Rapid Identification of Vicatia thibetica de Boiss and Quantitative Analysis of the Content of Six Chemical Components Based on Near-Infrared Spectroscopy
Abstract
:1. Introduction
2. Results and Discussions
2.1. V. thibetica Identification Based on NIR Spectroscopy and Chemometrics
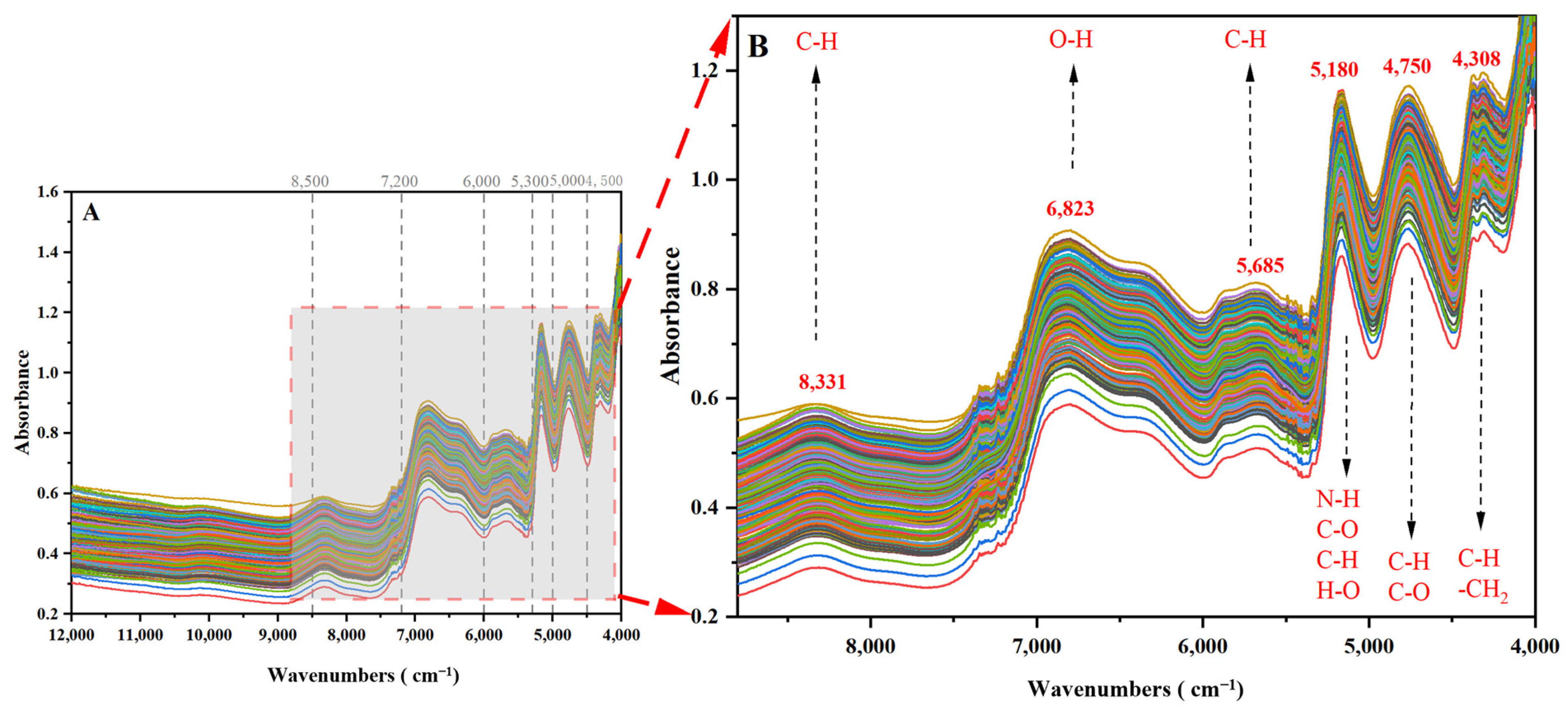
2.1.1. NIR Spectral Properties of V. thibetica
2.1.2. Identification of V. thibetica by OPLS-DA
2.1.3. ANN Modeling to Identify V. thibetica from Different Origins
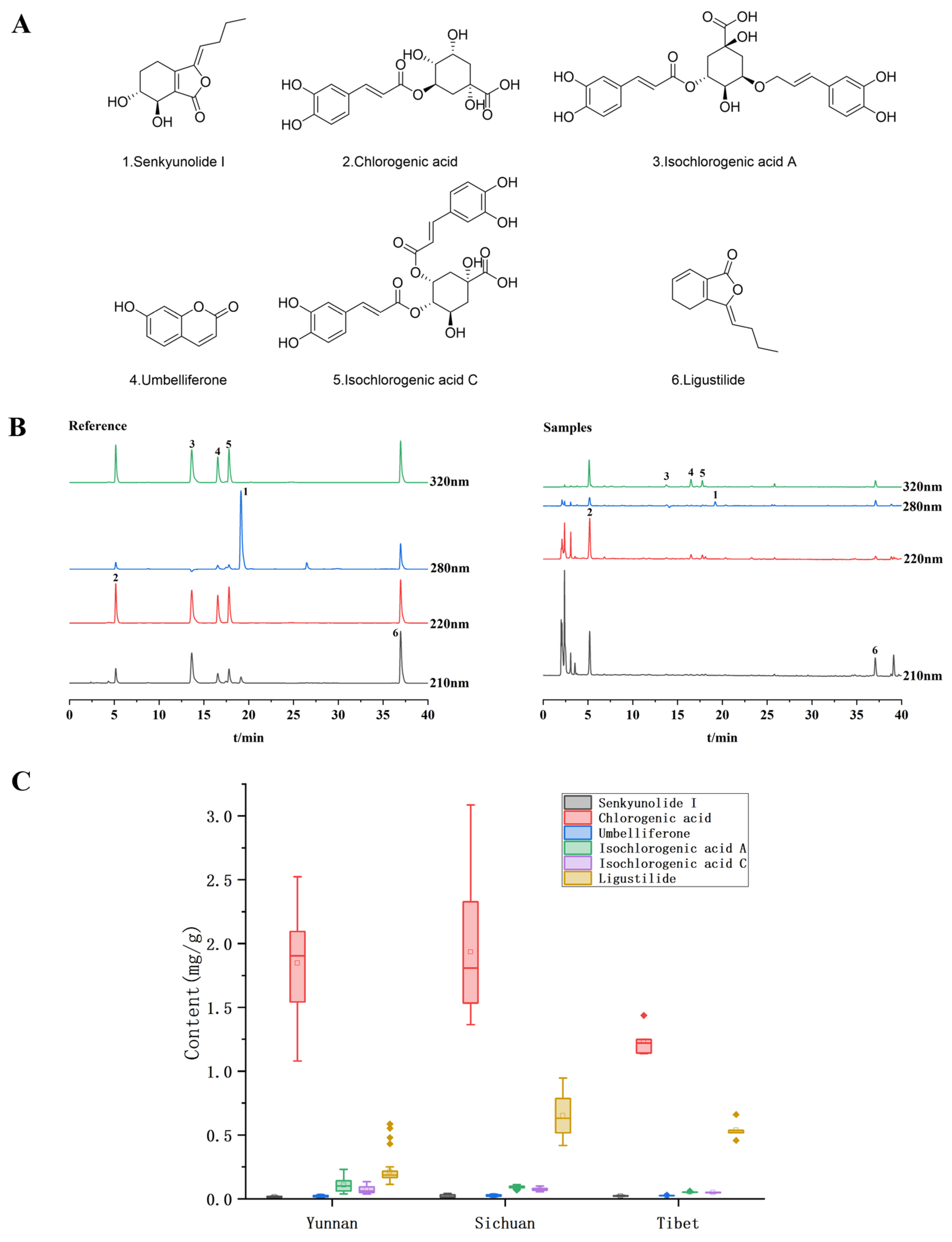
2.2. Simultaneous Determination of Six Compounds in V. thibetica by HPLC-UV
2.3. Quantitative Modeling of Six Chemical Components in V. thibetica Based on NIR Spectra
2.3.1. Selection of Spectral Pre-Processing Methods
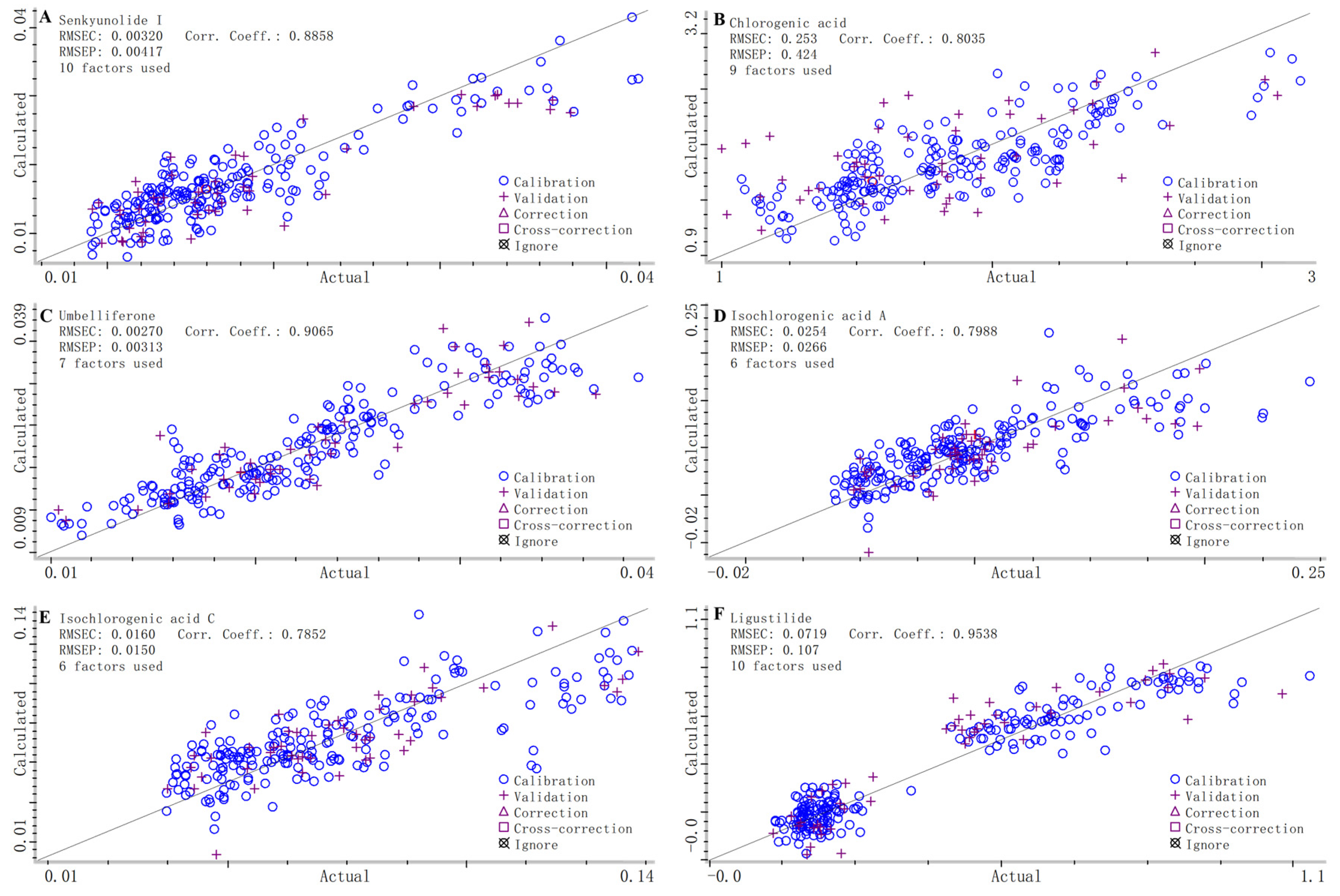
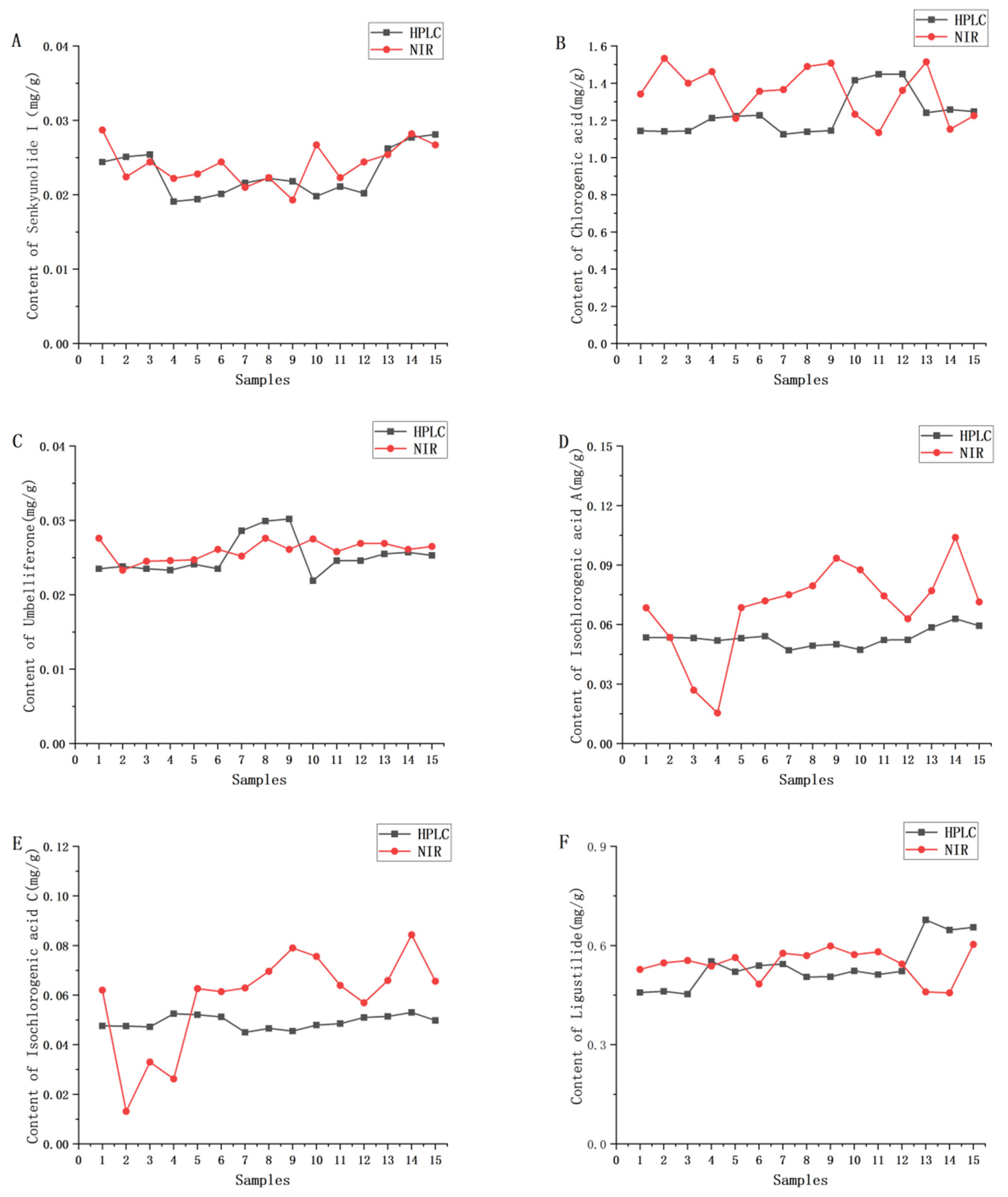
2.3.2. Build and Validate NIR Calibration Models
3. Materials and Methods
3.1. Samples of V. thibetica
3.2. Instruments and Reagents
3.3. NIR Spectral Acquisition
3.4. Determination of Six Compounds by High Performance Liquid Chromatography
3.4.1. Chromatographic Conditions
3.4.2. Sample Solution Preparation and Method Validation
3.5. Chemometrics Analysis
3.5.1. Preprocessing of NIR Spectra
3.5.2. Discriminant Models
3.5.3. Quantitative Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, B.; Xiao, C.J. Modern Research and Applications of Bai Ethnic Medicinal Plants; China Press of Traditional Chinese Medicine: Beijing, China, 2021. [Google Scholar]
- Zhou, N.; Duan, Y.M.; Chen, Q.; Ma, X.K. Study on Pharmacognosy of Xigui. J. Anhui Agric. Sci. 2007, 8, 2307–2425. [Google Scholar]
- Dong, S.T.; Zhang, X.Q.; Hu, Y.; Gong, X.M.; Yang, H.G. Chemical constituents, quality control and pharmacology research progress of Xigui. Chin. J. Ethnomed. Ethnopharm. 2018, 27, 40–42. [Google Scholar]
- Zhang, W.M.; Duang, Z.H.; Sun, F.; Rao, G.H. The chemical constituents from the roots of Vicatia thibetica. Nat. Prod. Res. Dev. 2004, 3, 218–219. [Google Scholar]
- Zhou, P.; Wang, L.P.; Deng, L.; He, J.; Wang, C.Y.; Wang, C.J. Study on antioxidative activity of flavonoids extracted from Seselopsis in vitro. J. Anhui Agric. Sci. 2011, 39, 1359–1360. [Google Scholar]
- Li, Y.; Yu, X.H.; Guo, M.; Du, T. Optimization of ultrasound-assisted extraction process for total flavonoids from Vicatia thibetica. Chin. Tradit. Pat. Med. 2022, 44, 362–366. [Google Scholar]
- Li, Y.; Yu, X.H.; Du, T.; Zhu, X.Y.; Guo, M. Simultaneous determination of four components in Vicatia thibetica de Boiss by HPLC. J. Tradit. Chin. Vet. Med. 2023, 42, 78–80. [Google Scholar]
- Cairang, N.J.; Xiangcuo, Z.M.; Duojie, R.Q. Quality assessment of different sources of Tibetan medicine “Jiawa” based on antioxidant activity spectrum-effect relationship. Chin. J. Mod. Appl. Pharm. 2024, 41, 1686–1693. [Google Scholar]
- Huang, L.Q.; Wang, S.; Yuan, Q.J.; Jiang, C.; Liu, C.X. 20 Years of Molecular Pharmacognosy. In Proceedings of the 2016 First China Conference on Traditional Chinese Medicine Resources and the 12th Annual Conference of the CSNR Committee on the Research of Traditional Chinese Medicine and Natural Medicine Resources, Xi’an, China, 10 August 2016; pp. 1–11. [Google Scholar]
- Wang, Y.Y.; Xu, W.; Qi, J.L.; Gao, B.X.; Gou, Y.; Li, Q.; Gao, C.; Wang, Y.; Yang, L.; Lan, Z.Q. Comparative study on HPLC fingerprints of Vicatia thibetica de Boiss and its similar species. Chin. J. Pharm. Anal. 2023, 43, 1925–1934. [Google Scholar]
- OU, Z.; Baima, Y.Z.; Yang, B.; Tudan, J.R.; LU, W.L.; Li, S.P. Analysis of chemical constituents and quantitative analysis of Vicatia thibetica de Boiss by UPLC-Q-TOF-MS. FNC 2023, 29, 22–26. [Google Scholar]
- She, X.; Huang, J.; Cao, X.; Wu, M.; Yang, Y. Rapid Measurement of total saponins, mannitol, and naringenin in Dendrobium officinale by near-infrared spectroscopy and chemometrics. Foods 2024, 13, 1199–1216. [Google Scholar] [CrossRef]
- Zhao, L.L.; Zhao, W.Q.; Zhao, Z.Y.; Xian, R.; Jia, M.Y.; Jiang, Y.B.; Li, Z.; Pan, X.L.; Lan, Z.Q.; Li, M. Rapid discrimination of Alismatis Rhizoma and quantitative analysis of triterpenoids based on near-infrared spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 321, 124618. [Google Scholar] [CrossRef]
- Lauß, J.; Kappacher, C.; Isser, O.; Huck, C.; Huck, C.W.; Rainer, M. Speciess-pecific quantification of bioactive boswellic acids in Boswellia resin using NIR spectroscopy, HPLC and multivariate data analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 316, 124384. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, J.; Li, Q.; Gao, J.; Tan, X.; Bian, X. Rapid identification and quantification of Panax notoginseng with its adulterants by near infrared spectroscopy combined with chemometrics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 206, 23–30. [Google Scholar] [CrossRef]
- Xue, J.T.; Shi, Y.L.; Yang, L.M.; Yang, Q.W.; Li, C.Y.; Chen, X.Y.; Jing, Y. Near-infrared spectroscopy for rapid and simultaneous determination of five main active components in rhubarb of different geographical origins and processing. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 205, 419–427. [Google Scholar]
- Qiu, S.S.; Gao, L.P.; Wang, J. Classification and regression of ELM, LVQ and SVM for E-nose data of strawberry juice. J. Food Eng. 2015, 144, 77–85. [Google Scholar] [CrossRef]
- Du, H.; Tang, B.; Cao, S.R.; Xi, C.X.; Li, X.L.; Zhang, L.; Wang, G.M.; Lai, G.Y.; Chen, Z.Q. Combination of stable isotopes and multi-elements analysis with chemometric for determining the geographical origins of Rhizoma Coptidis. Microchem. J. 2020, 152, 104427. [Google Scholar] [CrossRef]
- Gu, H.W.; Yin, X.L.; Pan, T.Q.; Pan, Y.; Cui, H.N.; Li, Z.Q.; Sun, W.Q.; Ding, B.M.; Hu, X.C.; Zhang, Z.H.; et al. Geographical origin identification and chemical markers screening of Chinese green tea using two-dimensional fingerprints technique coupled with multivariate chemometric methods. Food Control 2021, 135, 108795. [Google Scholar] [CrossRef]
- Shakiba, N.; Gerdes, A.; Holz, N.; Wenck, S.; Bachmann, R.; Schneider, T.; Seifert, S.; Fischer, M.; Hackl, T. Determination of the geographical origin of hazelnuts (Corylus avellana L.) by near-infrared spectroscopy (NIR) and a low-level fusion with nuclear magnetic resonance (NMR). Microchem. J. 2021, 174, 107066. [Google Scholar] [CrossRef]
- Nagy, M.M.; Wang, S.P.; Farag, M.A. Quality analysis and authentication of nutraceuticals using near IR (NIR) spectroscopy: A comprehensive review of novel trends and applications. Trends Food Sci. Technol. 2022, 123, 290–309. [Google Scholar] [CrossRef]
- Wu, M.L.; Li, Y.H.; Yuan, Y.; Li, S.; Song, X.X.; Yin, J.Y. Comparison of NIR and Raman spectra combined with chemometrics for the classification and quantification of mung beans (Vigna radiata L.) of different origins. Food Control 2022, 145, 109498. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Du, G.R.; Huang, Y. Exploring the use of Near-infrared spectroscopy as a tool to predict quality attributes in prickly pear (Rosa roxburghii Tratt) with chemometrics variable strategy. J. Food Compost. Anal. 2021, 105, 104225. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Tian, S.J.; Xu, H.R. Identification of the geographic origin of peaches by VIS-NIR spectroscopy, fluorescence spectroscopy and image processing technology. J. Food Compost. Anal. 2022, 114, 104843. [Google Scholar] [CrossRef]
- Chen, R.; Li, S.Q.; Cao, H.J.; Xu, T.G.; Bai, Y.C.; Li, Z.M.; Leng, X.J.; Huang, Y. Rapid quality evaluation and geographical origin recognition of ginger powder by portable NIRS in tandem with chemometrics. Food Chem. 2023, 438, 137931. [Google Scholar] [CrossRef] [PubMed]
- Puleo, S.; Di Monaco, R.; Langellotti, A.L.; Masi, P. The origin of mussels (Mytilus galloprovincialis): NIRS explanatory identification and the effect on consumers. Food Chem. X 2022, 16, 100497. [Google Scholar] [CrossRef]
- Li, S.; Li, L.X.; Zhang, F.J.; Lin, Y.H. Nondestructive detection of Panax notoginseng saponins by using hyperspectral imaging. Int. J. Food Sci. Tech. 2022, 7, 4537–4546. [Google Scholar]
- Yan, H.; Li, P.H.; Zhou, G.S.; Wang, Y.J.; Bao, B.H.; Wu, Q.N.; Huang, S.L. Rapid and practical qualitative and quantitative evaluation of non-fumigated ginger and sulfur-fumigated ginger via Fourier-transform infrared spectroscopy and chemometric methods. Food Chem. 2020, 341, 128241. [Google Scholar] [CrossRef]
- Varrà, M.O.; Fasolato, L.; Serva, L.; Ghidini, S.; Novelli, E.; Zanardi, E. Use of near infrared spectroscopy coupled with chemometrics for fast detection of irradiated dry fermented sausages. Food Control 2020, 110, 107009. [Google Scholar] [CrossRef]
- Sampaio, P.S.; Almeida, A.S.; Brites, C.M. Use of artificial neural network model for rice quality prediction based on grain physical parameters. Foods 2021, 12, 3016. [Google Scholar] [CrossRef]
- Cui, M.; Duan, B.Z.; Cheng, L.; Zhang, M.C.; He, F.M.; Zhou, P. Origin traceability analysis of Crataegus pinnatifida var. major based on Near-infrared spectroscopy combined with chemometric methods. Chin. Tradit. Herb. Drugs 2024, 55, 4897–4906. [Google Scholar]
- Liang, N.; Sun, S.S.; Zhang, C.; He, Y.; Qiu, Z.J. Advances in infrared spectroscopy combined with artificial neural network for the authentication and traceability of food. Crit. Rev. Food Sci. Nutr. 2020, 62, 21–22. [Google Scholar] [CrossRef]
- Wang, H.H.; Wang, K.L.; Zhu, X.Y.; Zhang, P.; Yang, J.X.; Tan, M.Q. Integration of partial least squares regression and hyperspectral data processing for the nondestructive detection of the scaling rate of carp (Cyprinus carpio). Foods 2020, 4, 500. [Google Scholar] [CrossRef]
- Zhang, X.D.; Bian, F.; Wang, Y.F.; Hu, L.; Yang, N.; Mao, H.P. A method for capture and detection of crop airborne disease spores based on microfluidic chips and Micro Raman spectroscopy. Foods 2022, 21, 3462. [Google Scholar] [CrossRef] [PubMed]
- Anita, G.G.; Antje, D.M.; Basrai, M.; Bischoff, S. Changes in plasma acylcarnitine and lysophosphatidylcholine levels following a highfructose diet: A targeted metabolomics study in healthy women. Nutrients 2018, 9, 1254. [Google Scholar] [CrossRef]
- Ming, J.; Liu, M.J.; Lei, M.; Huang, B.S.; Chen, L. Rapid determination of the total content of oleanolic acid and ursolic acid in Chaenomelis Fructus using near-infrared spectroscopy. Front. Plant Sci. 2022, 13, 978937. [Google Scholar] [CrossRef]
- Huang, M.G.; Li, R.J.; Yang, M.; Zhou, A.; Wu, H.; Li, Z.G.; Wu, H. Discovering the potential active ingredients of Qi-Yu-San-Long decoction for anti-oxidation, inhibition of non-small cell lung cancer based on the spectrum-effect relationship combined with chemometric methods. Front. Pharmacol. 2022, 13, 989139. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.Y.; Zhao, Y.R.; Wang, Y.; Li, J.M.; Zhu, F.L.; Yu, K.Q. Surface-enhanced Raman scattering spectroscopy combined with chemical imaging analysis for detecting apple valsa canker at an early stage. Front. Plant Sci. 2022, 13, 802761. [Google Scholar] [CrossRef]
- Ye, J.L. A study on multi-classification method of classification and origin identification of traditional chinese medicine based on spectral characteristics. J. Shanxi Norm. Univ. (Nat. Sci. Ed) 2023, 37, 13–21. [Google Scholar]
- Hu, J.C.; Szymczak, S. A review on longitudinal data analysis with random forest. Brief. Bioinform. 2023, 24, 2. [Google Scholar] [CrossRef]


| Sample Set | Actual Origin | Predicted Origin | Accuracy (%) | ||
|---|---|---|---|---|---|
| Yunnan | Sichuan | Tibet | |||
| Training | Yunnan | 37 | 0 | 0 | 100.0 |
| Sichuan | 0 | 21 | 0 | 100.0 | |
| Tibet | 0 | 0 | 4 | 100.0 | |
| Total (%) | 59.7 | 33.9 | 6.5 | 100.0 | |
| Validating | Yunnan | 10 | 0 | 0 | 100.0 |
| Sichuan | 0 | 5 | 0 | 100.0 | |
| Tibet | 0 | 0 | 1 | 100.0 | |
| Accuracy (%) | 62.5 | 31.3 | 6.3 | 100.0 | |
| Testing | Yunnan | 8 | 0 | 0 | 100.0 |
| Sichuan | 0 | 2 | 0 | 100.0 | |
| Tibet | 0 | 0 | 0 | 0.0 | |
| Total (%) | 80.0 | 20.0 | 0.0 | 100.0 | |
| Component | Regression | Linear Range (μg/mL) | Precision (RSD) (%) | Repeatability (RSD) (%) | Stability (RSD) (%) | Recovery (%) |
|---|---|---|---|---|---|---|
| Senkyunolide I | Y = 75.213X + 3.4951 R2 = 0.9992 | 0.3~9.0 | 0.67 | 2.86 | 0.36 | 105.51 |
| Chlorogenic acid | Y = 15.35X − 30.69 R2 = 0.9992 | 22.2~244.2 | 1.14 | 0.93 | 0.33 | 111.31 |
| Umbelliferone | Y = 83.873X + 6.6367 R2 = 0.9994 | 0.4~4.0 | 2.18 | 0.99 | 1.39 | 98.93 |
| Isochlorogenic acid A | Y = 27.02X − 35.422 R2 = 0.9992 | 2.4~18.0 | 0.65 | 1.38 | 1.51 | 97.19 |
| Isochlorogenic acid C | Y = 33.148X − 37.143 R2 = 0.9992 | 2.0~15.0 | 0.88 | 1.27 | 1.53 | 90.71 |
| Ligustilide | Y = 14.813X + 0.8867 R2 = 0.9999 | 2.0~120.0 | 0.95 | 1.82 | 0.31 | 102.71 |
| Pre-Treatment Methods | - | Senkyunolide I | Chlorogenic Acid | Umbelliferone | Isochlorogenic Acid A | Isochlorogenic Acid C | Ligustilide |
|---|---|---|---|---|---|---|---|
| MSC | R2 | 0.8351 | 0.5525 | 0.9017 | 0.7026 | 0.6994 | 0.9447 |
| RMSEC | 0.0038 | 0.3540 | 0.0028 | 0.0300 | 0.0185 | 0.0787 | |
| RMSEP | 0.0041 | 0.4050 | 0.0033 | 0.0268 | 0.0163 | 0.1080 | |
| Factors | 10 | 3 | 9 | 3 | 3 | 10 | |
| Wavelength | 11,539.95–4034.35 cm−1 | ||||||
| MSC + FD | R2 | 0.7286 | 0.4563 | 0.8834 | 0.7891 | 0.8902 | 0.9460 |
| RMSEC | 0.0047 | 0.3780 | 0.0030 | 0.0259 | 0.0118 | 0.0776 | |
| RMSEP | 0.0058 | 0.4300 | 0.0038 | 0.0274 | 0.0155 | 0.1450 | |
| Factors | 4 | 2 | 4 | 4 | 6 | 6 | |
| Wavelength | 4524.18–4346.76 cm−1; 4767.17–4535.75 cm−1; 6375.51–4771.03 cm−1; 7146.90–6834.49 cm−1 | ||||||
| MSC + SD | R2 | 0.3148 | 0.1848 | 0.2753 | 0.2037 | 0.3369 | 0.7941 |
| RMSEC | 0.0066 | 0.4170 | 0.0067 | 0.0413 | 0.0243 | 0.1460 | |
| RMSEP | 0.0083 | 0.4820 | 0.0067 | 0.0389 | 0.0228 | 0.1620 | |
| Factors | 1 | 1 | 1 | 1 | 1 | 1 | |
| Wavelength | 11,748.22–4045.92 cm−1 | ||||||
| MSC + SGF | R2 | 0.8218 | 0.7925 | 0.8919 | 0.7023 | 0.6993 | 0.9409 |
| RMSEC | 0.0039 | 0.2590 | 0.0029 | 0.0300 | 0.0185 | 0.0811 | |
| RMSEP | 0.0041 | 0.3760 | 0.0033 | 0.0266 | 0.0163 | 0.1090 | |
| Factors | 9 | 10 | 8 | 3 | 3 | 9 | |
| Wavelength | 11,547.66–4042.07 cm−1 | ||||||
| MSC + FD + NDF | R2 | 0.8858 | 0.8035 | 0.9065 | 0.7988 | 0.7852 | 0.9538 |
| RMSEC | 0.0032 | 0.2530 | 0.0027 | 0.0254 | 0.0160 | 0.0719 | |
| RMSEP | 0.0042 | 0.4240 | 0.0031 | 0.0266 | 0.0150 | 0.1070 | |
| Factors | 10 | 9 | 7 | 6 | 6 | 10 | |
| Wavelength | 7393.74–4315.91 cm−1 | ||||||
| MSC + SD + NDF | R2 | 0.8494 | 0.7088 | 0.8885 | 0.7744 | 0.7755 | 0.9083 |
| RMSEC | 0.0036 | 0.3000 | 0.0029 | 0.0267 | 0.0163 | 0.1000 | |
| RMSEP | 0.0044 | 0.4090 | 0.0033 | 0.0284 | 0.0165 | 0.1170 | |
| Factors | 8 | 8 | 7 | 3 | 8 | 5 | |
| Wavelength | 6159.52–4308.19 cm−1; 7335.89–6680.21 cm−1 | ||||||
| MSC + FD + SGF | R2 | 0.7100 | 0.4540 | 0.8810 | 0.9450 | 0.9444 | 0.9442 |
| RMSEC | 0.0049 | 0.3780 | 0.0030 | 0.0138 | 0.0085 | 0.0789 | |
| RMSEP | 0.0059 | 0.4310 | 0.0038 | 0.0272 | 0.0015 | 0.1430 | |
| Factors | 4 | 2 | 4 | 10 | 10 | 6 | |
| Wavelength | 4504.90–4342.91 cm−1; 4767.17–4539.61 cm−1; 6101.67–4774.88 cm−1; 7189.32–6846.06 cm−1 | ||||||
| MSC + SD + SGF | R2 | 0.3014 | 0.5873 | 0.9371 | 0.6707 | 0.6875 | 0.8895 |
| RMSEC | 0.0066 | 0.3440 | 0.0022 | 0.0313 | 0.0188 | 0.1090 | |
| RMSEP | 0.0083 | 0.4360 | 0.0050 | 0.0377 | 0.0197 | 0.1610 | |
| Factors | 10 | 9 | 7 | 6 | 6 | 10 | |
| Wavelength | 9534.34–4412.33 cm−1; 11,343.24–9538.20 cm−1; 11,794.50–11347.10 cm−1 | ||||||
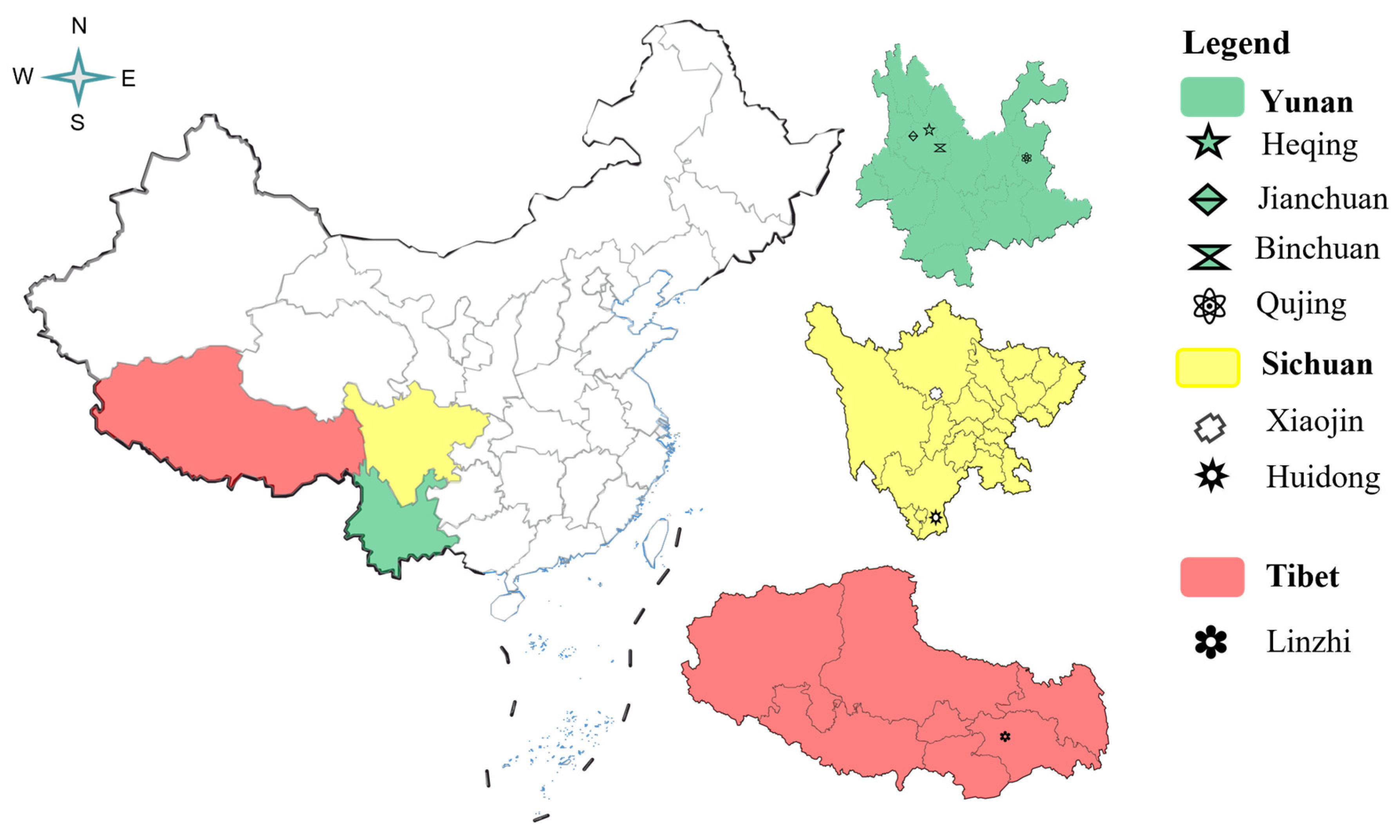
| Region (Province) | Location | Number |
|---|---|---|
| Yunnan | Machang Village, Heqing County, Dali Bai Autonomous Prefecture | 10 |
| Jianchuan County, Dali Bai Autonomous Prefecture, Yunnan | 10 | |
| Binchuan County, Dali Bai Autonomous Prefecture, Yunnan | 5 | |
| Bole Village, ZhanYi District, Qujing City | 30 | |
| Sichuan | Xiaojin County in Aba Autonomous Prefecture, Sichuan | 10 |
| Huidong County, Liangshan Yi Autonomous Prefecture | 8 | |
| Tangtang Township, Huidong County, Liangshan Yi Autonomous Prefecture, China | 5 | |
| Gaji Township, Huidong County, Liangshan Yi Autonomous Prefecture, China | 5 | |
| Tibet | Bomi County, Linzhi City, Tibet | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, J.; Sun, J.; Xia, T.; Hai, Y.; Li, J.; Yang, Y.; Xia, C. Rapid Identification of Vicatia thibetica de Boiss and Quantitative Analysis of the Content of Six Chemical Components Based on Near-Infrared Spectroscopy. Molecules 2025, 30, 1867. https://doi.org/10.3390/molecules30091867
Zhang Y, Li J, Sun J, Xia T, Hai Y, Li J, Yang Y, Xia C. Rapid Identification of Vicatia thibetica de Boiss and Quantitative Analysis of the Content of Six Chemical Components Based on Near-Infrared Spectroscopy. Molecules. 2025; 30(9):1867. https://doi.org/10.3390/molecules30091867
Chicago/Turabian StyleZhang, Yunta, Jian Li, Jin Sun, Tian Xia, Yonglin Hai, Jian Li, Yongcheng Yang, and Conglong Xia. 2025. "Rapid Identification of Vicatia thibetica de Boiss and Quantitative Analysis of the Content of Six Chemical Components Based on Near-Infrared Spectroscopy" Molecules 30, no. 9: 1867. https://doi.org/10.3390/molecules30091867
APA StyleZhang, Y., Li, J., Sun, J., Xia, T., Hai, Y., Li, J., Yang, Y., & Xia, C. (2025). Rapid Identification of Vicatia thibetica de Boiss and Quantitative Analysis of the Content of Six Chemical Components Based on Near-Infrared Spectroscopy. Molecules, 30(9), 1867. https://doi.org/10.3390/molecules30091867







