRef]
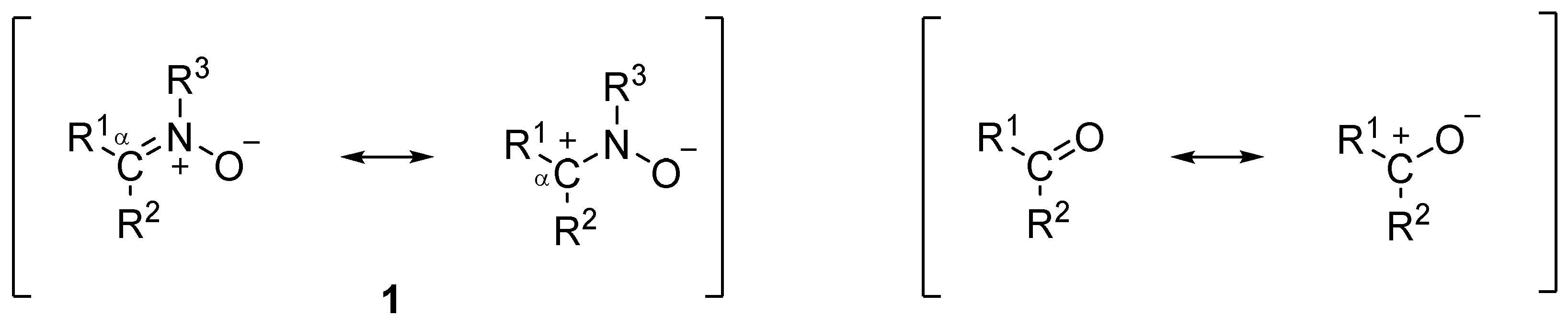
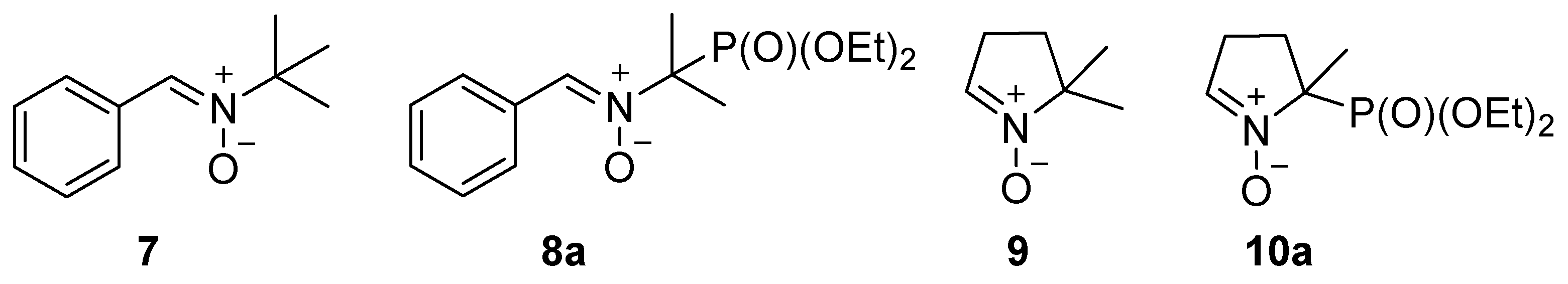

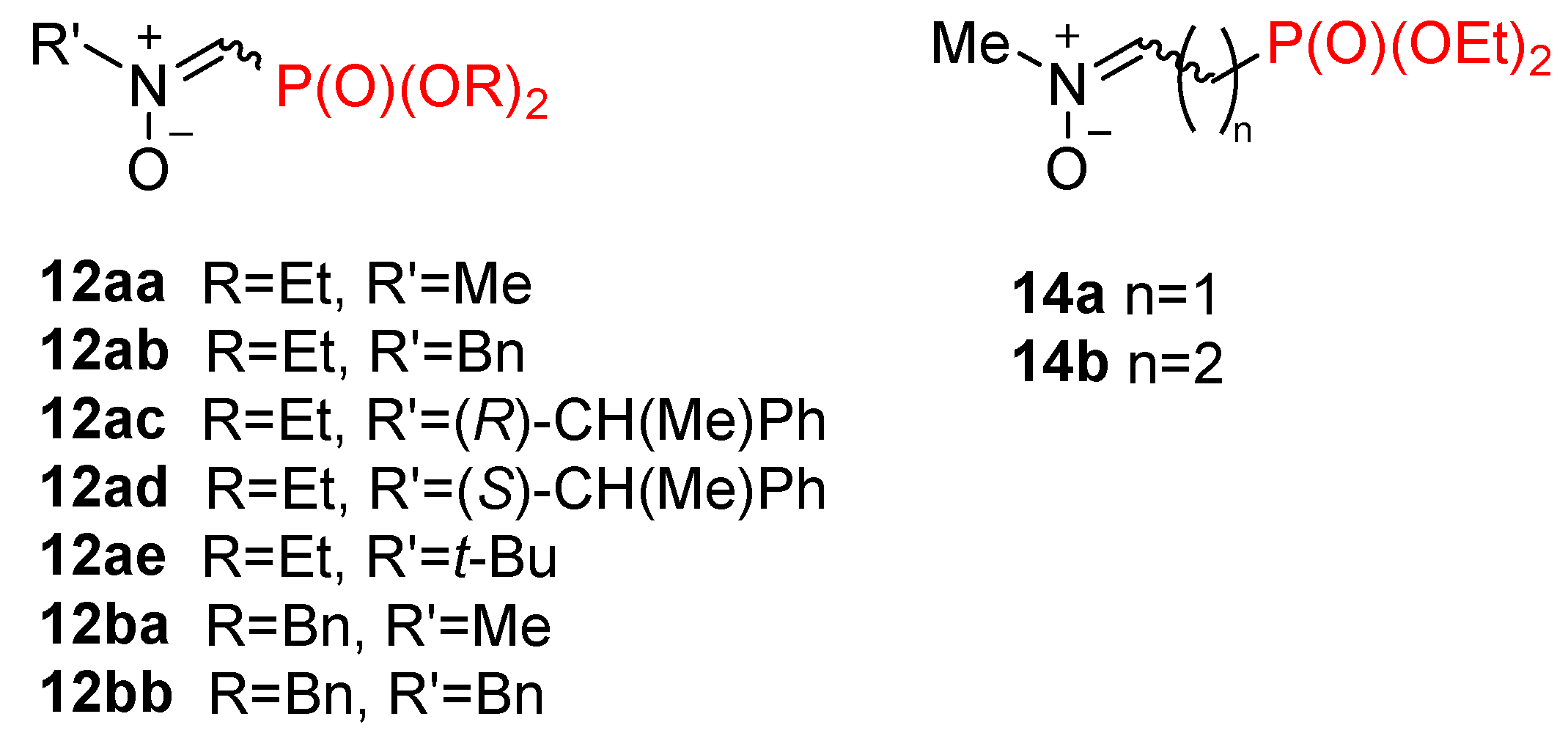

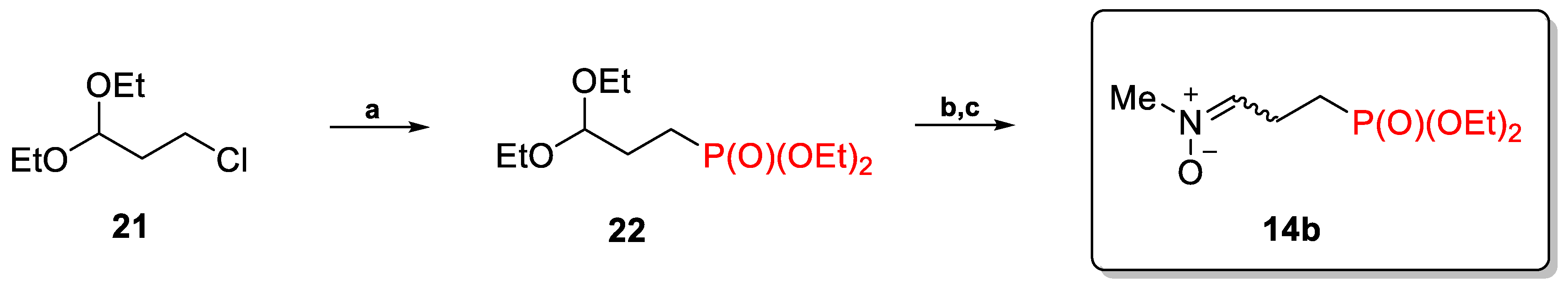











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozpara, I.; Marco-Contelles, J.; Piotrowska, D.G.; Głowacka, I.E. Phosphorylated Nitrones—Synthesis and Applications. Molecules 2025, 30, 1333. https://doi.org/10.3390/molecules30061333
Rozpara I, Marco-Contelles J, Piotrowska DG, Głowacka IE. Phosphorylated Nitrones—Synthesis and Applications. Molecules. 2025; 30(6):1333. https://doi.org/10.3390/molecules30061333
Chicago/Turabian StyleRozpara, Iwona, José Marco-Contelles, Dorota G. Piotrowska, and Iwona E. Głowacka. 2025. "Phosphorylated Nitrones—Synthesis and Applications" Molecules 30, no. 6: 1333. https://doi.org/10.3390/molecules30061333
APA StyleRozpara, I., Marco-Contelles, J., Piotrowska, D. G., & Głowacka, I. E. (2025). Phosphorylated Nitrones—Synthesis and Applications. Molecules, 30(6), 1333. https://doi.org/10.3390/molecules30061333






