Local Interactions in Aqueous Ethanol Solution Revealed by the C=O Stretching Probe
Abstract
:1. Introduction
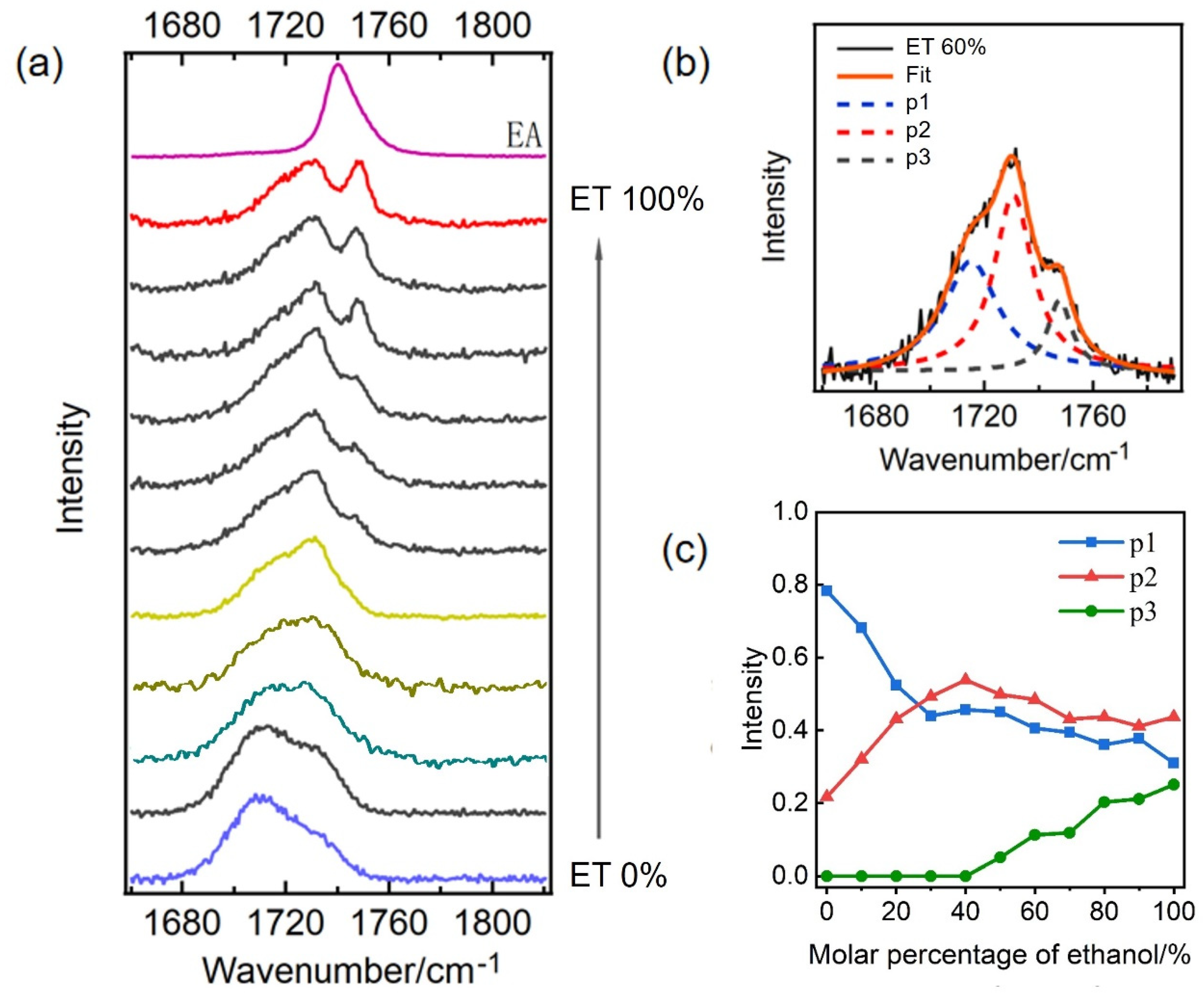
2. Results and Discussion
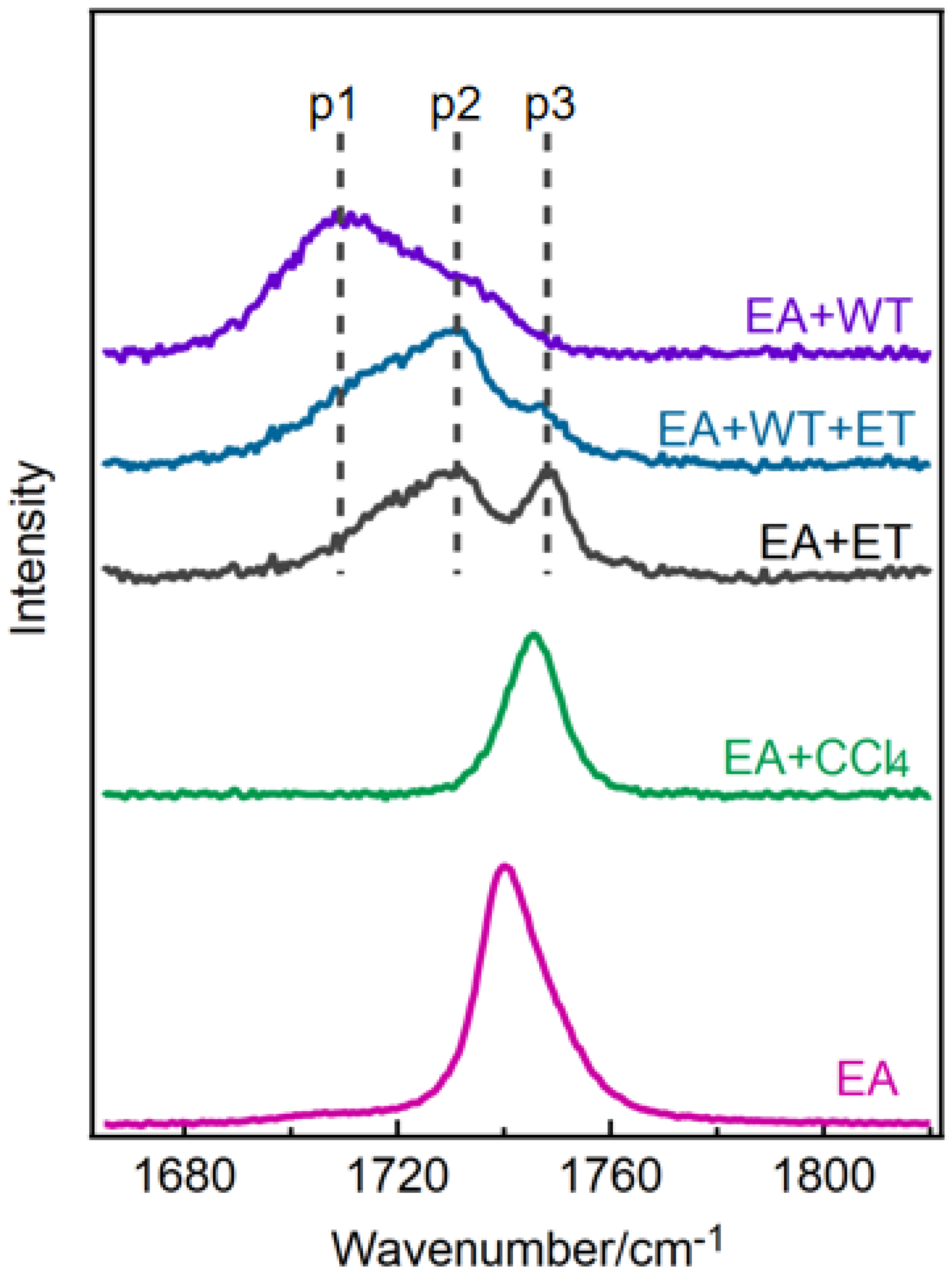
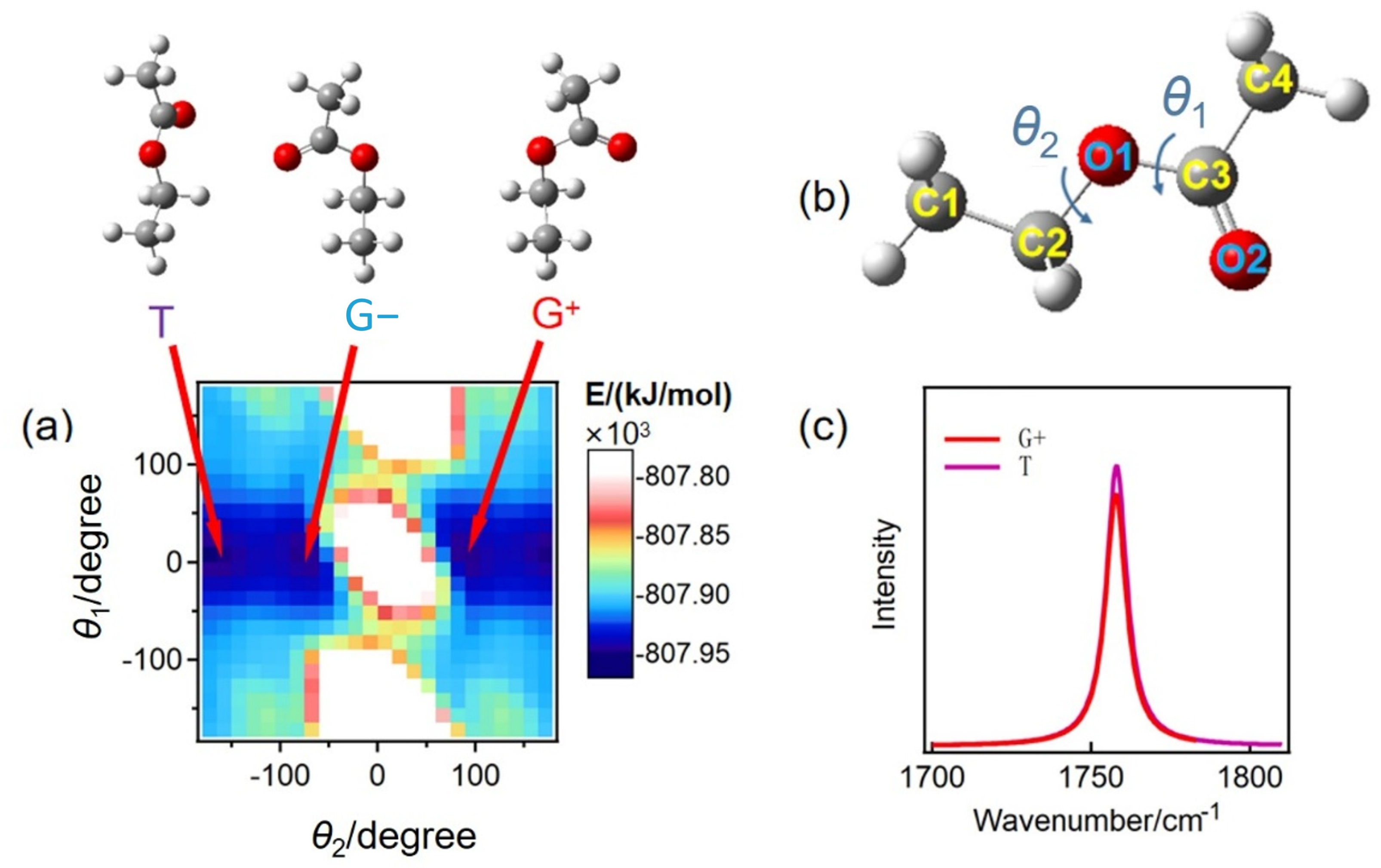
2.1. The Effect of Different Ethyl Acetate Conformers
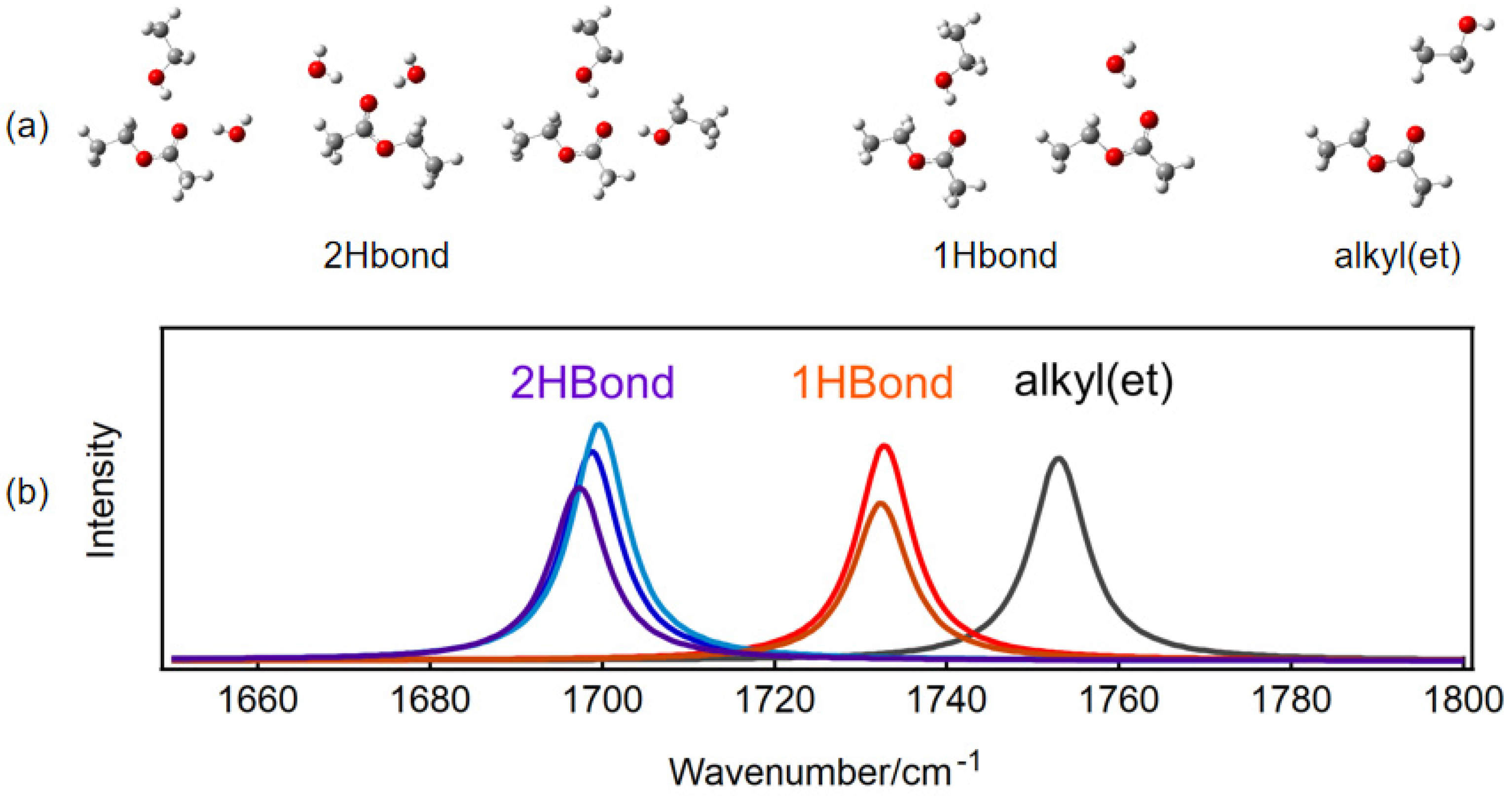
2.2. The Effect of Hydrophobic Alkyl Groups of Ethanol
3. Methods
3.1. Experimental Sections
3.2. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nature 2005, 437, 640–647. [Google Scholar] [PubMed]
- Sun, Q. The Hydrophobic Effects: Our Current Understanding. Molecules 2022, 27, 7009. [Google Scholar] [CrossRef]
- Pant, S.; Wainberg, Z.A.; Weekes, C.D.; Furqan, M.; Kasi, P.M.; Devoe, C.E.; Leal, A.D.; Chung, V.; Basturk, O.; VanWyk, H.; et al. Lymph-node-targeted, mKRAS-specific amphiphile vaccine in pancreatic and colorectal cancer: The phase 1 AMPLIFY-201 trial. Nat. Med. 2024, 30, 531–542. [Google Scholar]
- Li, Z.; Lin, Z. Self-Assembly of Bolaamphiphiles into 2D Nanosheets via Synergistic and Meticulous Tailoring of Multiple Noncovalent Interactions. ACS Nano 2021, 15, 3152–3160. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, X.; Zeng, X. Multiscale simulation and kinetic network model analysis of the self-assembly of amphiphilic systems. Sci. Sin. Chim. 2020, 50, 1118–1131. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Magazù, S.; Calandra, P. Amphiphiles Self-Assembly: Basic Concepts and Future Perspectives of Supramolecular Approaches. Adv. Condens. Matter Phys. 2015, 2015, 151683. [Google Scholar] [CrossRef]
- Hu, X.; Liu, G.; Li, Y.; Wang, X.; Liu, S. Cell-Penetrating Hyperbranched Polyprodrug Amphiphiles for Synergistic Reductive Milieu-Triggered Drug Release and Enhanced Magnetic Resonance Signals. J. Am. Chem. Soc. 2014, 137, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.; Ulijn, R.V. Design of nanostructures based on aromatic peptide amphiphiles. Chem. Soc. Rev. 2014, 43, 8150–8177. [Google Scholar]
- Meyer, E.E.; Rosenberg, K.J.; Israelachvili, J. Recent progress in understanding hydrophobic interactions. Proc. Natl. Acad. Sci. USA 2006, 103, 15739–15746. [Google Scholar]
- Long, J.A.; Rankin, B.M.; Ben-Amotz, D. Micelle Structure and Hydrophobic Hydration. J. Am. Chem. Soc. 2015, 137, 10809–10815. [Google Scholar]
- Egli, J.; Siebler, C.; Kohler, M.; Zenobi, R.; Wennemers, H. Hydrophobic Moieties Bestow Fast-Folding and Hyperstability on Collagen Triple Helices. J. Am. Chem. Soc. 2019, 141, 5607–5611. [Google Scholar] [CrossRef] [PubMed]
- Honciuc, A.; Schwartz, D.K. Probing Hydrophobic Interactions Using Trajectories of Amphiphilic Molecules at a Hydrophobic/Water Interface. J. Am. Chem. Soc. 2009, 131, 5973–5979. [Google Scholar] [PubMed]
- Matysiak, S.; Debenedetti, P.G.; Rossky, P.J. Role of hydrophobic hydration in protein stability: A 3D water-explicit protein model exhibiting cold and heat denaturation. J. Phys. Chem. B 2012, 116, 8095–8104. [Google Scholar]
- Honeycutt, J.D.; Thirumalai, D. The nature of folded states of globular proteins. Biopolymers 1992, 32, 695–709. [Google Scholar]
- Zhong, Y.; Patel, S. Electrostatic Polarization Effects and Hydrophobic Hydration in Ethanol-Water Solutions from Molecular Dynamics Simulations. J. Phys. Chem. B 2009, 113, 767–778. [Google Scholar]
- Juurinen, I.; Pylkkänen, T.; Sahle, C.J.; Simonelli, L.; Hämäläinen, K.; Huotari, S.; Hakala, M. Effect of the Hydrophobic Alcohol Chain Length on the Hydrogen-Bond Network of Water. J. Phys. Chem. B 2014, 118, 8750–8755. [Google Scholar]
- Tan, M.L.; Cendagorta, J.R.; Ichiye, T. Effects of Microcomplexity on Hydrophobic Hydration in Amphiphiles. J. Am. Chem. Soc. 2013, 135, 4918–4921. [Google Scholar] [PubMed]
- Robalo, J.R.; Streacker, L.M.; de Oliveira, D.M.; Imhof, P.; Ben-Amotz, D.; Verde, A.V. Hydrophobic but Water-Friendly: Favorable Water-Perfluoromethyl Interactions Promote Hydration Shell Defects. J. Am. Chem. Soc. 2019, 141, 15856–15868. [Google Scholar]
- Hazra, M.K.; Bagchi, B. Collective excitations and ultrafast dipolar solvation dynamics in water-ethanol binary mixture. J. Chem. Phys. 2018, 148, 114506. [Google Scholar]
- Matisz, G.; Kelterer, A.M.; Fabian, W.M.; Kunsagi-Mate, S. Application of the Quantum Cluster Equilibrium (QCE) model for the liquid phase of primary alcohols using B3LYP and B3LYP-D DFT methods. J. Phys. Chem. B 2011, 115, 3936–3941. [Google Scholar]
- Sum, A.K.; Sandler, S.I. Ab initio calculations of cooperativity effects on clusters of methanol, ethanol, 1-propanol and methanethiol. J. Phys. Chem. A 2000, 104, 1121–1129. [Google Scholar]
- Böhm, F.; Schwaab, G.; Havenith, M. Kartierung des Hydratwassers um Alkoholketten mittels THz-Kalorimetrie. Angew. Chem. 2017, 129, 10113–10117. [Google Scholar]
- Galamba, N. Water’s structure around hydrophobic solutes and the iceberg model. J. Phys. Chem. B 2013, 117, 2153–2159. [Google Scholar]
- Chen, L.; Zhu, W.; Lin, K.; Hu, N.; Yu, Y.; Zhou, X.; Yuan, L.F.; Hu, S.M.; Luo, Y. Identification of alcohol conformers by Raman spectra in the C-H stretching region. J. Phys. Chem. A 2015, 119, 3209–3217. [Google Scholar]
- Losada, M.; Tran, H.; Xu, Y. Lactic acid in solution: Investigations of lactic acid self-aggregation and hydrogen bonding interactions with water and methanol using vibrational absorption and vibrational circular dichroism spectroscopies. J. Chem. Phys. 2008, 128, 014508. [Google Scholar] [PubMed]
- Losada, M.; Xu, Y. Chirality transfer through hydrogen-bonding: Experimental and ab initio analyses of vibrational circular dichroism spectra of methyl lactate in water. Phys. Chem. Chem. Phys. 2007, 9, 3127–3135. [Google Scholar]
- Kaminsky, J.; Horackova, F.; Biackova, N.; Hubackova, T.; Socha, O.; Kubelka, J. Double Hydrogen Bonding Dimerization Propensity of Aqueous Hydroxy Acids Investigated Using Vibrational Optical Activity. J. Phys. Chem. B 2021, 125, 11350–11363. [Google Scholar] [PubMed]
- Heisler, I.A.; Mazur, K.; Yamaguchi, S.; Tominaga, K.; Meech, S.R. Measuring acetic acid dimer modes by ultrafast time-domain Raman spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 15573–15579. [Google Scholar] [CrossRef]
- Polavarapu, P.L.; Johnson, J.L.; Raghavan, V.; Zając, G.; Baranska, M. Vibrational Raman optical activity of diacetyl L-tartaric acid and corresponding surfactants: Sodium salts and shorter analogs of surfactants simplify the interpretations. J. Raman Spectrosc. 2022, 53, 1102–1114. [Google Scholar]
- Weirich, L.; Merten, C. Solvation and self-aggregation of chiral alcohols: How hydrogen bonding affects their VCD spectral signatures. Phys. Chem. Chem. Phys. 2019, 21, 13494–13503. [Google Scholar]
- Bunnemann, K.; Merten, C. Solvation of a chiral carboxylic acid: Effects of hydrogen bonding on the IR and VCD spectra of alpha-methoxyphenylacetic acid. Phys. Chem. Chem. Phys. 2017, 19, 18948–18956. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari-Bafrooei, A.; Nihonyanagi, S.; Borguet, E. Spectroscopy and Dynamics of the Multiple Free OH Species at an Aqueous/Hydrophobic Interface. J. Phys. Chem. C 2012, 116, 21734–21741. [Google Scholar] [CrossRef]
- Mishra, A.; Mohapatra, H.; Pradhan, M. Study of the perturbed hydrogen-bonded water structure of the hydration shell of N-Methyl-Acetamide by Raman multivariate curve resolution spectroscopy. J. Mol. Liq. 2024, 410, 125574. [Google Scholar] [CrossRef]
- Xu, X.; Jin, X.; Kuehne, M.; Bao, D.-L.; Martis, J.; Tu, Y.-M.; Ritt, C.L.; Idrobo, J.C.; Strano, M.S.; Majumdar, A.; et al. Hydrogen bonding in water under extreme confinement unveiled by nanoscale vibrational spectroscopy and simulations. arXiv 2024. [Google Scholar] [CrossRef]
- Pazos, I.M.; Ghosh, A.; Tucker, M.J.; Gai, F. Ester carbonyl vibration as a sensitive probe of protein local electric field. Angew. Chem. Int. Ed. Engl. 2014, 53, 6080–6084. [Google Scholar] [CrossRef]
- Chuntonov, L.; Pazos, I.M.; Ma, J.; Gai, F. Kinetics of exchange between zero-, one-, and two-hydrogen-bonded states of methyl and ethyl acetate in methanol. J. Phys. Chem. B 2015, 119, 4512–4520. [Google Scholar] [CrossRef]
- Jumabaev, A.; Koyambo-Konzapa, S.-J.; Hushvaktov, H.; Absanov, A.; Khudaykulov, B.; Holikulov, U.; Ernazarov, Z.; Issaoui, N.; Al-Dossary, O.M.; Nsangou, M. Intermolecular interactions in water and ethanol solution of ethyl acetate: Raman, DFT, MEP, FMO, AIM, NCI-RDG, ELF, and LOL analyses. J. Mol. Model. 2024, 30, 349. [Google Scholar] [CrossRef]
- Dahlqvist, M.; Hotokka, M.; Räsänen, M. UV-Induced Rotamerization and Vibrational Spectra of the Conformers of Cyanomethyl Formate: Matrix Isolation Infrared and ab Initio Studies. J. Phys. Chem. A 1997, 101, 1260–1266. [Google Scholar] [CrossRef]
- Kolling, O.W. FTIR study of the solvent influence on the carbonyl absorption peak of ethyl acetate. J. Phys. Chem. 1992, 96, 6217–6220. [Google Scholar] [CrossRef]
- Kagarise, R.E.; Whetsel, K.B. Solvent effects on infrared frequencies—III. Spectrochim. Acta 1962, 18, 341–352. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. PhRvB 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Musso, M.; Giorgini, M.G.; Asenbaum, A. The non-coincidence effect in highly diluted acetone-CCl I. Experimental results and theoretical predictions 4 binary mixtures. Mol. Phys. 2010, 92, 97–104. [Google Scholar]
- Tukhvatullin, F.H.; Andrews, D.L.; Jumabaev, A.; Tashkenbaev, U.N.; Hushvaktov, H.A.; Absanov, A.A. Polarized components of C=O vibrations Raman spectra for ethylacetate, acetone, and aggregation of molecules. In Proceedings of the International Symposium on Optical Science and Technology, Proceedings of SPIE, Seattle, WA, USA, 7–11 July 2002; Volume 4812, pp. 132–138. [Google Scholar]
- Murphy, W.F. The Rayleigh depolarization ratio and rotational Raman spectrum of water vapor and the polarizability components for the water molecule. J. Chem. Phys. 1977, 67, 5877–5882. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Chen, C.; Zhang, R.; Ma, L.; Lin, K. Local Interactions in Aqueous Ethanol Solution Revealed by the C=O Stretching Probe. Molecules 2025, 30, 1524. https://doi.org/10.3390/molecules30071524
Wang Z, Chen C, Zhang R, Ma L, Lin K. Local Interactions in Aqueous Ethanol Solution Revealed by the C=O Stretching Probe. Molecules. 2025; 30(7):1524. https://doi.org/10.3390/molecules30071524
Chicago/Turabian StyleWang, Zhiqiang, Chi Chen, Ruiting Zhang, Lin Ma, and Ke Lin. 2025. "Local Interactions in Aqueous Ethanol Solution Revealed by the C=O Stretching Probe" Molecules 30, no. 7: 1524. https://doi.org/10.3390/molecules30071524
APA StyleWang, Z., Chen, C., Zhang, R., Ma, L., & Lin, K. (2025). Local Interactions in Aqueous Ethanol Solution Revealed by the C=O Stretching Probe. Molecules, 30(7), 1524. https://doi.org/10.3390/molecules30071524




