Exploring the Influence of a Pomegranate Extract on the Functionality of Healthy and Diseased Human Gut Microbiota: An In Vitro Study
Abstract
:1. Introduction
2. Results
2.1. TPC Evaluation of Pomegranate Extract Before and After Digestion and Fermentation Processes
2.2. Radical Scavenging Activity of the Pomegranate Extract Before and After Digestion and Fermentation Processes
2.3. Reducing Power of the Pomegranate Extract Before and After Digestion and Fermentation Processes
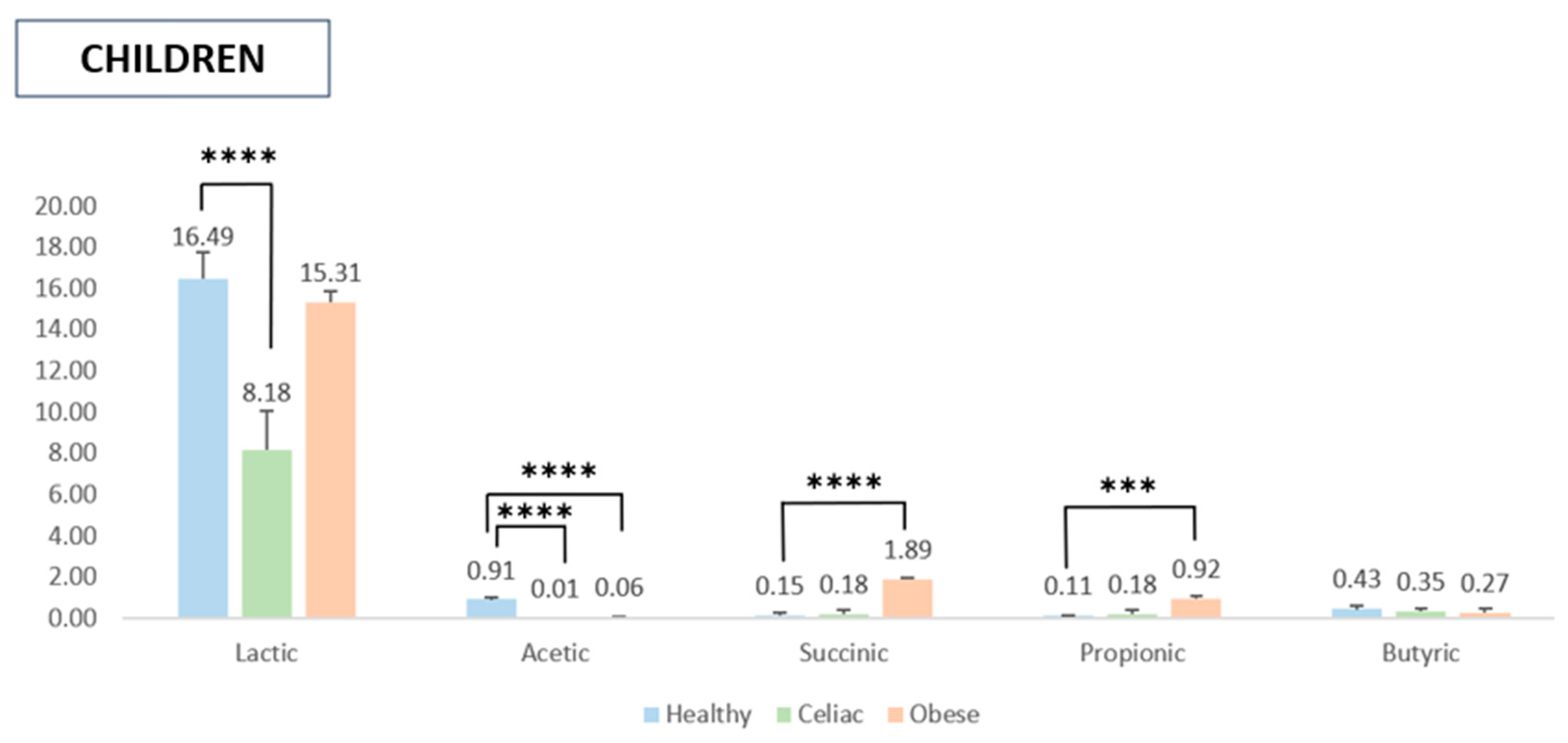
2.4. SCFA Quantification After In Vitro Digestion and Fermentation
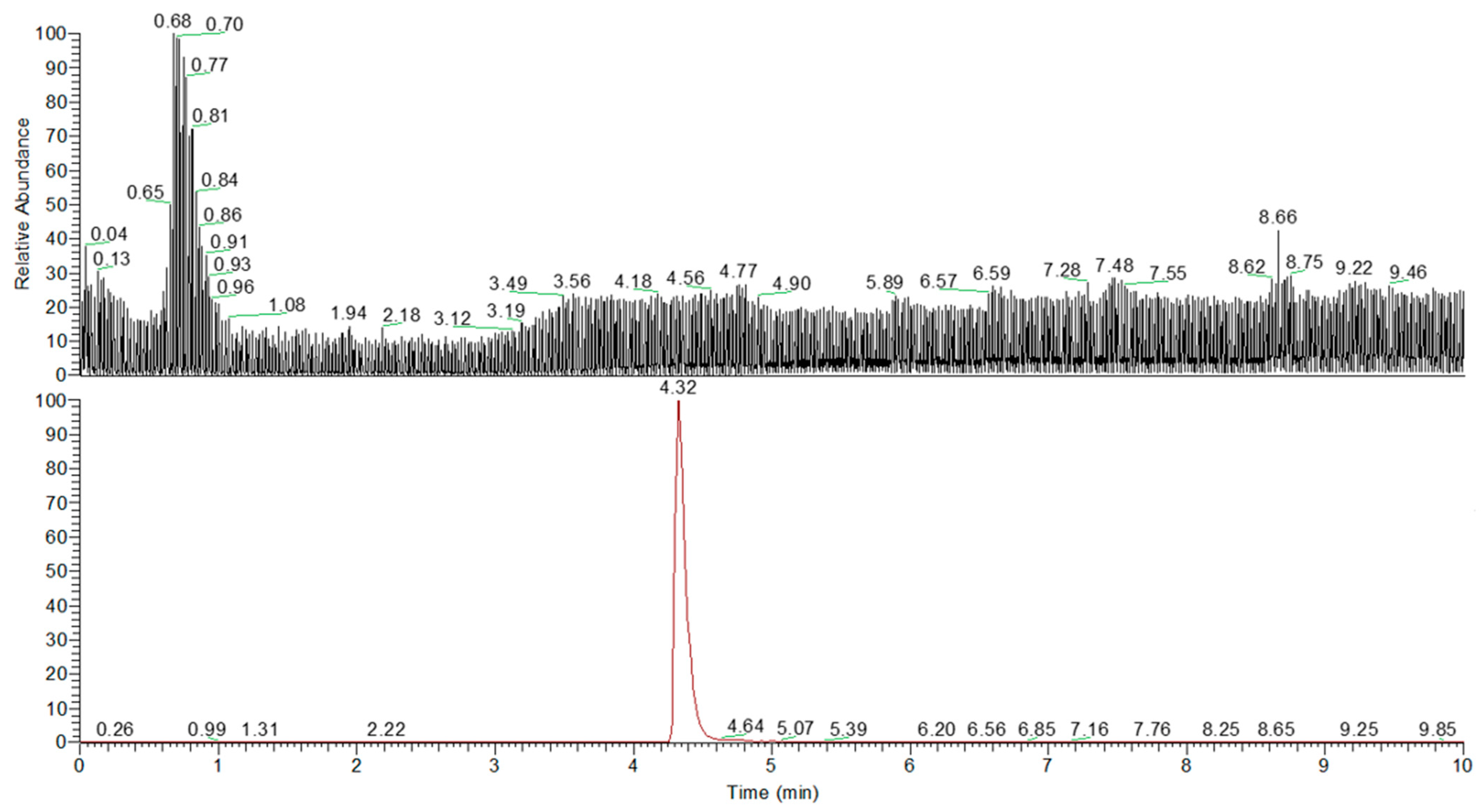
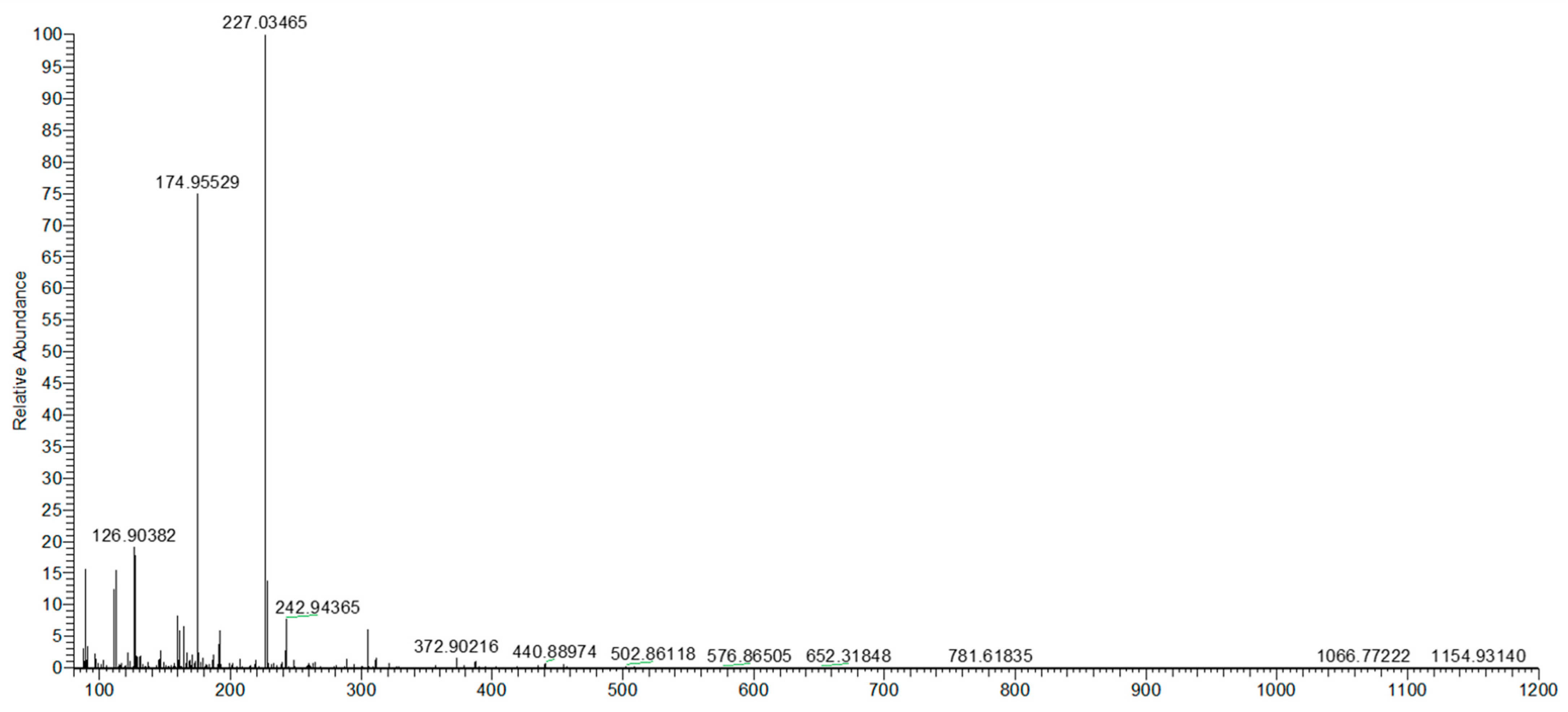
2.5. Quali–Quantitative Analysis of Urolithin A After In Vitro Fermentation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. In Vitro Digestion
4.3. In Vitro Fermentation
4.4. Antioxidant Assays
4.4.1. Total Phenolic Content (TPC)
4.4.2. DPPH Assay
4.4.3. ABTS Assay
4.4.4. FRAP Assay
4.5. Analysis of Short-Chain Fatty Acids (SCFAs)
4.6. Extraction of Urolithin A
4.7. UHPLC Q-Orbitrap HRMS Analysis of Urolithin A
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azinobis(3-ethylbenzothiazoline)-6-sulfonic acid |
| AP-1 | Activator protein |
| COX | Cyclooxygenase |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl hydrate |
| EA | Ellagic acid |
| H2O2 | Hydrogen peroxide |
| IL | Interleukins |
| NF-κB | Nuclear factor kappa B |
| PPARs | Peroxisome proliferator-activated receptors |
| SCFAs | Short-chain fatty acids |
| STAT | Signal transducer and activator of transcription |
| TNF-α | Tumor necrosis factor |
| TPC | Total phenolic content |
| TPTZ | 2,4,6-tri(2-pyridyl)-s-triazine |
| Trolox | 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid |
| UHPLC Q-Orbitrap HRMS | Ultrahigh-performance liquid chromatography coupled with high-resolution mass spectrometry |
| Uro-A | Urolithin A |
References
- Celik, I.; Temur, A.; Isik, I. Hepatoprotective role and antioxidant capacity of pomegranate (Punica granatum) flowers infusion against trichloroacetic acid-exposed in rats. Food Chem. Toxicol. 2009, 47, 145–149. [Google Scholar] [CrossRef]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef] [PubMed]
- USDA. Pomegranates, Raw. Available online: https://fdc.nal.usda.gov/food-details/169134/nutrients (accessed on 25 March 2025).
- Ullah, H.; Sommella, E.; Minno, A.D.; Piccinocchi, R.; Buccato, D.G.; De Lellis, L.F.; Riccioni, C.; Baldi, A.; El-Seedi, H.R.; Khalifa, S.A.; et al. Combination of chemically characterized pomegranate extract and hydrophilic vitamins against prolonged fatigue: A monocentric, randomized, double-blind, placebo-controlled clinical trial. Nutrients 2023, 15, 2883. [Google Scholar] [CrossRef]
- Coronado-Reyes, J.A.; Cortes-penagos, C.D.J.; González-Hernández, J.C. Chemical composition and great applications to the fruit of the pomegranate (Punica granatum): A review. Food Sci. Technol. 2021, 42, e29420. [Google Scholar] [CrossRef]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012, 143, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Rodrigo, R.; Miranda, A.; Vergara, L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin. Chim. Acta 2011, 412, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-Y.; Ho, C.-T.; Chen, Y.-K. Biological actions and molecular effects of resveratrol, pterostilbene, and 3′-hydroxypterostilbene. Yao Wu Shi Pin Fen Xi 2017, 25, 134–147. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Aspects Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Aloqbi, A.; Omar, U.; Yousr, M.; Grace, M.; Lila, M.A.; Howell, N. Antioxidant activity of pomegranate juice and punicalagin. Nat. Sci. 2016, 8, 235–246. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef]
- Vučić, V.; Grabež, M.; Trchounian, A.; Arsić, A. Composition and potential health benefits of pomegranate: A review. Curr. Pharm. Des. 2019, 25, 1817–1827. [Google Scholar] [PubMed]
- Bialonska, D.; Ramnani, P.; Kasimsetty, S.G.; Muntha, K.R.; Gibson, G.R.; Ferreira, D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010, 140, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Bialonska, D.; Kasimsetty, S.G.; Schrader, K.K.; Ferreira, D. The effect of pomegranate (Punica granatum L.) byproducts and ellagitannins on the growth of human gut bacteria. J. Agric. Food Chem. 2009, 57, 8344–8349. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Huang, W.; Guo, H.L.; Deng, X.; Zhu, T.T.; Xiong, J.F.; Xu, Y.H.; Xu, Y. Short-chain fatty acids inhibit oxidative stress and inflammation in mesangial cells induced by high glucose and lipopolysaccharide. Exp. Clin. Endocrinol. Diabetes 2017, 125, 98–105. [Google Scholar] [CrossRef]
- Den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.J.; et al. Short-chain fatty acids protect against high-fat diet–induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Bowman, B.A.; Ford, E.S.; Vinicor, F.; Marks, J.S.; Koplan, J.P. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001, 286, 1195–1200. [Google Scholar] [CrossRef]
- Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr. Rev. 1999, 20, 649–688. [Google Scholar]
- Ricote, M.; Li, A.C.; Willson, T.M.; Kelly, C.J.; Glass, C.K. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998, 391, 79–82. [Google Scholar] [CrossRef]
- Jiménez-Zamora, A.; Delgado-Andrade, C.; Rufián-Henares, J.A. Antioxidant capacity, total phenols and color profile during the storage of selected plants used for infusion. Food Chem. 2016, 199, 339–346. [Google Scholar] [PubMed]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Conti, G.O.; Sadrabad, E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 2018, 114, 108–111. [Google Scholar] [PubMed]
- Ardekani, M.R.S.; Hajimahmoodi, M.; Oveisi, M.R.; Sadeghi, N.; Jannat, B.; Ranjbar, A.M.; Gholam, N.; Moridi, T. Comparative antioxidant activity and total flavonoid content of Persian pomegranate (Punica granatum L.) cultivars. Iranian J. Pharm. Res. 2011, 10, 519. [Google Scholar]
- Fawole, O.A.; Opara, U.L. Stability of total phenolic concentration and antioxidant capacity of extracts from pomegranate co-products subjected to in vitro digestion. BMC Complement. Altern. Med. 2016, 16, 358. [Google Scholar]
- Chen, G.L.; Chen, S.G.; Zhao, Y.Y.; Luo, C.X.; Li, J.; Gao, Y.Q. Total phenolic contents of 33 fruits and their antioxidant capacities before and after in vitro digestion. Ind. Crops Prod. 2014, 57, 150–157. [Google Scholar] [CrossRef]
- Chiang, C.J.; Kadouh, H.; Zhou, K. Phenolic compounds and antioxidant properties of gooseberry as affected by in vitro digestion. LWT-Food Sci. Technol. 2013, 51, 417–422. [Google Scholar]
- Pavan, V.; Sancho, R.A.S.; Pastore, G.M. The effect of in vitro digestion on the antioxidant activity of fruit extracts (Carica papaya, Artocarpus heterophillus and Annona marcgravii). LWT-Food Sci. Technol. 2014, 59, 1247–1251. [Google Scholar]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Arranz, S.; Silván, J.M.; Saura-Calixto, F. Nonextractable polyphenols, usually ignored, are the major part of dietary polyphenols: A study on the Spanish diet. Mol. Nutr. Food Res. 2010, 54, 1646–1658. [Google Scholar]
- Frontela-Saseta, C.; López-Nicolás, R.; González-Bermúdez, C.A.; Peso-Echarri, P.; Ros-Berruezo, G.; Martínez-Graciá, C.; Canali, R.; Virgili, F. Evaluation of antioxidant activity and antiproliferative effect of fruit juices enriched with Pycnogenol® in colon carcinoma cells. The effect of in vitro gastrointestinal digestion. Phytother. Res. 2011, 25, 1870–1875. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. In vitro gastrointestinal digestion of pomegranate peel (Punica granatum) flour obtained from co-products: Changes in the antioxidant potential and bioactive compounds stability. J. Funct. Foods 2015, 19, 617–628. [Google Scholar] [CrossRef]
- Pérez-Vicente, A.; Gil-Izquierdo, A.; García-Viguera, C. In vitro gastrointestinal digestion study of pomegranate juice phenolic compounds, anthocyanins, and vitamin C. J. Agric. Food Chem. 2002, 50, 2308–2312. [Google Scholar] [PubMed]
- Mosele, J.I.; Macià, A.; Romero, M.P.; Motilva, M.J.; Rubió, L. Application of in vitro gastrointestinal digestion and colonic fermentation models to pomegranate products (juice, pulp and peel extract) to study the stability and catabolism of phenolic compounds. J. Funct. Foods 2015, 14, 529–540. [Google Scholar]
- De Pinedo, A.T.; Peñalver, P.; Morales, J.C. Synthesis and evaluation of new phenolic-based antioxidants: Structure–activity relationship. Food Chem. 2007, 103, 55–61. [Google Scholar] [CrossRef]
- Popovici, C.; Saykova, I.; Tylkowski, B. Evaluation of the antioxidant activity of phenolic compounds through reactivity with the free radical DPPH. Rev. Genie Ind. 2010, 4, 131–887. [Google Scholar]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of fermentation on bioactivity and the composition of polyphenols contained in polyphenol-rich foods: A review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Mula, H.M.; Tomás-Barberán, F.A.; García-Villalba, R. Pomegranate fruit and juice (cv. Mollar), rich in ellagitannins and anthocyanins, also provide a significant content of a wide range of proanthocyanidins. J. Agric. Food Chem. 2019, 67, 9160–9167. [Google Scholar]
- Liu, Q.; Hua, Z.; Chen, M.; Liu, S.; Ahmed, S.; Hou, X.; Yang, G.; Fang, Y. Changes in polyphenols and antioxidant properties of pomegranate peels fermented by urolithin A-producing Streptococcus thermophilus FUA329. ACS Food Sci. Technol. 2023, 3, 1383–1392. [Google Scholar]
- Singh, V.; Ahlawat, S.; Mohan, H.; Gill, S.S.; Sharma, K.K. Balancing reactive oxygen species generation by rebooting gut microbiota. J. Appl. Microbiol. 2022, 132, 4112–4129. [Google Scholar]
- De Filippis, A.; Ullah, H.; Baldi, A.; Dacrema, M.; Esposito, C.; Garzarella, E.U.; Santarcangelo, C.; Tantipongpiradet, A.; Daglia, M. Gastrointestinal disorders and metabolic syndrome: Dysbiosis as a key link and common bioactive dietary components useful for their treatment. Int. J. Mol. Sci. 2020, 21, 4929. [Google Scholar] [CrossRef]
- Barbara, G.; Feinle-Bisset, C.; Ghoshal, U.C.; Santos, J.; Vanner, S.J.; Vergnolle, N.; Zoetendal, E.G.; Quigley, E.M. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology 2016, 150, 1305–1318. [Google Scholar] [CrossRef] [PubMed]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut microbiota interrelationship: A transition to a new generation of prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.W.; Chen, H.C.; Chen, C.Y.; Yen, C.Y.; Lin, C.J.; Prajnamitra, R.P.; Chen, L.L.; Ruan, S.C.; Lin, J.H.; Lin, P.J.; et al. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation 2019, 139, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Ecklu-Mensah, G.; Choo-Kang, C.; Maseng, M.G.; Donato, S.; Bovet, P.; Viswanathan, B.; Bedu-Addo, K.; Plange-Rhule, J.; Oti Boateng, P.; Forrester, T.E.; et al. Gut microbiota and fecal short chain fatty acids differ with adiposity and country of origin: The METS-microbiome study. Nat. Commun. 2023, 14, 5160. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Henagan, T.M.; Stefanska, B.; Fang, Z.; Navard, A.M.; Ye, J.; Lenard, N.R.; Devarshi, P.P. Sodium butyrate epigenetically modulates high-fat diet-induced skeletal muscle mitochondrial adaptation, obesity and insulin resistance through nucleosome positioning. Br. J. Pharmacol. 2015, 172, 2782–2798. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef]
- Bonomo, R.R.; Cook, T.M.; Gavini, C.K.; White, C.R.; Jones, J.R.; Bovo, E.; Zima, A.V.; Brown, I.A.; Dugas, L.R.; Zakharian, E.; et al. Fecal transplantation and butyrate improve neuropathic pain, modify immune cell profile, and gene expression in the PNS of obese mice. Proc. Natl. Acad. Sci. USA 2020, 117, 26482–26493. [Google Scholar] [CrossRef]
- Nistal, E.; Caminero, A.; Vivas, S.; de Morales, J.M.R.; de Miera, L.E.S.; Rodríguez-Aparicio, L.B.; Casqueiro, J. Differences in faecal bacteria populations and faecal bacteria metabolism in healthy adults and celiac disease patients. Biochimie 2012, 94, 1724–1729. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; De Pasquale, I.; Ndagijimana, M.; Vernocchi, P.; Ricciuti, P.; Gagliardi, F.; Laghi, L.; Crecchio, C.; Guerzoni, M.E.; et al. Duodenal and faecal microbiota of celiac children: Molecular, phenotype and metabolome characterization. BMC Microbiol. 2011, 11, 219. [Google Scholar]
- Di Cagno, R.; Rizzello, C.G.; Gagliardi, F.; Ricciuti, P.; Ndagijimana, M.; Francavilla, R.; Guerzoni, M.E.; Crecchio, C.; Gobbetti, M.; De Angelis, M. Different fecal microbiotas and volatile organic compounds in treated and untreated children with celiac disease. Appl. Environ. Microbiol. 2009, 75, 3963–3971. [Google Scholar]
- Lerner, A.; Patricia, J.; Matthias, T. Nutrients, bugs and us: The short-chain fatty acids story in celiac disease. Int. J. Celiac Dis. 2016, 4, 92–94. [Google Scholar]
- Abedi, E.; Hashemi, S.M.B. Lactic acid production–producing microorganisms and substrates sources-state of art. Heliyon 2020, 6, e04974. [Google Scholar] [PubMed]
- Mosele, J.I.; Gosalbes, M.J.; Macia, A.; Rubio, L.; Vázquez-Castellanos, J.F.; Jimenez Hernandez, N.; Moya, A.; Latorre, A.; Motilva, M.J. Effect of daily intake of pomegranate juice on fecal microbiota and feces metabolites from healthy volunteers. Mol. Nutr. Food Res. 2015, 59, 1942–1953. [Google Scholar] [PubMed]
- Song, H.; Shen, X.; Deng, R.; Chu, Q.; Zheng, X. Pomegranate peel anthocyanins prevent diet-induced obesity and insulin resistance in association with modulation of the gut microbiota in mice. Eur. J. Nutr. 2022, 61, 1837–1847. [Google Scholar]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A comprehensive update on their metabolism, bioactivity, and associated gut microbiota. Mol. Nutr. Food Res. 2022, 66, e2101019. [Google Scholar]
- Mena, P.; Dall’Asta, M.; Calani, L.; Brighenti, F.; Del Rio, D. Gastrointestinal stability of urolithins: An in vitro approach. Eur. J. Nutr. 2015, 56, 99–106. [Google Scholar]
- He, F.; Bian, Y.; Zhao, Y.; Xia, M.; Liu, S.; Gui, J.; Hou, X.; Fang, Y. In vitro conversion of ellagic acid to urolithin A by different gut microbiota of urolithin metabotype A. Appl. Microbiol. Biotechnol. 2024, 108, 215. [Google Scholar]
- González-Barrio, R.; Edwards, C.A.; Crozier, A. Colonic catabolism of ellagitannins, ellagic acid, and raspberry anthocyanins: In vivo and in vitro studies. Drug Metab. Dispos. 2011, 39, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Beltrán, D.; Espín, J.C.; Selma, M.V.; Tomás-Barberán, F.A. Time course production of urolithins from ellagic acid by human gut microbiota. J. Agric. Food Chem. 2013, 61, 8797–8806. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Periago, P.; Espín, J.C.; Tomás-Barberán, F.A. Identification of urolithin a as a metabolite produced by human colon microflora from ellagic acid and related compounds. J. Agric. Food Chem. 2005, 53, 5571–5576. [Google Scholar] [CrossRef]
- Tan, J.; Ma, Q.; Li, J.; Liu, Q.; Zhuang, Y. Bioavailability and antioxidant activity of Rambutan (Nephelium lappaceum) peel polyphenols during in vitro simulated gastrointestinal digestion, Caco-2 monolayer cell model application, and colonic fermentation. J. Agric. Food Chem. 2023, 71, 15829–15841. [Google Scholar] [CrossRef]
- Dacrema, M.; Sommella, E.; Santarcangelo, C.; Bruno, B.; Marano, M.G.; Insolia, V.; Saviano, A.; Campiglia, P.; Stornaiuolo, M.; Daglia, M. Metabolic profiling, in vitro bioaccessibility and in vivo bioavailability of a commercial bioactive Epilobium angustifolium L. extract. Biomed. Pharmacother. 2020, 131, 110670. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2016, 61, 1500901. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Aguirre, C.; Villalba, R.; Beltrán Riquelme, D.; Frutos Lisón, M.; Espín, J.C.; Tomás-Barberán, F.; Selma, M. Gut bacteria involved in ellagic acid metabolism to yield human urolithin metabotypes revealed. J. Agric. Food Chem. 2023, 71, 4029–4035. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Molino, S.; Navajas-Porras, B.; Valverde-Moya, Á.J.; Hinojosa-Nogueira, D.; López-Maldonado, A.; Pastoriza, S.; Rufián-Henares, J.Á. An in vitro batch fermentation protocol for studying the contribution of food to gut microbiota composition and functionality. Nat. Protoc. 2021, 16, 3186–3209. [Google Scholar] [CrossRef]
- Moreno-Montoro, M.; Olalla-Herrera, M.; Gimenez-Martinez, R.; Navarro-Alarcon, M.; Rufián-Henares, J.A. Phenolic compounds and antioxidant activity of Spanish commercial grape juices. J. Food Compos. Anal. 2015, 38, 19–26. [Google Scholar]
- Yen, G.; Chen, H. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Panzella, L.; Pérez-Burillo, S.; Pastoriza, S.; Martín, M.Á.; Cerruti, P.; Goya, L.; Ramos, S.; Rufián-Henares, J.Á.; Napolitano, A.; d’Ischia, M. High Antioxidant action and prebiotic activity of hydrolyzed spent coffee grounds (HSCG) in a simulated digestion–fermentation model: Toward the development of a novel food supplement. J. Agric. Food Chem. 2017, 65, 6452–6459. [Google Scholar] [CrossRef]
- García-Villalba, R.; Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Identification of novel urolithin metabolites in human feces and urine after the intake of a pomegranate extract. J. Agric. Food Chem. 2019, 67, 11099–11107. [Google Scholar] [CrossRef] [PubMed]

| Assay | Before In Vitro Digestion | After In Vitro Digestion | p-Value |
|---|---|---|---|
| TPC (g of GAE/kg of extract) | 232 ± 16 | 1657 ± 34 | <0.0001 |
| TEACDPPH (g of Trolox/kg of extract) | 1996 ± 54 | 2504 ± 23 | 0.0013 |
| TEACABTS (g of Trolox/kg of extract) | 4994 ± 127 | 2198 ± 76 | 0.0001 |
| TEACFRAP (g of Trolox/kg of extract) | 1924 ± 31 | 2491 ± 175 | 0.0373 |
| Assay | In Vitro Digested Samples | After In Vitro Fermentation | |||||
|---|---|---|---|---|---|---|---|
| Adults | Children | ||||||
| Healthy | Obese | Celiac | Healthy | Obese | Celiac | ||
| TPC | 1657 ± 34 | 6139 ± 458 **** | 5889 ± 259 ns | 2914 ± 367 **** | 8375 ± 1388 *** | 8945 ± 1120 ns | 8195 ± 1180 ns |
| TEACDPPH | 2504 ± 23 | 175 ± 11 **** | 233 ± 30 ns | 241 ± 37 ns | 198 ± 31 **** | 195 ± 31 ns | 203 ± 31 ns |
| TEACABTS | 2198 ± 76 | 162 ± 29 **** | 107 ± 29 ns | 155 ± 23 ns | 199 ± 21 **** | 193 ± 23 ns | 200 ± 31 ns |
| TEACFRAP | 2491 ± 175 | 251 ± 15 **** | 201 ± 18 * | 188 ± 21 ** | 281 ± 28 **** | 284 ± 31 ns | 267 ± 10 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buccato, D.G.; Delgado-Osorio, A.; De Lellis, L.F.; Morone, M.V.; Ullah, H.; Izzo, L.; Lombardi, S.; Di Minno, A.; Riccioni, C.V.; Moriki, D.; et al. Exploring the Influence of a Pomegranate Extract on the Functionality of Healthy and Diseased Human Gut Microbiota: An In Vitro Study. Molecules 2025, 30, 1634. https://doi.org/10.3390/molecules30071634
Buccato DG, Delgado-Osorio A, De Lellis LF, Morone MV, Ullah H, Izzo L, Lombardi S, Di Minno A, Riccioni CV, Moriki D, et al. Exploring the Influence of a Pomegranate Extract on the Functionality of Healthy and Diseased Human Gut Microbiota: An In Vitro Study. Molecules. 2025; 30(7):1634. https://doi.org/10.3390/molecules30071634
Chicago/Turabian StyleBuccato, Daniele Giuseppe, Adriana Delgado-Osorio, Lorenza Francesca De Lellis, Maria Vittoria Morone, Hammad Ullah, Luana Izzo, Sonia Lombardi, Alessandro Di Minno, Costanza Valentina Riccioni, Dafni Moriki, and et al. 2025. "Exploring the Influence of a Pomegranate Extract on the Functionality of Healthy and Diseased Human Gut Microbiota: An In Vitro Study" Molecules 30, no. 7: 1634. https://doi.org/10.3390/molecules30071634
APA StyleBuccato, D. G., Delgado-Osorio, A., De Lellis, L. F., Morone, M. V., Ullah, H., Izzo, L., Lombardi, S., Di Minno, A., Riccioni, C. V., Moriki, D., Rufián-Henares, J. Á., & Daglia, M. (2025). Exploring the Influence of a Pomegranate Extract on the Functionality of Healthy and Diseased Human Gut Microbiota: An In Vitro Study. Molecules, 30(7), 1634. https://doi.org/10.3390/molecules30071634











