Recent Advances in Preparation and Application of BOPP Film for Energy Storage and Dielectric Capacitors
Abstract
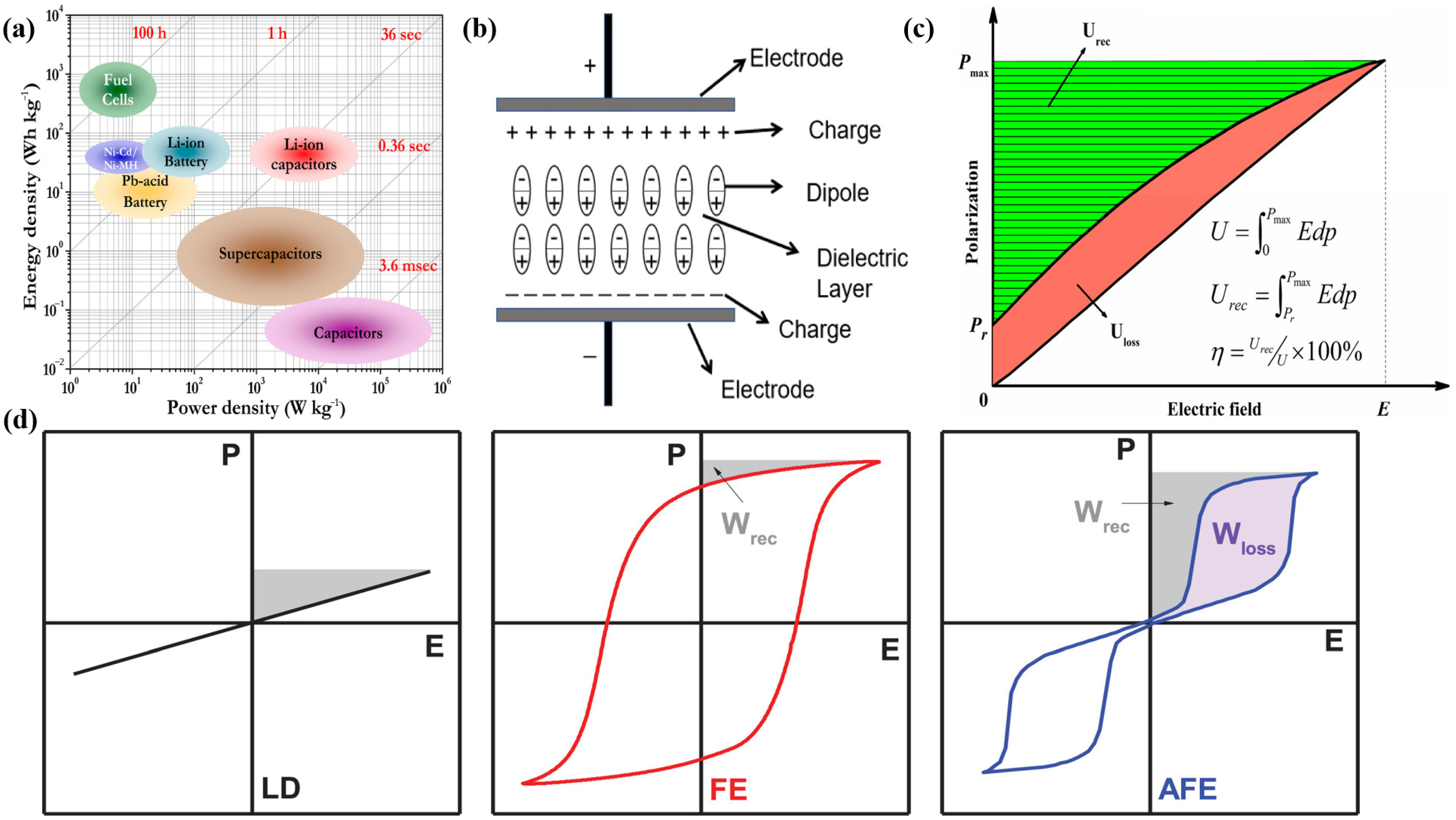
:1. Introduction
2. Polypropylene with High Energy Storage and Dielectric Stability
2.1. Co-Dopant
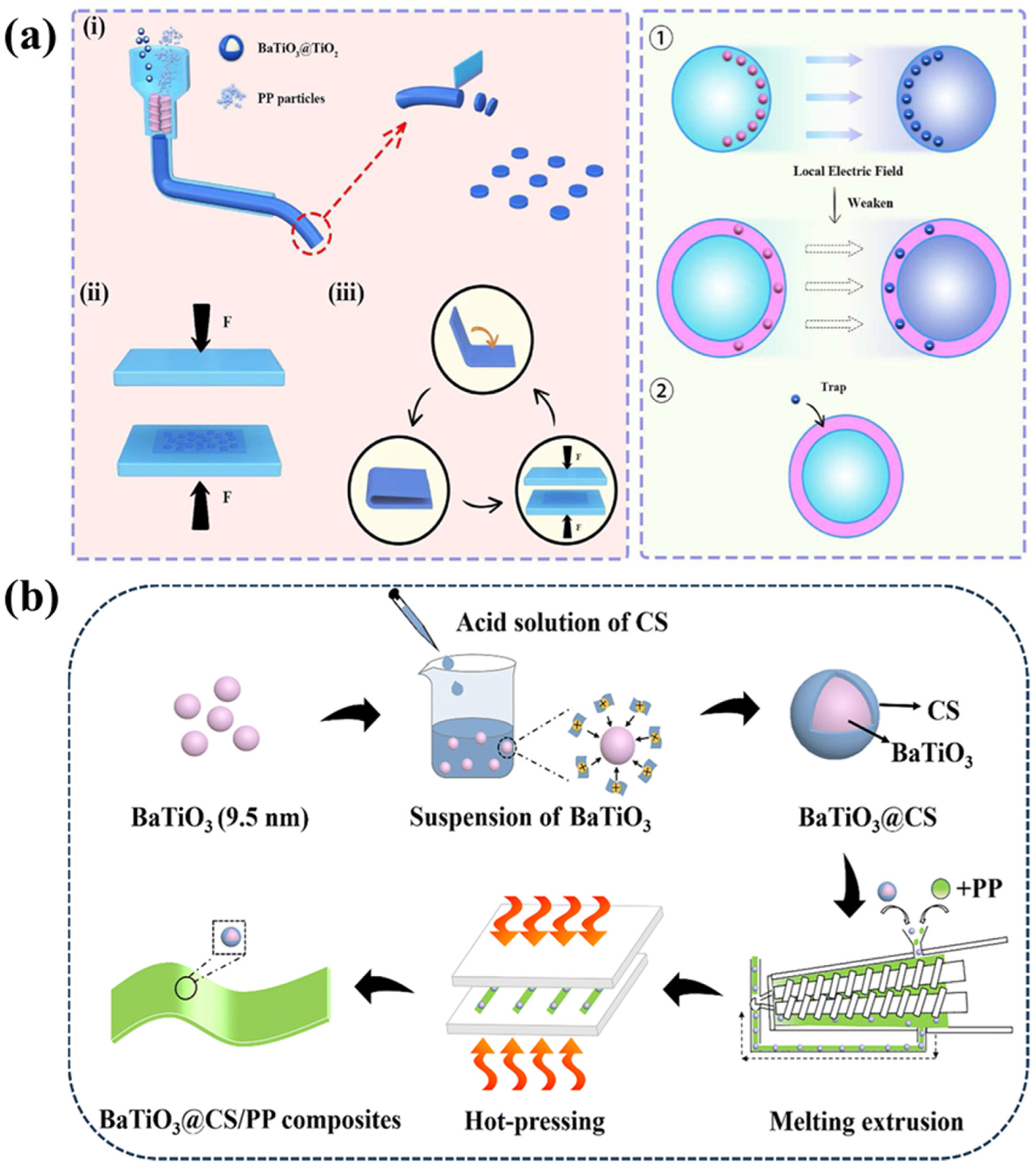
2.1.1. Incorporation of Inorganic Filler
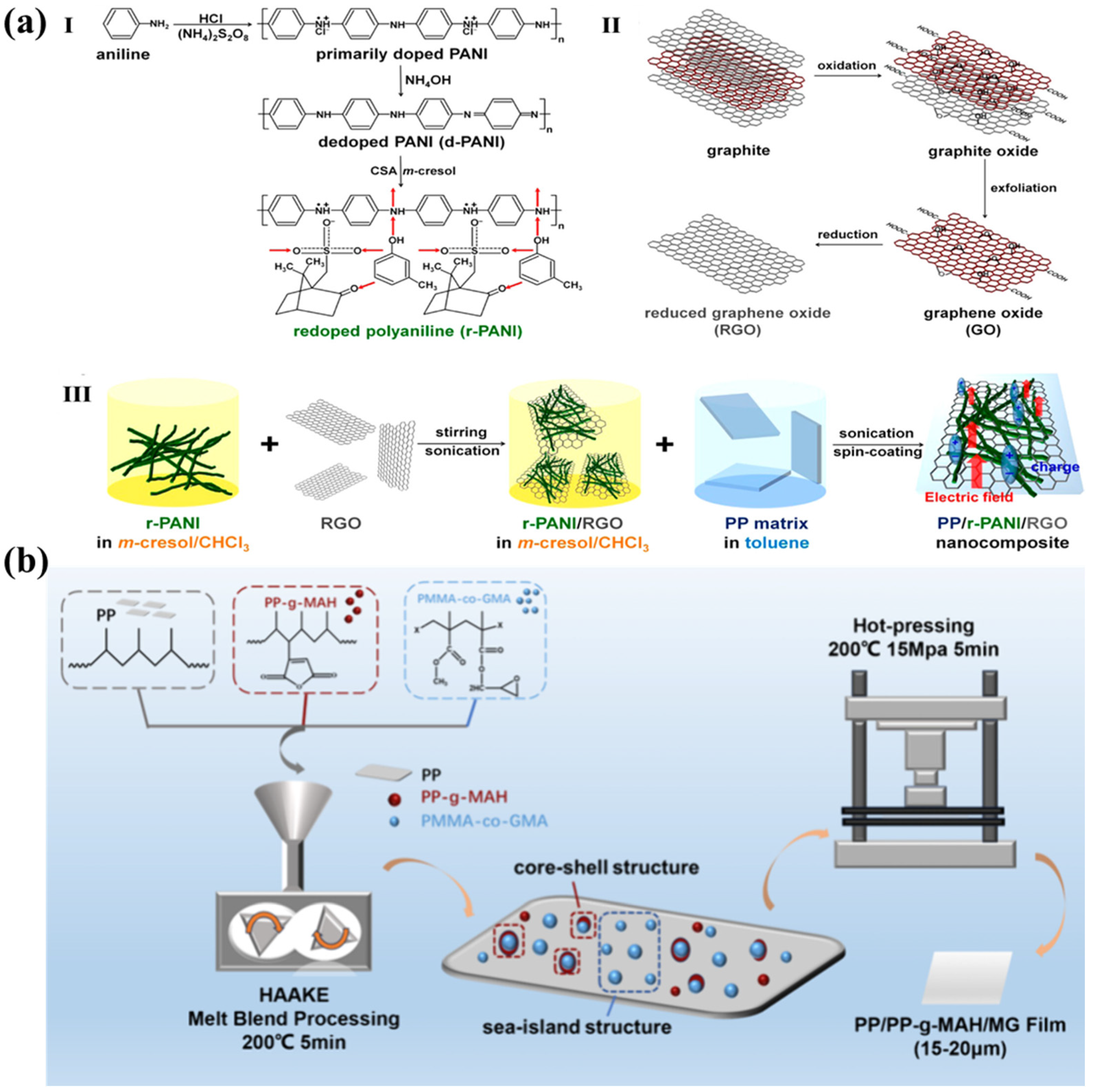
2.1.2. Blending with Organics
2.1.3. Multi-Blending
2.2. Surface Modification
2.2.1. Surface Coating
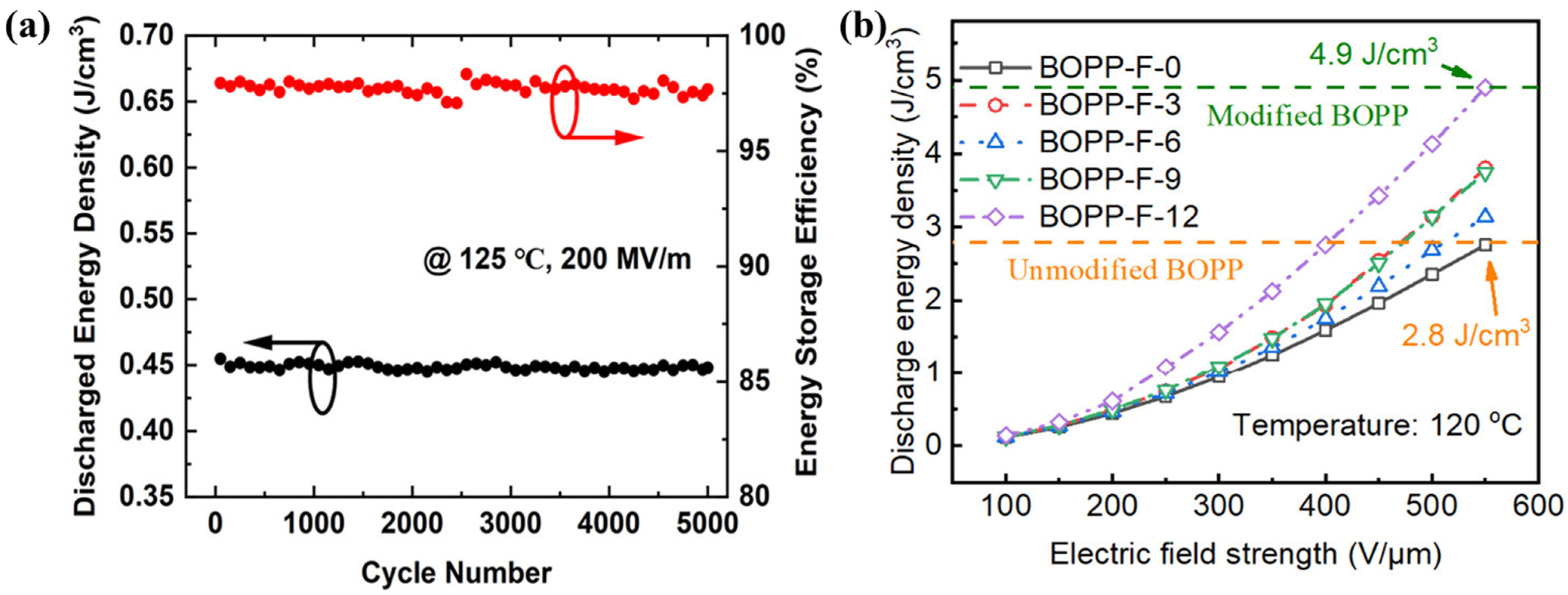
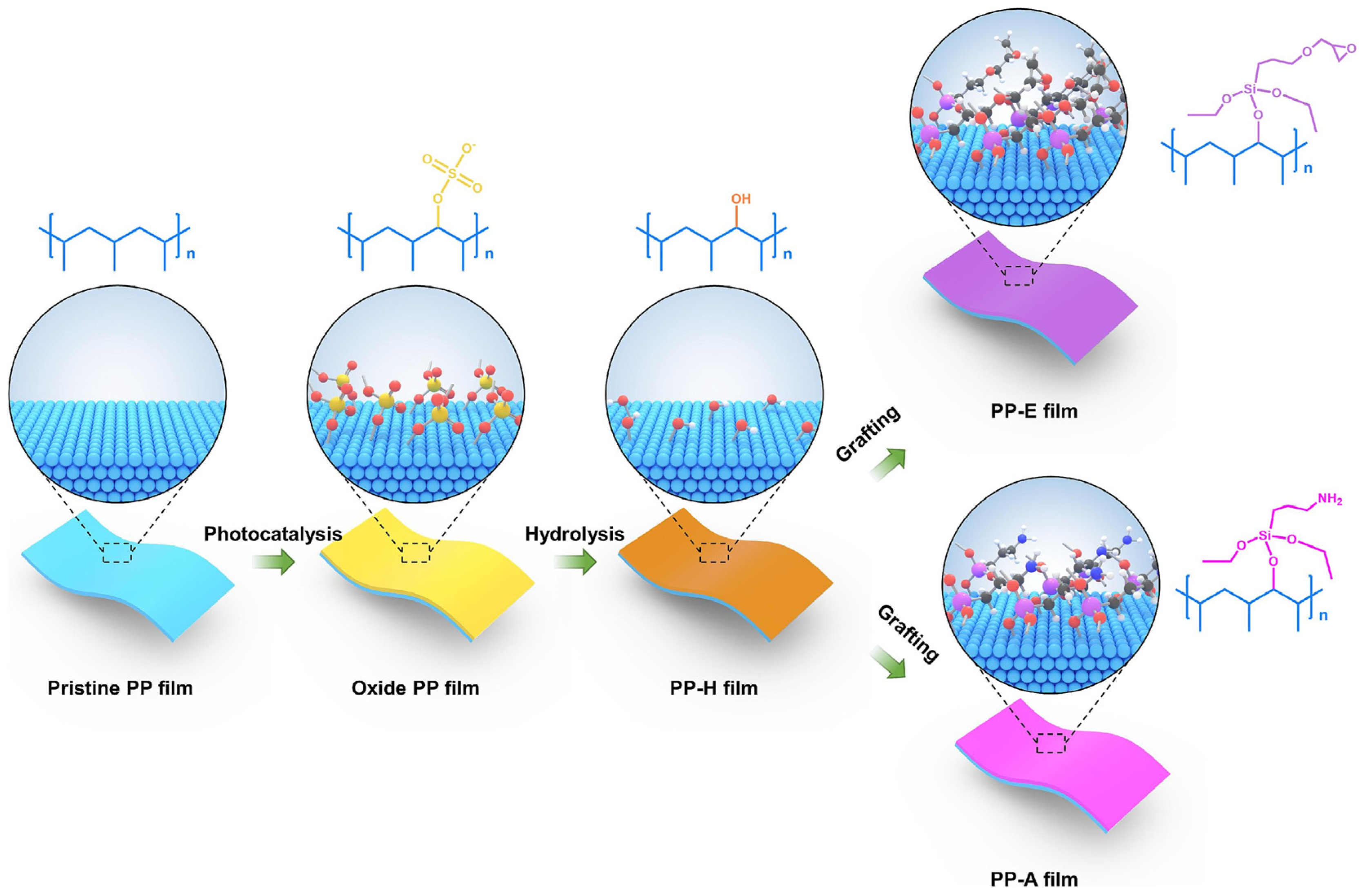
2.2.2. Surface Chemical Modification
2.3. Design of Molecular Structures
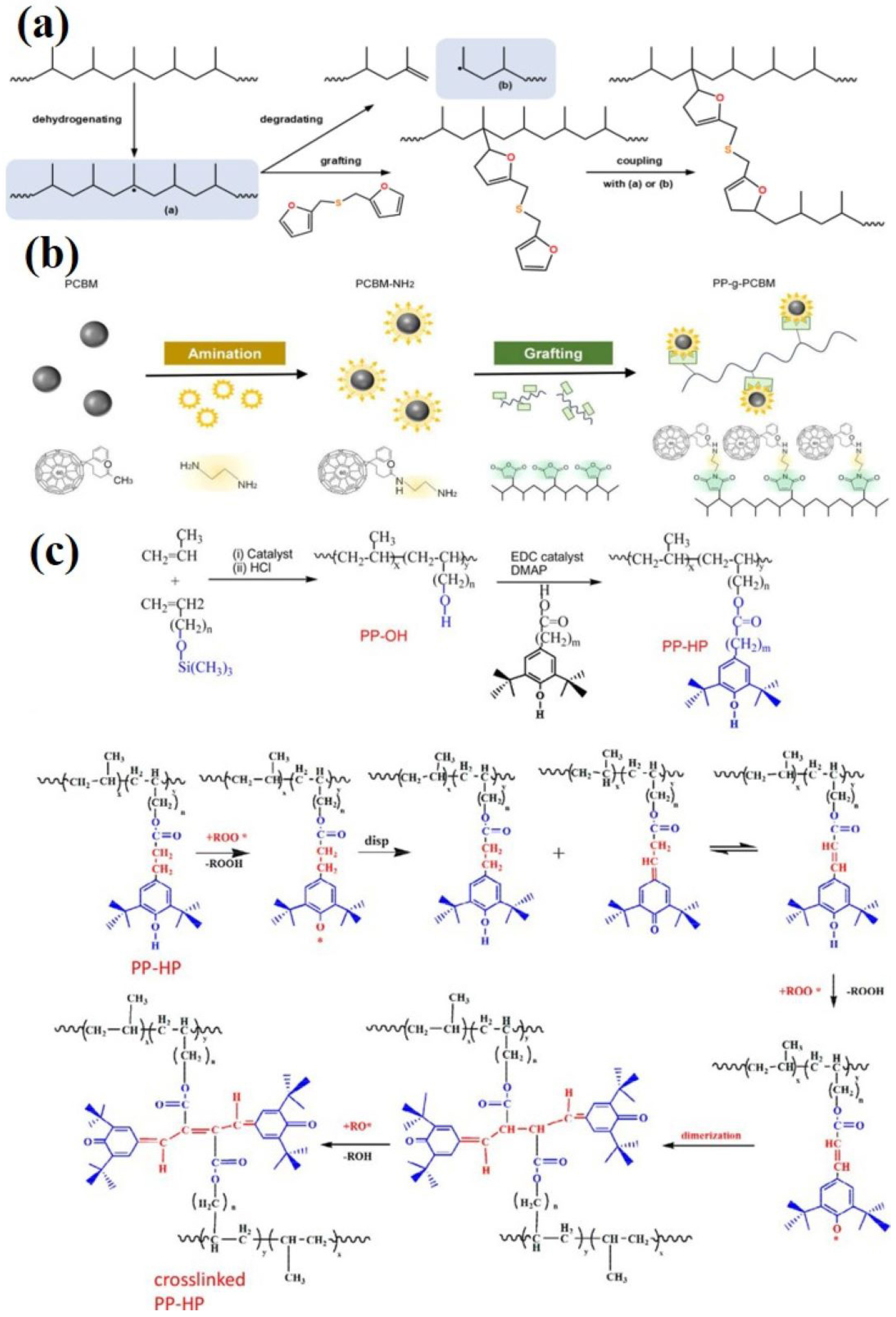
2.3.1. Construction of Crosslinked Structures
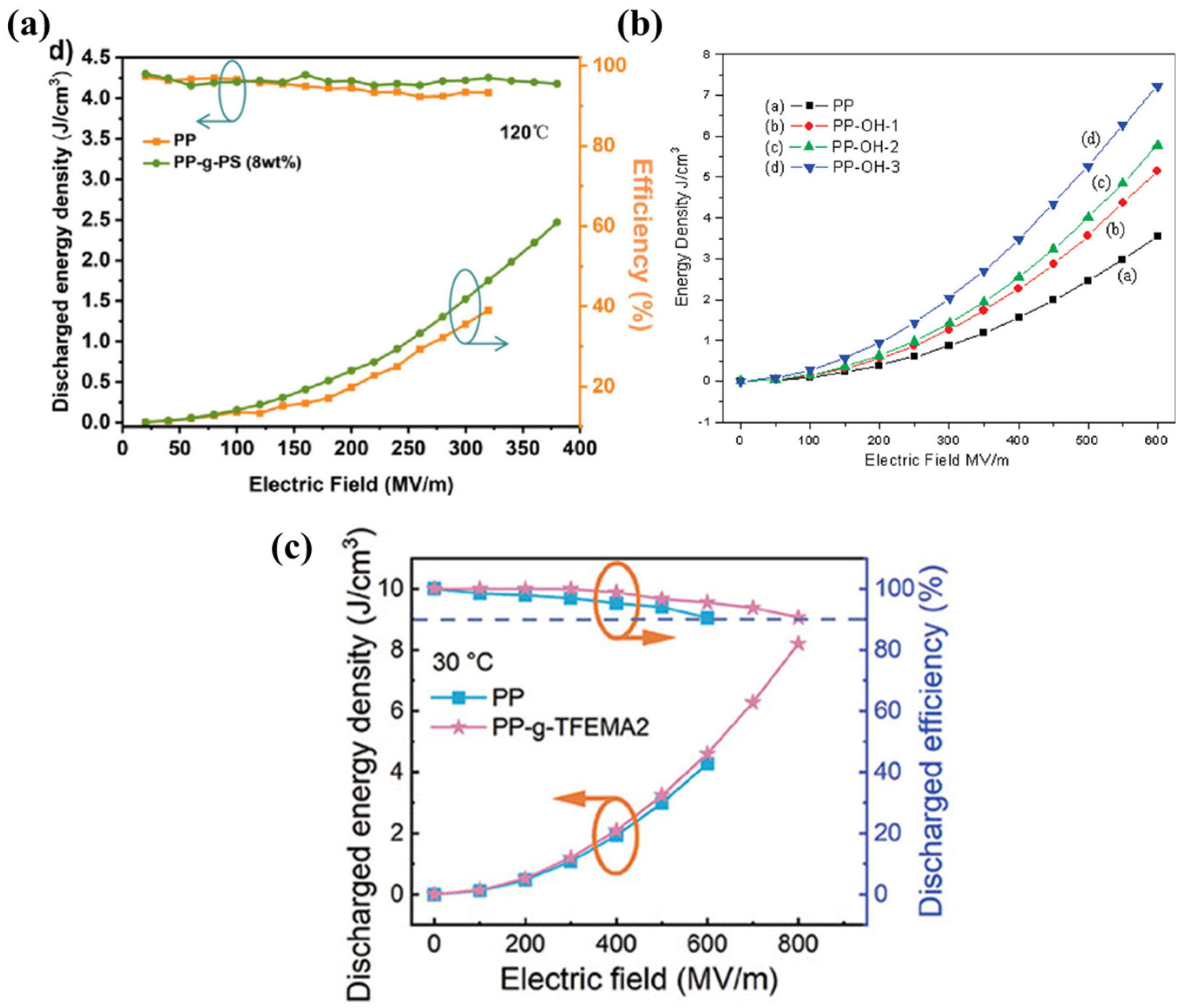
2.3.2. Grafting Organic Functional Groups
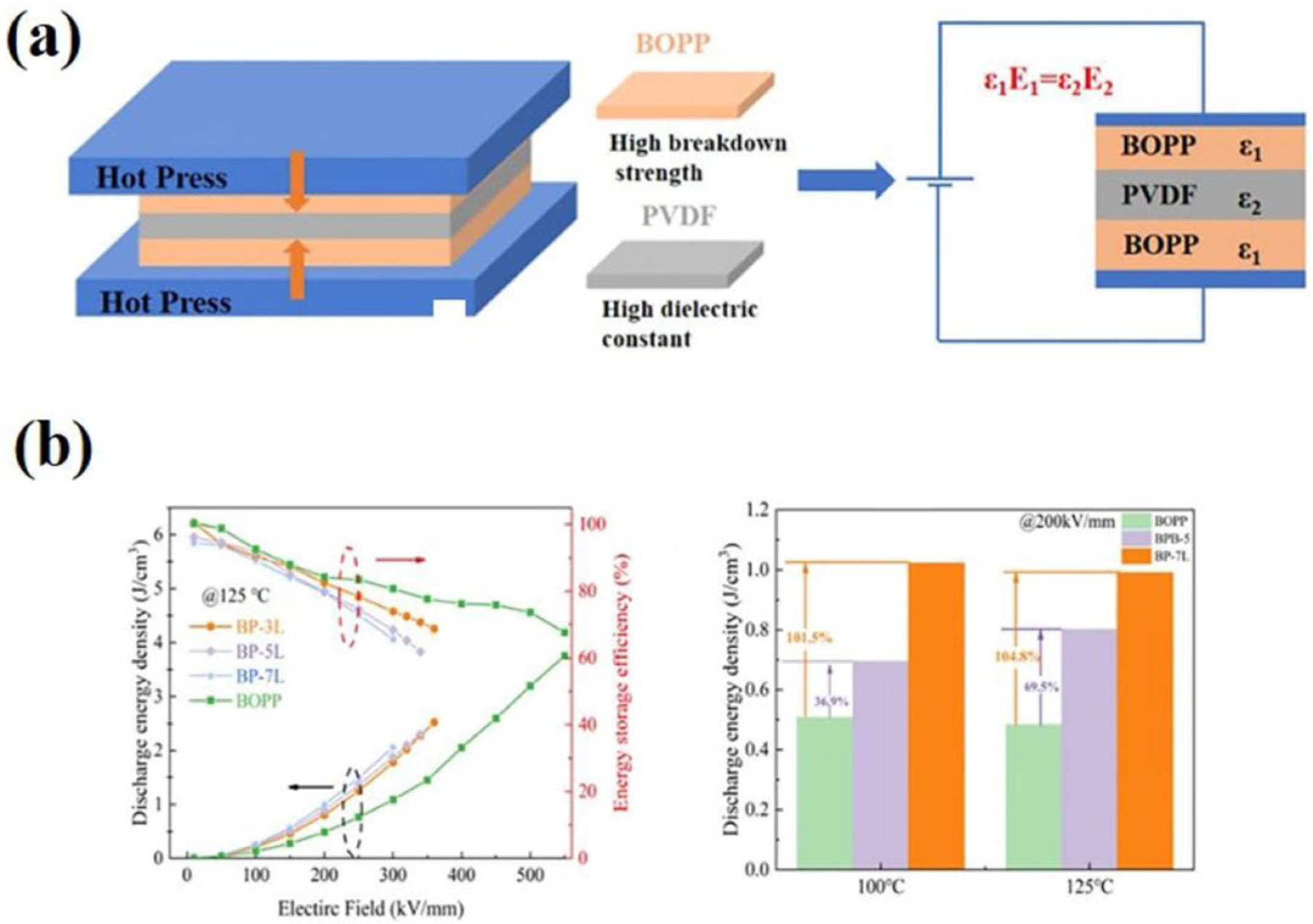
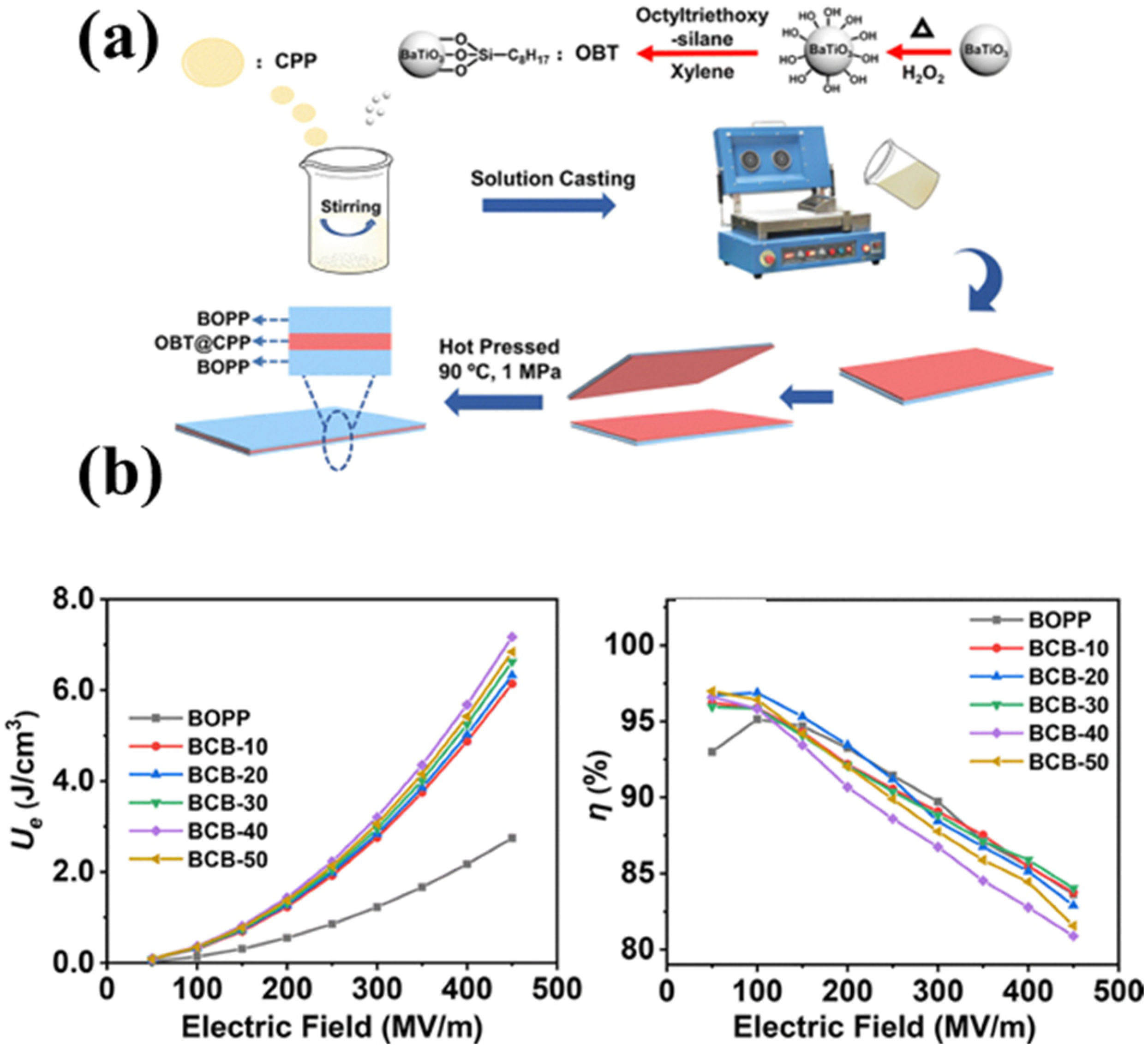
2.4. Multilayer Structure Design
3. Processing Affects Polypropylene Energy Storage and Dielectric Properties
3.1. Processing Technology
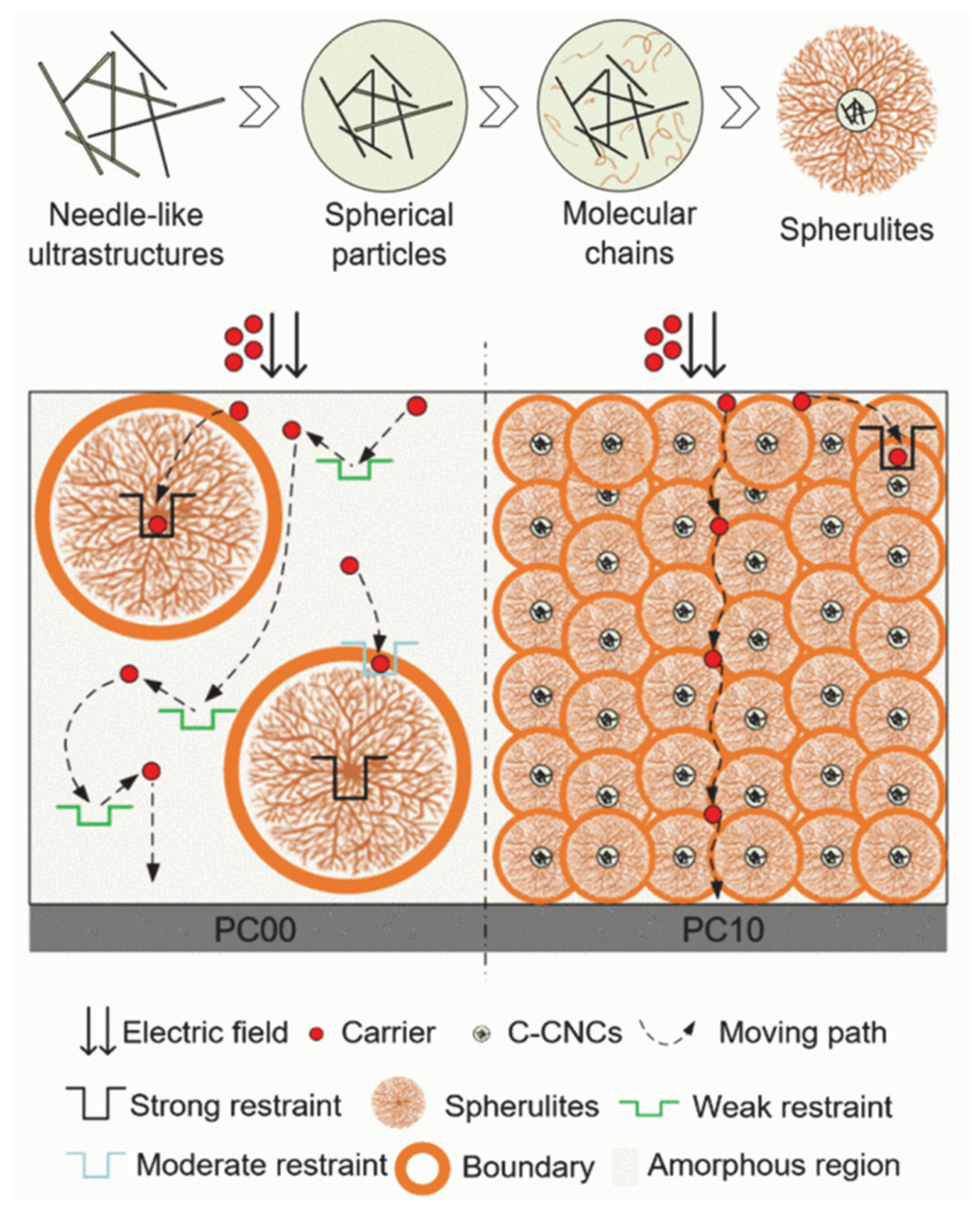
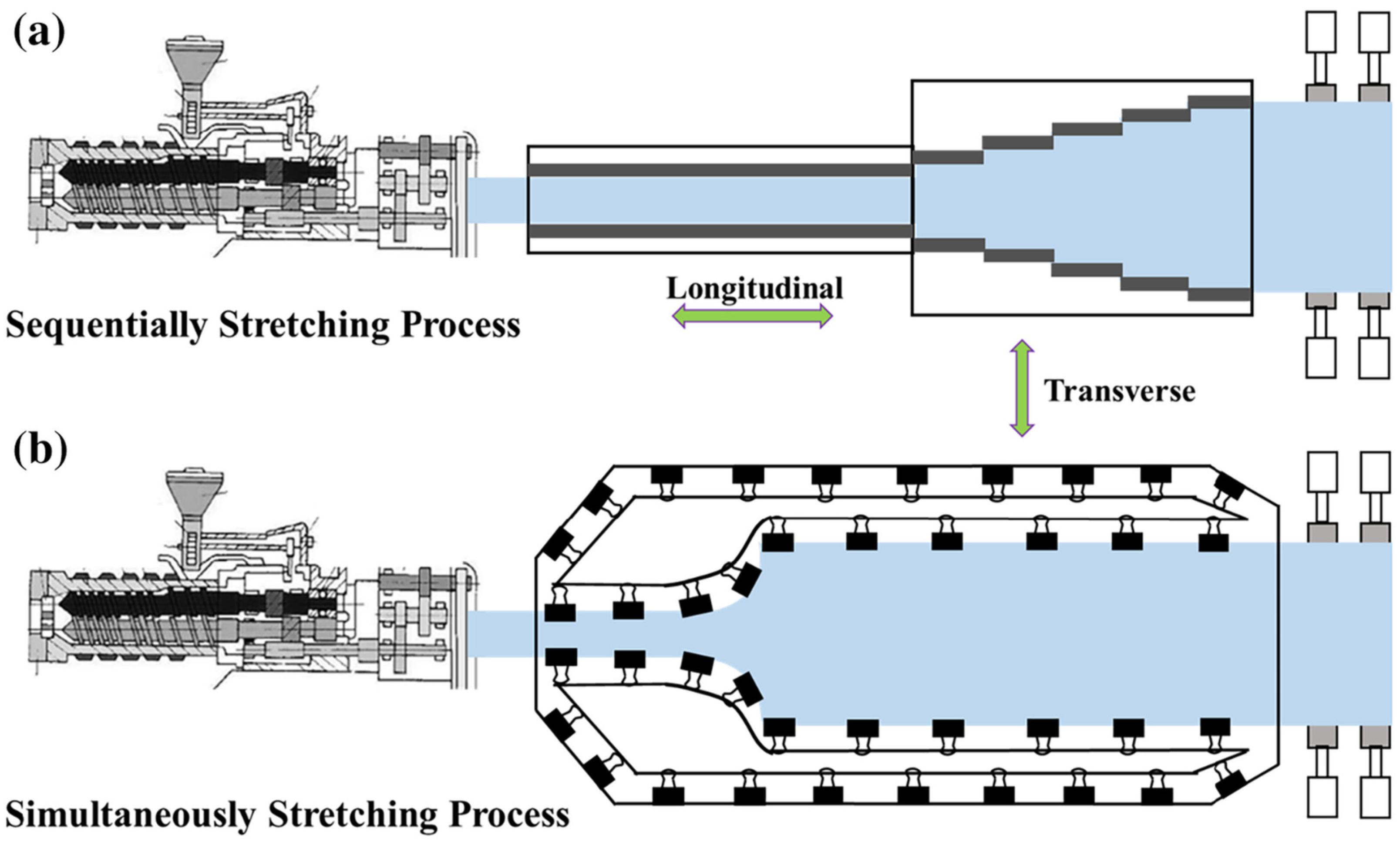
3.2. Stretching Technique
4. Outlook and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Yao, S.; Pan, L.; Liu, Y.; Zhu, C. A Review of Capacitive Power Transfer Technology for Electric Vehicle Applications. Electronics 2023, 12, 3534. [Google Scholar] [CrossRef]
- Ren, M.; Di, J.; Chen, W. Recent Progress and Application Challenges of Wearable Supercapacitors. Batter. Supercaps 2021, 4, 1279–1290. [Google Scholar]
- Zhang, L.; Pu, Y.; Chen, M. Complex impedance spectroscopy for capacitive energy-storage ceramics: A review and prospects. Mater. Today Chem. 2023, 28, 101353. [Google Scholar]
- Wang, X.; Zhang, Y.; Jia, S.; Deng, H.; Xu, W.; Shi, J. Unraveling lignin’s structural features for catalytic oxidative depolymerization and enhanced renewable resource utilization. Ind. Crops Prod. 2024, 207, 117750. [Google Scholar]
- Rey, S.O.; Romero, J.A.; Romero, L.T.; Martínez, À.F.; Roger, X.S.; Qamar, M.A.; Domínguez-García, J.L.; Gevorkov, L. Powering the Future: A Comprehensive Review of Battery Energy Storage Systems. Energies 2023, 16, 6344. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, Y.L.; Cao, P.F.; Lin, M.C. Energy storage system: Current studies on batteries and power condition system. Renew. Sustain. Energy Rev. 2018, 82, 3091–3106. [Google Scholar]
- Wang, C.; Zhai, S.; Yuan, Z.; Chen, J.; Yu, Z.; Pei, Z.; Liu, F.; Li, X.; Wei, L.; Chen, Y. Drying graphene hydrogel fibers for capacitive energy storage. Carbon 2020, 164, 100–110. [Google Scholar]
- Meng, Z.; Zhang, T.; Zhang, C.; Shang, Y.; Lei, Q.; Chi, Q. Advances in Polymer Dielectrics with High Energy Storage Performance by Designing Electric Charge Trap Structures. Adv. Mater. 2024, 36, 2310272. [Google Scholar]
- Singh, M.; Apata, I.E.; Samant, S.; Wu, W.; Tawade, B.V.; Pradhan, N.; Raghavan, D.; Karim, A. Nanoscale Strategies to Enhance the Energy Storage Capacity of Polymeric Dielectric Capacitors: Review of Recent Advances. Polym. Rev. 2022, 62, 211–260. [Google Scholar]
- Cheng, Y.; Ji, Q.; Zhu, B.; Zhang, X.; Gong, H.; Zhang, Z. Manipulating fluorine induced bulky dipoles and their strong interaction to achieve high efficiency electric energy storage performance in polymer dielectrics. Chem. Eng. J. 2023, 476, 146738. [Google Scholar]
- Li, Q.; Liu, J.; Zhang, X.; Tan, S.; Lu, J.; Zhang, Z. Tuning the dielectric and energy storage properties of polystyrene-based polymer dielectric by manipulating dipoles and their polarizing behavior. Phys. Chem. Chem. Phys. 2019, 21, 15712–15724. [Google Scholar] [PubMed]
- Yi, S.H.; Chan, Y.C.; Mo, C.L.; Lin, H.C.; Chen, M.J. Enhancement of energy storage for electrostatic supercapacitors through built-in electric field engineering. Nano Energy 2022, 99, 107342. [Google Scholar]
- Chen, C.; Nie, R.-P.; Shi, S.-C.; Jia, L.-C.; Li, Y.; Li, X.; Huang, Y.-C.; Han, D.-L.; Huang, H.-D.; Li, Z.-M. Simultaneous enhancement of breakdown strength and discharged energy efficiency of tri-layered polymer nanocomposite films by incorporating modified graphene oxide nanosheets. J. Mater. Sci. 2021, 56, 13165–13177. [Google Scholar]
- Aravindan, V.; Gnanaraj, J.; Lee, Y.S.; Madhavi, S. Insertion-Type Electrodes for Nonaqueous Li-Ion Capacitors. Chem. Rev. 2014, 114, 11619–11635. [Google Scholar]
- Liu, W.; Sun, X.; Yan, X.; Gao, Y.; Zhang, X.; Wang, K.; Ma, Y. Review of Energy Storage Capacitor Technology. Batteries 2024, 10, 271. [Google Scholar] [CrossRef]
- Diao, C.; Wang, H.; Wang, B.; He, Y.; Hou, Y.; Zheng, H. Overviews of dielectric energy storage materials and methods to improve energy storage density. J. Mater. Sci. Mater. Electron. 2022, 33, 21199–21222. [Google Scholar]
- Qi, H.; Xie, A.; Zuo, R. Local structure engineered lead-free ferroic dielectrics for superior energy-storage capacitors: A review. Energy Storage Mater. 2022, 45, 541–567. [Google Scholar]
- Huan, T.D.; Boggs, S.; Teyssedre, G.; Laurent, C.; Cakmak, M.; Kumar, S.; Ramprasad, R. Advanced polymeric dielectrics for high energy density applications. Prog. Mater. Sci. 2016, 83, 236–269. [Google Scholar]
- Fan, J.; Hu, T.; Ma, C.; Ma, C.; Lu, R.; Jin, J.; Hu, G.; Liu, M.; Jia, C. Ultrahigh Temperature Lead-Free Film Capacitors via Strain and Dielectric Constant Double Gradient Design. Small 2022, 18, 2105780. [Google Scholar]
- Guo, M.; Jiang, J.; Shen, Z.; Lin, Y.; Nan, C.W.; Shen, Y. High-Energy-Density Ferroelectric Polymer Nanocomposites for Capacitive Energy Storage: Enhanced Breakdown Strength and Improved Discharge Efficiency. Mater. Today 2019, 29, 49–67. [Google Scholar]
- Deng, Y.Y.; Zhou, D.L.; Han, D.; Zhang, Q.; Chen, F.; Fu, Q. Fluoride ion encapsulated polyhedral oligomeric silsesquioxane: A novel filler for polymer nanocomposites with enhanced dielectric constant and reduced dielectric loss. Compos. Sci. Technol. 2020, 189, 108035. [Google Scholar]
- Michelazzi, M.; Fabiani, D. Electrical Conduction in Thin-Film Polypropylene Capacitors. Energies 2023, 16, 6631. [Google Scholar] [CrossRef]
- González-Gutiérrez, A.G.; Cedano Villavicencio, K.G.; Sebastian, P.J. An innovative process to improve the end-contacts of metallic polypropylene capacitors by arc spray and PVD. Surf. Interfaces 2023, 38, 102766. [Google Scholar]
- Du, B.X.; Chen, K.; Liu, H.; Xiao, M. High energy density of biaxially oriented polypropylene film in cryogenic environment for advanced capacitor. J. Phys. D Appl. Phys. 2024, 57, 445502. [Google Scholar]
- Li, S.; Wu, W.; Yan, X. Structural and Performance Regulation of Binary Transition-Metal Oxides Toward Supercapacitors: Advances and Prospects. Renewables 2023, 1, 142–168. [Google Scholar]
- Xiao, M.; Zhang, M.; Du, B.; Ran, Z.; Liu, H.; Qin, Y. Breakdown strength improvement of polypropylene by introducing long-chain branched structures for film capacitors. J. Phys. D Appl. Phys. 2022, 55, 245501. [Google Scholar]
- Xiao, M.; Wang, K.; Song, Y.; Du, B. Dielectric Performance of Polypropylene Films Modified by UV Irradiation for HVDC Capacitors. IEEE Trans. Dielectr. Electr. Insul. 2024, 31, 176–183. [Google Scholar]
- Rytöluoto, I.; Gitsas, A.; Pasanen, S.; Lahti, K. Effect of film structure and morphology on the dielectric breakdown characteristics of cast and biaxially oriented polypropylene films. Eur. Polym. J. 2017, 95, 606–624. [Google Scholar]
- Du, B.; Zhang, J.; Xiao, M.; Liu, H.; Ran, Z.; Xing, J. Improvement of dielectric properties of polypropylene films for capacitors based on metal-ash suppression. Mater. Today Commun. 2022, 32, 104189. [Google Scholar]
- Wang, X.; Zhang, X.; Zhu, B. Influence of Surface Fluorination Treatment on Properties of Polypropylene Film. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 2058–2064. [Google Scholar]
- Ran, Z.; Yang, M.; Wang, R.; Li, J.; Li, M.; Meng, L.; Liu, Y.; Hu, J.; He, J.; Li, Q. Surface-gradient-structured polymer films with restricted thermal expansion for high-temperature capacitive energy storage. Energy Storage Mater. 2025, 74, 103952. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, M.; Liu, H.; Du, B.; Qin, Y. Dielectric Property and Breakdown Strength Performance of Long-Chain Branched Polypropylene for Metallized Film Capacitors. Materials 2022, 15, 3071. [Google Scholar] [CrossRef]
- Janakiraman, S.; Khalifa, M.; Biswal, R.; Ghosh, S.; Anandhan, S.; Venimadhav, A. High performance electrospun nanofiber coated polypropylene membrane as a separator for sodium ion batteries. J. Power Sources 2020, 460, 228060. [Google Scholar] [CrossRef]
- Qiao, Y.; Yin, X.; Zhu, T.; Li, H.; Tang, C. Dielectric polymers with novel chemistry, compositions and architectures. Prog. Polym. Sci. 2018, 80, 153–162. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, D.; Wu, K.; Fu, Q. Improved dielectric and energy storage properties of polypropylene by adding hybrid fillers and high-speed extrusion. Polymer 2021, 214, 123348. [Google Scholar] [CrossRef]
- Li, Z.; Cao, W.; Sheng, G.; Jiang, X.; Danikas, M.G. Experimental study on space charge and electrical strength of MgO nano-particles/polypropylene composite. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1812–1819. [Google Scholar] [CrossRef]
- Montanari, G.C.; Fabiani, D.; Palmieri, F.; Kaempfer, D.; Thomann, R.; Mulhaupt, R. Modification of electrical properties and performance of EVA and PP insulation through nanostructure by organophilic silicates. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 754–762. [Google Scholar] [CrossRef]
- Guan, F.; Yang, L.; Wang, J.; Guan, B.; Han, K.; Wang, Q.; Zhu, L. Confined Ferroelectric Properties in Poly(Vinylidene Fluoride-co-Chlorotrifluoroethylene)-graft-Polystyrene Graft Copolymers for Electric Energy Storage Applications. Adv. Funct. Mater. 2011, 21, 3176–3188. [Google Scholar] [CrossRef]
- Azmi, A.; Lau, K.Y.; Ahmad, N.A.; Abdul-Malek, Z.; Tan, C.W.; Ching, K.Y.; Vaughan, A.S. Dielectric Properties of Thermally Aged Polypropylene Nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 543–550. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, Y.; Xie, M.; Ma, G.; Li, P.; Qin, J. The effects of TiO2 nanoparticles on the temperature-dependent electrical and dielectric properties of polypropylene. Front. Energy Res. 2022, 10, 999438. [Google Scholar] [CrossRef]
- Yao, J.; Hu, L.; Zhou, M.; You, F.; Jiang, X.; Gao, L.; Wang, Q.; Sun, Z.; Wang, J. Synergistic Enhancement of Thermal Conductivity and Dielectric Properties in Al2O3/BaTiO3/PP Composites. Materials 2018, 11, 1536. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liang, W.; Nie, M.; Xu, D. A microwave-assisted, solvent-free approach for effective grafting of high-permittivity fillers to construct homogeneous polymer-based dielectric film with high energy density. Compos. Sci. Technol. 2024, 257, 110848. [Google Scholar] [CrossRef]
- Xie, H.; Luo, H.; Pei, Z.; Chen, S.; Zhang, D. Improved discharge energy density and efficiency of polypropylene-based dielectric nanocomposites utilizing BaTiO3@TiO2 nanoparticles. Mater. Today Energy 2022, 30, 101160. [Google Scholar] [CrossRef]
- Tian, W.; Yan, F.; Cai, C.; Wu, Z.; Zhang, C.; Yin, T.; Lao, S.; Hu, L. Freeze-drying and hot-pressing strategy to embed two-dimensional Ti0.87O2 monolayers in commercial polypropylene films with enhanced dielectric properties. J. Adv. Ceram. 2021, 10, 368–376. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, H.; Zhang, N.; Wang, H.; Zhang, C.; Yin, L.; Bai, J. Improved energy storage properties of polypropylene-based composite dielectrics by introducing surface-charged BaTiO3@chitisan ultrafine constructions. J. Mater. Chem. C 2024, 12, 2993–3004. [Google Scholar] [CrossRef]
- Xiao, M.; Fan, K.; Zhang, M.; Du, B.; Ran, Z.; Xing, Z. Effect of Cyclic Olefin Copolymer on Dielectric Performance of Polypropylene Films for Capacitors. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 2266–2273. [Google Scholar] [CrossRef]
- Liu, H.; Du, B.; Xiao, M.; Ran, Z.; Ma, Y. Aromatic Compound Improving Polypropylene Film Performance for Power Capacitor in Magnetic Field. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 1754–1762. [Google Scholar] [CrossRef]
- Ran, Z.Y.; Du, B.X.; Xiao, M. High-temperature Breakdown Performance Improvement of Polypropylene Films Based on Micromorphology Control. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1547–1554. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, M.; Du, B. Crystallisation regulation of long-chain branched polypropylene on dielectric performance and energy density for metallised film capacitors. High Volt. 2023, 8, 921–930. [Google Scholar] [CrossRef]
- Petersson, L.; Viertel, J.; Hillborg, H.; Zhang, G.; Chung, T.C.M. Electrical properties of polypropylene-bonded hindered phenol blends. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 590–596. [Google Scholar] [CrossRef]
- Cho, S.; Kim, M.; Lee, J.S.; Jang, J. Polypropylene/Polyaniline Nanofiber/Reduced Graphene Oxide Nanocomposite with Enhanced Electrical, Dielectric, and Ferroelectric Properties for a High Energy Density Capacitor. ACS Appl. Mater. Interfaces 2015, 7, 22301–22314. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Xie, J.; Liu, X.; Zheng, S.; Sun, S. Improved Dielectric and Energy Storage Properties of Polypropylene-Based Organic Films through Construction of a Microcompatible “Sea–Island” Structure. ACS Appl. Polym. Mater. 2024, 6, 7449–7457. [Google Scholar]
- Krache, R.; Benavente, R.; López-Majada, J.M.; Pereña, J.M.; Cerrada, M.L.; Pérez, E. Competition between α, β, and γ Polymorphs in a β-Nucleated Metallocenic Isotactic Polypropylene. Macromolecules 2007, 40, 6871–6878. [Google Scholar] [CrossRef]
- Zhu, K.; Ding, Q.; Hua, C.; Fu, H.; Yao, J. Effect of β-nucleating agent on crystallization of post-consumer polypropylene. Thermochim. Acta 2019, 675, 63–68. [Google Scholar] [CrossRef]
- Mohmeyer, N.; Schmidt, H.W.; Kristiansen, P.M.; Altstädt, V. Influence of Chemical Structure and Solubility of Bisamide Additives on the Nucleation of Isotactic Polypropylene and the Improvement of Its Charge Storage Properties. Macromolecules 2006, 39, 5760–5767. [Google Scholar]
- Zhu, X.; Chen, W.; Pan, M.; Zhou, X.; Zhang, Y.; Dong, L. Enhanced Energy Storage Properties of Polypropylene through Crystallization Modulation. ACS Appl. Polym. Mater. 2024, 6, 4808–4817. [Google Scholar]
- Xu, R.; Du, B.; Xiao, M.; Li, J.; Liu, H.; Ran, Z.; Xing, J. Dielectric properties dependent on crystalline morphology of PP film for HVDC capacitors application. Polymer 2021, 213, 123204. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, W.; Liu, H.; Li, S. Evolution of dielectric relaxation under elevated electric field of polypropylene-based films. J. Phys. D Appl. Phys. 2020, 53, 445502. [Google Scholar]
- Cheng, L.; Liu, W.; Liu, C.; Liu, X.; Li, S. Enhanced energy storage properties of polypropylene/maleic anhydride-grafted polypropylene/nano-ZrO2 ternary system. J. Appl. Polym. Sci. 2019, 136, 48211. [Google Scholar]
- Zhou, Y.; Yuan, C.; Wang, S.; Zhu, Y.; Cheng, S.; Yang, X.; Yang, Y.; Hu, J.; He, J.; Li, Q. Interface-modulated nanocomposites based on polypropylene for high-temperature energy storage. Energy Storage Mater. 2020, 28, 255–263. [Google Scholar]
- Liu, W.; Liu, H.; Cheng, L.; Li, S. Enhanced energy storage performance in polypropylene-acrylic acid grafted polypropylene-ZrO2 ternary nanocomposites. J. Phys. D Appl. Phys. 2023, 56, 244001. [Google Scholar]
- Liu, W.; Li, Z.; Liu, H.; Zhou, Y.; Zeng, J.; Zhao, Y.; Cheng, L.; Zhou, Y.; Li, S. Bi-axially oriented ternary polypropylene composite film with enhanced energy storage property at elevated temperature. J. Mater. Chem. A 2024, 12, 28531–28540. [Google Scholar]
- Tan, D.Q.; Liu, Y.; Lin, X.; Huang, E.; Lin, X.; Wu, X.; Lin, J.; Luo, R.; Wang, T. Exploration of Breakdown Strength Decrease and Mitigation of Ultrathin Polypropylene. Polymers 2023, 15, 2257. [Google Scholar] [CrossRef]
- Wu, X.; Tang, S.; Song, G.; Zhang, Z.; Tan, D.Q. High-temperature resistant polypropylene films enhanced by atomic layer deposition. Nano Express 2021, 2, 010025. [Google Scholar]
- Zhou, Y.; Wang, Q. Advanced polymer dielectrics for high temperature capacitive energy storage. J. Appl. Phys. 2020, 127, 240902. [Google Scholar]
- Li, H.; Zhou, Y.; Liu, Y.; Li, L.; Liu, Y.; Wang, Q. Dielectric polymers for high-temperature capacitive energy storage. Chem. Soc. Rev. 2021, 50, 6369–6400. [Google Scholar] [PubMed]
- Zhang, T.; Yu, H.; Jung, Y.H.; Zhang, C.; Feng, Y.; Chen, Q.; Lee, K.J. Significantly Improved High-Temperature Energy Storage Performance of BOPP Films by Coating Nanoscale Inorganic Layer. Energy Environ. Mater. 2024, 7, e12549. [Google Scholar]
- Bao, Z.; Du, X.; Ding, S.; Chen, J.; Dai, Z.; Liu, C.; Wang, Y.; Yin, Y.; Li, X. Improved Working Temperature and Capacitive Energy Density of Biaxially Oriented Polypropylene Films with Alumina Coating Layers. ACS Appl. Energy Mater. 2022, 5, 3119–3128. [Google Scholar]
- Zhang, C.; Ren, C.; Fu, J.; Dou, L.; Wang, H.; He, P.; Zhang, C.; Li, Q.; Zha, J.-W.; Shao, T. Improving Energy Storage Density of Biaxially Oriented Polypropylene Film by Quickly Repairing Surface Insulation Defects. ACS Appl. Polym. Mater. 2024, 6, 11110–11117. [Google Scholar]
- Umran, H.M.; Alesary, H.F.; Ismail, H.K.; Wang, F.; Barton, S. Influence of surface chemical modifications on enhancing the aging behavior of capacitor biaxially-oriented polypropylene thin film. Polym. Degrad. Stab. 2025, 231, 111105. [Google Scholar]
- Matsumoto, T.; Okumura, Y.; Ichimura, M.; Nakamura, H.; Honda, K.; Shibahara, M.; Hirano, T.; Yamada, H.; Tanaka, R.; Sakai, I.; et al. Surface Modification and Adhesion Mechanism of Isotactic Polypropylene with Low-Energy Electron-Beam Treatments. Langmuir 2020, 36, 10846–10852. [Google Scholar] [PubMed]
- Krause, B.; Stephan, M.; Volkland, S.; Voigt, D.; Häußler, L.; Dorschner, H. Long-chain branching of polypropylene by electron-beam irradiation in the molten state. J. Appl. Polym. Sci. 2006, 99, 260–265. [Google Scholar]
- Hu, J.; Zhao, X.; Xie, J.; Liu, Y.; Sun, S. Enhanced dielectric and energy storage properties of polypropylene by high-energy electron beam irradiation. Polym. Eng. Sci. 2022, 62, 1756–1763. [Google Scholar]
- Chen, K.; Du, B.X.; Liu, H.; Xiao, M. Enhanced high-temperatures energy storage performance of BOPP film via surface grafting modification. Chem. Eng. J. 2025, 505, 159724. [Google Scholar]
- Chen, J.Q.; Wang, X.; Sun, W.F.; Zhao, H. Improved Water-Tree Resistances of SEBS/PP Semi-Crystalline Composites under Crystallization Modifications. Molecules 2020, 25, 3669. [Google Scholar] [CrossRef] [PubMed]
- Muljana, H.; Arends, S.; Remerie, K.; Boven, G.; Picchioni, F.; Bose, R.K. Cross-Linking of Polypropylene via the Diels–Alder Reaction. Polymers 2022, 14, 1176. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Wang, K.; Chen, K.; Du, B. Effect of Photoinitiator on Dielectric Properties of Polypropylene Films Modified by UV Irradiation. IEEE Trans. Dielectr. Electr. Insul. 2024, 31, 168–175. [Google Scholar]
- Xiao, M.; Zhao, Y.; Song, Y.; Du, B. Polypropylene Films for HVDC Capacitors With Enhanced Dielectric Performance by Irradiation Crosslinking Modification. IEEE Trans. Dielectr. Electr. Insul. 2024, 31, 666–673. [Google Scholar]
- Xiao, M.; Wang, K.; Song, Y.; Du, B. Dielectric performance improvement of polypropylene film modified by γ-ray irradiation for HVDC capacitors. J. Phys. D Appl. Phys. 2024, 57, 125503. [Google Scholar]
- Xiao, M.; Song, Y.; Liu, H.; Zhang, M.; Du, B.; Xing, Z. Dielectric Properties of Cross-Linked Polypropylene by Gamma-Ray Irradiation for Film Capacitor. IEEE Trans. Dielectr. Electr. Insul. 2024, 31, 625–632. [Google Scholar]
- Yuan, X.; Chung, T.C.M. Cross-linking effect on dielectric properties of polypropylene thin films and applications in electric energy storage. Appl. Phys. Lett. 2011, 98, 062901. [Google Scholar]
- Girones, J.; Vo, L.T.T.; Haudin, J.M.; Freire, L.; Navard, P. Crystallization of polypropylene in the presence of biomass-based fillers of different compositions. Polymer 2017, 127, 220–231. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, W.; Wang, Q.; Si, Z.; Yang, Y.; Liao, Y.; Zhang, F.; Hiziroglu, H.R. Effect of Nano-Clay Filler on the Thermal Breakdown Mechanism and Lifespan of Polypropylene Film Under AC Fields. IEEE Access 2020, 8, 36055–36060. [Google Scholar]
- Mao, J.; Wang, S.; Cheng, Y.; Xiao, B.; Zhang, L.; Ai, D.; Chen, Y.; Sun, W.; Luo, J. High energy storage density and efficiency achieved in dielectric films functionalized with strong electronegative asymmetric halogen-phenyl groups. Chem. Eng. J. 2022, 444, 136331. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Zhu, Y.; Luo, Z.; Zhang, Y.-R.; Shao, Q.; Quan, H.; Wang, M.; Hu, S.; Yang, M.; et al. Biaxially oriented films of grafted-polypropylene with giant energy density and high efficiency at 125 °C. J. Mater. Chem. A 2023, 11, 10659–10668. [Google Scholar] [CrossRef]
- Yuan, C.; Zhou, Y.; Zhu, Y.; Hu, S.; Liang, J.; Luo, Z.; Gao, B.; Zeng, T.; Zhang, Y.; Li, J.; et al. Improved High-Temperature Electrical Properties of Polymeric Material by Grafting Modification. ACS Sustain. Chem. Eng. 2022, 10, 8685–8693. [Google Scholar]
- Xiao, M.; Zhao, Y.; Du, B. Dielectric Performance of Furan-Functionalized Polypropylene Film for HVDC Capacitors. IEEE Trans. Dielectr. Electr. Insul. 2024, 31, 160–167. [Google Scholar]
- Ran, Z.; Zhang, Y.; Luo, Z.; Zhu, Y.; Wang, M.; Wang, R.; Fu, J.; Shao, Q.; Quan, H.; Yuan, H.; et al. Significantly improved high-temperature capacitive performance in polypropylene based on molecular semiconductor grafting. Mater. Today Energy 2023, 38, 101429. [Google Scholar] [CrossRef]
- Drabek, J.; Zatloukal, M. Evaluation of Thermally Induced Degradation of Branched Polypropylene by Using Rheology and Different Constitutive Equations. Polymers 2016, 8, 317. [Google Scholar] [CrossRef]
- Teixeira, M.; Del Hoyo, I.; Wandrowelsti, F.; Swinka-Filho, V.; Munaro, M. Evaluation of thermal degradation in isotactic polypropylene films used in power capacitors. J. Therm. Anal. Calorim. 2017, 130, 997–1002. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, G.; Li, B.; Chung, T.C.M.; Rajagopalan, R.; Lanagan, M.T. Thermally Stable Low-Loss Polymer Dielectrics Enabled by Attaching Cross-Linkable Antioxidant to Polypropylene. ACS Appl. Mater. Interfaces 2020, 12, 14154–14164. [Google Scholar]
- Plaza-González, D.; Blázquez-Blázquez, E.; Gómez-Elvira, J.M.; Hoyos, M. Thermal and Dielectric Stability of Functional Photo-cross-linkable Carbazole-Modified Polypropylene. ACS Appl. Polym. Mater. 2023, 5, 7958–7967. [Google Scholar]
- Ding, J.; Wang, Q.; Jiang, Z.; Zhang, Y. Enhanced energy density at elevated temperature in polyetherimide based all-organic dielectrics via UV irradiation. Appl. Phys. Lett. 2023, 122, 112903. [Google Scholar]
- Wang, T.; Li, X.; Zhang, B.; Li, D.; Liu, J.; Zhang, G. Basic reason for the accumulation of charge on the surface of polymer dielectrics. Sci. China Mater. 2022, 65, 2884–2888. [Google Scholar]
- Zhong, S.; Liu, X.; Zheng, S.; Sun, S. Achieving High Energy Storage Capability of Polypropylene Films through Clean Electron Beam Irradiation Induced Grafting Strategy. ACS Appl. Polym. Mater. 2025, 7, 247–257. [Google Scholar]
- Yuan, X.; Matsuyama, Y.; Chung, T.C.M. Synthesis of Functionalized Isotactic Polypropylene Dielectrics for Electric Energy Storage Applications. Macromolecules 2010, 43, 4011–4015. [Google Scholar]
- Li, W.; Wang, Q.; Zhang, G.; He, Y.; Qin, B.; Zhang, X.; Liu, Z.; Gong, H.; Zhang, Z. Ultrahigh Energy Density Achieved at High Efficiency in Dielectric Capacitors by Regulating α-Phase Crystallization in Polypropylene Films with Fluorinated Groups. Adv. Funct. Mater. 2024, 34, 2410959. [Google Scholar] [CrossRef]
- Zhao, X.; Suo, Z. Electromechanical instability in semicrystalline polymers. Appl. Phys. Lett. 2009, 95, 031904. [Google Scholar]
- Feng, Q.K.; Zhang, Y.X.; Liu, D.F.; Song, Y.H.; Huang, L.; Dang, Z.M. Dielectric and energy storage properties of all-organic sandwich-structured films used for high-temperature film capacitors. Mater. Today Energy 2022, 29, 101132. [Google Scholar]
- Zhang, T.; Liang, S.; Yu, H.; Zhang, C.; Tang, C.; Li, H.; Chi, Q. Improved high-temperature energy storage density at low-electric field in BOPP/PVDF multilayer films. J. Appl. Polym. Sci. 2023, 140, e54729. [Google Scholar]
- Gong, Y.; Chen, D.; Duan, J.; Zhang, X.; Ma, Y.; Zhao, C.; Yang, W. Largely enhanced energy density of BOPP–OBT@CPP–BOPP sandwich-structured dielectric composites. J. Mater. Chem. C 2022, 10, 13074–13083. [Google Scholar]
- Gong, Y.; Chen, D.; Ma, Y.; Yang, W. Enhancement of Energy Density in the BOPP-Based Sandwich-Structured Film by the Synergistic Effect of BaTiO3 @Polyaniline Hybrid Dielectric Fillers. ACS Appl. Electron. Mater. 2023, 5, 5555–5563. [Google Scholar]
- Bai, Y.; Zhao, H.; Yin, L.; Bai, J. Enhancing energy storage property of polypropylene-based sandwich composites with surface-modified nonzero dimensional nanomaterials. J. Polym. Sci. 2024, 62, 3897–3908. [Google Scholar]
- Wang, J.; Guo, H.; Huang, X.; Du, J.; Zhang, Q.; Wang, K. Stretching-Regulated Homogenization of Lamellae Thickness in Biaxially Oriented Polypropylene Capacitor Films, and Its Effect on Breakdown Strength. Macromol. Rapid Commun. 2024, 46, 2400760. [Google Scholar]
- Dai, X.; Xing, Z.; Yang, W.; Zhang, C.; Li, F.; Chen, X.; Li, C.; Zhou, J.; Li, L. The Effect of Annealing on the Structure and Electric Performance of Polypropylene Films. Int. J. Polym. Sci. 2022, 2022, 1–12. [Google Scholar]
- Liu, D.; Wu, L.; Wu, K.; Xu, S.; Sui, G.; Jing, M.; Zhao, J.; Wei, Y.; Fu, Q. Largely enhanced energy density of polypropylene based nanocomposites via synergistic hybrid fillers and high shear extrusion assisted dispersion. Compos. Part A Appl. Sci. Manuf. 2019, 119, 134–144. [Google Scholar]
- Lee, B.; Hwang, U.; Kim, J.; Kim, S.-H.; Choi, K.; Park, I.-K.; Choi, C.; Suhr, J.; Nam, J.-D. Highly dispersed graphene nanoplatelets in polypropylene composites by employing high-shear stress for enhanced dielectric properties and frequency-selective electromagnetic interference shielding capability. Compos. Commun. 2023, 37, 101409. [Google Scholar]
- Wu, P.; Yang, Q.; Zhao, Z.; Sun, H.; Zhang, T.; Huang, Y.; Liao, X. Structure evolution and orientation mechanism of isotactic polypropylene during the two-stage solid die drawing process. J. Appl. Polym. Sci. 2018, 135, 46581. [Google Scholar]
- An, H.; Guo, J.; Gao, J.; Zhong, Y.; Li, Y.; Wang, Z. Structural evolution of biaxially oriented polypropylene films upon asynchronous stretching. J. Polym. Sci. 2024, 62, 3947–3958. [Google Scholar]
- Xiong, J.; Wang, X.; Zhang, X.; Xie, Y.; Lu, J.; Zhang, Z. How the biaxially stretching mode influence dielectric and energy storage properties of polypropylene films. J. Appl. Polym. Sci. 2021, 138, 50029. [Google Scholar]
- Kanai, T.; Egoshi, K.; Ohno, S.; Takebe, T. The evaluation of stretchability and its applications for biaxially oriented polypropylene film. Adv. Polym. Technol. 2018, 37, 2253–2260. [Google Scholar]
- Xiao, M.; Zhao, Y.; Wang, K.; Du, B. Effect of Biaxial Stretching on the Dielectric Performance of Long-Chain Branched Polypropylene for Metallized Film Capacitors. IEEE Trans. Dielectr. Electr. Insul. 2024, 31, 2381–2388. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Yao, J.; Zhu, F.; Gao, Y.; Gu, Y.; Guo, Y.; Sun, Y.; An, Y. Recent Advances in Preparation and Application of BOPP Film for Energy Storage and Dielectric Capacitors. Molecules 2025, 30, 1596. https://doi.org/10.3390/molecules30071596
Zhang K, Yao J, Zhu F, Gao Y, Gu Y, Guo Y, Sun Y, An Y. Recent Advances in Preparation and Application of BOPP Film for Energy Storage and Dielectric Capacitors. Molecules. 2025; 30(7):1596. https://doi.org/10.3390/molecules30071596
Chicago/Turabian StyleZhang, Kelei, Junlong Yao, Fangju Zhu, Yuan Gao, Yixi Gu, Yani Guo, Yimin Sun, and Yu An. 2025. "Recent Advances in Preparation and Application of BOPP Film for Energy Storage and Dielectric Capacitors" Molecules 30, no. 7: 1596. https://doi.org/10.3390/molecules30071596
APA StyleZhang, K., Yao, J., Zhu, F., Gao, Y., Gu, Y., Guo, Y., Sun, Y., & An, Y. (2025). Recent Advances in Preparation and Application of BOPP Film for Energy Storage and Dielectric Capacitors. Molecules, 30(7), 1596. https://doi.org/10.3390/molecules30071596




