Highly Efficient Catalytic Oxidation of Glucose to Formic Acid over Mn-Mo Doped Carbon Nanotube
Abstract
1. Introduction
2. Results
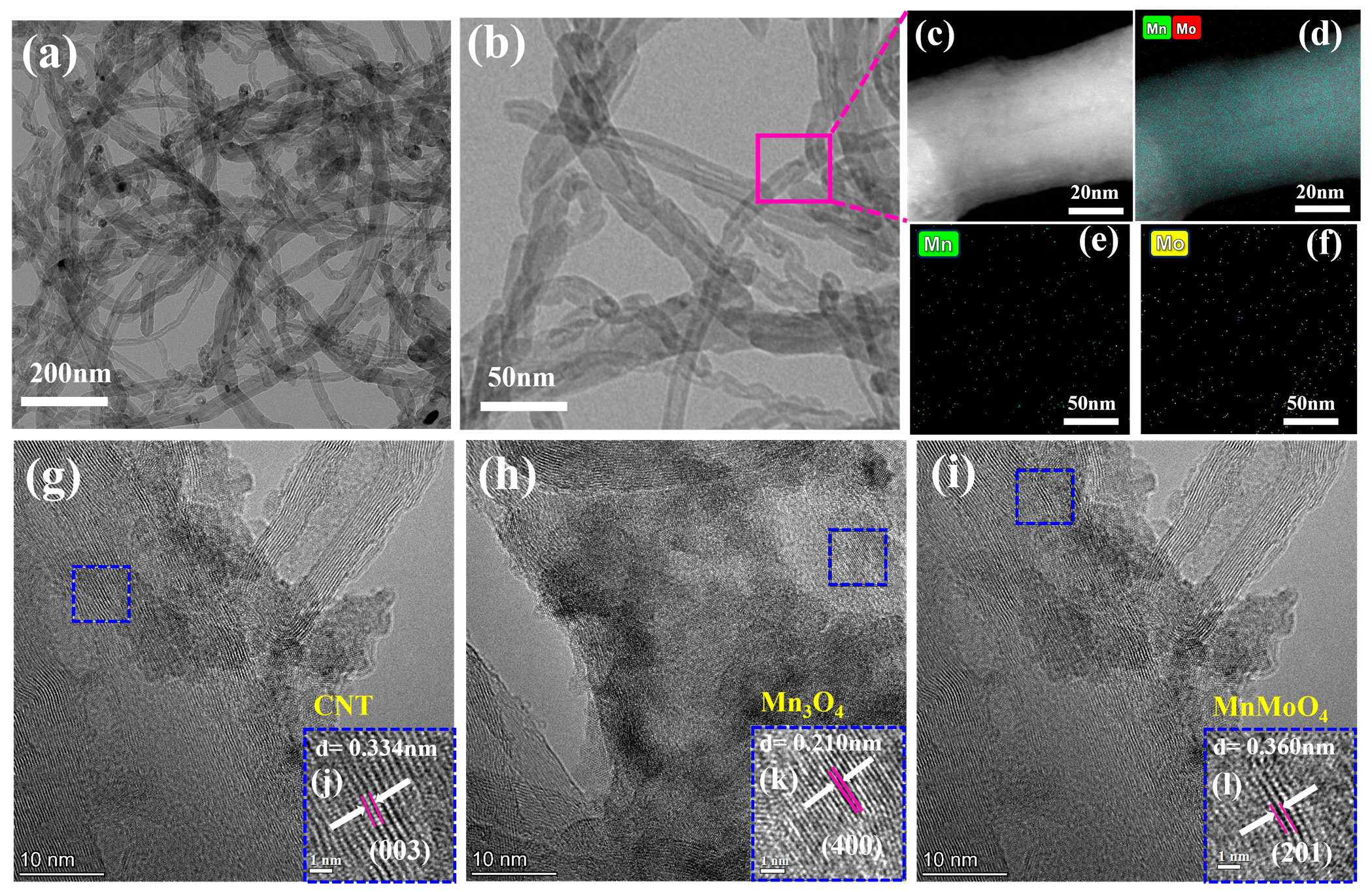
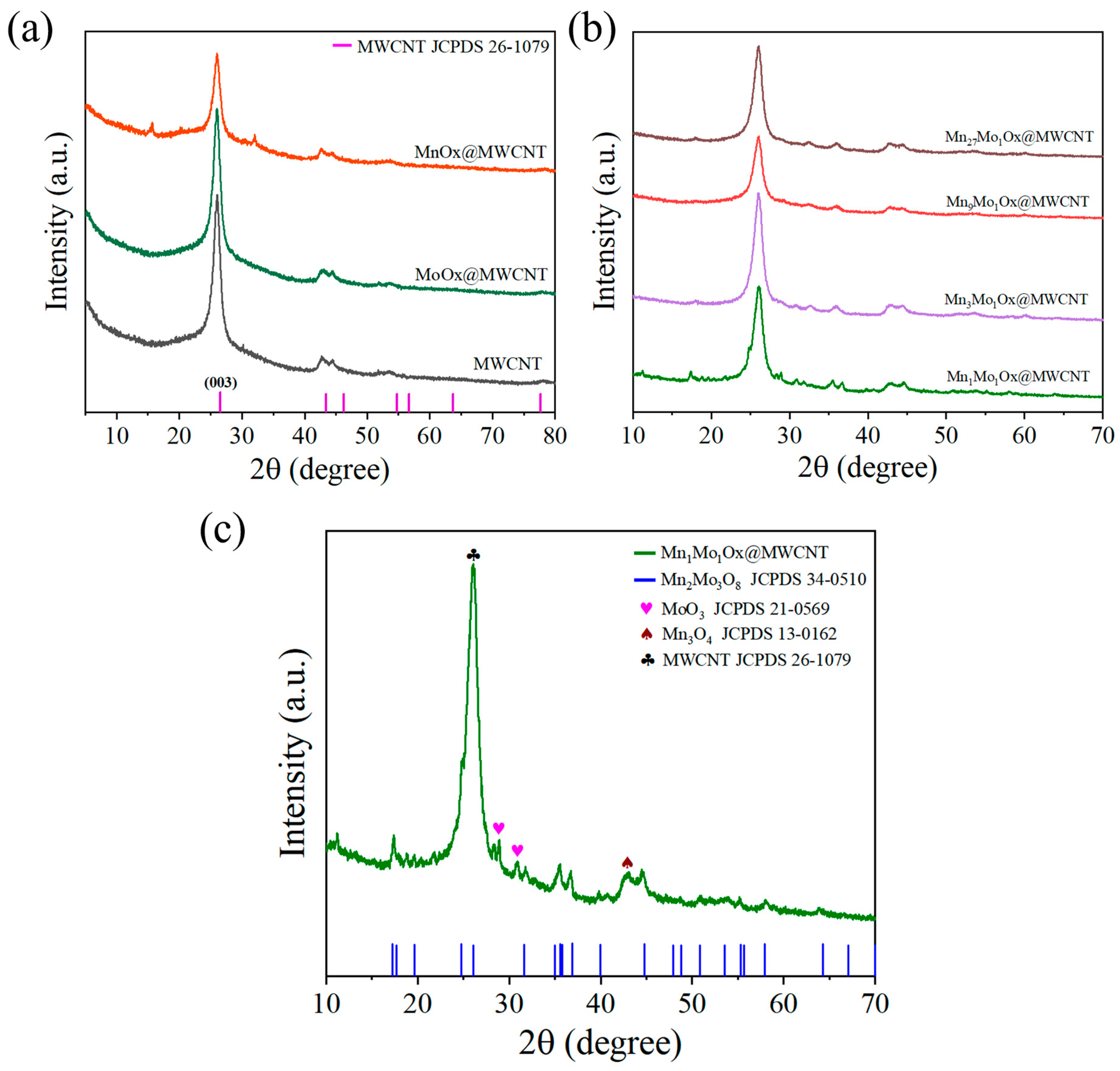
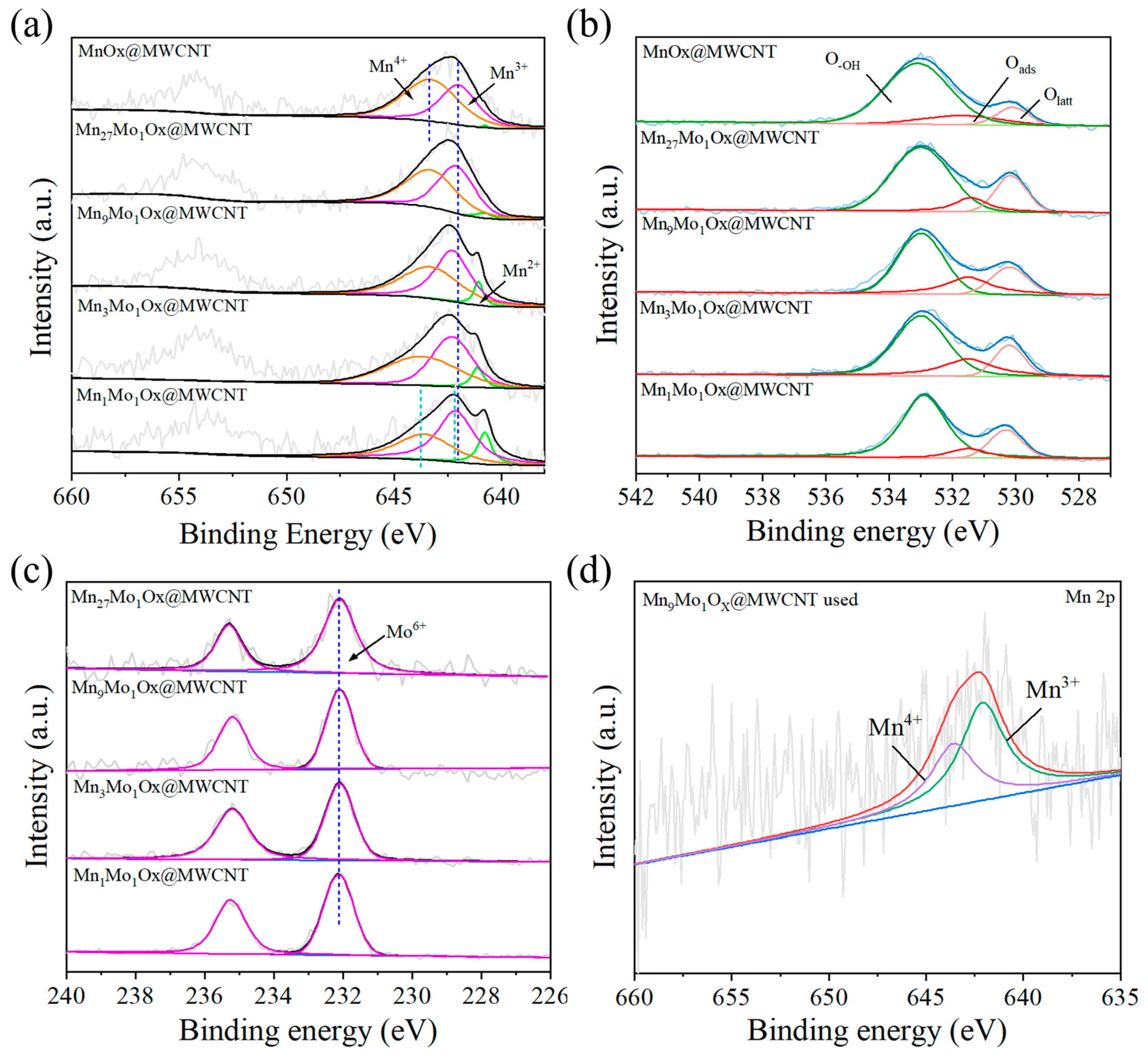
2.1. Characterizations of Catalysts
2.2. Catalytic Oxidation of Glucose to FA
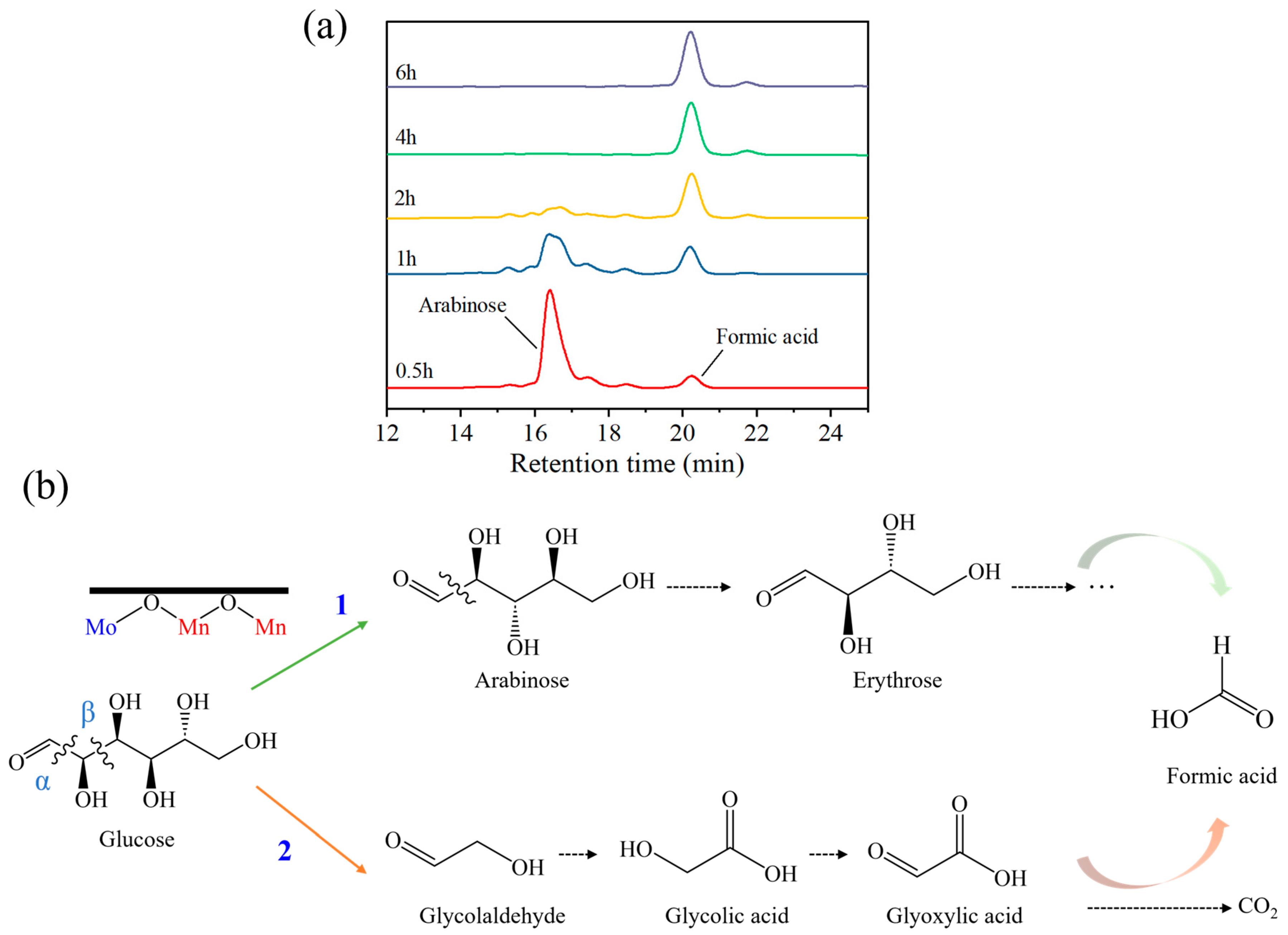
2.3. Possible Pathways of Glucose Oxidation to FA over Mn9Mo1OX@MWCNT
3. Materials and Methods
3.1. Materials
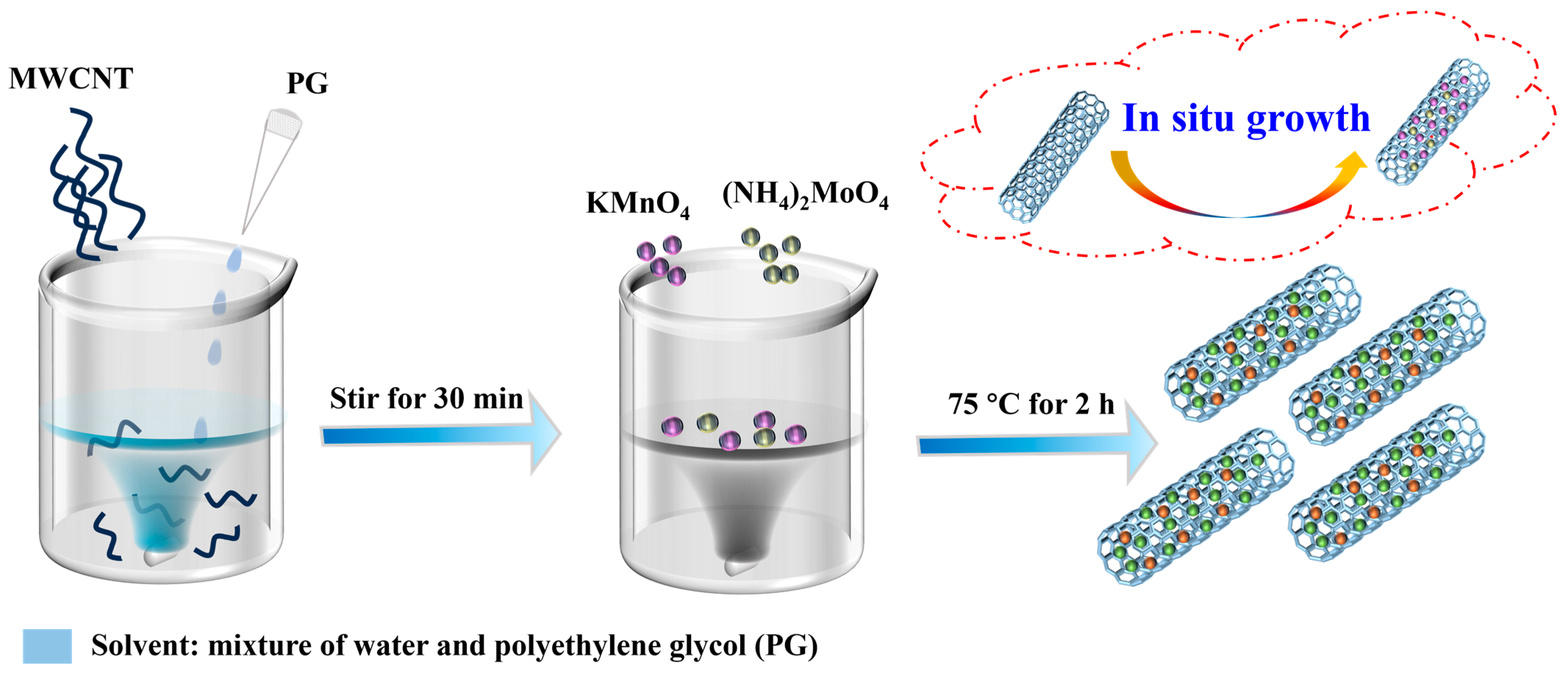
3.2. Synthesis of MnMoOX@MWCNT
3.3. Catalyst Characterization
3.4. Catalytic Reaction and Product Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Wang, T.; Chen, X.; Ma, X. Conversion study from lignocellulosic biomass and electric energy to H2 and chemicals. Int. J. Hydrogen Energy 2023, 48, 21004–21017. [Google Scholar] [CrossRef]
- Radosits, F.K.; Ajanovic, A.; Harasek, M. The relevance of biomass-based gases as energy carriers: A review. WIREs Energy Environ. 2024, 13, e527. [Google Scholar] [CrossRef]
- Alipour, S.; Omidvarborna, H. Enzymatic and catalytic hybrid method for levulinic acid synthesis from biomass sugars. J. Clean. Prod. 2017, 143, 490–496. [Google Scholar] [CrossRef]
- Liu, S.; Wang, K.; Yu, H.; Li, B.; Yu, S. Catalytic preparation of levulinic acid from cellobiose via Brønsted-Lewis acidic ionic liquids functional catalysts. Sci. Rep. 2019, 9, 1810. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Y.; Gao, Y.; Qian, K.; Wang, Y.; Li, N.; Wang, Y.; Ran, S.; Hou, X.; Zhu, Y. Catalytic pyrolysis of cellulose and hemicellulose: Investigation on furans selectivity with different zeolite structures at microporous scale. J. Anal. Appl. Pyrolysis 2023, 173, 106102. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, Z.; Liu, B.; Chen, S.; Zhang, Z. Catalytic oxidation of biomass derived 5-hydroxymethylfurfural (HMF) over RuIII-incorporated zirconium phosphate catalyst. J. Ind. Eng. Chem. 2016, 38, 181–185. [Google Scholar] [CrossRef]
- Castro, R.C.d.A.; Fonseca, B.G.; dos Santos, H.T.L.; Ferreira, I.S.; Mussatto, S.I.; Roberto, I.C. Alkaline deacetylation as a strategy to improve sugars recovery and ethanol production from rice straw hemicellulose and cellulose. Ind. Crops Prod. 2017, 106, 65–73. [Google Scholar] [CrossRef]
- Yu, H.; Guo, J.; Chen, Y.; Fu, G.; Li, B.; Guo, X.; Xiao, D. Efficient utilization of hemicellulose and cellulose in alkali liquor-pretreated corncob for bioethanol production at high solid loading by Spathaspora passalidarum U1-58. Bioresour. Technol. 2017, 232, 168–175. [Google Scholar] [CrossRef]
- Wang, Y.; Du, J.; Li, Q.; Tao, Y.; Cheng, Y.; Lu, J.; Wang, H. Bioconversion of cellulose and hemicellulose in corn cob into L-lactic acid and xylo-oligosaccharides. Int. J. Biol. Macromol. 2023, 253, 126775. [Google Scholar] [CrossRef]
- Wan, H.; Chen, X.; Cao, K.; Han, Y.; Duan, X.; Sun, Z.; Shi, J. High-efficient conversion from biomass to formic acid by isopolyacid Na6[α-Mo6V2O26] with strong oxidizing ability. Ind. Crops Prod. 2024, 222, 119466. [Google Scholar] [CrossRef]
- Guo, Y.-J.; Li, S.-J.; Sun, Y.-L.; Wang, L.; Zhang, W.-M.; Zhang, P.; Lan, Y.; Li, Y. Practical DMSO-promoted selective hydrolysis–oxidation of lignocellulosic biomass to formic acid attributed to hydrogen bonds. Green Chem. 2021, 23, 7041–7052. [Google Scholar]
- Eppinger, J.; Huang, K.-W. Formic acid as a hydrogen energy carrier. ACS Energy Lett. 2017, 2, 188–195. [Google Scholar]
- Singh, A.K.; Singh, S.; Kumar, A. Hydrogen energy future with formic acid: A renewable chemical hydrogen storage system. Catal. Sci. Technol. 2016, 6, 12–40. [Google Scholar]
- El-Nagar, G.A.; Hassan, M.A.; Lauermann, I.; Roth, C. Efficient direct formic acid fuel cells (DFAFCs) anode derived from seafood waste: Migration mechanism. Sci. Rep. 2017, 7, 17818. [Google Scholar]
- Shen, F.; Smith, R.L., Jr.; Li, J.; Guo, H.; Zhang, X.; Qi, X. Critical assessment of reaction pathways for conversion of agricultural waste biomass into formic acid. Green Chem. 2021, 23, 1536–1561. [Google Scholar]
- Kovács, I.; Kiss, J.; Kónya, Z. The Potassium-Induced Decomposition Pathway of HCOOH on Rh(111). Catalysts 2020, 10, 675. [Google Scholar] [CrossRef]
- Solakidou, M.; Gemenetzi, A.; Koutsikou, G.; Theodorakopoulos, M.; Deligiannakis, Y.; Louloudi, M. Cost Efficiency Analysis of H2 Production from Formic Acid by Molecular Catalysts. Energies 2023, 16, 1723. [Google Scholar] [CrossRef]
- Thijs, B.; Rongé, J.; Martens, J.A. Matching emerging formic acid synthesis processes with application requirements. Green Chem. 2022, 24, 2287–2295. [Google Scholar]
- Bulushev, D.A.; Ross, J.R.H. Towards Sustainable Production of Formic Acid. ChemSusChem 2018, 11, 821–836. [Google Scholar]
- Lu, T.; Hou, Y.; Wu, W.; Niu, M.; Wang, Y. Formic acid and acetic acid production from corn cob by catalytic oxidation using O2. Fuel Process. Technol. 2018, 171, 133–139. [Google Scholar]
- Gromov, N.V.; Taran, O.P.; Delidovich, I.V.; Pestunov, A.V.; Rodikova, Y.A.; Yatsenko, D.A.; Zhizhina, E.G.; Parmon, V.N. Hydrolytic oxidation of cellulose to formic acid in the presence of Mo-V-P heteropoly acid catalysts. Catal. Today 2016, 278, 74–81. [Google Scholar] [CrossRef]
- Li, J.; Ding, D.-J.; Deng, L.; Guo, Q.-X.; Fu, Y. Catalytic air oxidation of biomass-derived carbohydrates to formic acid. ChemSusChem 2012, 5, 1313–1318. [Google Scholar] [CrossRef]
- Wang, W.; Niu, M.; Hou, Y.; Wu, W.; Liu, Z.; Liu, Q.; Ren, S.; Marsh, K.N. Catalytic conversion of biomass-derived carbohydrates to formic acid using molecular oxygen. Green Chem. 2014, 16, 2614–2618. [Google Scholar] [CrossRef]
- Tang, Z.; Deng, W.; Wang, Y.; Zhu, E.; Wan, X.; Zhang, Q.; Wang, Y. Transformation of cellulose and its derived carbohydrates into formic and lactic acids catalyzed by vanadyl cations. ChemSusChem 2014, 7, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Choudhary, H.; Nishimura, S.; Ebitani, K. Synthesis of formic acid from monosaccharides using calcined Mg-Al hydrotalcite as reusable catalyst in the presence of aqueous hydrogen peroxide. Org. Process Res. Dev. 2015, 19, 449–453. [Google Scholar] [CrossRef]
- Choudhary, H.; Nishimura, S.; Ebitani, K. Synthesis of high-value organic acids from sugars promoted by hydrothermally loaded Cu oxide species on magnesia. Appl. Catal. B 2015, 162, 1–10. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, P.; Chu, Y.; Kang, F.; Cheng, Y.; Repo, E.; Feng, M.; Yu, X.; Zeng, H. Redox property of coordinated iron ion enables activation of O2 via in-situ generated H2O2 and additionally added H2O2 in EDTA-chelated Fenton reaction. Water Res. 2024, 248, 120826. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Z.; Chen, Y.; Wu, S.; Zheng, J.; Qian, Z. Advanced Oxidant Process with Fe(II)-Catalyzed Alkaline H2O2 Systems for Highly Efficient Concurrent Scavenging of NO and SO2 in High Gravitational Fields. Ind. Eng. Chem. Res. 2022, 61, 16257–16264. [Google Scholar] [CrossRef]
- Yu, K.; Guan, S.; Zhang, W.; Zhang, W.; Meng, Y.; Lin, H.; Gao, Q. Engineering Asymmetric Electronic Structure of Co─N─C Single-Atomic Sites Toward Excellent Electrochemical H2O2 Production and Biomass Upgrading. Angew. Chem. Int. Ed. 2025, e202502383. [Google Scholar]
- Jin, B.; Yao, G.; Wang, X.; Ding, K.; Jin, F. Photocatalytic oxidation of glucose into formate on nano TiO2 catalyst. ACS Sustain. Chem. Eng. 2017, 5, 6377–6381. [Google Scholar] [CrossRef]
- Mo, S.; Zhang, Q.; Li, J.; Sun, Y.; Ren, Q.; Zou, S.; Zhang, Q.; Lu, J.; Fu, M.; Mo, D.; et al. Highly efficient mesoporous MnO2 catalysts for the total toluene oxidation: Oxygen-Vacancy defect engineering and involved intermediates using in situ DRIFTS. Appl. Catal. B 2020, 264, 118464. [Google Scholar] [CrossRef]
- Tan, X.; Zhou, Y.; Qi, S.; Cheng, G.; Yi, C.; Yang, B. NO catalytic reduction on urea-loaded ferromanganese catalysts: Performance, characterization, and mechanism. Energy Fuels 2023, 37, 14213–14221. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, C.; Chen, C.; Bian, C.; Zhou, Y.; Lu, H. Construction of MnOx with abundant surface hydroxyl groups for efficient ozone decomposition. J. Environ. Chem. Eng. 2025, 13, 115048. [Google Scholar] [CrossRef]
- Xia, L.; Lu, Y.; Meng, H.; Li, C. Preparation of C-MOx nanocomposite for efficient adsorption of heavy metal ions via mechanochemical reaction of CaC2 and transitional metal oxides. J. Hazard. Mater. 2020, 393, 122487. [Google Scholar] [CrossRef]
- Li, H.; Yuan, N.; Qian, J.; Pan, B. Mn2O3 as an electron shuttle between peroxymonosulfate and organic pollutants: The dominant role of surface reactive Mn(IV) species. Environ. Sci. Technol. 2022, 56, 4498–4506. [Google Scholar] [CrossRef] [PubMed]
- Colman-Lerner, E.; Peluso, M.A.; Sambeth, J.; Thomas, H. Cerium, manganese and cerium/manganese ceramic monolithic catalysts. Study of VOCs and PM removal. J. Rare Earths 2016, 34, 675–682. [Google Scholar] [CrossRef]
- Lu, T.; Hou, Y.; Wu, W.; Niu, M.; Li, W.; Ren, S. Catalytic oxidation of cellulose to formic acid in V(V)-Fe(III)-H2SO4 aqueous solution with O2. Fuel Process. Technol. 2018, 173, 197–204. [Google Scholar] [CrossRef]
- Guo, H.; Li, J.; Xu, S.; Yang, J.; Chong, G.-H.; Shen, F. Mo-modified MnOx for the efficient oxidation of high-concentration glucose to formic acid in water. Fuel Process. Technol. 2023, 242, 107662. [Google Scholar] [CrossRef]
- Xu, S.; Yang, J.; Li, J.; Shen, F. Highly efficient oxidation of biomass xylose to formic acid with CeOx-promoted MnOx catalyst in water. ACS Sustain. Chem. Eng. 2023, 11, 921–930. [Google Scholar] [CrossRef]
- Zheng, N.; Stucky, G.D. A general synthetic strategy for oxide-supported metal nanoparticle catalysts. J. Am. Chem. Soc. 2006, 128, 14278–14280. [Google Scholar] [CrossRef]
- Gao, M.; Wang, L.; Yang, Y.; Sun, Y.; Zhao, X.; Wan, Y. Metal and metal oxide supported on ordered mesoporous carbon as heterogeneous catalysts. ACS Catal. 2023, 13, 4060–4090. [Google Scholar] [CrossRef]
- Tan, H.T.; Chen, Y.; Zhou, C.; Jia, X.; Zhu, J.; Chen, J.; Rui, X.; Yan, Q.; Yang, Y. Palladium nanoparticles supported on manganese oxide–CNT composites for solvent-free aerobic oxidation of alcohols: Tuning the properties of Pd active sites using MnOx. Appl. Catal. B 2012, 119–120, 166–174. [Google Scholar]
- Zhou, H.; Zhan, Y.; Guo, F.; Du, S.; Tian, B.; Dong, Y.; Qian, L. Synthesis of biomass-derived carbon aerogel/MnOx composite as electrode material for high-performance supercapacitors. Electrochim. Acta 2021, 390, 138817. [Google Scholar] [CrossRef]
- Meng, Q.; Du, L.; Yang, J.; Tang, Y.; Han, Z.; Zhao, K.; Zhang, G. Well-dispersed small-sized MnOx nanoparticles and porous carbon composites for effective methylene blue degradation. Colloids Surf. A 2018, 548, 142–149. [Google Scholar]
- Mehmood, U.; Ahmad, W.; Ahmed, S. Nickel impregnated multi-walled carbon nanotubes (Ni/MWCNT) as active catalyst materials for efficient and platinum-free dye-sensitized solar cells (DSSCs). Sustain. Energy Fuels 2019, 3, 3473–3480. [Google Scholar] [CrossRef]
- Peng, S.; Yang, X.; Strong, J.; Sarkar, B.; Jiang, Q.; Peng, F.; Liu, D.; Wang, H. MnO2-decorated N-doped carbon nanotube with boosted activity for low-temperature oxidation of formaldehyde. J. Hazard. Mater. 2020, 396, 122750. [Google Scholar]
- Pavithra, K.; Kumar, S.M.S. Embedding oxygen vacancies at SnO2–CNT surfaces via a microwave polyol strategy towards effective electrocatalytic reduction of carbon-dioxide to formate. Catal. Sci. Technol. 2020, 10, 1311–1322. [Google Scholar]
- Shano, A.M.; Habeeb, A.A.; Khodair, Z.T.; Adnan, S.K. Effects of Thickness on the Structural and Optical Properties of Mn3O4 Nanostructure Thin Films. J. Phys. Conf. Ser. 2021, 1818, 012049. [Google Scholar]
- Cui, D.; Li, Y.; Li, Y.; Fan, Y.; Chen, H.; Xu, H.; Xue, C. Co3O4@MnMoO4 Nanorod Clusters as an Electrode Material for Superior Supercapacitors. Int. J. Electrochem. Sci. 2020, 15, 2776–2791. [Google Scholar] [CrossRef]
- Abega, A.V.; Ngomo, H.M.; Nongwe, I.; Mukaya, H.E.; Kouoh Sone, P.-M.A.; Yangkou Mbianda, X. Easy and convenient synthesis of CNT/TiO2 nanohybrid by in-surface oxidation of Ti3+ ions and application in the photocatalytic degradation of organic contaminants in water. Synth. Met. 2019, 251, 1–14. [Google Scholar]
- Sun, Q.; Sun, L.; Ming, H.; Zhou, L.; Xue, H.; Wu, Y.; Wang, L.; Ming, J. Crystal reconstruction of binary oxide hexagonal nanoplates: Monocrystalline formation mechanism and high rate lithium-ion battery applications. Nanoscale 2020, 12, 4366–4373. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, Z.; Xu, X.; Wei, P.; Xie, G.; Zhang, X. The Growth Process and Photocatalytic Properties of h-MoO3 and α-MoO3 under Different Conditions. Crystals 2023, 13, 603. [Google Scholar] [CrossRef]
- Zhanga, W.; Chena, J.; Nia, J.; Yanga, Y.; Wanga, Y.; Chena, J.; Lia, J.; Yua, H.; Guana, R.; Yuea, L. Synthesis of Cage-like ZnO/Mn3O4 hollow nanospheres as anode materials for lithium-ion batteries. Mater. Lett. 2020, 260, 126917. [Google Scholar] [CrossRef]
- Grifasi, N.; Sartoretti, E.; Montesi, D.; Bensaid, S.; Russo, N.; Deorsola, F.A.; Fino, D.; Novara, C.; Giorgis, F.; Piumetti, M. Mesostructured manganese oxides for efficient catalytic oxidation of CO, ethylene, and propylene at mild temperatures: Insight into the role of crystalline phases and physico-chemical properties. Appl. Catal. B Environ. Energy 2025, 362, 124696. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, M.; Geng, H.; Dong, H.; Wu, P.; Li, X.; Guan, G.; Wang, T. Synergistically Tuning Electronic Structure of Porous β-Mo2C Spheres by Co Doping and Mo-Vacancies Defect Engineering for Optimizing Hydrogen Evolution Reaction Activity. Adv. Funct. Mater. 2020, 30, 2000561. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, J.; Zhang, J.-Y.; Chen, Y.; Yang, X.; Song, W.; Wei, L.; Li, W. Synergy of Mn and Ni enhanced catalytic performance for toluene combustion over Ni-doped α-MnO2 catalysts. Chem. Eng. J. 2020, 388, 124244. [Google Scholar] [CrossRef]
- Li, J.; Smith, R.L.; Xu, S.; Li, D.; Yang, J.; Zhang, K.; Shen, F. Manganese oxide as an alternative to vanadium-based catalysts for effective conversion of glucose to formic acid in water. Green Chem. 2022, 24, 315–324. [Google Scholar] [CrossRef]
- Sivkov, D.; Nekipelov, S.; Petrova, O.; Vinogradov, A.; Mingaleva, A.; Isaenko, S.; Makarov, P.; Ob’edkov, A.; Kaverin, B.; Gusev, S.; et al. Studies of buried layers and interfaces of tungsten carbide coatings on the MWCNT surface by XPS and NEXAFS spectroscopy. Appl. Sci. 2020, 10, 4736. [Google Scholar] [CrossRef]
- Hayashi, E.; Yamaguchi, Y.; Kamata, K.; Tsunoda, N.; Kumagai, Y.; Oba, F.; Hara, M. Effect of MnO2 crystal structure on aerobic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. J. Am. Chem. Soc. 2019, 141, 890–900. [Google Scholar] [CrossRef]
- Chen, D.; He, D.; Lu, J.; Zhong, L.; Liu, F.; Liu, J.; Yu, J.; Wan, G.; He, S.; Luo, Y. Investigation of the role of surface lattice oxygen and bulk lattice oxygen migration of cerium-based oxygen carriers: XPS and designed H2-TPR characterization. Appl. Catal. B 2017, 218, 249–259. [Google Scholar] [CrossRef]
- Du, H.; Luo, H.; Jiang, M.; Yan, X.; Jiang, F.; Chen, H. A review of activating lattice oxygen of metal oxides for catalytic reactions: Reaction mechanisms, modulation strategies of activity and their practical applications. Appl. Catal. A 2023, 664, 119348. [Google Scholar] [CrossRef]
- Liu, W.; Yang, S.; Yu, H.; Liu, S.; Li, H.; Shen, Z.; Song, Z.; Chen, X.; Zhang, X. Boosting the total oxidation of toluene by regulating the reactivity of lattice oxygen species and the concentration of surface adsorbed oxygen. Sep. Purif. Technol. 2023, 325, 124597. [Google Scholar]
- Guo, L.; Tan, X.; Liu, S.; Wu, J.; Ren, J.; Zhao, T.; Kang, X.; Wang, H.; Chu, W. Considerable capacity increase of high-nickel lithium-rich cathode materials by effectively reducing oxygen loss and activating Mn4+/3+ redox couples via Mo doping. J. Alloys Compd. 2019, 790, 170–178. [Google Scholar]
- Jang, W.-J.; Jeong, D.-W.; Shim, J.-O.; Kim, H.-M.; Roh, H.-S.; Son, I.H.; Lee, S.J. Combined steam and carbon dioxide reforming of methane and side reactions: Thermodynamic equilibrium analysis and experimental application. Appl. Energy 2016, 173, 80–91. [Google Scholar]
- Wu, H.; Guo, H.; Shen, B.; Zhang, X.; Shen, F. Enhanced glucose conversion to formic acid with deep eutectic solvents-mediated bimetallic oxides: Morphology and valence state regulation. Biomass Bioenergy 2025, 194, 107698. [Google Scholar]
- Jin, X.; Zhao, M.; Shen, J.; Yan, W.; He, L.; Thapa, P.S.; Ren, S.; Subramaniam, B.; Chaudhari, R.V. Exceptional performance of bimetallic Pt1Cu3/TiO2 nanocatalysts for oxidation of gluconic acid and glucose with O2 to glucaric acid. J. Catal. 2015, 330, 323–329. [Google Scholar] [CrossRef]
- Niu, M.; Hou, Y.; Ren, S.; Wang, W.; Zheng, Q.; Wu, W. The relationship between oxidation and hydrolysis in the conversion of cellulose in NaVO3–H2SO4 aqueous solution with O2. Green Chem. 2015, 17, 335–342. [Google Scholar]
- Álvarez-Hernández, D.; Ivanova, S.; Penkova, A.; Centeno, M.Á. Influence of vanadium species on the catalytic oxidation of glucose for formic acid production. Catal. Today 2024, 441, 114906. [Google Scholar]
- Maerten, S.; Kumpidet, C.; Voß, D.; Bukowski, A.; Wasserscheid, P.; Albert, J. Glucose oxidation to formic acid and methyl formate in perfect selectivity. Green Chem. 2020, 22, 4311–4320. [Google Scholar]
- Liu, G.; Wang, S.; Zhou, C.; Zhao, Q.; Hu, J.; Gui, Z.; Chen, Y.; Huang, Y.; Zhang, P.; Wang, F. Boosting the activity of BiVOx via vanadium-promotion for highly selective oxidation of biomass-derived xylose toward formic acid. Fuel 2024, 374, 132420. [Google Scholar]
- Reichert, J.; Albert, J. Detailed kinetic investigations on the selective oxidation of biomass to formic acid (OxFA process) using model substrates and real biomass. ACS Sustain. Chem. Eng. 2017, 5, 7383–7392. [Google Scholar] [CrossRef]
- Varničić, M.; Zasheva, I.N.; Haak, E.; Sundmacher, K.; Vidaković-Koch, T. Selectivity and sustainability of electroenzymatic process for glucose conversion to gluconic acid. Catalysts 2020, 10, 269. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Sun, M.; Ma, X.; Han, Y. Direct conversion of cellulose to glycolic acid with a phosphomolybdic acid catalyst in a water medium. ACS Catal. 2012, 2, 1698–1702. [Google Scholar] [CrossRef]
- Schandel, C.B.; Høj, M.; Osmundsen, C.M.; Jensen, A.D.; Taarning, E. Thermal cracking of sugars for the production of glycolaldehyde and other small oxygenates. ChemSusChem 2020, 13, 688–692. [Google Scholar] [PubMed]


| Catalyst | Ratio of Mn/Mo | |
|---|---|---|
| Theoretical Value | Actual Value a | |
| Mn1Mo1OX@MWCNT | 1.0 | 1.0 |
| Mn3Mo1OX@MWCNT | 3.0 | 2.8 |
| Mn9Mo1OX@MWCNT | 9.0 | 8.2 |
| Mn27Mo1OX@MWCNT | 27.0 | 20.4 |
| Catalyst | Mn | O | ||
|---|---|---|---|---|
| (Mn2+ + Mn3+)/Mntot (%) | Mn4+/Mntot (%) | Oads/Otot (%) | Olatt/Otot (%) | |
| MnOX@MWCNT | 41.3 | 58.7 | 5.2 | 8.8 |
| Mn27Mo1OX@MWCNT | 47.9 | 52.1 | 13.3 | 19.3 |
| Mn9Mo1OX@MWCNT | 54.2 | 45.8 | 23.6 | 19.2 |
| Mn3Mo1OX@MWCNT | 56.2 | 43.8 | 22.8 | 16.3 |
| Mn1Mo1OX@MWCNT | 64.3 | 35.7 | 14.9 | 19.5 |
| Reaction Conditions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Substrate | Qlty (g) | Cat (g) | H2O (mL) | T (°C) | Time (h) | p (O2) (Mpa) | FA Yield (%) |
| 1 | Fructose | 0.1 | 0.05 | 5 | 140 | 6 | 3 | 49.9 |
| 2 | Arabinose | 0.1 | 0.05 | 5 | 140 | 6 | 3 | 56.8 |
| 3 | Xylose | 0.1 | 0.05 | 5 | 140 | 6 | 3 | 57.3 |
| 4 | Cellobiose | 0.1 | 0.05 | 5 | 140 | 6 | 3 | 34.4 |
| 5 | Cellobiose | 0.1 | 0.05 | 5 | 160 | 4 | 3 | 52.3 |
| 6 | Xylan | 0.1 | 0.05 | 5 | 140 | 6 | 3 | 50.7 |
| 7 | Cellulose a | 0.1 | 0.05 | 5 | 170 | 6 | 3 | 20.5 |
| 8 | Cellulose a | 0.1 | 0.05 | 5 | 180 | 4 | 3 | 23.5 |
| Catalyst | Concentration (g/L) | Cat (g) | p (O2)(Mpa) | T (°C) | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Mn9Mo1OX@MWCNT | 20 | 0.05 | O2 (3) | 140 | 6 | 58.8 | ♣ |
| Mn9Mo1OX@MWCNT | 60 | 0.05 | O2 (3) | 140 | 6 | 54.3 | ♣ |
| Mn9Mo1OX@MWCNT | 20 | 0.05 | O2 (3) | 130 | 10 | 54.4 | ♣ |
| Mo(1)-MnOX | 20 | 0.05 | O2 (3) | 160 | 1.5 | 65.8 | [38] |
| MnOX-100 | 20 | 0.05 | O2 (3) | 160 | 2.5 | 67.2 | [57] |
| VOX/TiO2 | 20 | 0.20 | O2 (3) | 150 | 2.5 | 42.0 | [68] |
| H8[PV5Mo7O40] | 18 | 0.16 | O2 (2) | 90 | 24 | 55.0 | [69] |
| VOSO4 | 9 | 0.01 | O2 (2) | 140 | 1 | 45.0 | [24] |
| Bi1V2OX | 8.4 | 0.12 | O2 (1) | 170 | 0.34 | 62.0 | [70] |
| HPA-5 | 22.5 | 1.74 | O2 (6) | 80 | 8 | 58.3 | [71] |
| H5PV2Mo10O40 | 1 wt% | 5 mol % | O2 (2) | 100 | 3 | 52.0 | [22] |
| Recycle Times | Catalyst Mass (mg) | Glucose Conversion (%) | FA Yield (%) |
|---|---|---|---|
| 1 | 50 (fresh) | 91.8 | 46.8 |
| 2 | 50 (used) | 77.4 | 34.2 |
| 3 | 50 (used) | 60.7 | 21.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Yang, F.; Chen, S.; Wu, H.; Yang, J.; Shen, F. Highly Efficient Catalytic Oxidation of Glucose to Formic Acid over Mn-Mo Doped Carbon Nanotube. Molecules 2025, 30, 1639. https://doi.org/10.3390/molecules30071639
Guo H, Yang F, Chen S, Wu H, Yang J, Shen F. Highly Efficient Catalytic Oxidation of Glucose to Formic Acid over Mn-Mo Doped Carbon Nanotube. Molecules. 2025; 30(7):1639. https://doi.org/10.3390/molecules30071639
Chicago/Turabian StyleGuo, Hongrui, Fan Yang, Siwei Chen, Hejuan Wu, Jirui Yang, and Feng Shen. 2025. "Highly Efficient Catalytic Oxidation of Glucose to Formic Acid over Mn-Mo Doped Carbon Nanotube" Molecules 30, no. 7: 1639. https://doi.org/10.3390/molecules30071639
APA StyleGuo, H., Yang, F., Chen, S., Wu, H., Yang, J., & Shen, F. (2025). Highly Efficient Catalytic Oxidation of Glucose to Formic Acid over Mn-Mo Doped Carbon Nanotube. Molecules, 30(7), 1639. https://doi.org/10.3390/molecules30071639







