Eucommiae cortex Comprehensive Phytochemical Analysis Connected with Its In Vitro Anti-Inflammatory Activity in Human Immune Cells
Abstract
1. Introduction
2. Results
2.1. HPLC-DAD-MS/MS Analysis
| Compound | UV (nm) | Rt (min) | [M-H]− [M + COOH]− | Product Mass Peaks | Group | Infusion | Tincture | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Aucubin | 245 | 3.2 | 391.20 * | 345.11 182.82 | Irydoids | + | − | [12,15] |
| 2. | Geniposidic acid | 240 | 7.7 | 373.21 | 210.76 166.72 122.79 | Irydoids | + | + | [12,15] |
| 3. | Unknown | 195 | 10.7 | 447.31 | 409.10 378.81 314.94 160.91 | − | + | + | − |
| 4. | 3-O-caffeoylquinic acid | 322 | 11.1 | 353.47 | 190.77 | Caffeic acid derivatives | + | − | [12,15] |
| 5. | Caffeoyl-O-(glucosyl) quinic acid | 320 | 12.6 | 515.29 | 353.02 190.73 | Caffeic acid derivatives | + | − | [12,14] |
| 6. | Unknown | − | 13.7 | 551.29 | 541.38 487.18 380.18 267.80 | − | + | + | − |
| 7. | Unknown | − | 15.3 | 599.27 * | 553.20 391.06 373.13 361.06 | − | + | + | − |
| 8. | Unknown | − | 16.1 | 599.27 * | 553.20 391.06 373.13 361.06 | − | + | + | − |
| 9. | Olivil-di-O-glucoside | 275 | 16.8 | 745.63 * | 699.22 537.17 375.10 | Lignans | + | + | [12,14] |
| 10. | 5-O-caffeoylquinic acid | 325 | 17.9 | 353.16 | 190.93 | Caffeic acid derivatives | + | + | [12,14] |
| 11. | 4-O-caffeoylquinic acid | 325 | 18.4 | 353.41 | 190.70 178.50 172.75 | Caffeic acid derivatives | + | + | [12,14] |
| 12. | Geniposide | 225 | 20.0 | 433.61 | 387.07 224.82 122.84 | Irydoids | + | − | [12,15] |
| 13. | Unknown | 200 | 20.7 | 461.40 * | 415.11 265.28 | − | + | + | − |
| 14. | Olivil-O-glucoside (I) | 270 | 21.0 | 583.30 * | 537.19 375.09 357.06 | Lignans | + | + | [12] |
| 15. | Coniferin | − | 21.9 | 387.52 * | 340.91 284.94 206.78 178.72 | Phenolics | + | + | [16,17] |
| 16. | Hydroxypinoresinol di-O-glucoside | 270 | 22.3 | 743.29 * | 697.29 535.14 373.19 | Lignans | + | + | [12,14] |
| 17. | Olivil-O-glucoside (II) | 270 | 22.8 | 583.29 * | 537.16 375.08 194.77 | Lignans | + | + | [12,14] |
| 18. | Genipin | 200 | 24.1 | 225.10 | 207.08 122.79 | Irydoids | + | + | [12,14] |
| 19. | Pinoresinol di-O-glucoside (I) | 276 | 25.5 | 727.21 * | 681.22 519.19 357.20 | Lignans | + | + | [12,18] |
| 20. | Pinoresinol di-O-glucoside (II) | 276 | 26.4 | 727.37 * | 681.30 519.09 357.13 341.08 | Lignans | + | + | [12,18] |
| 21. | Citrusin B | 275 | 27.0 | 613.30 * | 567.30 405.12 357.11 208.85 | Lignans | + | + | [19] |
| 22. | Syringaresinol-di-O- glucoside | 275 | 27.7 | 787.75 * | 741.33 579.30 417.14 | Lignans | − | + | [12,14] |
| 23. | Hydroxypinoresinol-O-glucoside | 275 | 28.4 | 581.30 * | 535.14 373.07 343.09 313.31 | Lignans | + | + | [12] |
| 24. | Olivil | 272 | 29.5 | 375.64 | 357.04 327.08 194.72 | Lignans | + | + | [14,15] |
| 25. | Unknown | 273 | 30.4 | 565.36 | 339.06 327.07 | − | + | + | |
| 26. | Eucomoside B | 203 | 31.1 | 520.29 | 355.03 233.83 190.75 | Irydoids | + | − | [12,14] |
| 27. | Pinoresinol-O-glucoside (I) | 275 | 33.4 | 519.38 | 357.34 | Lignans | + | + | [12,15] |
| 28. | Pinoresinol-O-glucoside (II) | 275 | 33.9 | 565.29 * | 519.14 | Lignans | + | + | [12,15] |
| 29. | Syringaresinol-O-glucoside | 275 | 34.3 | 579.33 | 417.11 | Lignans | + | + | [12,14] |
| 30. | Unknown | 203 | 34.7 | 419.39 * | 373.06 | − | − | + | − |
| 31. | Unknown | 343 | 35.5 | 563.25 | 503.08 337.06 | − | + | + | − |
| 32. | Dicaffeoylquinic acid | 312 | 37.7 | 515.34 | 353.01 | Caffeic acid derivatives | + | − | [14,15] |
| 33. | Pinoresinol vanillic acid ether diglucoside | 275 | 38.6 | 831.37 | 669.23 519.39 343.57 311.01 | Lignans | + | + | [12,14] |
| 34. | Unknown | 270 | 44.3 | 583.37 | 535.16 369.08 357.28 | − | + | + | − |
| 35. | Unknown | 345 | 47.0 | 337.46 | 321.98 | − | + | + | − |
| 36. | Pinoresinol vanillic acid ether glucoside | − | 47.4 | 699.24 | 357.13 343.06 310.97 | Lignans | + | + | [11,12,14] |
| 37. | Medioresinol-O-guaiacylglycerol ether | − | 49.2 | 583.35 | 535.20 505.17 387.10 357.15 | Lignans | + | + | [11,12,14] |
| 38. | Unknown | 320 | 49.7 | 581.34 | 533.16 367.08 355.13 | − | + | + | − |
2.2. Content of Leading Compounds in the Tested Extracts
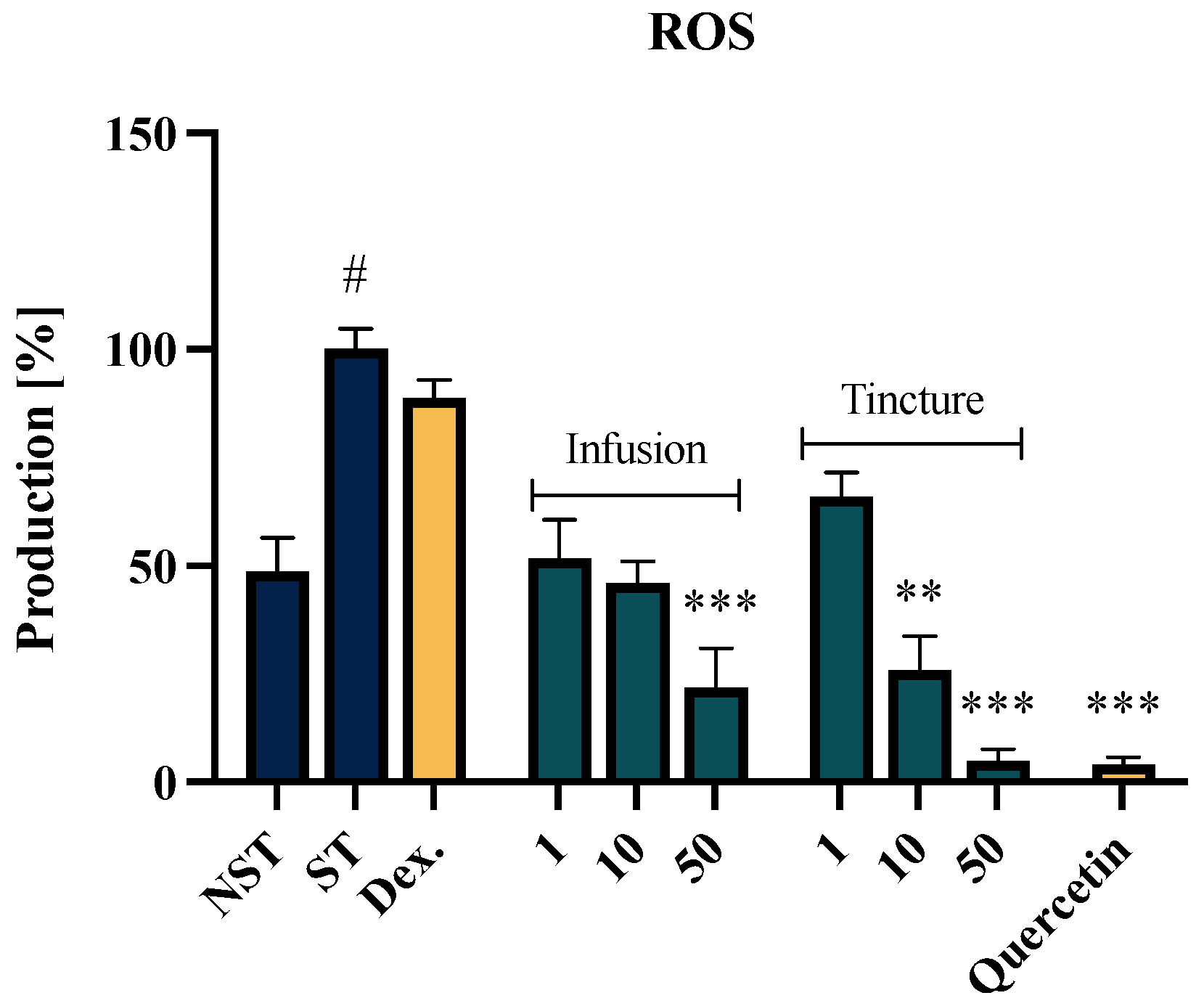
2.3. Effect of EC Extracts on the ROS Release of f-MLP-Stimulated Neutrophils
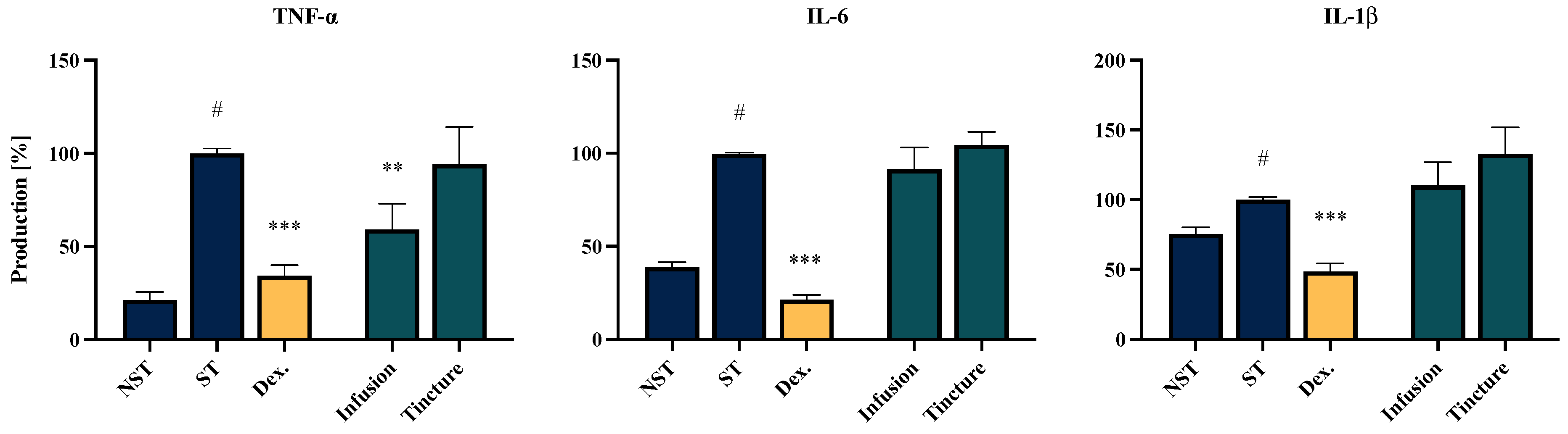
2.4. Effects of EC Extracts and Leading Compounds on the Proinflammatory Functions of LPS-Stimulated Neutrophils
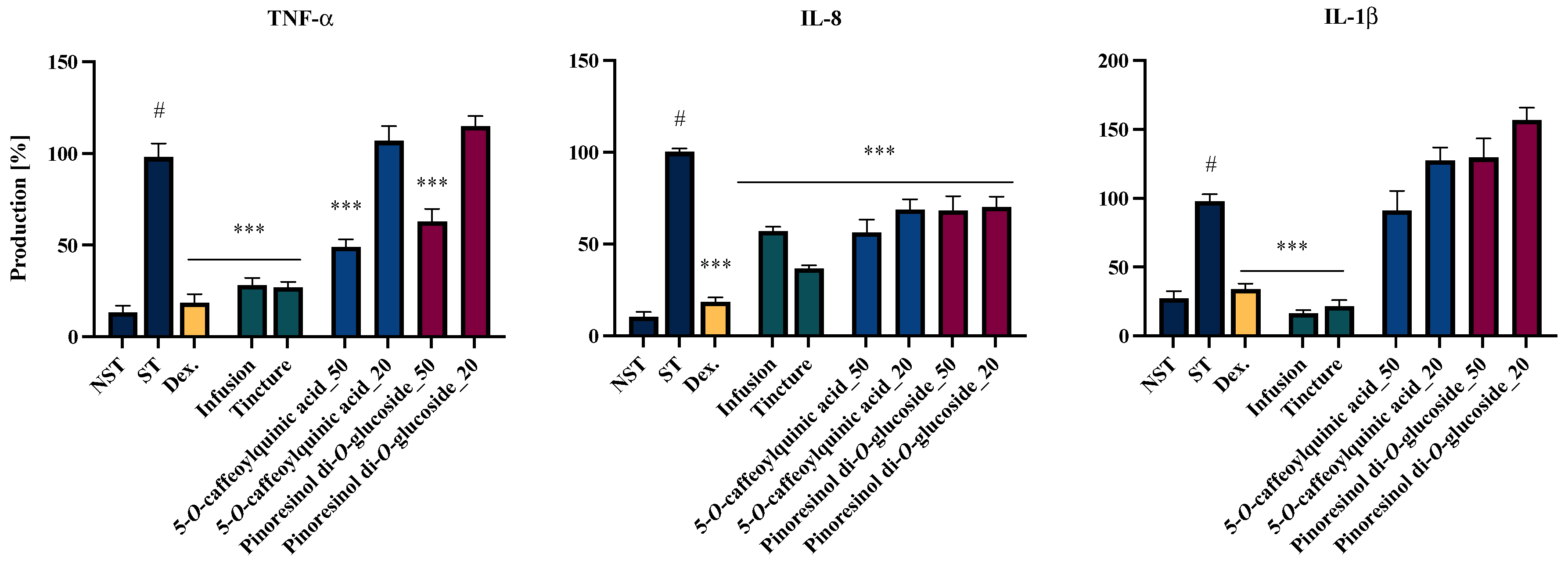
2.5. Effect of EC Extracts and Compounds on the Proinflammatory Function of LPS-Stimulated PMBCs Monocytes/Macrophages
2.6. Effect of EC Extracts and Compounds on the Proinflammatory Function of LPS-LPS-Stimulated THP-1-Derived Macrophages
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents Used in the Study
4.2. Plant Material
4.3. Extracts Preparation
4.4. UHPLC-DAD-ESI-MS/MS Analysis
4.5. Extract Fractionation and Isolation of Active Compounds
4.6. Determination of the Content of Predominant Compounds in the Analyzed Extracts
4.7. Preparation of Samples for Bioassay
4.8. Isolation of Human Neutrophiles and PBMCs Monocytes/Macrophages
4.9. Human Monocytic Cell Line (THP-1) Cell Culture
4.10. Cytotoxicity and Metabolic Activity Determination
4.11. ROS Secretion by Human Neutrophils
4.12. TNF-α, IL-6, IL8, MCP-1 and IL-1β Secretion
4.13. Statistics and Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| fMLP | N Formylmethionyl-leucyl-phenylalanine |
| LDH | Lactate dehydrogenase |
| LPS | Lipopolysaccharide |
| ROS | Reactive oxygen species |
| TCM | Traditional Chinese Medicine |
References
- Royal Botanic Gardens, K. Plants of the World Online. Eucommiaceae. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:60451288-2 (accessed on 2 January 2025).
- Wang, X.-S.; Peng, M.-J.; He, C.-T. The antihypertensive effects of Eucommia ulmoides leaf water/ethanol extracts are chlorogenic acid dependent. J. Funct. Foods 2022, 94, 105129. [Google Scholar] [CrossRef]
- Ding, S.; Xu, S.; Ma, Y.; Liu, G.; Jang, H.; Fang, J. Modulatory Mechanisms of the NLRP3 Inflammasomes in Diabetes. Biomolecules 2019, 9, 850. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Yamaguchi, Y.; Ueda, T.; Kajimoto, O.; Nakazawa, Y.; Nakagawa, S.; Kajimoto, Y. Hypotensive effects of beverages containing Eucommia leaf glycosides on high normotensive and mild hypertensive subjects. In International Symposium on Eucommia ulmoides; Japanese Society of Eucommia: Osaka City, Japan, 2007; pp. 47–54. [Google Scholar]
- Do, M.H.; Hur, J.; Choi, J.; Kim, M.; Kim, M.J.; Kim, Y.; Ha, S.K. Eucommia ulmoides Ameliorates Glucotoxicity by Suppressing Advanced Glycation End-Products in Diabetic Mice Kidney. Nutrients 2018, 10, 265. [Google Scholar] [CrossRef]
- Fujikawa, T.; Hirata, T.; Wada, A.; Kawamura, N.; Yamaguchi, Y.; Fujimura, K.; Ueda, T.; Yurugi, Y.; Soya, H.; Nishibe, S. Chronic administration of Eucommia leaf stimulates metabolic function of rats across several organs. Br. J. Nutr. 2010, 104, 1868–1877. [Google Scholar] [CrossRef]
- Fang, C.; Chen, L.; He, M.; Luo, Y.; Zhou, M.; Zhang, N.; Yuan, J.; Wang, H.; Xie, Y. Molecular mechanistic insight into the anti-hyperuricemic effect of Eucommia ulmoides in mice and rats. Pharm. Biol. 2019, 57, 112–119. [Google Scholar] [CrossRef]
- Hosoo, S.; Koyama, M.; Watanabe, A.; Ishida, R.; Hirata, T.; Yamaguchi, Y.; Yamasaki, H.; Wada, K.; Higashi, Y.; Nakamura, K. Preventive effect of Eucommia leaf extract on aortic media hypertrophy in Wistar-Kyoto rats fed a high-fat diet. Hypertens. Res. 2017, 40, 546–551. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Cho, J.H.; Nam, D.; Kim, E.J.; Ha, I.H. Efficacy and safety of Cortex Eucommiae (Eucommia ulmoides Oliver) extract in subjects with mild osteoarthritis: Study protocol for a 12-week, multicenter, randomized, double-blind, placebo-controlled trial. Medicine 2019, 98, e18318. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, X.; Zong, W.; Wang, X.; Chen, L.; Zhou, L.; Li, C.; Huang, Q.; Huang, X.; Zeng, G.; et al. Aucubin Alleviates Seizures Activity in Li-Pilocarpine-Induced Epileptic Mice: Involvement of Inhibition of Neuroinflammation and Regulation of Neurotransmission. Neurochem. Res. 2019, 44, 472–484. [Google Scholar] [CrossRef]
- Jia, J.; Liu, M.; Wen, Q.; He, M.; Ouyang, H.; Chen, L.; Li, J.; Feng, Y.; Zhong, G.; Yang, S. Screening of anti-complement active ingredients from Eucommia ulmoides Oliv. branches and their metabolism in vivo based on UHPLC-Q-TOF/MS/MS. J. Chromatogr. B 2019, 1124, 26–36. [Google Scholar] [CrossRef]
- Guo, J.; Li, J.; Yang, X.; Wang, H.; He, J.; Liu, E.; Gao, X.; Chang, Y.-X. A Metabolomics Coupled With Chemometrics Strategy to Filter Combinatorial Discriminatory Quality Markers of Crude and Salt-Fired Eucommiae cortex. Front. Pharmacol. 2020, 11, 838. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Jia, J.; Li, J.; Wu, B.; Huang, W.; Liu, M.; Li, Y.; Yang, S.; Ouyang, H.; Feng, Y. Application of characteristic ion filtering with ultra-high performance liquid chromatography quadrupole time of flight tandem mass spectrometry for rapid detection and identification of chemical profiling in Eucommia ulmoides Oliv. J. Chromatogr. A 2018, 1554, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Zhai, P.; Chen, X.; Sun, W.; Wang, P.; Guo, Y.; Wang, Z.; Li, N.; Zhai, W.; Zheng, B. Comparison of Chemical comstituents and content analysis in different parts of Eucommia ulmoides based on UPLC-Q-Orbitrap-MS and RP-HPLC. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Gewali, M.B.; HATTORI, M.; NAMBA, T. Constituents of the Stems of Eucommia ulmoides OLIV. J. Nat. Med. 1988, 42, 247–248. [Google Scholar]
- Cheng, J.; Bai, Y.-j.; Zhao, Y.-y.; Wang, B.; Cheng, T.-m. Studies on the Phenylpropanoids from Eucommia ulmoides. China J. Chin. Mater. Medica 2002, 27, 38–40. [Google Scholar]
- Feng, S.; Ni, S.; Sun, W. Preparative isolation and purification of the lignan pinoresinol diglucoside and liriodendrin from the bark of Eucommia ulmoides Oliv. by high speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 135–145. [Google Scholar] [CrossRef]
- Deyama, T.; Ikawa, T.; Kitagawa, S.; Nishibe, S. The constituents of Eucommia ulmoides Oliv. VI. Isolation of a new sesquilignan and neolignan glycosides. Chem. Pharm. Bull. 1987, 35, 1803–1807. [Google Scholar] [CrossRef]
- Pu, Y.; Cai, Y.; Zhang, Q.; Hou, T.; Zhang, T.; Zhang, T.; Wang, B. Comparison of Pinoresinol and its Diglucoside on their ADME Properties and Vasorelaxant Effects on Phenylephrine-Induced Model. Front. Pharmacol. 2021, 12, 695530. [Google Scholar] [CrossRef]
- Huang, L.; Lyu, Q.; Zheng, W.; Yang, Q.; Cao, G. Traditional application and modern pharmacological research of Eucommia ulmoides Oliv. Chin. Med. 2021, 16, 73. [Google Scholar] [CrossRef]
- Peng, M.; Zhou, Y.; Liu, B. Biological properties and potential application of extracts and compounds from different medicinal parts (bark, leaf, staminate flower, and seed) of Eucommia ulmoides: A review. Heliyon 2024, 10, e27870. [Google Scholar] [CrossRef]
- Bao, L.; Sun, Y.; Wang, J.; Li, W.; Liu, J.; Li, T.; Liu, Z. A review of “plant gold” Eucommia ulmoides Oliv.: A medicinal and food homologous plant with economic value and prospect. Heliyon 2024, 10, e24851. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; Chen, L.W.; Heude, B.; Bernard, J.Y.; Harvey, N.C.; Duijts, L.; Mensink-Bout, S.M.; Polanska, K.; Mancano, G.; Suderman, M.; et al. Dietary Inflammatory Index and Non-Communicable Disease Risk: A Narrative Review. Nutrients 2019, 11, 1873. [Google Scholar] [CrossRef] [PubMed]
- Padrón-Monedero, A. A pathological convergence theory for non-communicable diseases. Aging Med. 2023, 6, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Kalinkovich, A. Targeting chronic inflammation as a potential adjuvant therapy for osteoporosis. Life Sci. 2022, 306, 120847. [Google Scholar] [CrossRef]
- Kim, M.J.; Kang, J.Y.; Kim, J.M.; Moon, J.H.; Lee, H.L.; Jeong, H.R.; Go, M.J.; Lee, U.; Heo, H.J. Effect of Ethyl Acetate Fraction from Eucommia ulmoides Leaves on PM(2.5)-Induced Inflammation and Cognitive Dysfunction. Oxid. Med. Cell Longev. 2022, 2022, 7157444. [Google Scholar] [CrossRef]
- Zhang, F.-L.; Hao, Q.; Zhang, Q.-s.; Lv, H.-Y.; Yang, Y.-L.; Chao, R.; Zhang, Z.; Zhou, Z.-G. Influences of dietary Eucommia ulmoides leaf extract on the hepatic lipid metabolism, inflammation response, intestinal antioxidant capacity, intestinal microbiota, and disease resistance of the channel catfish (Ictalurus punctatus). Fish Shellfish. Immunol. 2022, 123, 75–84. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, J.M.; Lee, H.L.; Go, M.J.; Lee, D.Y.; Kim, C.W.; Kim, H.J.; Heo, H.J. Eucommia ulmoides Leaves Alleviate Cognitive Dysfunction in Dextran Sulfate Sodium (DSS)-Induced Colitis Mice through Regulating JNK/TLR4 Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 4063. [Google Scholar] [CrossRef]
- Duan, Y.; Guo, F.; Li, C.; Xiang, D.; Gong, M.; Yi, H.; Chen, L.; Yan, L.; Zhang, D.; Dai, L.; et al. Aqueous extract of fermented Eucommia ulmoides leaves alleviates hyperlipidemia by maintaining gut homeostasis and modulating metabolism in high-fat diet fed rats. Phytomedicine 2024, 128, 155291. [Google Scholar] [CrossRef]
- Kaneko, M.; Iizuka, T.; Nakajima, T. Inhibition Effect of Eucommia ulmoides Leaf Extract on Interleukin 8 Production by A549 Cells. Biol. Pharm. Bull. 2021, 44, 1891–1893. [Google Scholar] [CrossRef]
- Li, R.; Huang, T.; Nie, L.; Jia, A.; Zhang, L.; Yuan, Y.; Hong, Y.; Wang, J.; Hu, X. Chemical Constituents from Staminate Flowers of Eucommia ulmoides Oliver and Their Anti-Inflammation Activity in Vitro. Chem. Biodivers. 2021, 18, e2100331. [Google Scholar] [CrossRef]
- Wang, J.Y.; Chen, X.J.; Zhang, L.; Pan, Y.Y.; Gu, Z.X.; Yuan, Y. Anti-inflammatory effects of Eucommia ulmoides Oliv. male flower extract on lipopolysaccharide-induced inflammation. Chin. Med. J. 2019, 132, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.Y.; Wang, H.; Chen, X.Y.; Zhang, L.; Yuan, Y. An alcohol extract prepared from the male flower of Eucommia ulmoides Oliv. promotes synoviocyte apoptosis and ameliorates bone destruction in rheumatoid arthritis. Chin. Med. 2021, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Chen, L.; Chen, C.; Zeng, J.; Xu, J.; He, X.; Wang, Y. Anti-inflammatory terpenoids from the seeds of Eucommia ulmoides. Phytochem. Lett. 2024, 61, 44–51. [Google Scholar] [CrossRef]
- Prince, L.R.; Allen, L.; Jones, E.C.; Hellewell, P.G.; Dower, S.K.; Whyte, M.K.; Sabroe, I. The role of interleukin-1beta in direct and toll-like receptor 4-mediated neutrophil activation and survival. Am. J. Pathol. 2004, 165, 1819–1826. [Google Scholar] [CrossRef]
- Bernhard, S.; Hug, S.; Stratmann, A.E.P.; Erber, M.; Vidoni, L.; Knapp, C.L.; Thomaß, B.D.; Fauler, M.; Nilsson, B.; Nilsson Ekdahl, K.; et al. Interleukin 8 Elicits Rapid Physiological Changes in Neutrophils That Are Altered by Inflammatory Conditions. J. Innate Immun. 2021, 13, 225–241. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor. Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Q. Uncoupled pyroptosis and IL-1β secretion downstream of inflammasome signaling. Front. Immunol. 2023, 14, 1128358. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Kim, M.-C.; Kim, D.-S.; Kim, S.-J.; Park, J.; Kim, H.-L.; Kim, S.-Y.; Ahn, K.S.; Jang, H.-J.; Lee, S.-G.; Lee, K.-M.; et al. Eucommiae cortex Inhibits TNF-α and IL-6 Through the Suppression of Caspase-1 in Lipopolysaccharide-Stimulated Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2012, 40, 135–149. [Google Scholar] [CrossRef]
- Koh, W.; Shin, J.-S.; Lee, J.; Lee, I.-H.; Lee, S.K.; Ha, I.-H.; Chung, H.-J. Anti-inflammatory effect of Cortex Eucommiae via modulation of the toll-like receptor 4 pathway in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2017, 209, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Tang, M.; Li, Y.; Liu, F.; Li, X.; Dai, R. Antioxidant Properties of Du-zhong (Eucommia ulmoides Oliv.) Extracts and Their Effects on Color Stability and Lipid Oxidation of Raw Pork Patties. J. Agric. Food Chem. 2010, 58, 7289–7296. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yu, D.; Zhang, Y.; Dong, J.; Li, D.; Wang, D. Metabolite Profiles, Bioactivity, and HPLC Fingerprint of Different Varieties of Eucommia ulmoides Oliv.: Towards the Utilization of Medicinal and Commercial Chinese Endemic Tree. Molecules 2018, 23, 1898. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Tian, C.; Xu, J.; Sun, Y. Studies on the antioxidant activity and phenolic compounds of enzyme-assisted water extracts from Du-zhong (Eucommia ulmoides Oliv.) leaves. J. Enzym. Inhib. Med. Chem. 2009, 24, 1280–1287. [Google Scholar] [CrossRef]
- Commission, C.P. Pharmacopoeia of People’s Republic of China; China Medical Science Press: Beijing, China, 2020; pp. 38–363. [Google Scholar]
- Osmakov, D.I.; Kalinovskii, A.P.; Belozerova, O.A.; Andreev, Y.A.; Kozlov, S.A. Lignans as Pharmacological Agents in Disorders Related to Oxidative Stress and Inflammation: Chemical Synthesis Approaches and Biological Activities. Int. J. Mol. Sci. 2022, 23, 6031. [Google Scholar] [CrossRef]
- Sun, J.; Song, X.; Wang, C.; Ruan, Q. Geniposidic acid alleviates osteoarthritis progression through inhibiting inflammation and chondrocytes ferroptosis. J. Cell Mol. Med. 2024, 28, e18228. [Google Scholar] [CrossRef]
- Tang, L.D.; Wang, J.Y.; Zhang, Y.; Chen, X.Y.; Zhang, L.; Yuan, Y. Iridoid from Eucommia ulmoides Oliv. Exerts Antiarthritis Effects by Inhibiting the JAK2/STAT3 Signaling Pathway In Vivo and In Vitro. Evid. Based Complement. Altern. Med. 2023, 2023, 4167906. [Google Scholar] [CrossRef]
- Duan, M.; Yuan, Y.; Liu, C.; Cai, Z.; Xie, Q.; Hu, T.; Tang, Q.; Wu, Q. Indigo Fruits Ingredient, Aucubin, Protects against LPS-Induced Cardiac Dysfunction in Mice. J. Pharmacol. Exp. Ther. 2019, 371, 348–359. [Google Scholar] [CrossRef]
- Qiu, Y.L.; Cheng, X.N.; Bai, F.; Fang, L.Y.; Hu, H.Z.; Sun, D.Q. Aucubin protects against lipopolysaccharide-induced acute pulmonary injury through regulating Nrf2 and AMPK pathways. Biomed. Pharmacother. 2018, 106, 192–199. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, X.; Lu, Y.; Wang, F.; Xu, Z.; Liu, S.; Zheng, H.; Liu, X. Geniposide inhibits NLRP3 inflammasome activation via autophagy in BV-2 microglial cells exposed to oxygen-glucose deprivation/reoxygenation. Int. Immunopharmacol. 2020, 84, 106547. [Google Scholar] [CrossRef]
- Woźniak, M.; Michalak, B.; Wyszomierska, J.; Dudek, M.K.; Kiss, A.K. Effects of Phytochemically Characterized Extracts From Syringa vulgaris and Isolated Secoiridoids on Mediators of Inflammation in a Human Neutrophil Model. Front. Pharmacol. 2018, 9, 349. [Google Scholar] [CrossRef]
- Zapolska-Downar, D.; Siennicka, A.; Chełstowski, K.; Widecka, K.; Goracy, I.; Hałasa, M.; Machaliński, B.; Naruszewicz, M. Is there an association between angiotensin-converting enzyme gene polymorphism and functional activation of monocytes and macrophage in young patients with essential hypertension? J. Hypertens. 2006, 24, 1565–1573. [Google Scholar] [CrossRef] [PubMed]

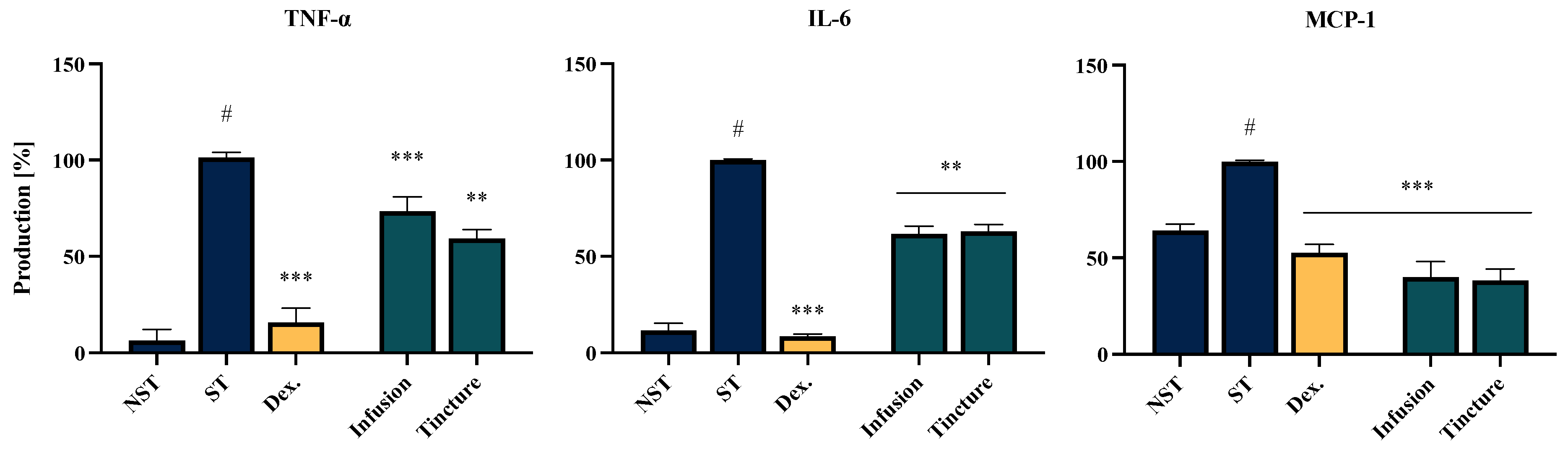
| No. | Analytes | Calibration Curves a | R2 | Mean Concentration (mg/g) | |
|---|---|---|---|---|---|
| Infusion | Ethanolic Extract | ||||
| 1. | 5-O-caffeoylquinic | y = 5161.5x − 94.68 | 0.99 | 12.0 ± 2.6 | 5.2 ± 2.8 |
| 2. | Pinoresinol di-O-glucoside | y = 1781x − 2.87 | 1.00 | 23.6 ± 0.6 | 11.7 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kołtun-Jasion, M.; Dudek, M.K.; Kiss, A.K. Eucommiae cortex Comprehensive Phytochemical Analysis Connected with Its In Vitro Anti-Inflammatory Activity in Human Immune Cells. Molecules 2025, 30, 1364. https://doi.org/10.3390/molecules30061364
Kołtun-Jasion M, Dudek MK, Kiss AK. Eucommiae cortex Comprehensive Phytochemical Analysis Connected with Its In Vitro Anti-Inflammatory Activity in Human Immune Cells. Molecules. 2025; 30(6):1364. https://doi.org/10.3390/molecules30061364
Chicago/Turabian StyleKołtun-Jasion, Małgorzata, Marta Katarzyna Dudek, and Anna Karolina Kiss. 2025. "Eucommiae cortex Comprehensive Phytochemical Analysis Connected with Its In Vitro Anti-Inflammatory Activity in Human Immune Cells" Molecules 30, no. 6: 1364. https://doi.org/10.3390/molecules30061364
APA StyleKołtun-Jasion, M., Dudek, M. K., & Kiss, A. K. (2025). Eucommiae cortex Comprehensive Phytochemical Analysis Connected with Its In Vitro Anti-Inflammatory Activity in Human Immune Cells. Molecules, 30(6), 1364. https://doi.org/10.3390/molecules30061364






