Facile Synthesis and Characterization of Novel Analcime/Sodium Magnesium Aluminum Silicon Silicate Nanocomposite for Efficient Removal of Methylene Blue Dye from Aqueous Media
Abstract
1. Introduction
2. Results and Discussion
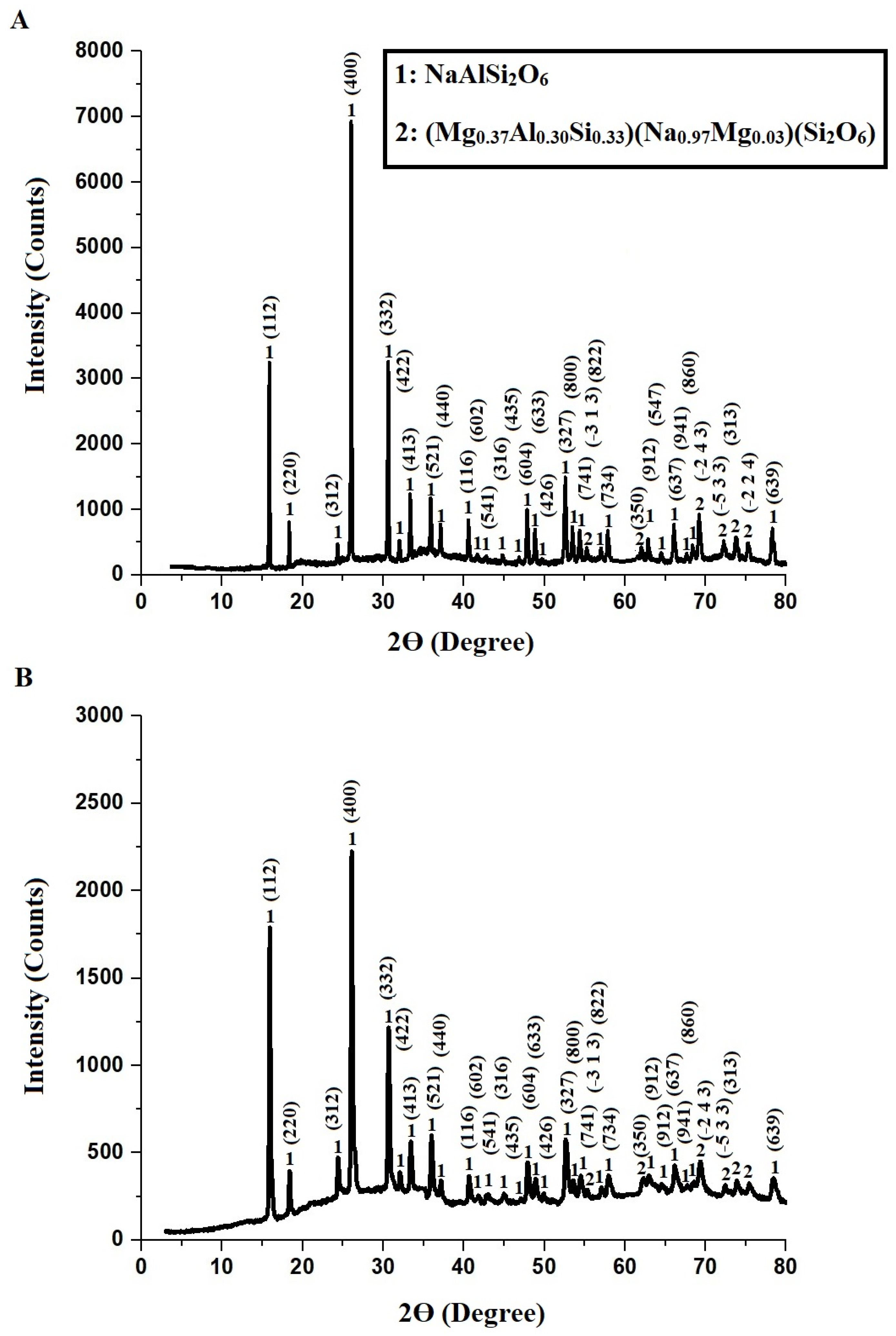
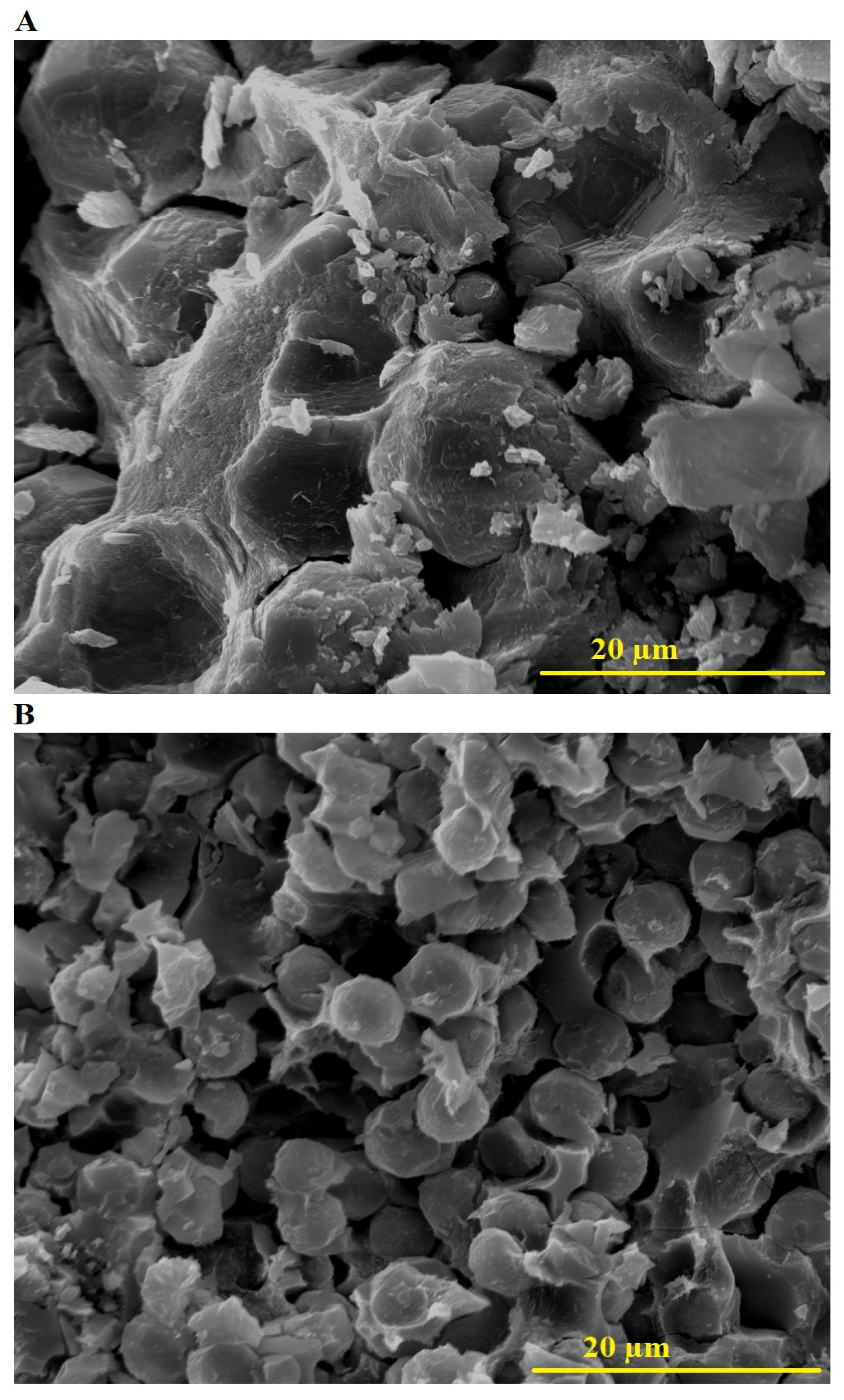
2.1. Characterization
2.2. Removal of Methylene Blue Dye from Aqueous Media
2.2.1. Effect of pH
2.2.2. Effect of Contact Time
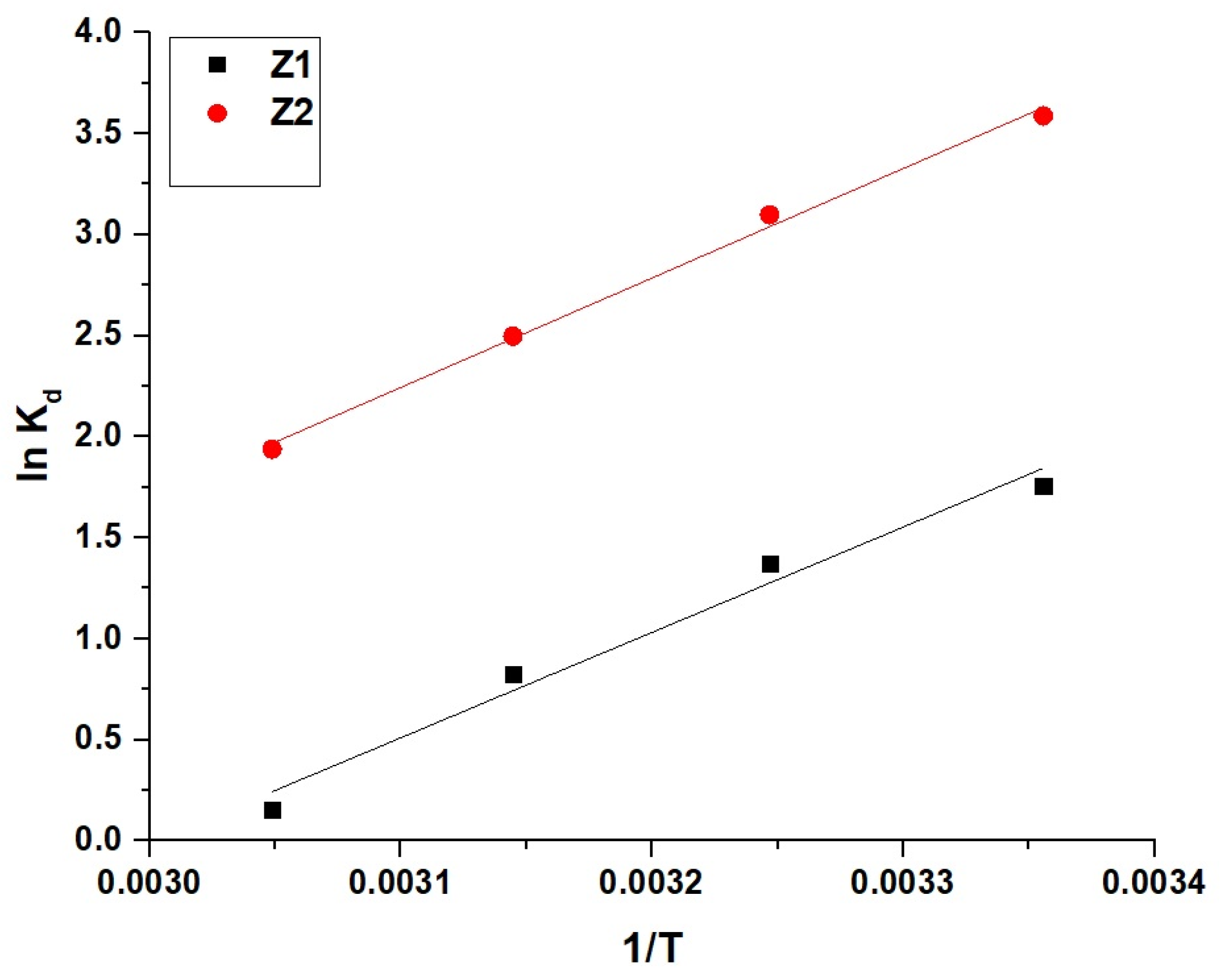
2.2.3. Effect of Temperature
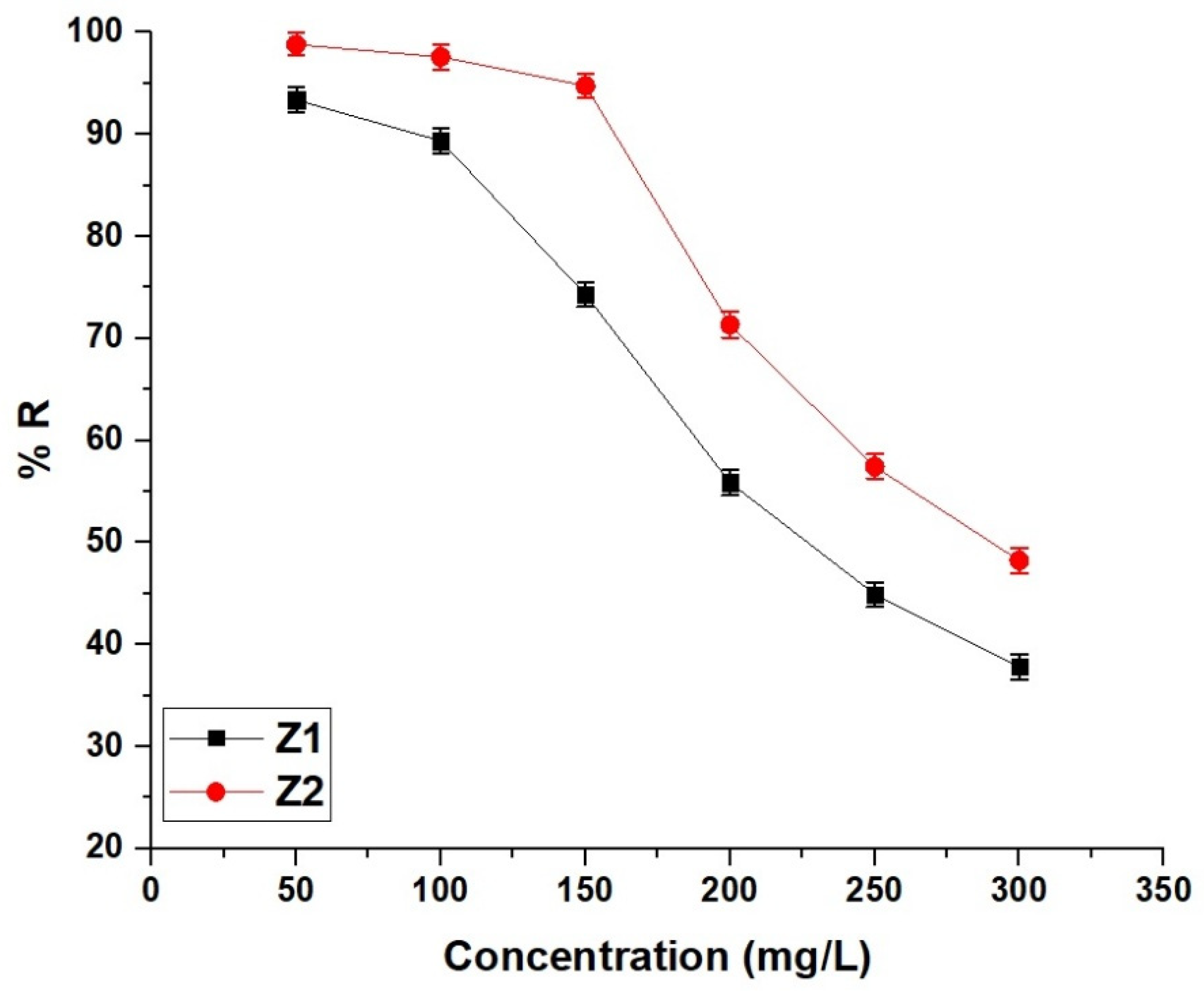
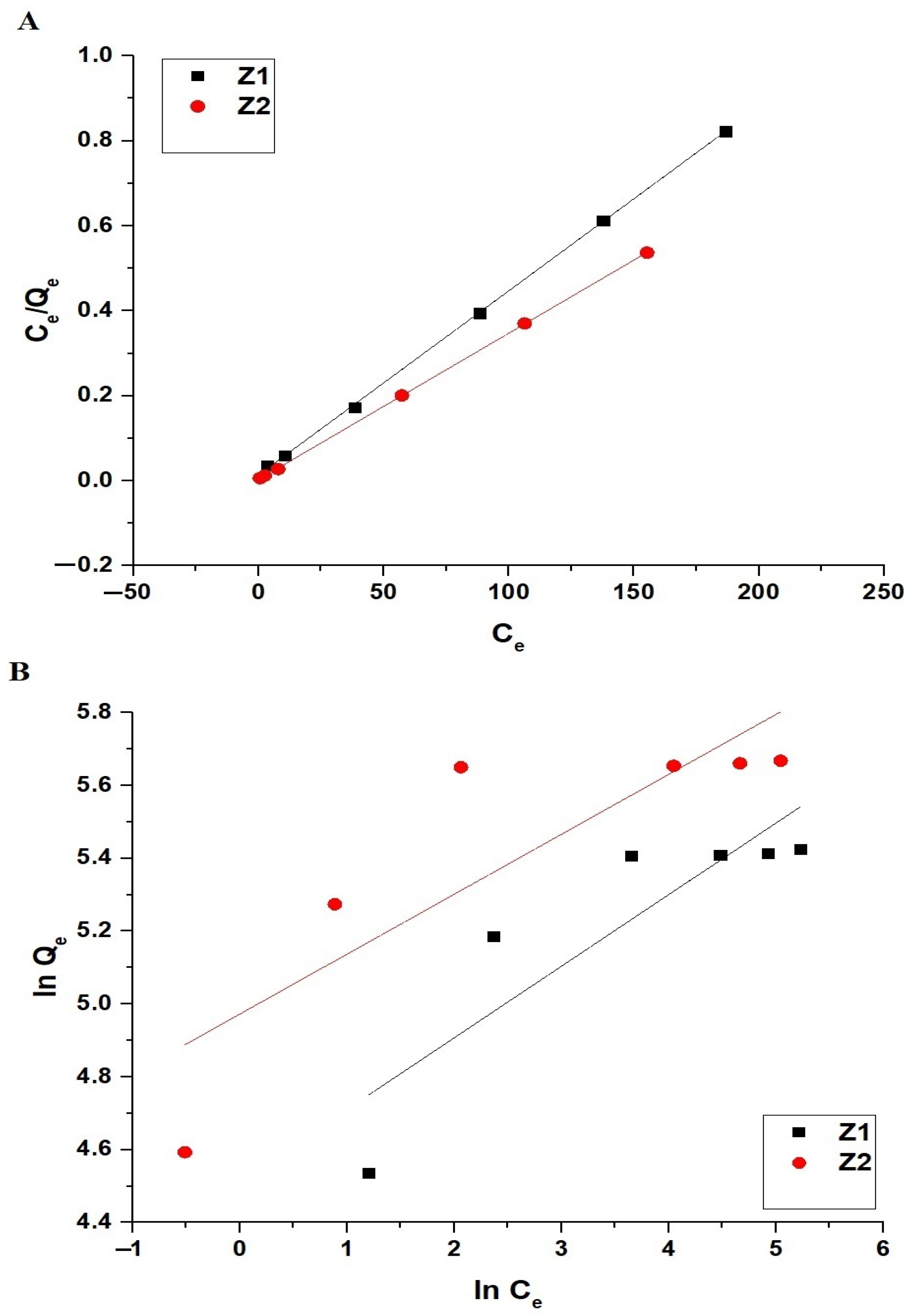
2.2.4. Effect of Concentration
3. Experimental
3.1. Materials
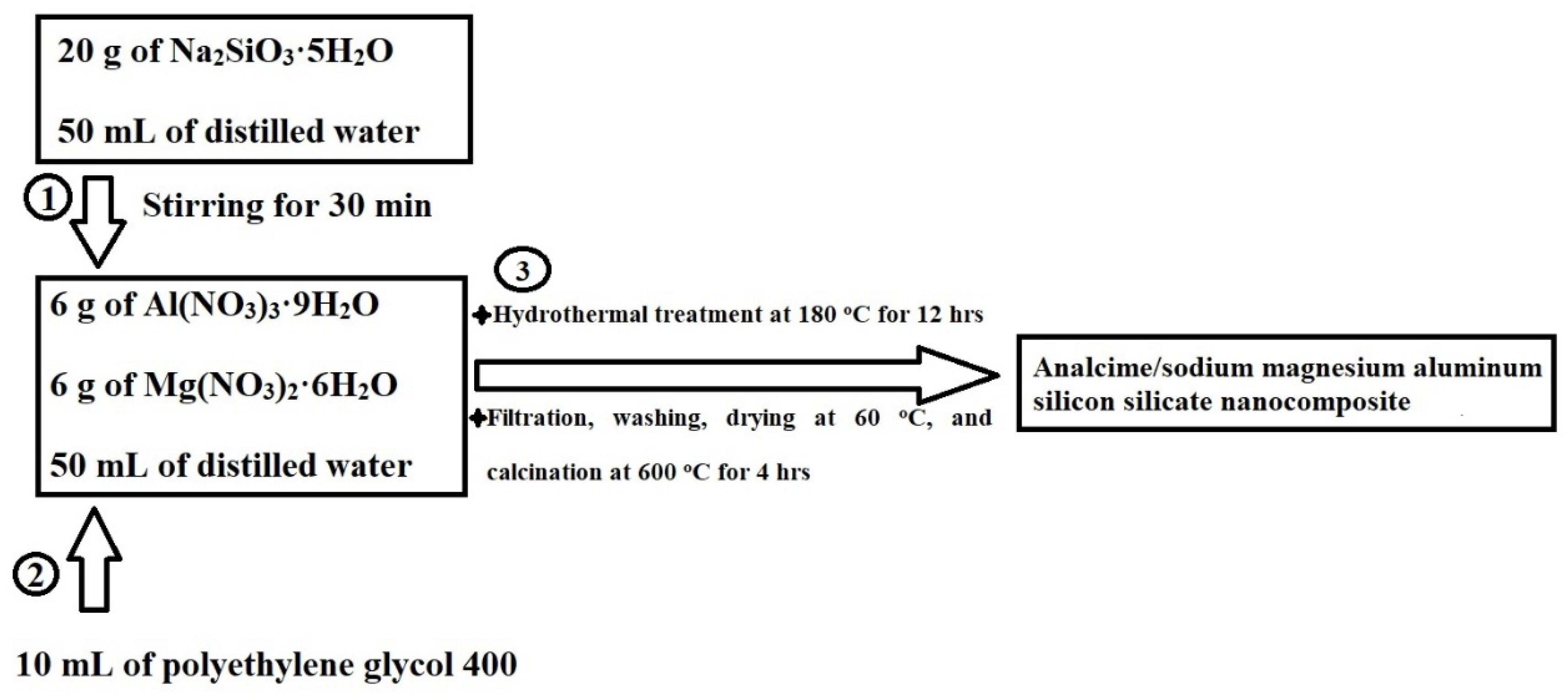
3.2. Synthesis of Analcime/Sodium Magnesium Aluminum Silicon Silicate Nanocomposites
3.3. Instrumentation
3.4. Removal of Methylene Blue Dye from Aqueous Media
3.5. Point of Zero Charge (pHPZC) of Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Firmansyah, M.L.; Ashraf, M.; Ullah, N. A Critical Review on the Removal of Anionic Dyes by Cross-Linked Resin: Recent Progress, Challenges and Future Perspective. Sep. Purif. Technol. 2025, 360, 131111. [Google Scholar] [CrossRef]
- Fahira, N.; Gareso, P.L.; Tahir, D. Exploring Wood-Based Strategies for Dye Removal: A Comprehensive Literature Review. Bioresour. Technol. Rep. 2025, 29, 102048. [Google Scholar] [CrossRef]
- Al Rubai, H.F.; Sultan, M.S.; Abed, G.M. Enhancing the Removal Efficiency of Azo Dye Using Sodium Borohydride in the Presence of Inorganic Nano Catalysts. Green Anal. Chem. 2025, 12, 100194. [Google Scholar] [CrossRef]
- Ahmad, S.; Mujtaba, G.; Zubair, M.; Shah, M.U.H.; Daud, M.; Mu’azu, N.D.; Al-Harthi, M.A. A Review of Performance, Mechanism, and Challenges of Layered Double Hydroxide-Based Biocomposites for the Adsorptive Removal of Dye Contaminants from Water and Wastewater. J. Water Process Eng. 2025, 70, 106837. [Google Scholar] [CrossRef]
- Silina, A.; El Achari, A.; Salaün, F. Metal-Organic Framework Electrospun Nanofibers in Application to Dye Removal from Textile Wastewaters: A Review. J. Environ. Chem. Eng. 2024, 12, 114819. [Google Scholar] [CrossRef]
- Ciğeroğlu, Z.; El Messaoudi, N.; Miyah, Y.; Georgin, J.; Franco, D.S.; Benjelloun, M.; Şenol, Z.M.; Kazan-Kaya, E.S.; Ergan, B.T. Recent Advances in the Removal of Sunset Yellow Dye from Wastewater: A Review. Sustain. Mater. Technol. 2024, 42, e01187. [Google Scholar] [CrossRef]
- Loura, N.; Rathee, K.; Dhull, R.; Singh, M.; Dhull, V. Carbon Nanotubes for Dye Removal: A Comprehensive Study of Batch and Fixed-Bed Adsorption, Toxicity, and Functionalization Approaches. J. Water Process Eng. 2024, 67, 106193. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, Z.U.H.; Sabahat, S.; Sun, J.; Shah, N.S.; Khan, Z.U.; Muhammad, N.; Mir, S.; Rahim, A.; Nadeem, M.; et al. Innovations in Metal Oxides-Biochar Nanoparticles for Dye Removal. Nano-Struct. Nano-Objects 2024, 39, 101269. [Google Scholar] [CrossRef]
- Pattanayak, D.S.; Surana, M.; Kumar, A.; Singh, D.; Pal, D. Graphitic Carbon Nitride(g-C3N4)-Based Photocatalysts for Dye Removal: Current Status. Sustain. Chem. Environ. 2024, 7, 100141. [Google Scholar] [CrossRef]
- Anulaya, S.V.; Subash, A.; Gholap, V.; Kandasubramanian, B. Electrospinning of Cellulose Acetate for Methylene Blue Dye Removal. Hybrid. Adv. 2024, 6, 100205. [Google Scholar] [CrossRef]
- Park, D.; Nam, S.N.; Jung, B.; Soo Choi, J.; Min Park, C.; Earn Choong, C.; Jang, M.; Cho, K.S.; Jun, B.M.; Yoon, Y. Removal of Selected Contaminants of Dyes and Pharmaceuticals Using MXene-Based Nanoadsorbents: A Review. Sep. Purif. Technol. 2024, 341, 126864. [Google Scholar] [CrossRef]
- Ciğeroğlu, Z.; El Messaoudi, N.; Şenol, Z.M.; Başkan, G.; Georgin, J.; Gubernat, S. Clay-Based Nanomaterials and Their Adsorptive Removal Efficiency for Dyes and Antibiotics: A Review. Mater. Today Sustain. 2024, 26, 100735. [Google Scholar] [CrossRef]
- Martínez, F.; Leo, P.; Orcajo, G.; Díaz-García, M.; Sanchez-Sanchez, M.; Calleja, G. Sustainable Fe-BTC Catalyst for Efficient Removal of Mehylene Blue by Advanced Fenton Oxidation. Catal. Today 2018, 313, 6–11. [Google Scholar] [CrossRef]
- Javaid, H.; Mustafa, K.; Khan, M.; Iqbal, S.; Ahmad, S.; Rani, M.; Musaddiq, S. Fabrication and Kinetic Evaluation of Dye Adsorption Capability of Metal Oxide@RGO Nanocomposites Integrated Cellulose Triacetate Membranes. Chem. Phys. 2023, 573, 112019. [Google Scholar] [CrossRef]
- Hossain, M.S.; Hossain, M.S.; Ahmed, S.; Bin Mobarak, M. Characterization and Adsorption Performance of Nano-Hydroxyapatite Synthesized from Conus Litteratus Waste Seashells for Congo Red Dye Removal. RSC Adv. 2024, 14, 38560–38577. [Google Scholar] [CrossRef]
- Pu, C.; Wang, J.; Liu, Q.; Lu, L.; Ahmad, A.; Alshammari, M.B.; Ansari, I.A.; Afzal, M.; Alarifi, A. Fabrication of Co(II)-Based Coordination Polymer for Photocatalytic Degradation: Selective Removal of Cationic Methyl Violet Dye. Polyhedron 2025, 267, 117321. [Google Scholar] [CrossRef]
- Cui, T.; Wang, X.; Chen, Y.; Chen, Y.; Fu, B.; Tu, Y. Reverse Osmosis Coupling Multi-Catalytic Ozonation (RO-MCO) in Treating Printing and Dyeing Wastewater and Membrane Concentrate: Removal Performance and Mechanism. Water Resour. Ind. 2023, 30, 100217. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Hosamani, K.M.; Aminabhavi, T.M. Nanofiltration and Reverse Osmosis Thin Film Composite Membrane Module for the Removal of Dye and Salts from the Simulated Mixtures. Desalination 2009, 249, 12–17. [Google Scholar] [CrossRef]
- Anantha, M.S.; Olivera, S.; Hu, C.; Jayanna, B.K.; Reddy, N.; Venkatesh, K.; Muralidhara, H.B.; Naidu, R. Comparison of the Photocatalytic, Adsorption and Electrochemical Methods for the Removal of Cationic Dyes from Aqueous Solutions. Environ. Technol. Innov. 2020, 17, 100612. [Google Scholar] [CrossRef]
- Salman, A.B.; Al-khateeb, R.T.; Abdulqahar, S.N. Electrochemical Removal of Crystal Violet Dye from Simulated Wastewater by Stainless Steel Rotating Cylinder Anode: COD Reduction and Decolorization. Desalin. Water Treat. 2024, 320, 100787. [Google Scholar] [CrossRef]
- Pratap Singh, V.; Godara, P.; Srivastava, A. Sustainable Microalgal Bioremediation of Heavy Metals and Dyes from Synthetic Wastewater: Progressing towards United Nations Sustainable Development Goals. Waste Manag. Bull. 2024, 2, 123–135. [Google Scholar] [CrossRef]
- Ariaeenejad, S.; Kavousi, K.; Zeinalabedini, M.; Afshar Jahanshahi, D.; Beh-Afarin, S.R. Enhanced Bioremediation of Saline Azo Dye Effluents Using PersiLac3: A Thermo-Halotolerant Laccase from a Tannery Metagenome. Results Eng. 2024, 24, 103172. [Google Scholar] [CrossRef]
- Saheed, I.O.; Zairuddin, N.I.; Nizar, S.A.; Hanafiah, M.A.K.M.; Latip, A.F.A.; Suah, F.B.M. Adsorption Potential of CuO-Embedded Chitosan Bead for the Removal of Acid Blue 25 Dye. Ain Shams Eng. J. 2024, 15, 103125. [Google Scholar] [CrossRef]
- Elhadj-Daouadji, B.; Zaoui, F.; Zorgani, M.A.; Abubakar, S.; Siddig, L.A.; Abdelhamid, A.S.; Bhardwaj, M.; Hachemaoui, M.; Guezzoul, M.; Kumar, A.; et al. Efficient Removal of Asphaltene and Organic Dyes Using Hybrid Magnetic Nanomaterials: Adsorption Selectivity and Comparative Study. Fuel 2025, 381, 133284. [Google Scholar] [CrossRef]
- Arunkumar, G.; Deviga, G.; Mariappan, M.; Pannipara, M.; Al-Sehemi, A.G.; Soliman, U.A.; Anthony, S.P. Carbon Encapsulated ZnO Nanoplates for Efficient Removal of Organic Dyes from Aqueous Medium by Adsorption: Role of Organic Ligand and Calcination Temperature. J. Mol. Liq. 2024, 403, 124852. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Omran, H.A.; Hassan, M.K.; Mohamed, A.F.; Backar, A.H.; Irfan, O.M.; Mohamed, O.A. Graphite-TiO2-Doped Coated Sand Granules for Efficient Continuous Removal of Methylene Blue Dye: Combining Adsorption and Photocatalytic Degradation. Results Eng. 2023, 20, 101646. [Google Scholar] [CrossRef]
- Jiang, R.; Shen, T.T.; Zhu, H.Y.; Fu, Y.Q.; Jiang, S.T.; Li, J.B.; Wang, J.L. Magnetic Fe3O4 Embedded Chitosan–Crosslinked-Polyacrylamide Composites with Enhanced Removal of Food Dye: Characterization, Adsorption and Mechanism. Int. J. Biol. Macromol. 2023, 227, 1234–1244. [Google Scholar] [CrossRef]
- Salah Elbanna, E.; Farghali, A.A.; Khedr, M.H.; Taha, M. Nano Clinoptilolite Zeolite as a Sustainable Adsorbent for Dyes Removal: Adsorption and Computational Mechanistic Studies. J. Mol. Liq. 2024, 409, 125538. [Google Scholar] [CrossRef]
- Jia, X.; Kanbaiguli, M.; Zhang, B.; Huang, Y.; Peydayesh, M.; Huang, Q. Anisotropic Chitosan-Nanocellulose/Zeolite Imidazolate Frameworks-8 Aerogel for Sustainable Dye Removal. J. Colloid Interface Sci. 2024, 676, 298–309. [Google Scholar] [CrossRef]
- Katowah, D.F.; Al-zahrani, H.K. A New Ternary Nanocomposites-Based Cellulose Derivatives-CuFe2O4-Zeolite with Ultra-High Adsorption Capacity for Brilliant Green Dye Treatment and Removal from the Aquatic Environment. J. Saudi Chem. Soc. 2023, 27, 101764. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, L.; Wang, L.; Li, X.; Yang, Z.; Yuan, M.; Li, W.; Ma, X.; Gao, Y.; Xiong, H.; et al. Adsorption of Cationic Dyes in Wastewater with Magnetic κ-Carrageenan Nanoparticles. Process Saf. Environ. Prot. 2024, 189, 177–187. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Abdelrahman, E.A.; Shah, R.K.; Abdelhakim, N.A.; Saad, F.A. Facile Synthesis of Novel Nanocomposite Composed of Co3O4, MgO, and Mg3B2O6 for Malachite Green Dye Decontamination from Aqueous Media. Sci. Rep. 2024, 14, 30570. [Google Scholar] [CrossRef]
- Al-Kadhi, N.S.; Abdelrahman, E.A.; Alamro, F.S.; Saad, F.A.; Al-Raimi, D.S. Facile Synthesis of MnCO3/ZrO2/MgCO3 Nanocomposite for High- Efficiency Malachite Green Dye Removal. J. Inorg. Organomet. Polym. Mater. 2025, in press. [Google Scholar] [CrossRef]
- Abbas, M.; Trari, M. Adsorption Behavior of Methylene Blue Onto Activated Coconut Shells: Kinetic, Thermodynamic, Mechanism and Regeneration of the Adsorbent. Dose-Response 2024, 22, 1–18. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, X.; Zhou, S. Adsorption of Methylene Blue in Water onto Activated Carbon by Surfactant Modification. Water 2020, 12, 587. [Google Scholar] [CrossRef]
- Mennas, N.; Lahreche, S.; Chouli, F.; Sabantina, L.; Benyoucef, A. Adsorption of Methylene Blue Dye by Cetyltrimethylammonium Bromide Intercalated Polyaniline-Functionalized Montmorillonite Clay Nanocomposite: Kinetics, Isotherms, and Mechanism Study. Polymers 2023, 15, 3518. [Google Scholar] [CrossRef] [PubMed]
- Zango, Z.U.; Dennis, J.O.; Aljameel, A.I.; Usman, F.; Ali, M.K.M.; Abdulkadir, B.A.; Algessair, S.; Aldaghri, O.A.; Ibnaouf, K.H. Effective Removal of Methylene Blue from Simulated Wastewater Using ZnO-Chitosan Nanocomposites: Optimization, Kinetics, and Isotherm Studies. Molecules 2022, 27, 4746. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Cuenca, G. Enhancing Methylene Blue Removal through Adsorption and Photocatalysis—A Study on the GO/ZnTiO3/TiO2 Composite. Int. J. Mol. Sci. 2024, 25, 4367. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, F.; Wei, W.; Gao, H.; Zhang, K.; Zhang, G.; Xu, Y.; Zhang, P. Efficient and Fast Adsorption of Methylene Blue Dye onto a Nanosheet MFI Zeolite. J. Solid. State Chem. 2021, 295, 121917. [Google Scholar] [CrossRef]
- Hadda Aya, H.; Djamel, N.; Samira, A.; Otero, M.; Ali Khan, M. Optimizing Methylene Blue Adsorption Conditions on Hydrothermally Synthesized NaX Zeolite through a Full Two-Level Factorial Design. RSC Adv. 2024, 14, 23816–23827. [Google Scholar] [CrossRef]







| Sample | Atomic Percentages | ||||
|---|---|---|---|---|---|
| % O | % Na | % Mg | % Al | % Si | |
| Z1 | 59.3 | 8.1 | 8.6 | 3.4 | 20.6 |
| Z2 | 55.2 | 11.5 | 6.0 | 4.9 | 22.4 |
| Sample | QExp (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| K1 (1/min) | R2 | Qe (mg/g) | K2 (g/mg·min) | R2 | Qe (mg/g) | ||
| Z1 | 225.66 | 0.0262 | 0.9438 | 91.71 | 0.0007979 | 0.9999 | 220.75 |
| Z2 | 285.28 | 0.0476 | 0.9894 | 62.59 | 0.0018770 | 0.9999 | 286.53 |
| Sample | ∆So (KJ/molK) | ∆Ho (KJ/mol) | ∆Go (KJ/mol) | |||
|---|---|---|---|---|---|---|
| 298 | 308 | 318 | 328 | |||
| Z1 | 0.1308 | −43.57 | −82.55 | −83.85 | −85.16 | −86.47 |
| Z2 | 0.1215 | −45.25 | −81.46 | −82.89 | −83.89 | −85.11 |
| Sample | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| Qmax (mg/g) | R2 | K3 (L/mg) | K4 (mg/g)(L/mg)1/n | Qmax (mg/g) | 1/n | R2 | |
| Z1 | 230.95 | 0.9997 | 0.3077 | 91.31 | 258.53 | 0.1964 | 0.7219 |
| Z2 | 290.69 | 0.9999 | 1.0886 | 144.36 | 345.05 | 0.1645 | 0.6641 |
| Adsorbent | Qmax (mg/g) | Ref. |
|---|---|---|
| Activated coconut shells | 30.30 | [34] |
| Activated carbon/sodium lauryl sulfate composite | 232.5 | [35] |
| Cetyltrimethylammonium bromide/montmorillonite/polyaniline composite | 61.30 | [36] |
| ZnO/chitosan composite | 97.93 | [37] |
| Graphene oxide/ZnTiO3/TiO2 composite | 78.00 | [38] |
| ZSM-5 zeolite | 105.82 | [39] |
| NaX zeolite | 24.39 | [40] |
| Z1 | 230.95 | This study |
| Z2 | 290.69 | This study |
| Effect | V (L) | Co (mg/L) | W (mg) | T (K) | t (min) | pH |
|---|---|---|---|---|---|---|
| pH | 0.1 | 150 | 50 | 298 | 360 | 2.5–10.5 |
| Time | 0.1 | 150 | 50 | 298 | 10–100 | 10.5 |
| Temperature | 0.1 | 150 | 50 | 298–328 | 70 (Z1) 50 (Z2) | 10.5 |
| Concentration | 0.1 | 50–300 | 50 | 298 | 70 (Z1) 50 (Z2) | 10.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrahman, E.A.; Alqahtani, Z.; Abou-Krisha, M.M.; Saad, F.A.; Shah, R.K. Facile Synthesis and Characterization of Novel Analcime/Sodium Magnesium Aluminum Silicon Silicate Nanocomposite for Efficient Removal of Methylene Blue Dye from Aqueous Media. Molecules 2025, 30, 1488. https://doi.org/10.3390/molecules30071488
Abdelrahman EA, Alqahtani Z, Abou-Krisha MM, Saad FA, Shah RK. Facile Synthesis and Characterization of Novel Analcime/Sodium Magnesium Aluminum Silicon Silicate Nanocomposite for Efficient Removal of Methylene Blue Dye from Aqueous Media. Molecules. 2025; 30(7):1488. https://doi.org/10.3390/molecules30071488
Chicago/Turabian StyleAbdelrahman, Ehab A., Zahrah Alqahtani, Mortaga M. Abou-Krisha, Fawaz A. Saad, and Reem K. Shah. 2025. "Facile Synthesis and Characterization of Novel Analcime/Sodium Magnesium Aluminum Silicon Silicate Nanocomposite for Efficient Removal of Methylene Blue Dye from Aqueous Media" Molecules 30, no. 7: 1488. https://doi.org/10.3390/molecules30071488
APA StyleAbdelrahman, E. A., Alqahtani, Z., Abou-Krisha, M. M., Saad, F. A., & Shah, R. K. (2025). Facile Synthesis and Characterization of Novel Analcime/Sodium Magnesium Aluminum Silicon Silicate Nanocomposite for Efficient Removal of Methylene Blue Dye from Aqueous Media. Molecules, 30(7), 1488. https://doi.org/10.3390/molecules30071488







