DNA/BSA Binding Affinity and Cytotoxicity of Dinuclear Palladium(II) Complexes with Amino Acids as Ligands
Abstract
:1. Introduction
2. Results and Discussion
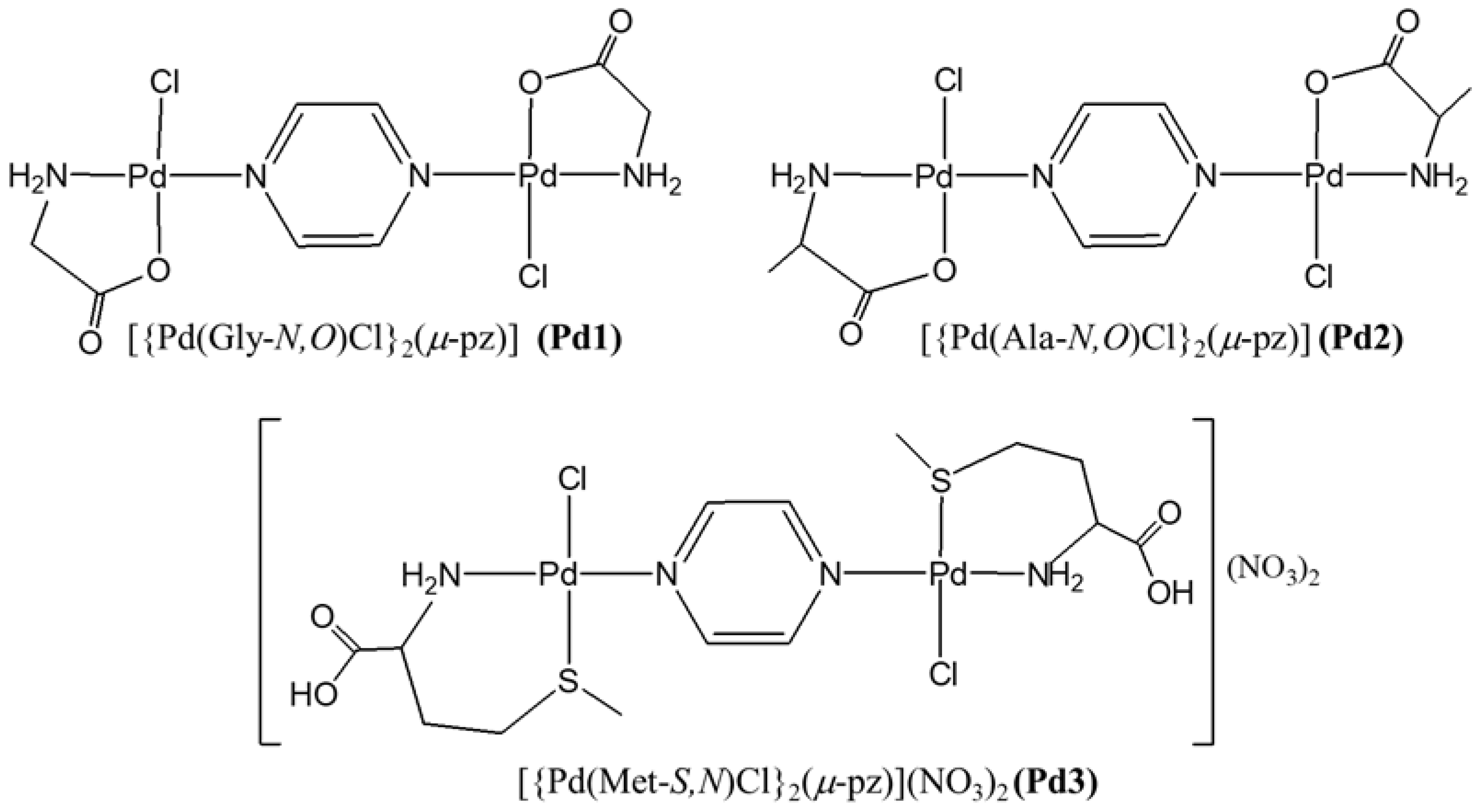
2.1. Synthesis and Characterization of [{Pd(Gly-N,O)Cl}2(µ-pz)], [{Pd(Ala-N,O)Cl}2(µ-pz)], and [{Pd(Met-S,N)Cl}2(µ-pz)](NO3)2 Complexes
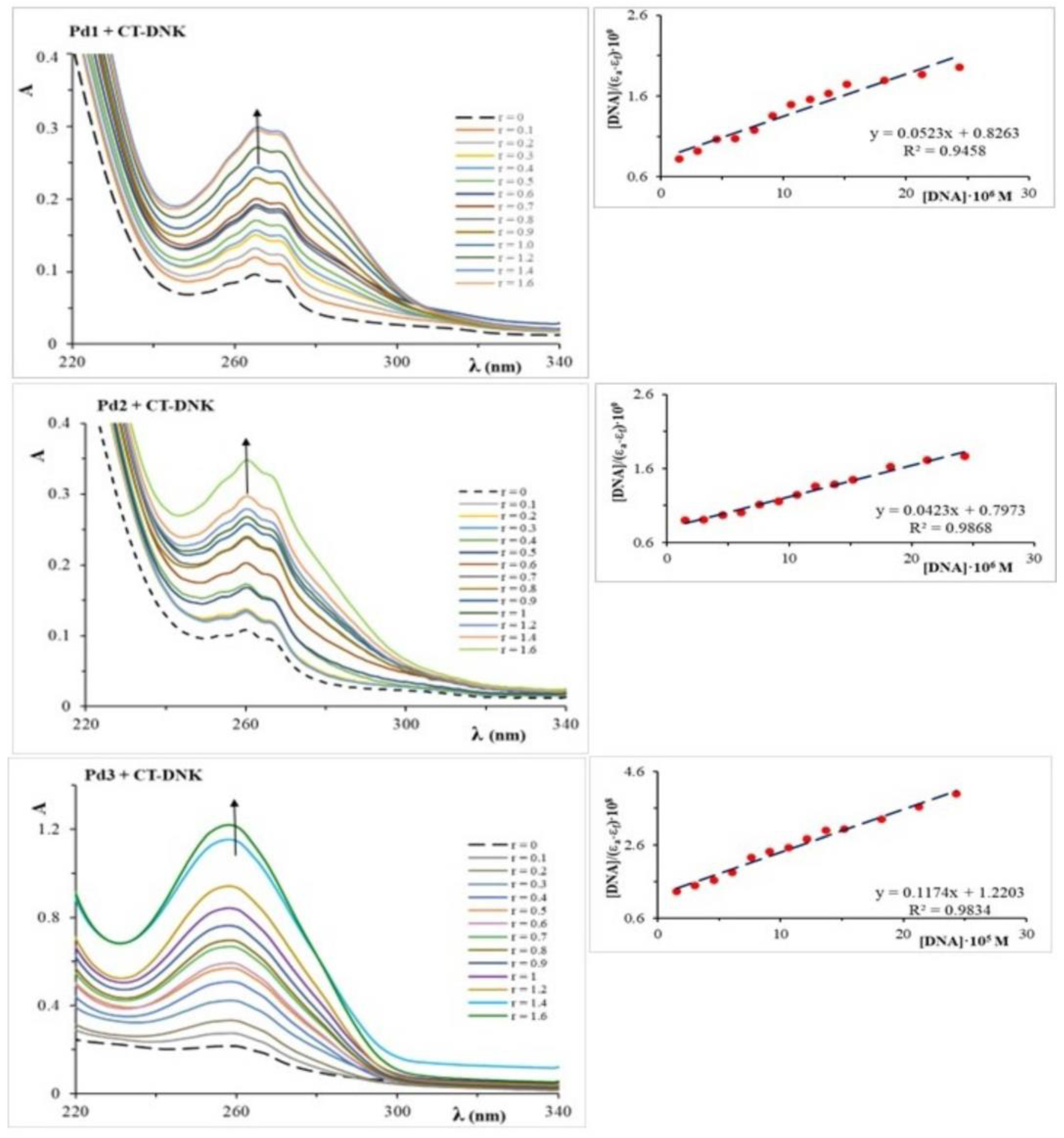
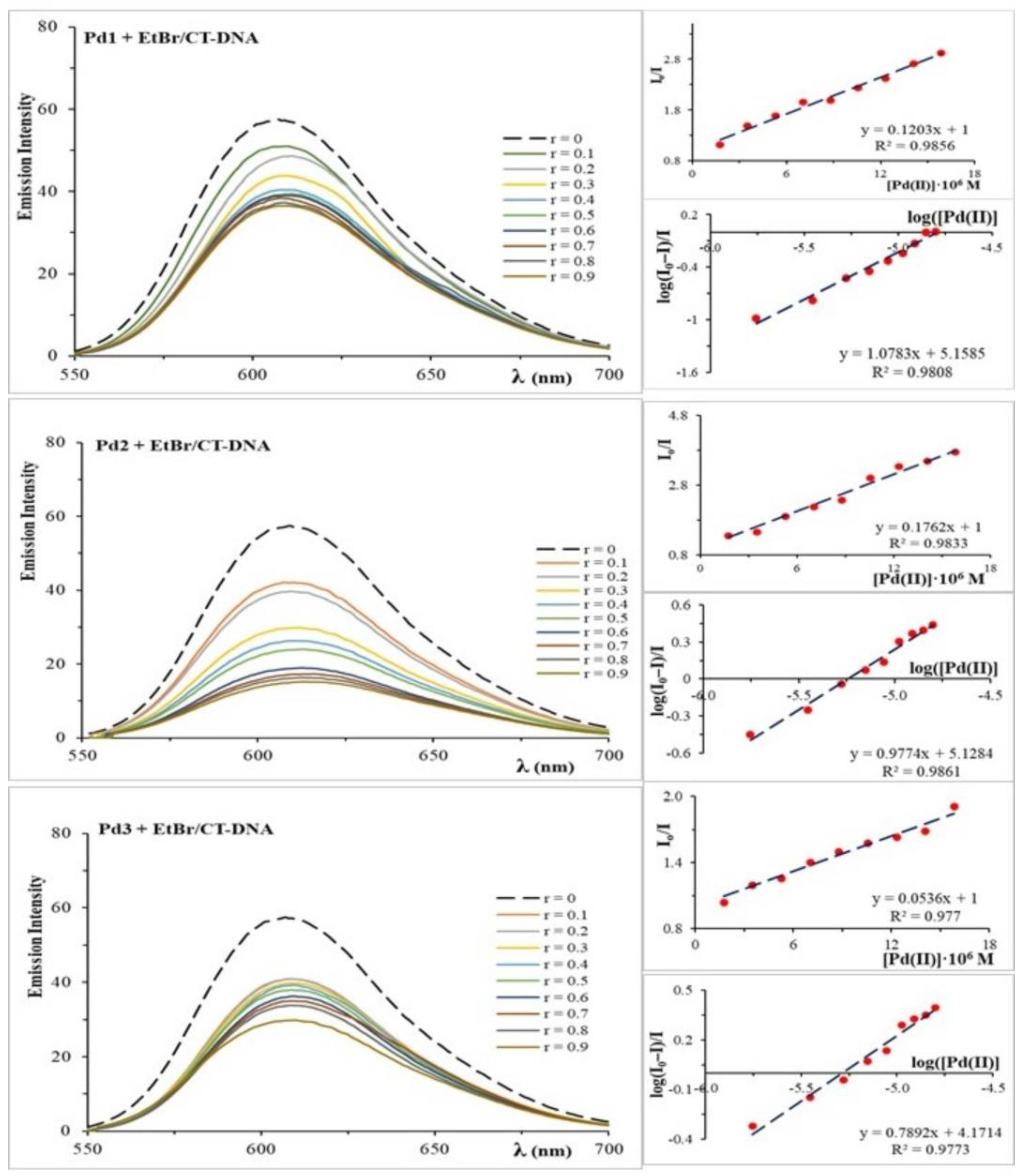
2.2. Analysis of DNA Binding and Interactions
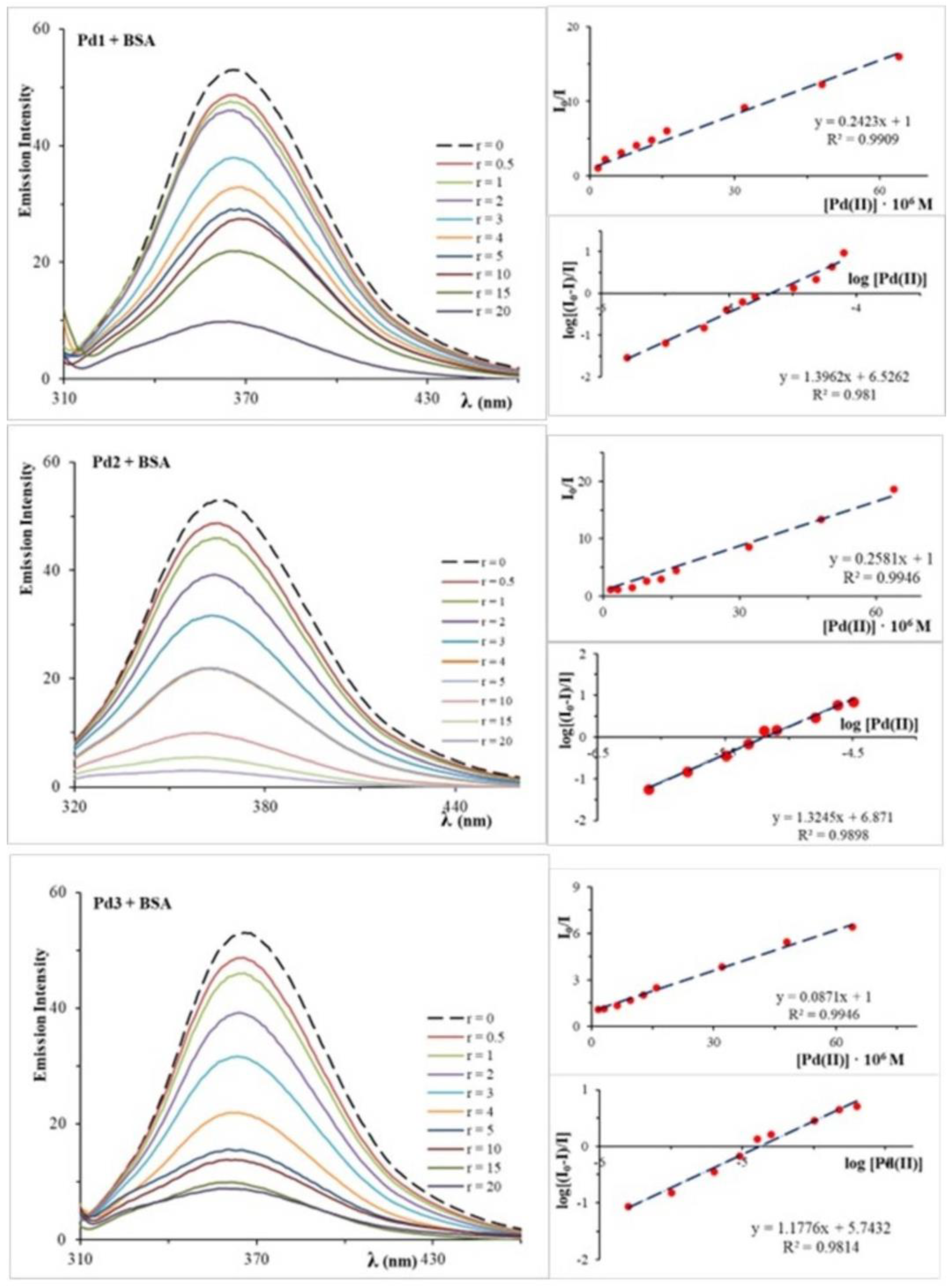
2.3. Analysis of BSA Interactions
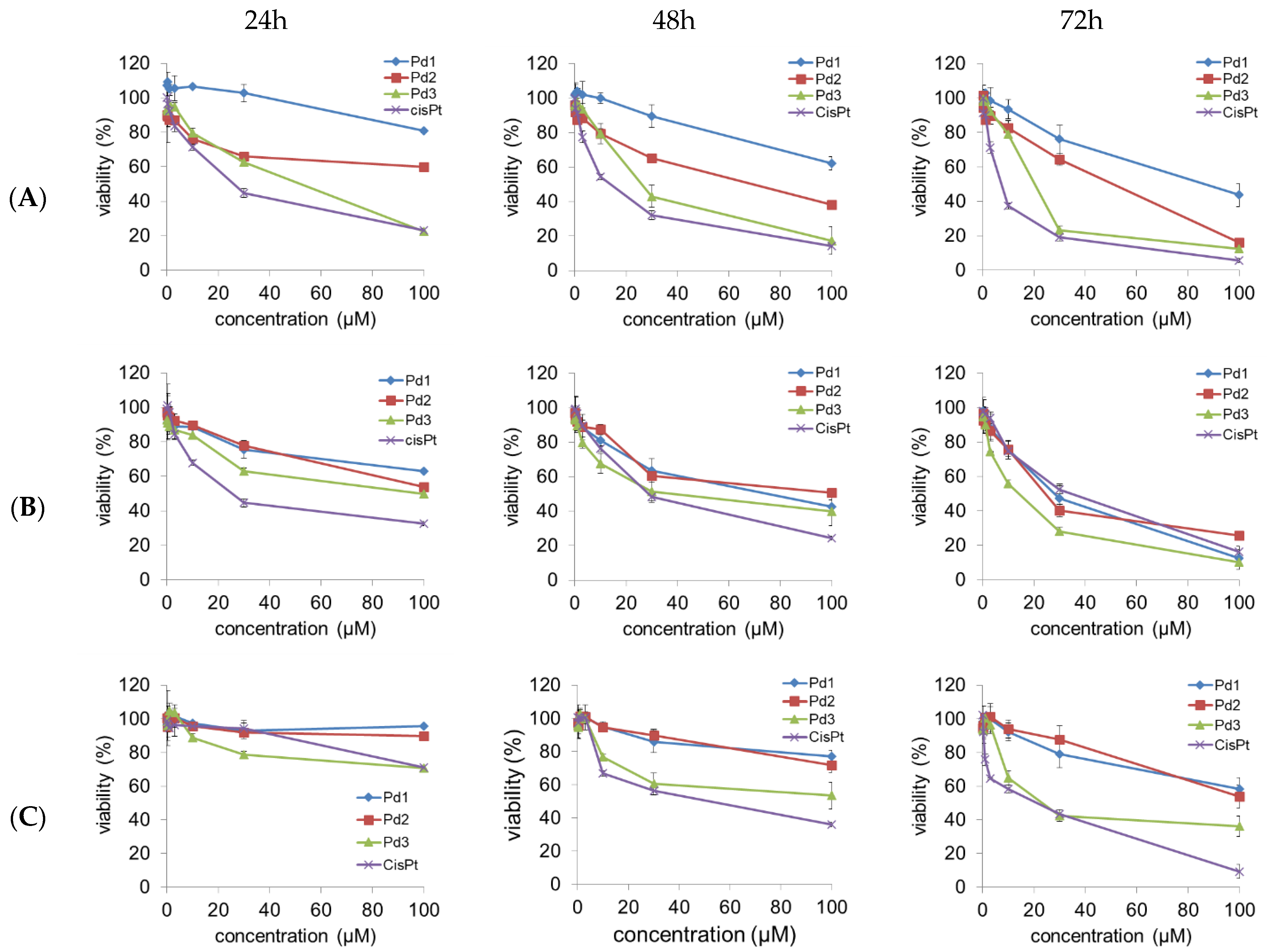
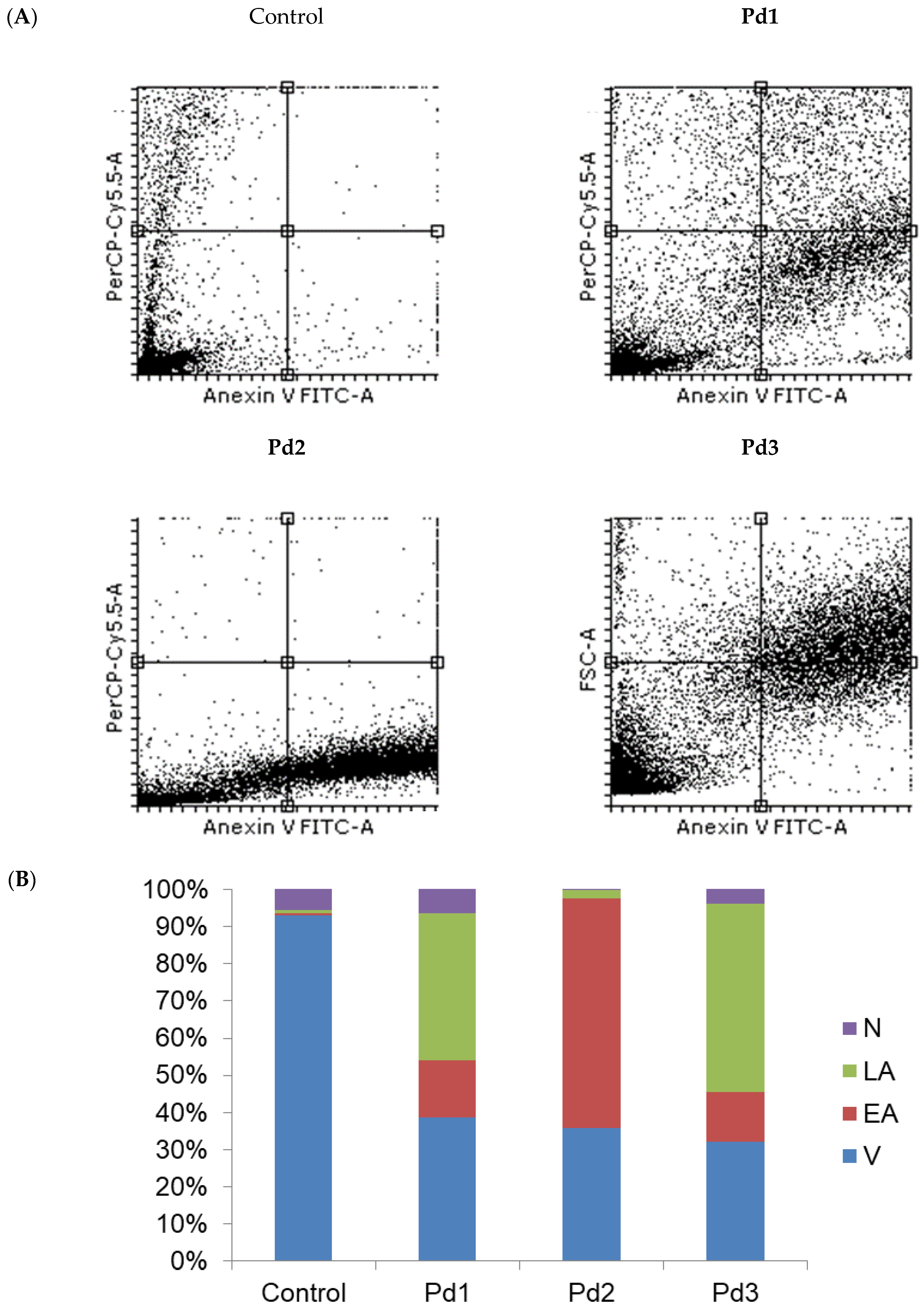
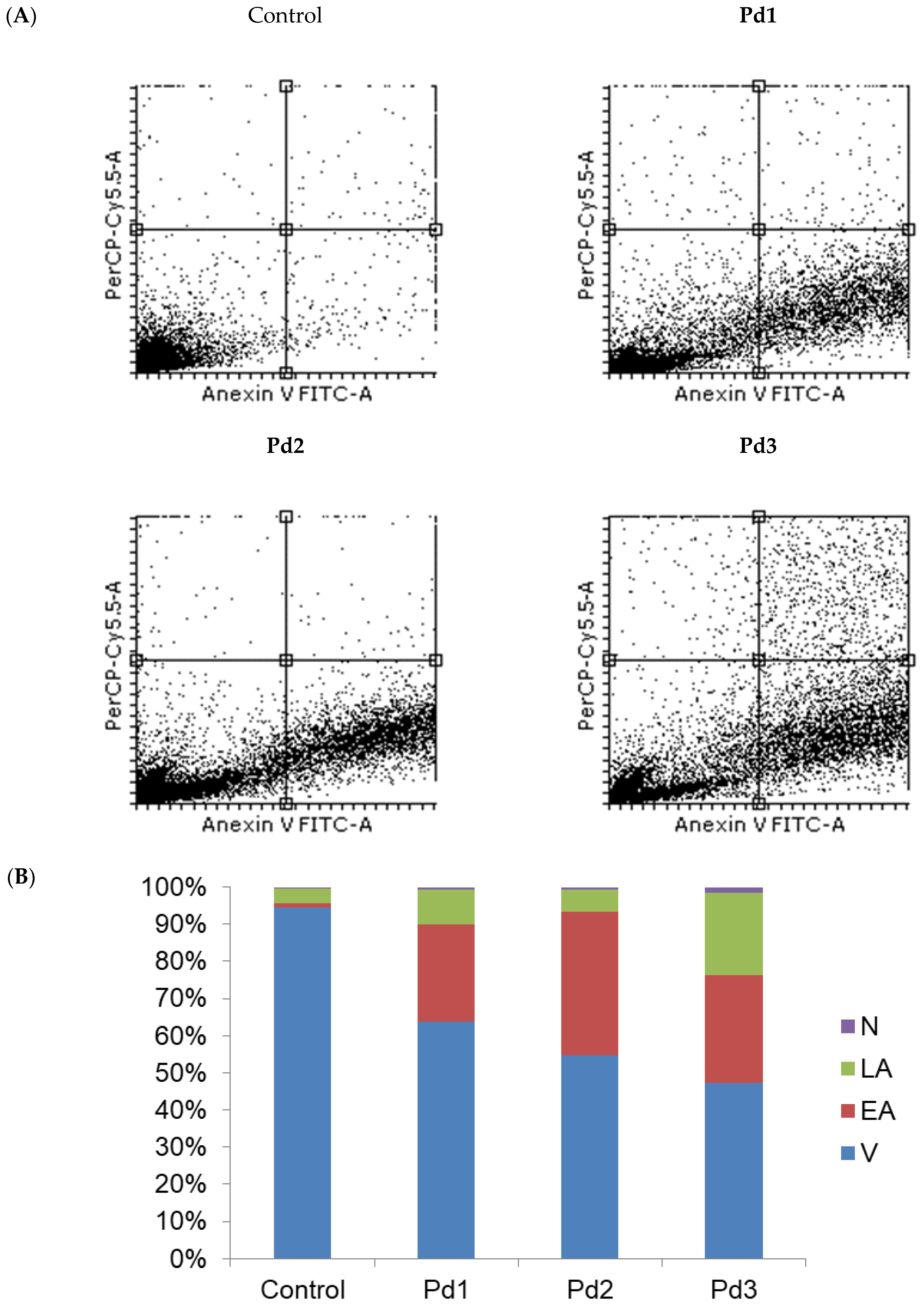
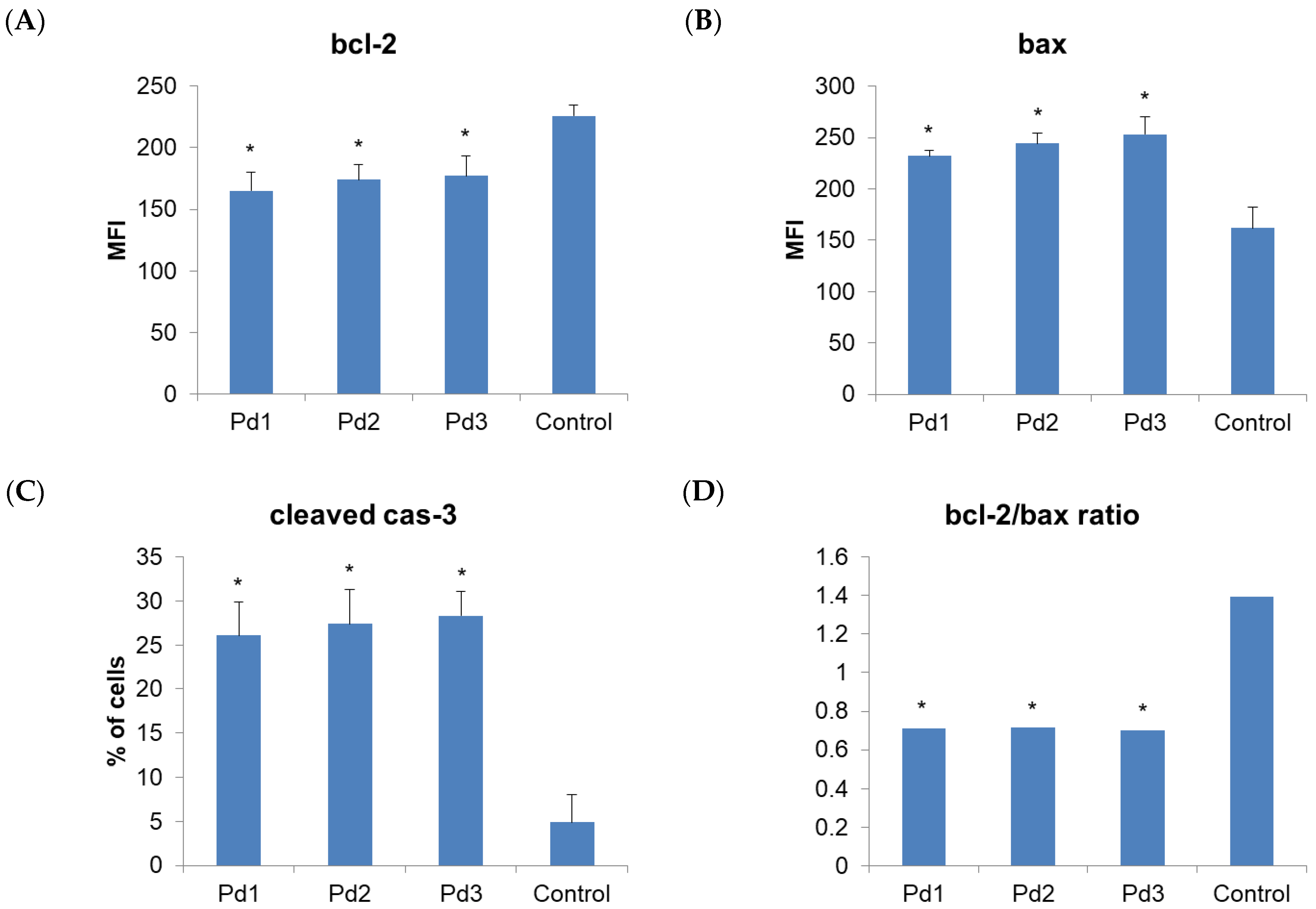
2.4. Cytotoxicity of Pd(II) Complexes, Effect on Apoptosis, and Effect on Cell Cycle
3. Materials and Methods
3.1. Chemicals and Equipment
3.2. Synthesis of [{Pd(Gly-N,O)Cl}2(µ-pz)], [{Pd(Ala-N,O)Cl}2(µ-pz)], and [{Pd(Met-S,N)Cl}2(µ-pz)](NO3)2 Complexes
3.3. Analysis of DNA Binding and Interactions
3.3.1. Absorption Spectroscopic Measurements
3.3.2. Competitive Fluorescence Measurements
3.4. Analysis of BSA Interactions
3.5. Cytotoxicity Assay
3.5.1. Cell Cultures
3.5.2. MTT Assay
3.5.3. Annexin V/7AAD Assay
3.5.4. Assessment of Apoptosis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Komeda, S.; Lutz, M.; Spek, A.L.; Chikuma, M.; Reedijk, J. New Antitumor-Active Azole-Bridged Dinuclear Platinum(II) Complexes: Synthesis, Characterization, Crystal Structures, and Cytotoxic Studies. J. Inorg. Chem. 2000, 39, 4230. [Google Scholar] [CrossRef]
- Wheate, N.J.; Cullinane, C.; Webster, L.K.; Collins, J.G. Synthesis, Cytotoxicity, Cell Uptake and DNA Interstrand Cross-Linking of 4,4′-Dipyrazolylmethane-Linked Multinuclear Platinum Anti-Cancer Complexes. Anti-Cancer Drug Des. 2001, 16, 91. [Google Scholar]
- Garoufis, A.; Hadjikakou, S.K.; Hadjiliadis, N. Palladium Coordination Compounds as Anti-Viral, Anti-Fungal, Anti-Microbial and Anti-Tumor Agents. Coord. Chem. Rev. 2009, 253, 1384. [Google Scholar] [CrossRef]
- Faraglia, G.; Fregona, D.; Sitran, S.; Giovagnini, L.; Marzano, C.; Baccichetti, F.; Casellato, U.; Graziani, R. Platinum(II) and Palladium(II) Complexes with Dithiocarbamates and Amines: Synthesis, Characterization and Cell Assay. J. Inorg. Biochem. 2001, 83, 31. [Google Scholar] [CrossRef]
- Franich, A.A.; Živković, M.D.; Ćoćić, D.; Petrović, B.; Milovanović, M.; Arsenijević, A.; Milovanović, J.; Arsenijević, D.; Stojanović, B.; Djuran, M.I.; et al. New Dinuclear Palladium(II) Complexes with Benzodiazines as Bridging Ligands: Interactions with CT-DNA and BSA, and Cytotoxic Activity. J. Biol. Inorg. Chem. 2019, 24, 1009. [Google Scholar] [CrossRef]
- Butour, S.; Wimmer, F.; Wimmer, F.; Castan, P. Palladium(II) Compounds with Potential Antitumor Properties and Their Platinum Analogues: A Comparative Study of the Reaction of Some Orotic Acid Derivatives with DNA in vitro. Chem. Biol. Interact. 1997, 104, 165. [Google Scholar] [CrossRef]
- Wimmer, F.Z.; Wimmer, S.; Castan, P.; Cros, S.; Johnson, N.; Colacio-Rodriguez, E. The Antitumor Activity of Some Palladium(II) Complexes with Chelating Ligands. Anticancer. Res. 1989, 9, 791. [Google Scholar]
- Zhao, G.; Lin, H.; Yu, P.; Sun, H.; Zhu, S.; Su, X.; Chen, Y. Ethylenediamine-Palladium(II) Complexes with Pyridine and Its Derivatives: Synthesis, Molecular Structure and Initial Antitumor Studies. J. Inorg. Biochem. 1999, 73, 145. [Google Scholar] [CrossRef]
- Rodrigues, E.G.; Silva, L.S.; Fausto, D.M.; Hayashi, M.S.; Dreher, S.; Santos, E.L.; Pesquero, J.B.; Travassos Caires, L.R. Cyclopalladated Compounds as Chemotherapeutic Agents: Antitumor Activity Against a Murine Melanoma Cell Line. Int. J. Cancer 2003, 107, 498. [Google Scholar] [CrossRef]
- Eddings, D.; Barnes, C.; Gerasimchuk, N.; Durham, P.; Domasevich, K. First Bivalent Palladium and Platinum Cyanoximates: Synthesis, Characterization, and Biological Activity. Inorg. Chem. 2004, 43, 3894. [Google Scholar] [CrossRef]
- Giovagnini, L.; Ronconi, L.; Aldinucci, D.; Lorenzon, D.; Sitran, S.; Fregona, D. Synthesis, Characterization, and Comparative in Vitro Cytotoxicity Studies of Platinum(II), Palladium(II), and Gold(III) Methylsarcosinedithiocarbamate Complexes. J. Med. Chem. 2005, 48, 1588. [Google Scholar] [CrossRef]
- Cheng, H.; Huq, F.; Beale, P.; Fisher, K. Synthesis, Characterisation, Activities, Cell Uptake and DNA Binding of a Trinuclear Complex: [{trans-PtCl(NH3)}2μ-{trans-Pd(NH3)(2-hydroxypyridine)-(H2N(CH2)6NH2)2]Cl4. Eur. J. Med. Chem. 2006, 41, 896. [Google Scholar] [CrossRef] [PubMed]
- Mavroidi, B.; Sagnou, M.; Stamatakis, K.; Paravatou-Petsotas, M.; Pelecanou, M.; Methenitis, C. Palladium(II) and Platinum(II) Complexes of Derivatives of 2-(4′-Aminophenyl)benzothiazole as Potential Anticancer Agents. Inorg. Chim. Acta 2016, 444, 63–75. [Google Scholar] [CrossRef]
- Abu-Surrah, A.S.; Kettunen, M. Platinum Group Antitumor Chemistry: Design and Development of New Anticancer Drugs Complementary to Cisplatin. Curr. Med. Chem. 2006, 13, 1337. [Google Scholar] [CrossRef]
- Franich, A.A.; Živković, M.D.; Milovanović, J.; Arsenijević, D.; Arsenijević, A.; Milovanović, M.; Djuran, M.I.; Rajković, S. In Vitro Cytotoxic Activities, DNA- and BSA-Binding Studies of Dinuclear Palladium(II) Complexes with Different Pyridine-Based Bridging Ligands. J. Inorg. Biochem. 2020, 210, 111158. [Google Scholar] [CrossRef]
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 Cancer Estimates: Data Sources, Methods, and a Snapshot of the Cancer Burden Worldwide. Int. J. Cancer 2025, 156, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, C.M.; Trougakos, I.P.; Erin, N.; Papageorgis, P. Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance. Cancers 2021, 13, 4363. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Garcia-Saez, A.J. Mechanisms of BCL-2 Family Proteins in Mitochondrial Apoptosis. Nat. Rev. Mol. Cell Biol. 2023, 24, 732–748. [Google Scholar] [CrossRef]
- Asadi, M.; Taghizadeh, S.; Kaviani, E.; Vakili, O.; Taheri-Anganeh, M.; Tahamtan, M.; Savardashtaki, A. Caspase-3: Structure, Function, and Biotechnological Aspects. Biotechnol. Appl. Biochem. 2022, 69, 1633–1645. [Google Scholar] [CrossRef]
- Romani, A.M.P. Cisplatin in Cancer Treatment. Biochem. Pharmacol. 2022, 206, 115323. [Google Scholar] [CrossRef]
- Živković, M.D.; Rajković, S.; Glišić, B.Đ.; Drašković, N.S.; Djuran, M.I. Hydrolysis of the Amide Bond in Histidine- and Methionine-Containing Dipeptides Promoted by Pyrazine and Pyridazine Palladium(II)-Aqua Dimers: Comparative Study with Platinum(II) Analogues. Bioorg. Chem. 2017, 72, 190. [Google Scholar] [CrossRef]
- Zhao, G.; Lin, H.; Zhu, S.; Sun, H.; Chen, Y. Dinuclear Palladium(II) Complexes Containing Two Monofunctional [Pd(en)(Pyridine)Cl]+ Units Bridged by Se or S: Synthesis, Characterization, Cytotoxicity and Kinetic Studies of DNA-Binding. J. Inorg. Biochem. 1998, 70, 219–226. [Google Scholar] [CrossRef]
- Novakova, O.; Chen, H.; Vrana, O.; Rodger, A.; Sadler, P.J.; Brabec, V. DNA Interactions of Monofunctional Organometallic Ruthenium(II) Antitumor Complexes in Cell-Free Media. Biochemistry 2003, 42, 11544–11554. [Google Scholar] [CrossRef]
- Rizvi, M.A.; Zaki, M.; Afzal, M.; Mane, M.; Kumar, M.; Shah, B.A.; Srivastav, S.; Srikrishna, S.; Peerzada, G.M.; Tabassum, S. Nuclear Blebbing of Biologically Active Organoselenium Compound Towards Human Cervical Cancer Cell (HeLa): In Vitro DNA/HSA Binding, Cleavage and Cell Imaging Studies. Eur. J. Med. Chem. 2015, 90, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Recio Despaigne, A.A.; Da Silva, J.G.; Da Costa, P.R.; Dos Santos, R.G.; Beraldo, H. ROS-Mediated Cytotoxic Effect of Copper(II) Hydrazone Complexes against Human Glioma Cells. Molecules 2014, 19, 17202–17220. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Nethaji, M.; Chakravarty, A.R. Effect of Charge Transfer Bands on the Photo-Induced DNA Cleavage Activity of [1-(2-Thiazolylazo)-2-Naphtholato]Copper(II) Complexes. J. Inorg. Biochem. 2005, 99, 805–812. [Google Scholar] [CrossRef]
- Konovalov, B.; Živković, M.D.; Milovanović, J.Z.; Djordjević, D.B.; Arsenijević, A.N.; Vasić, I.R.; Janjić, G.V.; Franich, A.; Manojlović, D.; Skrivanj, S.; et al. Synthesis, cytotoxic activity and DNA interaction studies of new dinuclear platinum(II) complexes with an aromatic 1,5-naphthyridine bridging ligand: DNA binding mode of polynuclear platinum(II) complexes in relation to the complex structure. Dalton Trans. 2018, 47, 15091. [Google Scholar] [CrossRef]
- Majorek, K.A.; Porebski, P.J.; Dayal, A.; Zimmerman, M.D.; Jablonska, K.; Stewart, A.J.; Chruszcz, M.; Minor, W. Structural and Immunologic Characterization of Bovine, Horse, and Rabbit Serum Albumins. Mol. Immunol. 2012, 52, 174–182. [Google Scholar] [CrossRef]
- Jalali, F.; Dorraji, P.S.; Mahdiuni, H. Binding of the Neuroleptic Drug, Gabapentin, to Bovine Serum Albumin: Insights from Experimental and Computational Studies. J. Lumin. 2014, 148, 347–352. [Google Scholar] [CrossRef]
- Adamczyk, O.; Szota, M.; Rakowski, K.; Prochownik, M.; Doveiko, D.; Chen, Y.; Jachimska, B. Bovine Serum Albumin as a Platform for Designing Biologically Active Nanocarriers—Experimental and Computational Studies. Int. J. Mol. Sci. 2024, 25, 37. [Google Scholar] [CrossRef]
- Golianová, K.; Havadej, S.; Verebová, V.; Uličný, J.; Holečková, B.; Staničová, J. Interaction of Conazole Pesticides Epoxiconazole and Prothioconazole with Human and Bovine Serum Albumin Studied Using Spectroscopic Methods and Molecular Modeling. Int. J. Mol. Sci. 2021, 22, 1925. [Google Scholar] [CrossRef] [PubMed]
- Topală, T.; Bodoki, A.; Oprean, L.; Oprean, R. Bovine Serum Albumin Interactions with Metal Complexes. Clujul Med. 2014, 87, 215–219. [Google Scholar] [CrossRef]
- Makarska-Bialokoz, M. Interactions of Hemin with Bovine Serum Albumin and Human Hemoglobin: A Fluorescence Quenching Study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 193, 23–32. [Google Scholar] [CrossRef]
- Rajendiran, V.; Karthik, R.; Palaniandavar, M.; Stoeckli-Evans, H.; Periasamy, V.S.; Akbarsha, M.A.; Srinag, B.S.; Krishnamurthy, H. Mixed-Ligand Copper(II)-Phenolate Complexes: Effect of Coligand on Enhanced DNA and Protein Binding, DNA Cleavage, and Anticancer Activity. Inorg. Chem. 2007, 46, 8208–8221. [Google Scholar] [CrossRef] [PubMed]
- Tarushi, A.; Totta, X.; Papadopoulos, A.; Kljun, J.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Antioxidant Activity and Interaction with DNA and Albumins of Zinc-Tolfenamato Complexes. Crystal Structure of [Zn(tolfenamato)2(2,2′-dipyridylketoneoxime)2]. Eur. J. Med. Chem. 2014, 74, 187–198. [Google Scholar] [CrossRef]
- Sathyadevi, P.; Krishnamoorthy, P.; Butorac, R.R.; Cowley, A.H.; Bhuvanesh, N.S.; Dharmaraj, N. Effect of Substitution and Planarity of the Ligand on DNA/BSA Interaction, Free Radical Scavenging, and Cytotoxicity of Diamagnetic Ni(II) Complexes: A Systematic Investigation. Dalton Trans. 2011, 40, 9690–9702. [Google Scholar] [CrossRef]
- Carneiro, T.J.; Martins, A.S.; Marques, M.P.M.; Gil, A.M. Metabolic Aspects of Palladium(II) Potential Anti-Cancer Drugs. Front. Oncol. 2020, 10, 590970. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.A.O.; Dias, I.S.; Sarto, L.E.; de Gois, E.P.; Torres, C.; de Almeida, E.T.; Gouvêa, C.M.C.P. Cytotoxicity Induced by Newly Synthesized Palladium (II) Complexes Leads to the Death of MCF-7 and MDA-MB-435 Cancer Cell Lines. Adv. Pharm. Bull. 2023, 13, 160–169. [Google Scholar] [CrossRef]
- Mavrova, A.; Dimov, S.; Sulikovska, I.; Yancheva, D.; Iliev, I.; Tsoneva, I.; Staneva, G.; Nikolova, B. Design, Cytotoxicity and Antiproliferative Activity of 4-Amino-5-methyl-thieno[2,3-d]pyrimidine-6-carboxylates against MCF-7 and MDA-MB-231 Breast Cancer Cell Lines. Molecules 2022, 27, 3314. [Google Scholar] [CrossRef]
- Czarnomysy, R.; Radomska, D.; Szewczyk, O.K.; Roszczenko, P.; Bielawski, K. Platinum and Palladium Complexes as Promising Sources for Antitumor Treatments. Int. J. Mol. Sci. 2021, 22, 8271. [Google Scholar] [CrossRef]
- Maciel, L.L.F.; Silva, M.B.; Moreira, R.O.; Cardoso, A.P.; Fernandes, C.; Horn, A., Jr.; de Aquino Almeida, J.C.; Kanashiro, M.M. In Vitro and In Vivo Relevant Antineoplastic Activity of Platinum(II) Complexes toward Triple-Negative MDA-MB-231 Breast Cancer Cell Line. Pharmaceutics 2022, 14, 2013. [Google Scholar] [CrossRef]
- Basaran, E.; Sogukomerogullari, H.G.; Cakmak, R.; Akkoc, S.; Taskin-Tok, T.; Köse, A. Novel Chiral Schiff Base Palladium(II), Nickel(II), Copper(II) and Iron(II) Complexes: Synthesis, Characterization, Anticancer Activity and Molecular Docking Studies. Bioorg. Chem. 2022, 129, 106176. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Hussain, S.; Zafar, M.N.; Hussain, I.; Khan, F.; Mughal, E.U.; Tahir, M.N. Preliminary Anticancer Evaluation of New Pd(II) Complexes Bearing NNO Donor Ligands. Saudi Pharm. J. 2024, 32, 101915. [Google Scholar] [CrossRef]
- Kazimir, A.; Schwarze, B.; Lönnecke, P.; Jelača, S.; Mijatović, S.; Maksimović-Ivanić, D.; Hey-Hawkins, E. Metallodrugs against Breast Cancer: Combining the Tamoxifen Vector with Platinum(II) and Palladium(II) Complexes. Pharmaceutics 2023, 15, 682, Erratum in Pharmaceutics 2023, 15, 1769. [Google Scholar] [CrossRef]
- Lasri, J.; Haukka, M.; Al-Rasheed, H.H.; Abutaha, N.; El-Faham, A.; Soliman, S.M. Synthesis, Structure and In Vitro Anticancer Activity of Pd(II) Complex of Pyrazolyl-s-Triazine Ligand; A New Example of Metal-Mediated Hydrolysis of s-Triazine Pincer Ligand. Crystals 2021, 11, 119. [Google Scholar] [CrossRef]
- Djuran, M.I.; Milinković, S.U. Hydrolysis of Amide Bond in Histidine-Containing Peptides Promoted by Chelated Amino Acid Palladium(II) Complexes: Dependence of Hydrolytic Pathway on the Coordination Modes of the Peptides. Polyhedron 1999, 18, 3611–3618. [Google Scholar] [CrossRef]
- Caubet, A.; Moreno, V.; Molins, E.; Miravitlles, C. Methionine and Histidine Pd(II) and Pt(II) Complexes: Crystal Structures and Spectroscopic Properties. J. Inorg. Biochem. 1992, 48, 135–145. [Google Scholar] [CrossRef]
- Dimiza, F.; Fountoulaki, S.; Papadopoulos, A.N.; Kontogiorgis, C.A.; Tangoulis, V.; Raptopoulou, C.P.; Psycharis, V.; Terzis, A.; Kessissoglou, D.P.; Psomas, G. Non-Steroidal Anti-Inflammatory Drug-Copper(II) Complexes: Structure and Biological Perspectives. Dalton Trans. 2011, 40, 8555–8568. [Google Scholar] [CrossRef]
- Dimiza, F.; Perdih, F.; Tangoulis, V.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Interaction of Copper(II) with the Non-Steroidal Anti-Inflammatory Drugs Naproxen and Diclofenac: Synthesis, Structure, DNA- and Albumin-Binding. J. Inorg. Biochem. 2011, 105, 476–489. [Google Scholar] [CrossRef]
- Bošković, M.; Franich, A.A.; Rajković, S.; Jovanović, M.; Jurisević, M.; Gajović, N.; Jovanović, M.; Arsenijević, N.; Jovanović, I.; Živković, M.D. Potential Antitumor Effect of Newly Synthesized Dinuclear1,5-Naphthyridine-Bridging Palladium(II) Complexes. ChemistrySelect 2020, 5, 10549–10555. [Google Scholar] [CrossRef]
- Bi, S.; Pang, B.; Zhao, T.; Wang, T.; Wang, Y.; Yan, L. Binding Characteristics of Salbutamol with DNA by Spectral Methods. Spectrochim. Acta Part A 2013, 111, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Ašanin, D.P.; Živković, M.D.; Rajković, S.; Warzajtis, B.; Rychlewska, U.; Djuran, M.I. Crystallographic evidence of anion⋯π interactions in the pyrazine bridged {[Pt(en)Cl]2(μ-pz)}Cl2 complex and a comparative study of the catalytic ability of mononuclear and binuclear platinum(II) complexes in the hydrolysis of N-acetylated L-methionylglycine. Polyhedron 2013, 51, 255. [Google Scholar] [CrossRef]
- Rajković, S.; Rychlewska, U.; Warzajtis, B.; Ašanin, D.P.; Živković, M.D.; Djuran, M.I. Disparate behavior of pyrazine and pyridazine platinum(II) dimers in the hydrolysis of histidine- and methionine-containing peptides and unique crystal structure of {[Pt(en)Cl]2(μ-pydz)}Cl2 with a pair of NH⋯Cl⋯HN hydrogen bonds supporting the pyridazine bridge. Polyhedron 2014, 67, 279. [Google Scholar] [CrossRef]
- Rajković, S.; Živković, M.D.; Warzajtis, B.; Rychlewska, U.; Djuran, M.I. Synthesis, spectroscopic and X-ray characterization of various pyrazine-bridged platinum(II) complexes: 1H NMR comparative study of their catalytic abilities in the hydrolysis of methionine- and histidine-containing dipeptides. Polyhedron 2016, 117, 367. [Google Scholar] [CrossRef]
- Rajković, S.; Warzajtis, B.; Živković, M.D.; Glišić, B.Đ.; Rychlewska, U.; Djuran, M.I. Hydrolysis of Methionine- and Histidine-Containing Peptides Promoted by Dinuclear Platinum(II) Complexes with Benzodiazines as Bridging Ligands: Influence of Ligand Structure on the Catalytic Ability of Platinum(II) Complexes. Bioinorgan. Chem. Appl. 2018, 2018, 3294948. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Shao, Y.; Zhu, J.; Zhu, Y.; Li, Y.; Xu, Q.; Guo, Z. A Trinuclear Copper(II) Complex of 2,4,6-Tris(di-2-pyridylamine)-1,3,5-triazine Shows Prominent DNA Cleavage Activity. Inorg. Chem. 2007, 46, 3306–3312. [Google Scholar] [CrossRef]
- Jing, J.; Jiang, M.; Li, Y.T.; Wu, Z.Y.; Yan, C.W. In Vitro Cytotoxic Activities, DNA-, and BSA-Binding Studies of a New Dinuclear Copper(II) Complex with N-[3-(Dimethylamino)propyl]-N′-(2-carboxylatophenyl)-Oxamide as Ligand. J. Biochem. Mol. Toxicol. 2014, 28, 47–55. [Google Scholar] [CrossRef]
- Ni, Y.; Zhu, R.; Kokot, S. Competitive Binding of Small Molecules with Biopolymers: A Fluorescence Spectroscopy and Chemometrics Study of the Interaction of Aspirin and Ibuprofen with BSA. Analyst 2011, 136, 4794–4801. [Google Scholar] [CrossRef]
- Ašanin, D.P.; Živković, M.D.; Rajković, S.; Warżajtis, B.; Rychlewska, U.; Djuran, M.I. Hydrolysis of the Amide Bond in L-Methionine- and L-Histidine-Containing Dipeptides in the Presence of Dinuclear Palladium(II) Complexes with Benzodiazines Bridging Ligands. Polyhedron 2013, 51, 255–262. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Simić, D.; Zarić, M.; Nikolić, I.; Živković-Zarić, R.; Čanović, P.; Kočović, A.; Radojević, I.; Raković, I.; Milić, S.J.; Petrović, Đ.; et al. Newly Synthesized Palladium(II) Complexes with Aminothiazole Derivatives: In Vitro Study of Antimicrobial Activity and Antitumor Activity on the Human Prostate Cancer Cell Line. Dalton Trans. 2022, 51, 1191–1205. [Google Scholar] [CrossRef] [PubMed]

| Ligand/Complex | 1H NMR (ppm) |
|---|---|
| Gly | 3.85 (s, CH2) |
| Pd1 | 3.52 (s, CH2); 8.83 (d, CH-pz) |
| L-Ala | 1.40 (d, CH3) 3.55 (q, CH) |
| Pd2 | 1.46 (d, CH3); 3.69 (q, CH); 8.92 (s, CH-pz) |
| L-Met | 2.14 (s, S-CH3); 2.17 (m, CH2-β); 2.65 (t, CH2-γ); 3.87 (m, CH) |
| Pd3 | 2.58 (s, S-CH3); 2.23 (m, CH2-β); 2.73 (m, CH2-γ); 3.94 (m, CH); 8.89 (s, CH-pz) |
| pz | 8.66 (s) |
| Pd1 | Pd2 | Pd3 | |||
|---|---|---|---|---|---|
| CT-DNA interactions | UV-Vis | Kb (⋅104 M−1) | 6.33 | 5.31 | 0.96 |
| ΔG298 (kJ/mol) | −28.49 | −28.04 | −23.63 | ||
| Fluorescence measurements | Ksv (⋅104 M−1) | 12.03 | 17.60 | 5.36 | |
| Ka (⋅104 M−1) | 14.40 | 15.20 | 1.48 | ||
| n | ≈1 | ≈1 | ≈0.9 | ||
| BSA interactions | Fluorescence measurements | Ksv (⋅105 M−1) | 2.42 | 2.58 | 0.87 |
| Ka (⋅106 M−1) | 3.36 | 7.43 | 0.56 | ||
| kq (⋅1013 M−1 s−1) | 2.42 | 2.58 | 0.87 | ||
| n | ≈1.4 | ≈1.3 | ≈1.2 | ||
| Cell Line | Time | Pd1 | Pd2 | Pd3 | CP |
|---|---|---|---|---|---|
| MCF-7 | 24 h | 220.2 ± 22.8 | >250 | 58.2 ± 4.8 | 52.5 ± 5.9 |
| 48 h | 129.1 ± 14.8 | 72.7 ± 7.9 | 28.3 ± 2.6 | 12.4 ± 1.1 | |
| 72 h | 86.2 ± 7.3 | 54.7 ± 5.1 | 16.1 ± 1.8 | 5.8 ± 0.5 | |
| MDA-MB-231 | 24 h | 119.2 ± 12.8 | 103.7 ± 8.5 | 98.7 ± 7.9 | 28.3 ± 3.1 |
| 48 h | 83.1 ± 7.5 | 90.4 ± 7.7 | 29.4 ± 2.7 | 24.8 ± 2.1 | |
| 72 h | 21.9 ± 2.4 | 27.6 ± 3.2 | 8.9 ± 0.7 | 20.1 ± 1.9 | |
| MRC-5 | 24 h | >250 | >250 | 48.4 ± 4.2 | 183.7 ± 21.9 |
| 48 h | 210.4 ± 19.1 | 179.3 ± 19.6 | 93.7 ± 11.2 | 43.1 ± 3.6 | |
| 72 h | 117.9 ± 12.1 | 110.1 ± 11.6 | 43.1 ± 3.6 | 19.7 ± 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakovljevic, S.; Canovic, P.; Spasic, M.; Zivkovic, M.; Zaric, M.; Zivkovic Zaric, R.; Franich, A.; Rajkovic, S.; Todorovic, Z.; Relic, N.; et al. DNA/BSA Binding Affinity and Cytotoxicity of Dinuclear Palladium(II) Complexes with Amino Acids as Ligands. Molecules 2025, 30, 1534. https://doi.org/10.3390/molecules30071534
Jakovljevic S, Canovic P, Spasic M, Zivkovic M, Zaric M, Zivkovic Zaric R, Franich A, Rajkovic S, Todorovic Z, Relic N, et al. DNA/BSA Binding Affinity and Cytotoxicity of Dinuclear Palladium(II) Complexes with Amino Acids as Ligands. Molecules. 2025; 30(7):1534. https://doi.org/10.3390/molecules30071534
Chicago/Turabian StyleJakovljevic, Stefan, Petar Canovic, Marko Spasic, Marija Zivkovic, Milan Zaric, Radica Zivkovic Zaric, Andjela Franich, Snezana Rajkovic, Zeljko Todorovic, Nenad Relic, and et al. 2025. "DNA/BSA Binding Affinity and Cytotoxicity of Dinuclear Palladium(II) Complexes with Amino Acids as Ligands" Molecules 30, no. 7: 1534. https://doi.org/10.3390/molecules30071534
APA StyleJakovljevic, S., Canovic, P., Spasic, M., Zivkovic, M., Zaric, M., Zivkovic Zaric, R., Franich, A., Rajkovic, S., Todorovic, Z., Relic, N., Zivic, M., & Mirkovic, N. (2025). DNA/BSA Binding Affinity and Cytotoxicity of Dinuclear Palladium(II) Complexes with Amino Acids as Ligands. Molecules, 30(7), 1534. https://doi.org/10.3390/molecules30071534







