Anti-HIV Activity of Tigliane Derivatives from Euphorbia nicaeensis Roots
Abstract
:1. Introduction
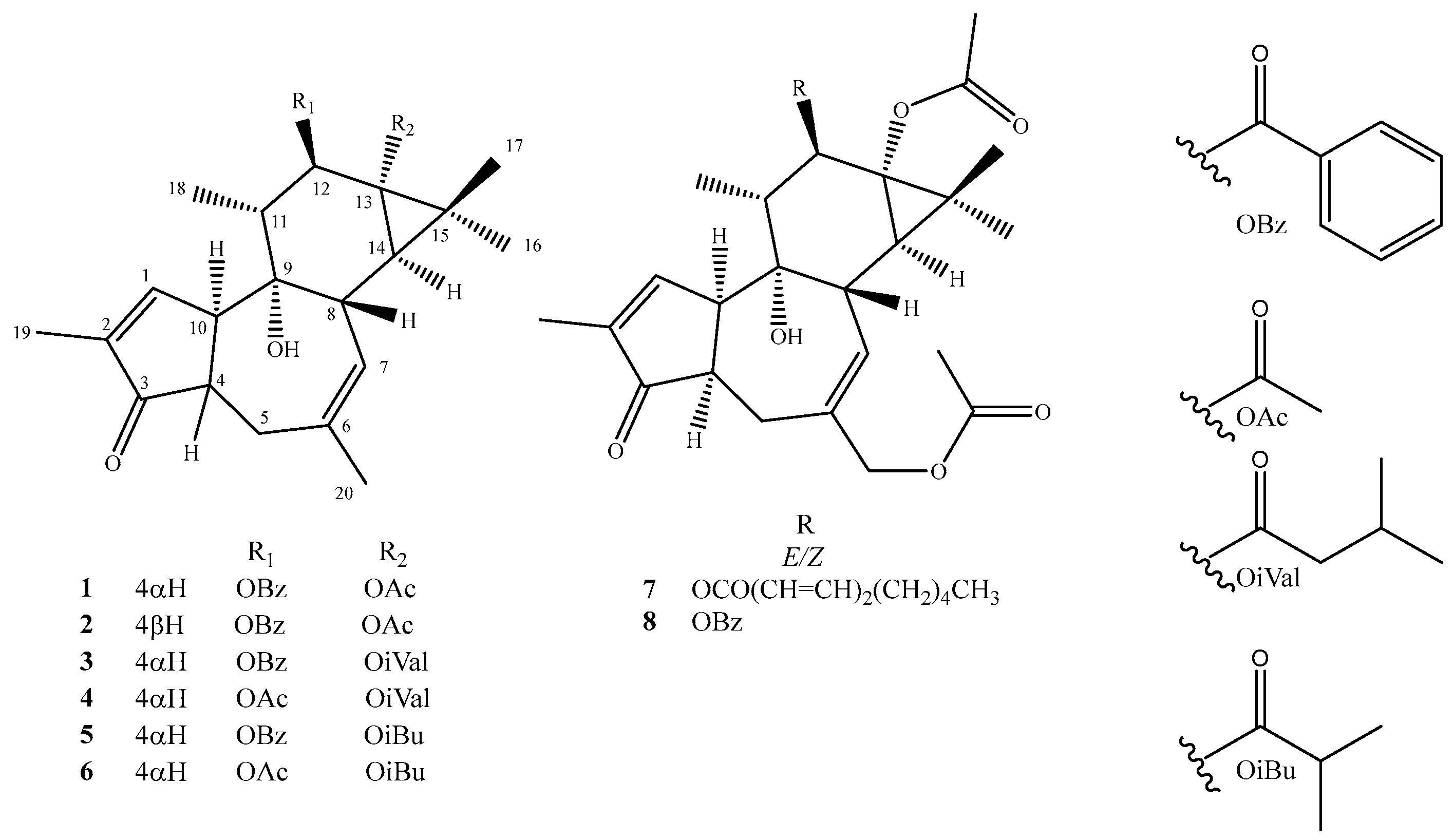
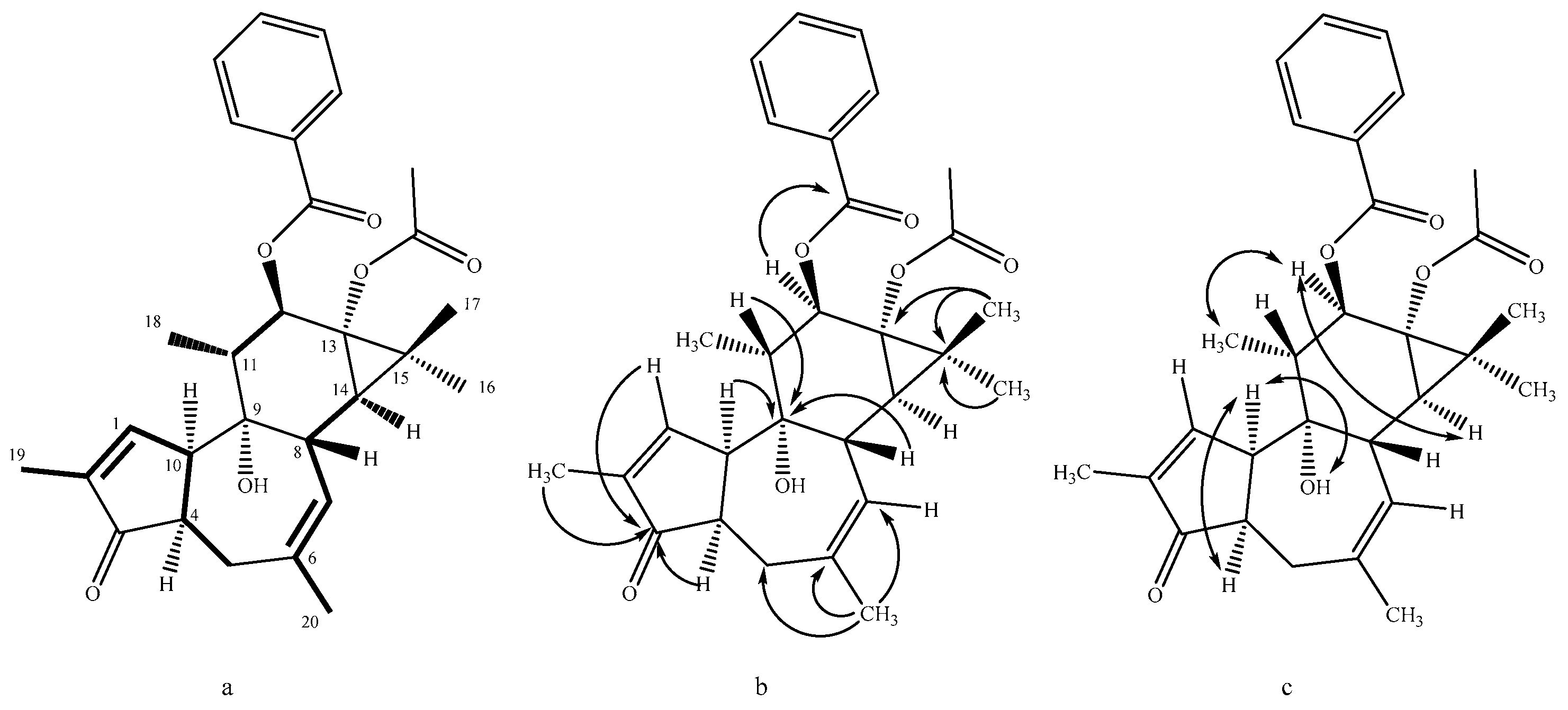
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
- Nicaeenin H (13α-Acetyloxy-12β-benzoyloxy-4-epi-4,20-dideoxyphorbol, 1): colorless, amorphous, solid substance; [α]D20 -14.0 (c 0.10, MeOH); UV (MeOH) λmax 195, 231 nm; IR (ATR) νmax 2970, 1729, 1244, 1120, 1015 cm−1; 1H and 13C NMR data in Table 1 and Table 2; HRESIMS m/z 501.2246 [M + Na]+ (calcd for C29H34NaO6+ 501.2248).
- Nicaeenin I (13α-Acetyloxy-12β-benzoyloxy-4,20-dideoxyphorbol, 2): colorless, amorphous, solid substance; [α]D20 +14.0 (c 0.10, MeOH); UV (MeOH) λmax 201, 231 nm; IR (ATR) νmax 2981, 1730, 1228, 1124, 1025 cm−1; 1H and 13C NMR data in Table 1 and Table 2; HRESIMS m/z 479.2416 [M + H]+ (calcd for C29H35O6+ 479.2428).
- Nicaeenin J (12β-Benzoyloxy-13α-isovaleryloxy-4-epi-4,20-dideoxyphorbol, 3): colorless, amorphous, solid substance; [α] D20 +9.9 (c 0.07, acetone); UV (MeOH) λmax 201, 232 nm; IR (ATR) νmax 2974, 1735, 1230, 1122, 1020 cm−1; 1H and 13C NMR data in Table 1 and Table 2; HRESIMS m/z 543.2714 [M + Na]+ (calcd for C32H40NaO6+ 543.2717).
- Nicaeenin K (12β-Acetyloxy-13α-isovaleryloxy-4-epi-4,20-dideoxyphorbol, 4): colorless, amorphous, solid substance; [α] D20 +7.8 (c 0.28, acetone); UV (MeOH) λmax 210, 236 nm; IR (ATR) νmax 2969, 1731, 1233, 1129, 1022 cm−1; 1H and 13C NMR data data in Table 1 and Table 2; HRESIMS m/z 481.2542 [M + Na]+ (calcd for C27H38NaO6+ 481.2561).
- Nicaeenin L (13α,20-Diacetyloxy-12β-(2′E,4′E-nonadienoyloxy)-4-epi-deoxyphorbol, 7): colorless, amorphous, solid; [α]D20 -1.3 (c 0.08, MeOH); UV (MeOH) λmax 199, 268 nm; IR (ATR) νmax 2974, 1733, 1235, 1126, 1024 cm−1; 1H and 13C NMR data in Table 1 and Table 2; HRESIMS m/z 583.3265 [M + H]+ (calcd for C34H47O8+ 583.3265).
3.4. Anti-HIV-Activity Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Q.W.; Su, X.H.; Kiyota, H. Chemical and pharmacological research of the plants in genus. Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [PubMed]
- Vasas, A.; Hohmann, J. Euphorbia diterpenes: Isolation, structure, biological activity, and synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar]
- Zhao, H.; Sun, L.; Kong, C.H.; Mei, W.L.; Dai, H.F.; Xu, F.Q.; Huang, S.Z. Phytochemical and pharmacological review of diterpenoids from the genus Euphorbia Linn (2012–2021). J. Ethnopharmacol. 2022, 298, 115574. [Google Scholar]
- Mwine, J.T.; Van Damme, P. Why do Euphorbiaceae tick as medicinal plants?: A review of Euphorbiaceae family and its medicinal features. J. Med. Plants Res. 2011, 5, 652–662. [Google Scholar]
- Huang, X.; Tang, C.; Huang, X.; Yang, Y.; Li, Q.; Ma, M.; Zhao, L.; Yang, L.; Cui, Y.; Zhang, Z.; et al. Synthesis, and anti-HIV activities of phorbol derivatives. Chin. J. Nat. Med. 2024, 22, 1–16. [Google Scholar]
- Emerit, I.; Cerutti, P.A. Tumor promoter phorbol 12-myristate 13-acetate induces a clastogenic factor in human lymphocytes. Proc. Natl. Acad. Sci. USA 1982, 79, 7509–7513. [Google Scholar]
- Otsuki, K.; Li, W. Tigliane and daphnane diterpenoids from Thymelaeaceae family: Chemistry, biological activity, and potential in drug discovery. J. Nat. Med. 2023, 77, 625–643. [Google Scholar]
- Cragg, G.M.; Newman, D.J.; Kingston, D.G.I. Comprehensive Natural Products II Chemistry and Biology; Mander, L., Lui, H.-W., Eds.; Elsevier: Oxford, UK, 2010; Volume 2, Chapter 2.02. [Google Scholar]
- Cashmore, A.R.; Seelye, R.N. The structure of prostratin: A toxic tetracyclic diterpene ester from Pimelea prostrata. Tetrahedron Lett. 1976, 20, 1737–1738. [Google Scholar]
- Wang, H.B.; Wang, X.Y.; Liu, L.P.; Qin, G.W.; Kang, T.G. Tigliane diterpenoids from the Euphorbiaceae and Thymelaeaceae families. Chem. Rev. 2015, 115, 2975–3011. [Google Scholar]
- El-Mekkawy, S.; Meselhy, M.R.; Nakamura, N.; Hattori, M.; Kawahata, T.; Otake, T. Anti-HIV-1 phorbol esters from the seeds of Croton tiglium. Phytochemistry 2000, 53, 457–464. [Google Scholar]
- Nothias-Scaglia, L.-F.; Pannecouque, C.; Renucci, F.; Delang, L.; Neyts, J.; Roussi, F.; Costa, J.; Leyssen, P.; Litaudon, M.; Paolini, J. Antiviral activity of diterpene esters on chikungunya virus and HIV replication. J. Nat. Prod. 2015, 78, 1277–1283. [Google Scholar]
- Chen, H.; Zhang, R.; Luo, R.-H.; Yang, L.-M.; Wang, R.-R.; Ha, X.-J.; Zheng, Y.-T. Anti-HIV Activities and mechanism of 12-O-tricosanoylphorbol-20-acetate, a novel phorbol ester from Ostodes katharinae. Molecules 2017, 22, 1498. [Google Scholar] [CrossRef] [PubMed]
- Hemmers, H.; Gülz, P.-G. Epicuticular waxes from leaves of five Euphorbia species. Phytochemistry 1986, 25, 2103–2107. [Google Scholar]
- Öksüz, S.; Shieh, H.-L.; Pezzuto, J.M.; Özhatay, N.; Cordell, G.A. Biologically active compounds from the Euphorbiaceae; Part 1. Triterpenoids of Euphorbia nicaeensis subsp. glareosa. Planta Med. 1993, 59, 472–473. [Google Scholar]
- Krstić, G.B.; Novaković, M.M.; Jadranin, M.B.; Tešević, V.V. Tetracyclic triterpenoids from Euphorbia nicaeensis All. Adv. Technol. 2019, 8, 37–45. [Google Scholar]
- Cateni, F.; Zilic, J.; Falsone, G.; Hollan, F.; Frausin, F.; Scarcia, V. Preliminary biological assay on cerebroside mixture from Euphorbia nicaeensis All. Isolation and structure determination of five glucocerebrosides. Il Farm. 2003, 58, 809–817. [Google Scholar]
- Cateni, F.; Falsone, G.; Zilic, J.; Bonivento, P.; Zacchigna, M.; Žigon, D.; Sosa, S.; Altinier, G. Glyceroglycolipids from Euphorbia nicaeensis All. with antiinflamatory activity. ARKIVOC 2004, 5, 54–65. [Google Scholar] [CrossRef]
- Krstić, G.; Jadranin, M.; Todorović, N.; Pešić, M.; Stanković, T.; Aljančić, I.; Tešević, V. Jatrophane diterpenoids with multidrug-resistance modulating activity from the latex of Euphorbia nicaeensis. Phytochemistry 2018, 148, 104–112. [Google Scholar]
- Krstić, G.; Kostić, A.; Jadranin, M.; Pešić, M.; Novaković, M.; Aljančić, I.; Vajs, V. Two new jatrophane diterpenes from the roots of Euphorbia nicaeensis. J. Serb. Chem. Soc. 2021, 86, 1219–1228. [Google Scholar]
- Aichour, S.; Haba, H.; Benkhaled, M.; Harakat, D.; Lavaud, C. Terpenoids and other constituents from Euphorbia bupleuroides. Phytochem. Lett. 2014, 10, 198–203. [Google Scholar]
- Benmerache, A.; Alabdul Magid, A.; Labed, A.; Kabouche, A.; Voutquenne-Nazabadioko, L.; Hubert, J.; Morjani, H.; Kabouche, Z. Isolation and characterisation of cytotoxic compounds from Euphorbia clementei Boiss. Nat. Prod. Res. 2017, 31, 2091–2098. [Google Scholar] [PubMed]
- Μiana, G.A.; Schmidt, R.; Hecker, E.; Shamma, M.; Moniot, J.L.; KlAmuddin, M. Notizen: 4α-Sapinine—A novel diterpene ester from Sapium indicum. Z. Naturforsch. B. 1977, 32, 727–728. [Google Scholar]
- Wang, L.-Y.; Wang, N.-L.; Yao, X.-S.; Miyata, S.; Kitanaka, S. Diterpenes from the roots of Euphorbia kansui and their in vitro effects on the cell division of Xenopus. J. Nat. Prod. 2002, 65, 1246–1251. [Google Scholar] [PubMed]
- Lu, Y.; Huang, Y.S.; Chen, C.H.; Akiyama, T.; Morris-Natschke, S.L.; Cheng, Y.Y.; Chen, I.S.; Yang, S.Z.; Chen, D.F.; Lee, K.H. Anti-HIV tigliane diterpenoids from Reutealis trisperma. Phytochemistry 2020, 174, 112360. [Google Scholar]
- Asada, Y.; Sukemori, A.; Watanabe, T.; Malla, K.J.; Yoshikawa, T.; Li, W.; Koike, K.; Chen, C.-H.; Akiyama, T.; Qian, K.; et al. Stelleralides A–C, Novel Potent Anti-HIV Daphnane-Type Diterpenoids from Stellera chamaejasme L. Org. Lett. 2011, 13, 2904–2907. [Google Scholar]
- Otsuki, K.; Zhang, M.; Yamamoto, A.; Tsuji, M.; Tejima, M.; Bai, Z.S.; Zhou, D.; Huang, L.; Chen, C.H.; Lee, K.H.; et al. Anti-HIV Tigliane Diterpenoids from Wikstroemia scytophylla. J. Nat. Prod. 2020, 83, 3584–3590. [Google Scholar] [PubMed]
- Zhang, M.; Otsuki, K.; Kikuchi, T.; Bai, Z.-S.; Zhou, D.; Huang, L.; Chen, C.-H.; Morris-Natschke, S.L.; Lee, K.-H.; Li, N.; et al. LC-MS identification, isolation, and structural elucidation of anti-HIV tigliane diterpenoids from Wikstroemia lamatsoensis. J. Nat. Prod. 2021, 84, 2366–2373. [Google Scholar]
- Ma, Q.-G.; Liu, W.-Z.; Wu, X.-Y.; Zhou, T.-X.; Qin, G.-W. Diterpenoids from Euphorbia fischeriana. Phytochemistry 1997, 44, 663–666. [Google Scholar]
- Shusterman, A.J.; McDougal, P.G.; Glasfeld, A. Dry-column flash chromatography. J. Chem. Educ. 1997, 10, 1222–1223. [Google Scholar]
| 1 | 2 | 3 | 4 | 7 | |
|---|---|---|---|---|---|
| 1 | 7.04, brs | 7.58, brs | 7.04, brs | 6.99, brs | 7.00, brs |
| 4 | 2.71, m | 2.49, m | 2.71, m | 2.68, m | 2.75, m |
| 5α | 3.41, d, 16 | 2.86, dd, 18, 10 | 3.47, m | 3.39, m | 3.38, brd, 16 |
| 5β | 2.38, dd, 16, 5 | 2.03, dd, 18, 10 | 2.38, dd, 15, 5 | 2.35, dd, 15, 5 | 2.47, dd, 16, 5 |
| 7 | 4.84, brs | 5.24, d, 5 | 4.85, brs | 4.81, brs | 5.18, brs |
| 8 | 1.97, brs | 2.41, m | 1.96, brs | 1.88, brs | 1.99, brs |
| 9(OH) | 5.13, s | 5.55, s | 5.24, s | 5.13, s | 5.17, brs |
| 10 | 3.46, m | 3.32, m | 3.48, m | 3.43, m | 3.47, m |
| 11 | 1.87, m | 1.72, m | 1.86, m | 1.68, m | 1.75, m |
| 12 | 5.74, d, 10 | 5.67, d, 10 | 5.73, d, 11 | 5.43, d, 10 | 5.55, d, 10 |
| 14 | 0.87, d, 5 | 1.07, d, 5 | 0.84, d, 5 | 0.77, d, 5 | 0.82, d, 5 |
| 16 | 1.18, s | 1.20, s | 1.18, s | 1.17, s | 1.25, s |
| 17 | 1.34, s | 1.32, s | 1.35, s | 1.19, s | 1.19, s |
| 18 | 1.12, d, 6 | 0.97, d, 7 | 1.11, d, 7 | 1.06, d, 7 | 1.08, d, 6 |
| 19 | 1.80, s | 1.73, brs | 1.80, s | 1.78, s | 1.77, s |
| 20 | 1.75, s | 1.75, brs | 1.76, s | 1.74, s | 4.47, d, 124.35, d, 12 |
| 12-OR | |||||
| 2′ | 2.11, s | 5.91, d, 15 | |||
| 3′ | 8.06, d, 7 | 8.02, d, 8 | 8.07, d, 8 | 7.66, dd, 15, 11 | |
| 4′ | 7.48, t, 7 | 7.46, t,8 | 7.48, t, 8 | 6.17, t, 11 | |
| 5′ | 7.60, t, 7 | 7.60, m | 7.60, t, 8 | - | 5.92, m |
| 6′ | 7.48, t, 7 | 7.46, t,8 | 7.48, t, 8 | - | 2.32, m |
| 7′ | 8.06, d, 7 | 8.02, d, 8 | 8.07, d, 8 | - | 1.44, m |
| 8′ | - | - | - | - | 1.33, m |
| 9′ | - | - | - | - | 1.32, m |
| 10′ | - | - | - | - | 0.91, t, 7 |
| 13-OR | |||||
| 2″ | 2.09, s | 2.13, s | 2.21, m | 2.18, m | 2.13, s |
| 3″ | - | - | 2.10, m | 2.10, m | - |
| 4″ | - | - | 0.97, d, 7 | 0.96, d, 7 | - |
| 5″ | - | - | 0.95, d, 7 | 0.94, d, 7 | - |
| 20-OR | |||||
| 2‴ | - | - | - | - | 2.09, s |
| 1 | 2 | 3 | 4 | 7 | |
|---|---|---|---|---|---|
| 1 | 155.5 | 160.2 | 155.7 | 155.7 | 155.4 |
| 2 | 143.2 | 136.7 | 143.4 | 143.3 | 143.6 |
| 3 | 211.7 | 210.2 | 212.0 | 211.9 | 211.2 |
| 4 | 49.2 | 44.8 | 49.5 | 49.4 | 49.1 |
| 5 | 30.0 | 34.3 | 30.2 | 30.1 | 26.6 |
| 6 | 134.9 | 139.3 | 135.1 | 134.9 | 133.0 |
| 7 | 124.2 | 125.9 | 124.5 | 124.4 | 129.0 |
| 8 | 40.9 | 42.5 | 41.2 | 41.0 | 41.2 |
| 9 | 78.1 | 78.2 | 78.3 | 78.1 | 78.0 |
| 10 | 47.1 | 54.6 | 47.3 | 47.2 | 47.1 |
| 11 | 43.3 | 42.8 | 43.8 | 43.4 | 43.4 |
| 12 | 76.7 | 78.1 | 77.0 | 76.3 | 75.7 |
| 13 | 65.4 | 65.7 | 65.3 | 65.2 | 65.5 |
| 14 | 37.7 | 36.2 | 38.1 | 37.8 | 37.1 |
| 15 | 25.2 | 25.6 | 25.7 | 25.6 | 25.4 |
| 16 | 24.2 | 24.0 | 24.5 | 24.4 | 16.6 |
| 17 | 16.6 | 17.2 | 16.9 | 16.5 | 24.3 |
| 18 | 11.9 | 15.4 | 12.2 | 12.1 | 12.1 |
| 19 | 10.5 | 10.4 | 10.7 | 10.6 | 10.7 |
| 20 | 29.7 | 26.0 | 29.2 | 29.0 | 70.4 |
| 12-OR | |||||
| 1′ | 166.2 | 166.5 | 166.3 | 170.8 | 167.2 |
| 2′ | 130.1 | 130.2 | 130.5 | 21.2 | 120.8 |
| 3′ | 129.7 | 128.7 | 130.0 | - | 140.5 |
| 4′ | 128.5 | 129.9 | 128.7 | - | 126.6 |
| 5′ | 133.1 | 133.4 | 133.3 | - | 142.6 |
| 6′ | 128.5 | 129.9 | 128.7 | - | 28.5 |
| 7′ | 129.7 | 128.7 | 130.0 | - | 29.2 |
| 8′ | - | - | - | - | 31.6 |
| 9′ | - | - | - | - | 22.7 |
| 10′ | - | - | - | - | 14.2 |
| 13-OR | |||||
| 1″ | 173.5 | 173.9 | 175.7 | 175.5 | 171.0 |
| 2″ | 21.1 | 21.4 | 43.7 | 43.6 | 21.3 |
| 3″ | - | - | 25.6 | 25.3 | - |
| 4″ | - | - | 22.7 | 22.5 | - |
| 5″ | - | - | 22.7 | 22.6 | - |
| 20-OR | |||||
| 1‴ | - | - | - | - | 173.7 |
| 2‴ | - | - | - | - | 21.3 |
| 1 | 2 | 3 | 4 | 5 | 6 | 8 | PMPA | AMD3100 | |
|---|---|---|---|---|---|---|---|---|---|
| HIV-1 | >42.0 ± 0.0 | 7.5 ± 0.2 | >18.0 ± 0.0 | >52.0 ± 0.0 | >34.0 ± 0.0 | >101.0 ± 0.0 | 3.3 ± 1.8 | 2.4 ± 0.7 | 0.008 ± 0.002 |
| HIV-2 | 9.4 ± 1.4 | 1.7 ± 1.3 | >18.0 ± 0.0 | >52.0 ± 0.0 | 4.6 ± 2.3 | >101.0 ± 0.0 | 1.1 ± 0.8 | 0.7 ± 0.5 | 0.0075 ± 0.0005 |
| Cellular toxicity | 42.0 ± 0.0 | 94.0 ± 0.0 | 17.6 ± 0.3 | 52.0 ± 0.0 | 33.5 ± 0.4 | 101.0 ± 0.0 | 20.8 ± 5.6 | >100.0 ± 0.0 | >10.0 ± 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krstić, G.; Jadranin, M.; Schols, D.; Claes, S.; Tešević, V.; Mandić, B.; Milosavljević, S.; Wittine, K. Anti-HIV Activity of Tigliane Derivatives from Euphorbia nicaeensis Roots. Molecules 2025, 30, 1452. https://doi.org/10.3390/molecules30071452
Krstić G, Jadranin M, Schols D, Claes S, Tešević V, Mandić B, Milosavljević S, Wittine K. Anti-HIV Activity of Tigliane Derivatives from Euphorbia nicaeensis Roots. Molecules. 2025; 30(7):1452. https://doi.org/10.3390/molecules30071452
Chicago/Turabian StyleKrstić, Gordana, Milka Jadranin, Dominique Schols, Sandra Claes, Vele Tešević, Boris Mandić, Slobodan Milosavljević, and Karlo Wittine. 2025. "Anti-HIV Activity of Tigliane Derivatives from Euphorbia nicaeensis Roots" Molecules 30, no. 7: 1452. https://doi.org/10.3390/molecules30071452
APA StyleKrstić, G., Jadranin, M., Schols, D., Claes, S., Tešević, V., Mandić, B., Milosavljević, S., & Wittine, K. (2025). Anti-HIV Activity of Tigliane Derivatives from Euphorbia nicaeensis Roots. Molecules, 30(7), 1452. https://doi.org/10.3390/molecules30071452






