Implications of Mucin-Type O-Glycosylation in Alzheimer’s Disease
Abstract
1. Introduction
1.1. Alzheimer’s Disease
1.2. Hypotheses Surrounding Alzheimer’s Disease Onset
1.2.1. Tau Hypothesis
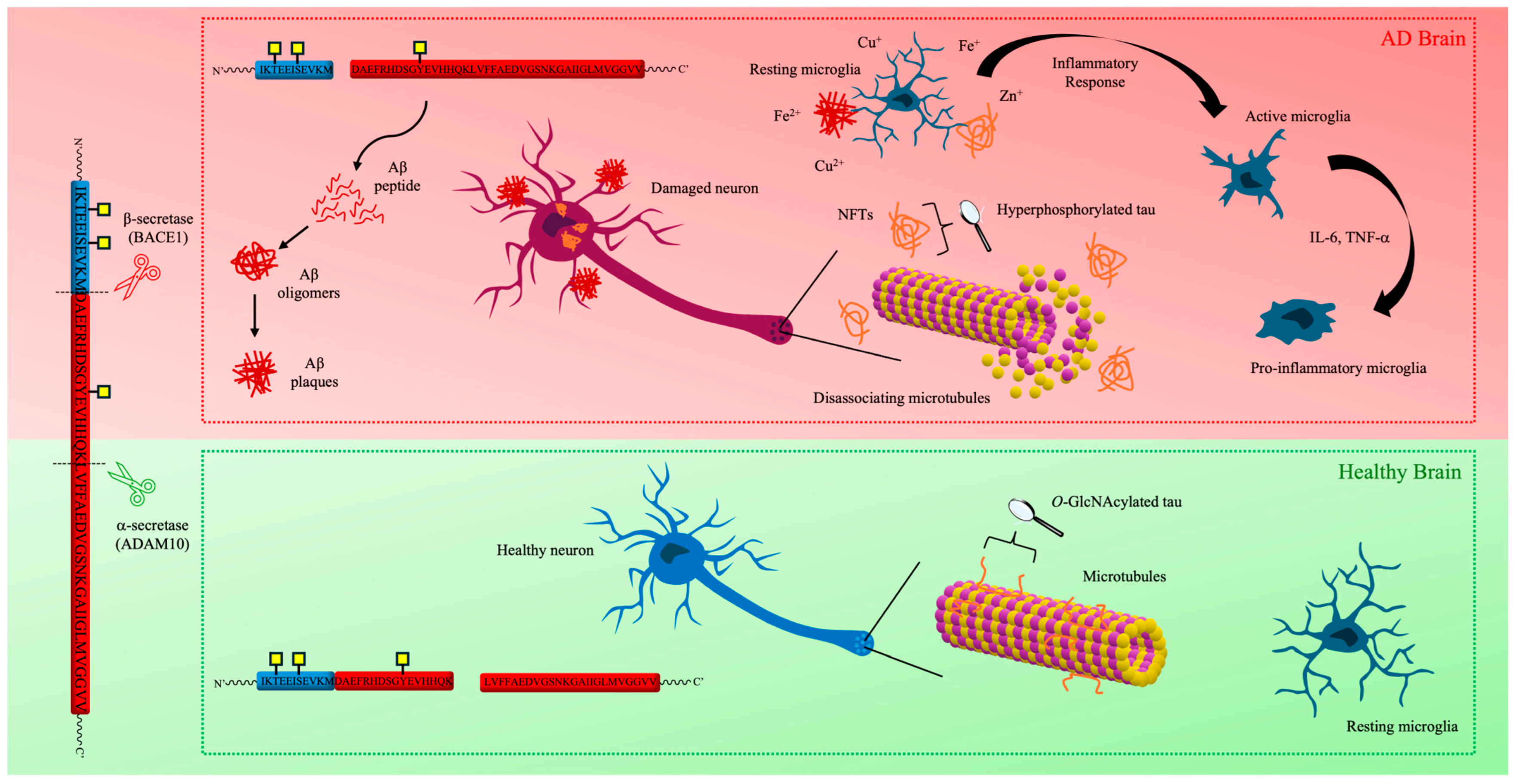
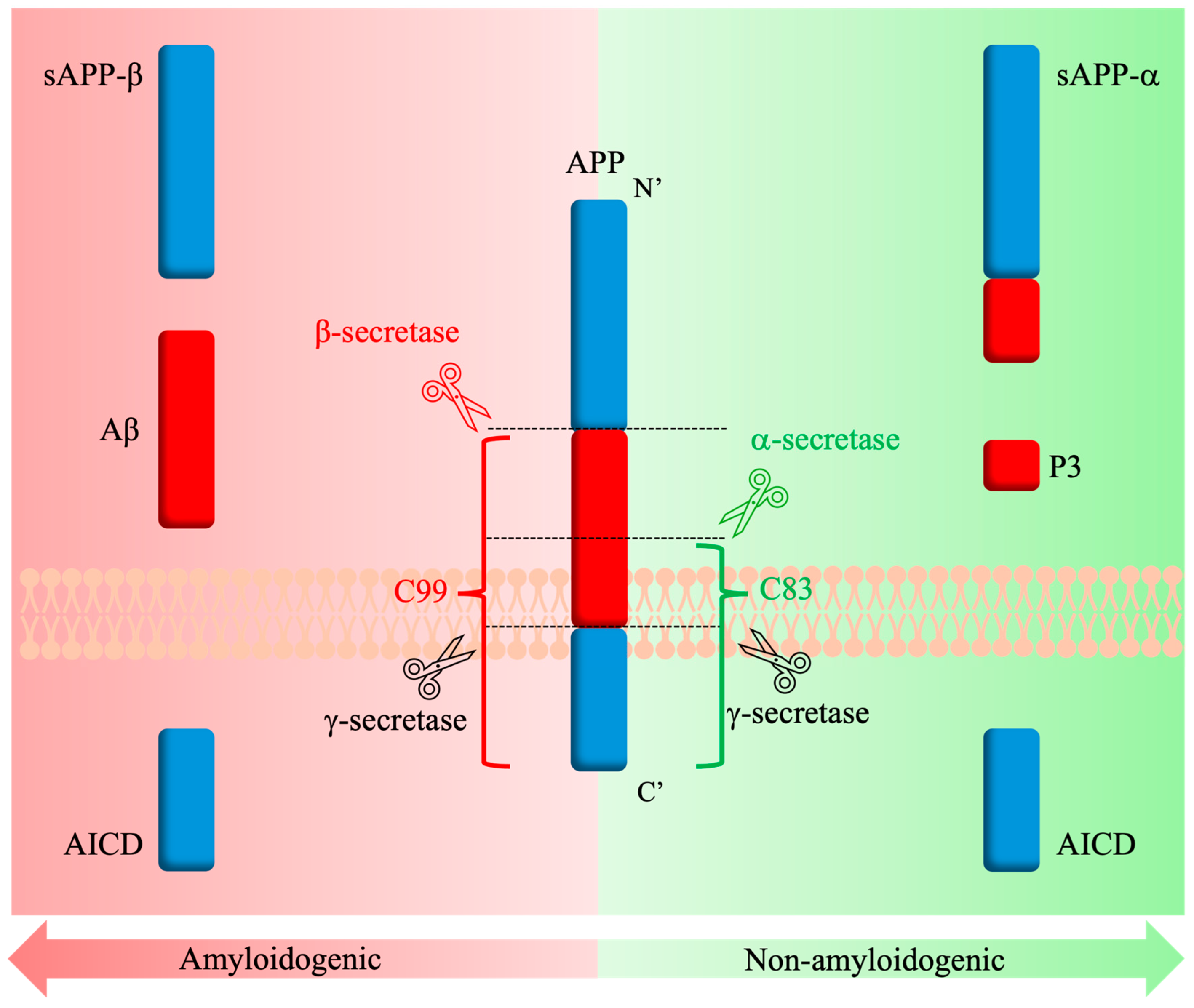
1.2.2. The Amyloid Cascade Hypothesis
1.2.3. Neuroinflammation Cascade
1.2.4. Metal Hypothesis
2. Post-Translational Modification in Alzheimer’s Disease
2.1. Methylation
2.2. Ubiquitylation
2.3. Acetylation
2.4. Phosphorylation
2.5. N-Glycosylation
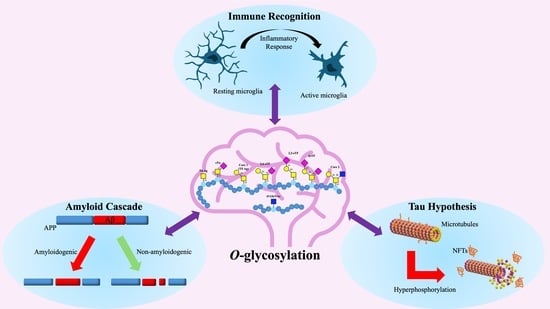
2.6. O-Glycosylation
3. Implications of APP Mucin-Type O-Glycosylation in APP Processing
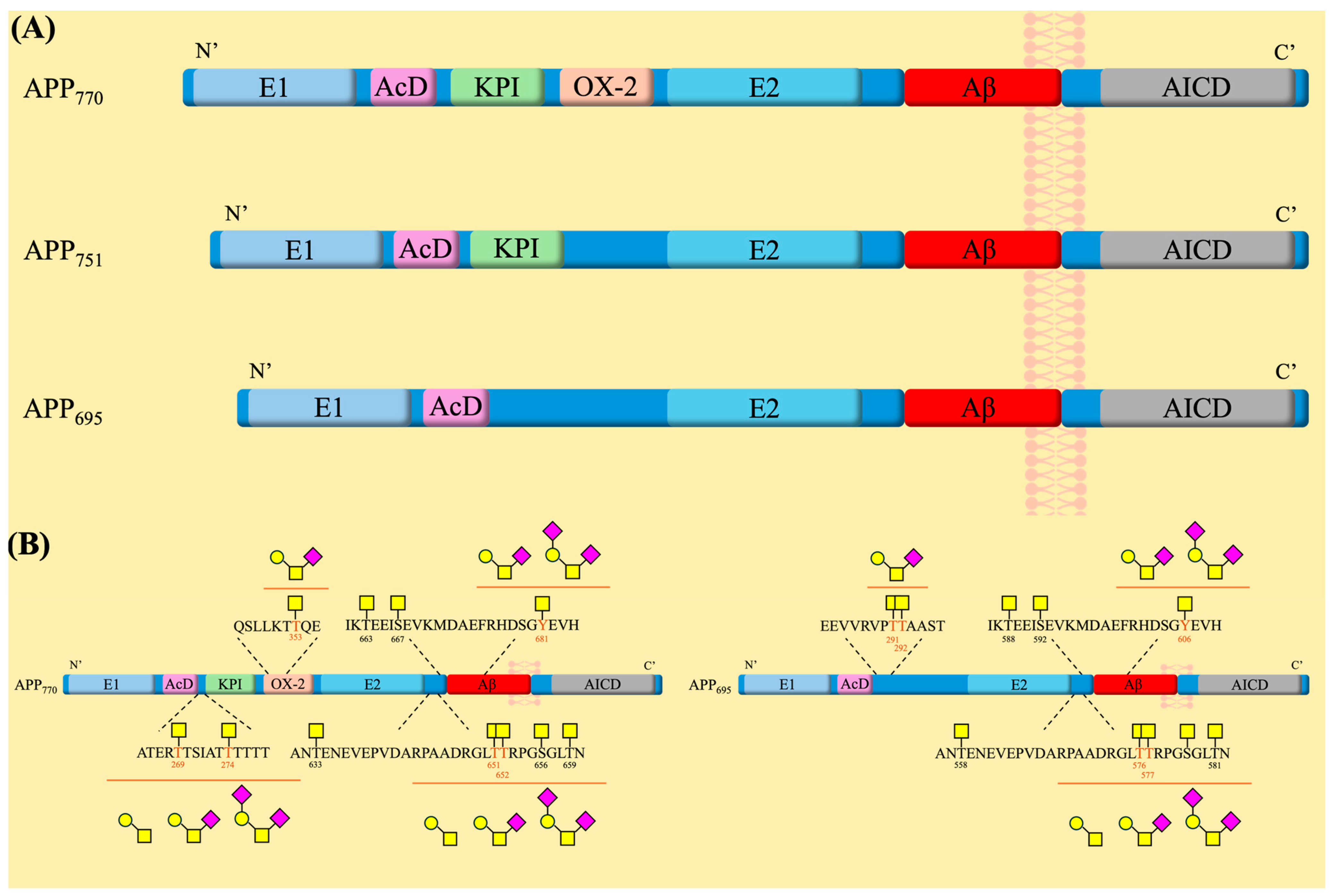
3.1. APP Structure and Physiological Functions
3.2. Role of Mucin-Type O-Glycosylation in APP Processing and AD Pathology
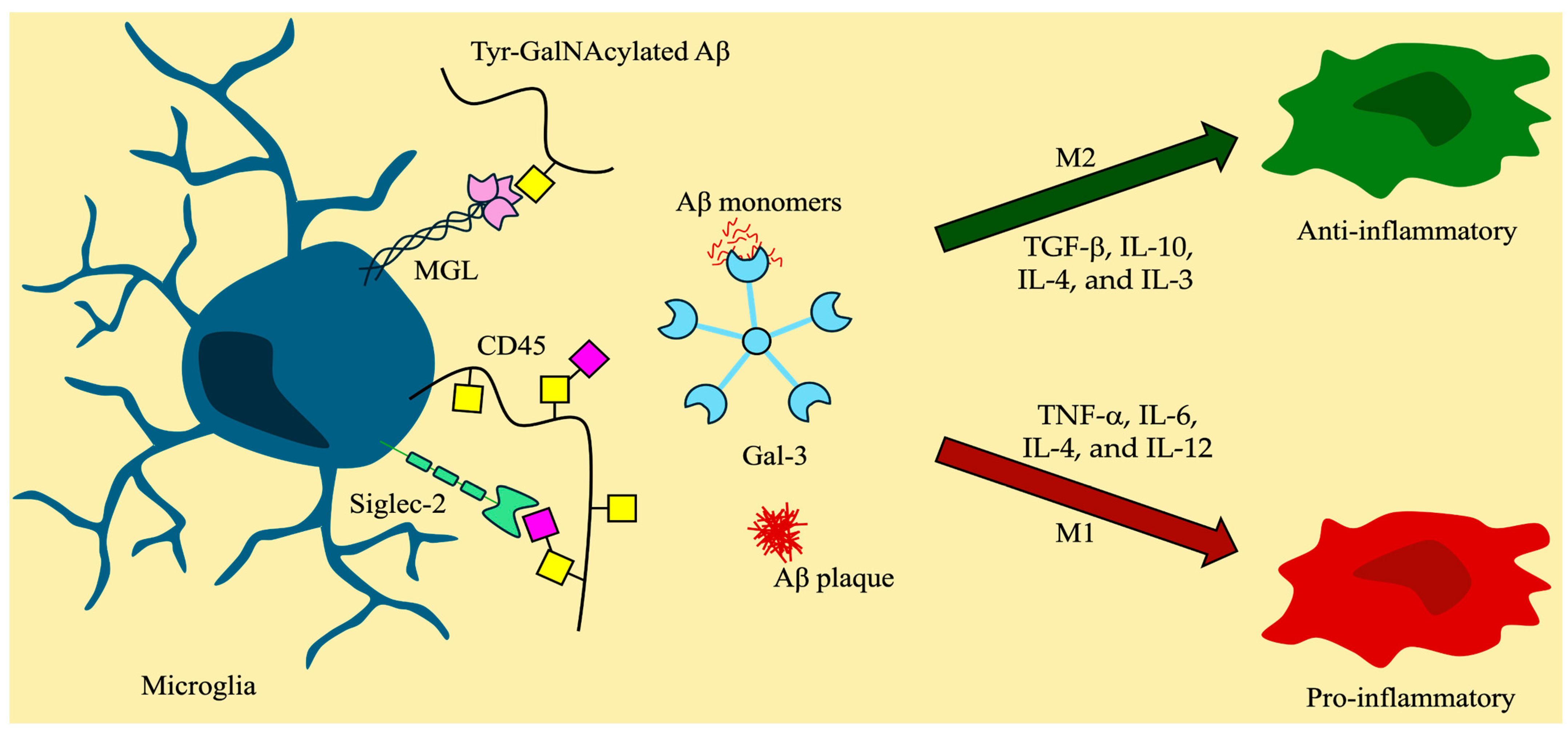
4. Regulation of Immune Responses and Neuroinflammation by O-Glycan Binding Proteins
4.1. Immune Homeostasis
4.2. The CNS and the Neuroimmune Signaling Interplay
4.3. Glycan-Binding Proteins and Immune Response
4.3.1. Galectins and O-Glycans
4.3.2. Siglecs and Sialylated O-Glycans
4.3.3. C-Type Lectins and O-Glycans
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, M.V.F.; de Loures, C.M.G.; Alves, L.C.V.; de Souza, L.C.; Borges, K.B.G.; Carvalho, M.d.G. Alzheimer’s Disease: Risk Factors and Potentially Protective Measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef] [PubMed]
- 2024 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [CrossRef] [PubMed]
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T.; et al. Global Estimates on the Number of Persons across the Alzheimer’s Disease Continuum. Alzheimer’s Dement. 2023, 19, 658–670. [Google Scholar] [CrossRef]
- Kingston, A.; Comas-Herrera, A.; Jagger, C. Forecasting the Care Needs of the Older Population in England over the next 20 Years: Estimates from the Population Ageing and Care Simulation (PACSim) Modelling Study. Lancet Public Health 2018, 3, e447–e455. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s Disease Drug Development Pipeline: 2019. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 272–293. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Osse, A.M.L.; Cammann, D.; Powell, J.; Chen, J. Anti-Amyloid Monoclonal Antibodies for the Treatment of Alzheimer’s Disease. BioDrugs 2024, 38, 5–22. [Google Scholar] [CrossRef]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease. JAMA 2023, 330, 512. [Google Scholar] [CrossRef]
- Karran, E.; De Strooper, B. The Amyloid Hypothesis in Alzheimer Disease: New Insights from New Therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318. [Google Scholar] [CrossRef]
- Stelzmann, R.A.; Norman Schnitzlein, H.; Reed Murtagh, F. An English Translation of Alzheimer’s 1907 Paper, “Über Eine Eigenartige Erkankung Der Hirnrinde”. Clin. Anat. 1995, 8, 429–431. [Google Scholar] [CrossRef]
- Tagarelli, A.; Piro, A.; Tagarelli, G.; Lagonia, P.; Quattrone, A. Alois Alzheimer: A Hundred Years after the Discovery of the Eponymous Disorder. Int. J. Biomed. Sci. 2006, 2, 196–204. [Google Scholar] [CrossRef]
- Glenner, G.G.; Wong, C.W. Alzheimer’s Disease: Initial Report of the Purification and Characterization of a Novel Cerebrovascular Amyloid Protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Masters, C.L.; Simms, G.; Weinman, N.A.; Multhaupt, G.; Mcdonald, B.L.; Beyreuthert, K. Amyloid Plaque Core Protein in Alzheimer Disease and Down Syndrome. Proc. Natl. Acad. Sci. USA 1985, 82, 4245–4249. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M. Alzheimer’s Disease-an Electron Microscopical Study. Brain 1964, 87, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M. Paired Helical Filaments in Electron Microscopy of Alzheimer’s Disease. Nature 1963, 197, 192–193. [Google Scholar] [CrossRef]
- Terry, R.D. The FIne Structure of Neurofibrillary Tangles in Alzheimer’s Disease. J. Neuropathol. Exp. Neurol. 1963, 22, 629–642. [Google Scholar] [CrossRef]
- Terry, R.; Gonatas, N.; Weiss, M. Ultrastructural Studies in Alzheimer’s Presenile Dementia. Am. J. Pathol. 1964, 44, 269–297. [Google Scholar]
- Iqbal, K.; Grundke-Iqbal, I. Discoveries of Tau, Abnormally Hyperphosphorylated Tau and Others of Neurofibrillary Degeneration: A Personal Historical Perspective. J. Alzheimer’s Dis. 2006, 9, 219–242. [Google Scholar] [CrossRef]
- Johnson, G.V.; Jenkins, S.M. Tau Protein in Normal and Alzheimer’s Disease Brain. J. Alzheimer’s Dis. 1999, 1, 307–328. [Google Scholar] [CrossRef]
- Montine, T.J.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; Mirra, S.S.; et al. National Institute on Aging-Alzheimer’s Association Guidelines for the Neuropathologic Assessment of Alzheimer’s Disease: A Practical Approach. Acta Neuropathol. 2012, 123, 1–11. [Google Scholar] [CrossRef]
- Deture, M.A.; Dickson, D.W. The Neuropathological Diagnosis of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Mandelkow, E.-M.; Mandelkow, E. Biochemistry and Cell Biology of Tau Protein in Neurofibrillary Degeneration. Cold Spring Harb. Perspect. Med. 2012, 2, a006247. [Google Scholar] [CrossRef]
- Kirschner, M. The Discovery of Tau Protein. Cytoskeleton 2024, 81, 78–82. [Google Scholar] [CrossRef]
- Weingarten, M.D.; Lockwood, A.H.; Hwo, S.Y.; Kirschner, M.W. A Protein Factor Essential for Microtubule Assembly. Proc. Natl. Acad. Sci. USA 1975, 72, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Neve, R.L.; Kosik, K.S. The Microtubule Binding Domain of Tau Protein. Neuron 1989, 2, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Brandt, R.; Léger, J.; Lee, G. Interaction of Tau with the Neural Plasma Membrane Mediated by Tau’s Amino-Terminal Projection Domain. J. Cell Biol. 1995, 131, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Alquezar, C.; Arya, S.; Kao, A.W. Tau Post-Translational Modifications: Dynamic Transformers of Tau Function, Degradation, and Aggregation. Front. Neurol. 2021, 11, 595532. [Google Scholar] [CrossRef]
- Neddens, J.; Temmel, M.; Flunkert, S.; Kerschbaumer, B.; Hoeller, C.; Loeffler, T.; Niederkofler, V.; Daum, G.; Attems, J.; Hutter-Paier, B. Phosphorylation of Different Tau Sites during Progression of Alzheimer’s Disease. Acta Neuropathol. Commun. 2018, 6, 52. [Google Scholar] [CrossRef]
- Köpke, E.; Tung, Y.C.; Shaikh, S.; Alonso, A.C.; Iqbal, K.; Grundke-Iqbal, I. Microtubule-Associated Protein Tau. Abnormal Phosphorylation of a Non-Paired Helical Filament Pool in Alzheimer Disease. J. Biol. Chem. 1993, 268, 24374–24384. [Google Scholar] [CrossRef]
- Cleveland, D.W.; Hwo, S.-Y.; Kirschner, M.W. Physical and Chemical Properties of Purified Tau Factor and the Role of Tau in Microtubule Assembly. J. Mol. Biol. 1977, 116, 227–247. [Google Scholar] [CrossRef]
- Lindwall, G.; Cole, R.D. Phosphorylation Affects the Ability of Tau Protein to Promote Microtubule Assembly. J. Biol. Chem. 1984, 259, 5301–5305. [Google Scholar] [CrossRef]
- Tenreiro, S.; Eckermann, K.; Outeiro, T.F. Protein Phosphorylation in Neurodegeneration: Friend or Foe? Front. Mol. Neurosci. 2014, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Selkoe, D.J. Aβ Oligomers—A Decade of Discovery. J. Neurochem. 2007, 101, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Guo, Z. Alzheimer’s Aβ42 and Aβ40 Peptides Form Interlaced Amyloid Fibrils. J. Neurochem. 2013, 126, 305–311. [Google Scholar] [CrossRef]
- Wang, L.; Eom, K.; Kwon, T. Different Aggregation Pathways and Structures for Aβ40 and Aβ42 Peptides. Biomolecules 2021, 11, 198. [Google Scholar] [CrossRef]
- Tolar, M.; Hey, J.; Power, A.; Abushakra, S. Neurotoxic Soluble Amyloid Oligomers Drive Alzheimer’s Pathogenesis and Represent a Clinically Validated Target for Slowing Disease Progression. Int. J. Mol. Sci. 2021, 22, 6355. [Google Scholar] [CrossRef]
- Chen, G.; Xu, T.; Yan, Y.; Zhou, Y.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid Beta: Structure, Biology and Structure-Based Therapeutic Development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Herrup, K. The Case for Rejecting the Amyloid Cascade Hypothesis. Nat. Neurosci. 2015, 18, 794–799. [Google Scholar] [CrossRef]
- Dickson, D.W. The Pathogenesis of Senile Plaques. J. Neuropathol. Exp. Neurol. 1997, 56, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Translating Cell Biology into Therapeutic Advances in Alzheimer’s Disease. Nature 1999, 399, A23–A31. [Google Scholar] [CrossRef]
- Barage, S.H.; Sonawane, K.D. Amyloid Cascade Hypothesis: Pathogenesis and Therapeutic Strategies in Alzheimer’s Disease. Neuropeptides 2015, 52, 1–18. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Kepp, K.P.; Robakis, N.K.; Høilund-Carlsen, P.F.; Sensi, S.L.; Vissel, B. The Amyloid Cascade Hypothesis: An Updated Critical Review. Brain 2023, 146, 3969–3990. [Google Scholar] [CrossRef]
- Shen, X.-N.; Niu, L.-D.; Wang, Y.-J.; Cao, X.-P.; Liu, Q.; Tan, L.; Zhang, C.; Yu, J.-T. Inflammatory Markers in Alzheimer’s Disease and Mild Cognitive Impairment: A Meta-Analysis and Systematic Review of 170 Studies. J. Neurol. Neurosurg. Psychiatry 2019, 90, 590–598. [Google Scholar] [CrossRef]
- Garland, E.F.; Hartnell, I.J.; Boche, D. Microglia and Astrocyte Function and Communication: What Do We Know in Humans? Front. Neurosci. 2022, 16, 824888. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Tago, H.; McGeer, E.G. Reactive Microglia in Patients with Senile Dementia of the Alzheimer Type Are Positive for the Histocompatibility Glycoprotein HLA-DR. Neurosci. Lett. 1987, 79, 195–200. [Google Scholar] [CrossRef]
- Tooyama, I.; Kimura, H.; Akiyama, H.; McGeer, P.L. Reactive Microglia Express Class I and Class II Major Histocompatibility Complex Antigens in Alzheimer’s Disease. Brain Res. 1990, 523, 273–280. [Google Scholar] [CrossRef]
- Dani, M.; Wood, M.; Mizoguchi, R.; Fan, Z.; Walker, Z.; Morgan, R.; Hinz, R.; Biju, M.; Kuruvilla, T.; Brooks, D.J.; et al. Microglial Activation Correlates in Vivo with Both Tau and Amyloid in Alzheimer’s Disease. Brain 2018, 141, 2740–2754. [Google Scholar] [CrossRef]
- Kabba, J.A.; Xu, Y.; Christian, H.; Ruan, W.; Chenai, K.; Xiang, Y.; Zhang, L.; Saavedra, J.M.; Pang, T. Microglia: Housekeeper of the Central Nervous System. Cell. Mol. Neurobiol. 2018, 38, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef]
- van Kooyk, Y.; Ilarregui, J.M.; van Vliet, S.J. Novel Insights into the Immunomodulatory Role of the Dendritic Cell and Macrophage-Expressed C-Type Lectin MGL. Immunobiology 2015, 220, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Ilarregui, J.M.; Kooij, G.; Rodríguez, E.; van der Pol, S.M.A.; Koning, N.; Kalay, H.; van der Horst, J.C.; van Vliet, S.J.; García-Vallejo, J.J.; de Vries, H.E.; et al. Macrophage Galactose-Type Lectin (MGL) Is Induced on M2 Microglia and Participates in the Resolution Phase of Autoimmune Neuroinflammation. J. Neuroinflamm. 2019, 16, 130. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, S.J.; Saeland, E.; van Kooyk, Y. Sweet Preferences of MGL: Carbohydrate Specificity and Function. Trends Immunol. 2008, 29, 83–90. [Google Scholar] [CrossRef]
- Hanisch, F.-G. O-Glycosylation of the Mucin Type. Biol. Chem. 2001, 382, 143–149. [Google Scholar] [CrossRef]
- Guevara, J.; Espinosa, B.; Zenteno, E.; Vázquez, L.; Luna, J.; Perry, G.; Mena, R. Altered Glycosylation Pattern of Proteins in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 1998, 57, 905–914. [Google Scholar] [CrossRef]
- Bush, A.I.; Tanzi, R.E. Therapeutics for Alzheimer’s Disease Based on the Metal Hypothesis. Neurotherapeutics 2008, 5, 421–432. [Google Scholar] [CrossRef]
- Babić Leko, M.; Langer Horvat, L.; Španić Popovački, E.; Zubčić, K.; Hof, P.R.; Šimić, G. Metals in Alzheimer’s Disease. Biomedicines 2023, 11, 1161. [Google Scholar] [CrossRef]
- Chen, L.-L.; Fan, Y.-G.; Zhao, L.-X.; Zhang, Q.; Wang, Z.-Y. The Metal Ion Hypothesis of Alzheimer’s Disease and the Anti-Neuroinflammatory Effect of Metal Chelators. Bioorg. Chem. 2023, 131, 106301. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, D.; Yu, Q.; Johnson, J.; Shipman, R.; Zhong, X.; Huang, J.; Yu, Q.; Zetterberg, H.; Asthana, S.; et al. In-Depth Site-Specific O-Glycosylation Analysis of Glycoproteins and Endogenous Peptides in Cerebrospinal Fluid (CSF) from Healthy Individuals, Mild Cognitive Impairment (MCI), and Alzheimer’s Disease (AD) Patients. ACS Chem. Biol. 2022, 17, 3059–3068. [Google Scholar] [CrossRef]
- Boix, C.P.; Lopez-Font, I.; Cuchillo-Ibañez, I.; Sáez-Valero, J. Amyloid Precursor Protein Glycosylation Is Altered in the Brain of Patients with Alzheimer’s Disease. Alzheimer’s Res. Ther. 2020, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, C.; Chin, L.-S.; Li, L. Integrative Glycoproteomics Reveals Protein N-Glycosylation Aberrations and Glycoproteomic Network Alterations in Alzheimer’s Disease. Sci. Adv. 2020, 6, eabc5802. [Google Scholar] [CrossRef]
- Suttapitugsakul, S.; Stavenhagen, K.; Donskaya, S.; Bennett, D.A.; Mealer, R.G.; Seyfried, N.T.; Cummings, R.D. Glycoproteomics Landscape of Asymptomatic and Symptomatic Human Alzheimer’s Disease Brain. Mol. Cell. Proteom. 2022, 21, 100433. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, D.M.; Montagna, D.R.; Gu, Y.; Selkoe, D.J.; Wolfe, M.S. Nicastrin Functions to Sterically Hinder γ-Secretase–Substrate Interactions Driven by Substrate Transmembrane Domain. Proc. Natl. Acad. Sci. USA 2016, 113, E509–E518. [Google Scholar] [CrossRef]
- Vanoni, O.; Paganetti, P.; Molinari, M. Consequences of Individual N-Glycan Deletions and of Proteasomal Inhibition on Secretion of Active BACE. Mol. Biol. Cell 2008, 19, 4086–4098. [Google Scholar] [CrossRef]
- Frenkel-Pinter, M.; Shmueli, M.D.; Raz, C.; Yanku, M.; Zilberzwige, S.; Gazit, E.; Segal, D. Interplay between Protein Glycosylation Pathways in Alzheimer’s Disease. Sci. Adv. 2017, 3, e1601576. [Google Scholar] [CrossRef]
- Müller, M.M. Post-Translational Modifications of Protein Backbones: Unique Functions, Mechanisms, and Challenges. Biochemistry 2018, 57, 177–185. [Google Scholar] [CrossRef]
- Keenan, E.K.; Zachman, D.K.; Hirschey, M.D. Discovering the Landscape of Protein Modifications. Mol. Cell 2021, 81, 1868–1878. [Google Scholar] [CrossRef]
- Fuchs, S.M. Chemically Modified Tandem Repeats in Proteins: Natural Combinatorial Peptide Libraries. ACS Chem. Biol. 2013, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.-J.; Dammer, E.B.; Wang, G.; Seyfried, N.T.; Levey, A.I. Proteomics of Protein Post-Translational Modifications Implicated in Neurodegeneration. Transl. Neurodegener. 2014, 3, 23. [Google Scholar] [CrossRef]
- Gupta, R.; Sahu, M.; Srivastava, D.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Post-Translational Modifications: Regulators of Neurodegenerative Proteinopathies. Ageing Res. Rev. 2021, 68, 101336. [Google Scholar] [CrossRef]
- Schaffert, L.-N.; Carter, W.G. Do Post-Translational Modifications Influence Protein Aggregation in Neurodegenerative Diseases: A Systematic Review. Brain Sci. 2020, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, H.-L.; Wang, J.-Z.; Liu, R.; Wang, X. Abnormal Protein Post-Translational Modifications Induces Aggregation and Abnormal Deposition of Protein, Mediating Neurodegenerative Diseases. Cell Biosci. 2024, 14, 22. [Google Scholar] [CrossRef]
- Lee, J.M.; Hammarén, H.M.; Savitski, M.M.; Baek, S.H. Control of Protein Stability by Post-Translational Modifications. Nat. Commun. 2023, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Rowe, E.M.; Xing, V.; Biggar, K.K. Lysine Methylation: Implications in Neurodegenerative Disease. Brain Res. 2019, 1707, 164–171. [Google Scholar] [CrossRef]
- Thomas, S.N.; Funk, K.E.; Wan, Y.; Liao, Z.; Davies, P.; Kuret, J.; Yang, A.J. Dual Modification of Alzheimer’s Disease PHF-Tau Protein by Lysine Methylation and Ubiquitylation: A Mass Spectrometry Approach. Acta Neuropathol. 2012, 123, 105–117. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, X.; Jiao, B. Epigenetics: Recent Advances and Its Role in the Treatment of Alzheimer’s Disease. Front. Neurol. 2020, 11, 538301. [Google Scholar] [CrossRef]
- Guan, P.-P.; Wang, P. The Involvement of Post-Translational Modifications in Regulating the Development and Progression of Alzheimer’s Disease. Mol. Neurobiol. 2023, 60, 3617–3632. [Google Scholar] [CrossRef]
- Choudhary, C.; Kumar, C.; Gnad, F.; Nielsen, M.L.; Rehman, M.; Walther, T.C.; Olsen, J.V.; Mann, M. Lysine Acetylation Targets Protein Complexes and Co-Regulates Major Cellular Functions. Science 2009, 325, 834–840. [Google Scholar] [CrossRef]
- Bai, N.; Li, N.; Cheng, R.; Guan, Y.; Zhao, X.; Song, Z.; Xu, H.; Yi, F.; Jiang, B.; Li, X.; et al. Inhibition of SIRT2 Promotes APP Acetylation and Ameliorates Cognitive Impairment in APP/PS1 Transgenic Mice. Cell Rep. 2022, 40, 111062. [Google Scholar] [CrossRef] [PubMed]
- Tracy, T.; Claiborn, K.C.; Gan, L. Regulation of Tau Homeostasis and Toxicity by Acetylation. In Tau Biology; Springer: Singapore, 2019; pp. 47–55. [Google Scholar]
- Rubin, C.S.; Rosen, O.M. Protein Phosphorylation. Annu. Rev. Biochem. 1975, 44, 831–887. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Ram, M.; Kumar, R.; Prasad, R.; Roy, B.K.; Singh, K.K. Phosphorylation: Implications in Cancer. Protein J. 2017, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Wang, W.; Qin, J.; Shi, Z.; Hao, L.; Ma, Y.; Xu, H.; Wu, Z.; Pan, D.; Chen, Z.; et al. Role of Protein Phosphorylation in Cell Signaling, Disease, and the Intervention Therapy. MedComm 2022, 3, e175. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, D.; Lee, T.H. Phosphorylation Signaling in APP Processing in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 21, 209. [Google Scholar] [CrossRef]
- Schneider, A.; Biernat, J.; von Bergen, M.; Mandelkow, E.; Mandelkow, E.-M. Phosphorylation That Detaches Tau Protein from Microtubules (Ser262, Ser214) Also Protects It against Aggregation into Alzheimer Paired Helical Filaments. Biochemistry 1999, 38, 3549–3558. [Google Scholar] [CrossRef]
- Schwarz, F.; Aebi, M. Mechanisms and Principles of N-Linked Protein Glycosylation. Curr. Opin. Struct. Biol. 2011, 21, 576–582. [Google Scholar] [CrossRef]
- Stanley, P.; Moremen, K.W.; Lewis, N.E.; Taniguchi, N.; Aebi, M. N-Glycans. In Essentials of Glycobiology, 4th ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2022; ISBN 9781621824213. [Google Scholar]
- Esmail, S.; Manolson, M.F. Advances in Understanding N-Glycosylation Structure, Function, and Regulation in Health and Disease. Eur. J. Cell Biol. 2021, 100, 151186. [Google Scholar] [CrossRef]
- Lin, T.; van Husen, L.S.; Yu, Y.; Tjernberg, L.O.; Schedin-Weiss, S. Lack of N-Glycosylation Increases Amyloidogenic Processing of the Amyloid Precursor Protein. Glycobiology 2022, 32, 506–517. [Google Scholar] [CrossRef]
- Sato, Y.; Naito, Y.; Grundke-Iqbal, I.; Iqbal, K.; Endo, T. Analysis of N-glycans of Pathological Tau: Possible Occurrence of Aberrant Processing of Tau in Alzheimer’s Disease. FEBS Lett. 2001, 496, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zaidi, T.; Iqbal, K.; Grundke-Iqbal, I.; Merkle, R.K.; Gong, C.-X. Role of Glycosylation in Hyperphosphorylation of Tau in Alzheimer’s Disease. FEBS Lett. 2002, 512, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Kizuka, Y.; Kitazume, S.; Taniguchi, N. N-Glycan and Alzheimer’s Disease. Biochim. Biophys. Acta—Gen. Subj. 2017, 1861, 2447–2454. [Google Scholar] [CrossRef]
- Haltiwanger, R.S.; Wells, L.; Freeze, H.H.; Jafar-Nejad, H.; Okajima, T.; Stanley, P. Other Classes of Eukaryotic Glycans. In Essentials of Glycobiology, 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; ISBN 9781621824213. [Google Scholar]
- Brockhausen, I.; Schachter, H.; Stanley, P. O-GalNAc Glycans. In Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2009; ISBN 9780879697709. [Google Scholar]
- Taherzadeh, G.; Dehzangi, A.; Golchin, M.; Zhou, Y.; Campbell, M.P. SPRINT-Gly: Predicting N- and O-Linked Glycosylation Sites of Human and Mouse Proteins by Using Sequence and Predicted Structural Properties. Bioinformatics 2019, 35, 4140–4146. [Google Scholar] [CrossRef]
- Pakhrin, S.C.; Chauhan, N.; Khan, S.; Upadhyaya, J.; Beck, M.R.; Blanco, E. Prediction of Human O-Linked Glycosylation Sites Using Stacked Generalization and Embeddings from Pre-Trained Protein Language Model. Bioinformatics 2024, 40, btae643. [Google Scholar] [CrossRef]
- Torres, C.R.; Hart, G.W. Topography and Polypeptide Distribution of Terminal N-Acetylglucosamine Residues on the Surfaces of Intact Lymphocytes. Evidence for O-Linked GlcNAc. J. Biol. Chem. 1984, 259, 3308–3317. [Google Scholar] [CrossRef]
- Lefebvre, T.; Ferreira, S.; Dupont-Wallois, L.; Bussière, T.; Dupire, M.-J.; Delacourte, A.; Michalski, J.-C.; Caillet-Boudin, M.-L. Evidence of a Balance between Phosphorylation and O-GlcNAc Glycosylation of Tau Proteins—A Role in Nuclear Localization. Biochim. Biophys. Acta—Gen. Subj. 2003, 1619, 167–176. [Google Scholar] [CrossRef]
- Liu, F.; Iqbal, K.; Grundke-Iqbal, I.; Hart, G.W.; Gong, C.-X. O-GlcNAcylation Regulates Phosphorylation of Tau: A Mechanism Involved in Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2004, 101, 10804–10809. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Liu, F.; Iqbal, K. O-GlcNAcylation: A Regulator of Tau Pathology and Neurodegeneration. Alzheimer’s Dement. 2016, 12, 1078–1089. [Google Scholar] [CrossRef]
- Griffith, L.S.; Mathes, M.; Schmitz, B. Β-Amyloid Precursor Protein Is Modified with O-linked N-acetylglucosamine. J. Neurosci. Res. 1995, 41, 270–278. [Google Scholar] [CrossRef]
- Jacobsen, K.T.; Iverfeldt, K. O-GlcNAcylation Increases Non-Amyloidogenic Processing of the Amyloid-β Precursor Protein (APP). Biochem. Biophys. Res. Commun. 2011, 404, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Kwon, O.-H.; Chung, S. O-GlcNAcylation of Amyloid-β Precursor Protein at Threonine 576 Residue Regulates Trafficking and Processing. Biochem. Biophys. Res. Commun. 2017, 490, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; Ten Hagen, K.G. Mucin-Type O-Glycosylation during Development. J. Biol. Chem. 2013, 288, 6921–6929. [Google Scholar] [CrossRef]
- Bagdonaite, I.; Pallesen, E.M.H.; Nielsen, M.I.; Bennett, E.P.; Wandall, H.H. Mucin-Type O-GalNAc Glycosylation in Health and Disease. In The Role of Glycosylation in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2021; pp. 25–60. [Google Scholar]
- Bennett, E.P.; Mandel, U.; Clausen, H.; Gerken, T.A.; Fritz, T.A.; Tabak, L.A. Control of Mucin-Type O-Glycosylation: A Classification of the Polypeptide GalNAc-Transferase Gene Family. Glycobiology 2012, 22, 736–756. [Google Scholar] [CrossRef]
- Beckwith, D.M.; Cudic, M. Tumor-Associated O-Glycans of MUC1: Carriers of the Glyco-Code and Targets for Cancer Vaccine Design. Semin. Immunol. 2020, 47, 101389. [Google Scholar] [CrossRef]
- Lohmueller, J.J.; Sato, S.; Popova, L.; Chu, I.M.; Tucker, M.A.; Barberena, R.; Innocenti, G.M.; Cudic, M.; Ham, J.D.; Cheung, W.C.; et al. Antibodies Elicited by the First Non-Viral Prophylactic Cancer Vaccine Show Tumor-Specificity and Immunotherapeutic Potential. Sci. Rep. 2016, 6, 31740. [Google Scholar] [CrossRef]
- Perdivara, I.; Petrovich, R.; Allinquant, B.; Deterding, L.J.; Tomer, K.B.; Przybylski, M. Elucidation of O-Glycosylation Structures of the β-Amyloid Precursor Protein by Liquid Chromatography−Mass Spectrometry Using Electron Transfer Dissociation and Collision Induced Dissociation. J. Proteome Res. 2009, 8, 631–642. [Google Scholar] [CrossRef]
- Halim, A.; Brinkmalm, G.; Rüetschi, U.; Westman-Brinkmalm, A.; Portelius, E.; Zetterberg, H.; Blennow, K.; Larson, G.; Nilsson, J. Site-Specific Characterization of Threonine, Serine, and Tyrosine Glycosylations of Amyloid Precursor Protein/Amyloid β-Peptides in Human Cerebrospinal Fluid. Proc. Natl. Acad. Sci. USA 2011, 108, 11848–11853. [Google Scholar] [CrossRef] [PubMed]
- Akasaka-Manya, K.; Manya, H. The Role of APP O-Glycosylation in Alzheimer’s Disease. Biomolecules 2020, 10, 1569. [Google Scholar] [CrossRef]
- Shi, J.; Ku, X.; Zou, X.; Hou, J.; Yan, W.; Zhang, Y. Comprehensive Analysis of O-Glycosylation of Amyloid Precursor Protein (APP) Using Targeted and Multi-Fragmentation MS Strategy. Biochim. Biophys. Acta—Gen. Subj. 2021, 1865, 129954. [Google Scholar] [CrossRef]
- Tachida, Y.; Iijima, J.; Takahashi, K.; Suzuki, H.; Kizuka, Y.; Yamaguchi, Y.; Tanaka, K.; Nakano, M.; Takakura, D.; Kawasaki, N.; et al. O-GalNAc Glycosylation Determines Intracellular Trafficking of APP and Aβ Production. J. Biol. Chem. 2023, 299, 104905. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Regmi, D.; Ormaza, D.; Ayyalasomayajula, R.; Vela, N.; Mundim, G.; Du, D.; Minond, D.; Cudic, M. Mucin-Type O-Glycosylation Proximal to β-Secretase Cleavage Site Affects APP Processing and Aggregation Fate. Front. Chem. 2022, 10, 859822. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wei, Q.; Xia, W.; He, C.; Zhang, Q.; Huang, L.; Wang, X.; Sun, Y.; Ma, Y.; Zhang, X.; et al. O-Glycosylation Induces Amyloid-β To Form New Fibril Polymorphs Vulnerable for Degradation. J. Am. Chem. Soc. 2021, 143, 20216–20223. [Google Scholar] [CrossRef]
- Rebelo, A.L.; Chevalier, M.T.; Russo, L.; Pandit, A. Role and Therapeutic Implications of Protein Glycosylation in Neuroinflammation. Trends Mol. Med. 2022, 28, 270–289. [Google Scholar] [CrossRef]
- Gabriele, R.M.C.; Abel, E.; Fox, N.C.; Wray, S.; Arber, C. Knockdown of Amyloid Precursor Protein: Biological Consequences and Clinical Opportunities. Front. Neurosci. 2022, 16, 835645. [Google Scholar] [CrossRef]
- Zhang, Y.; Thompson, R.; Zhang, H.; Xu, H. APP Processing in Alzheimer’s Disease. Mol. Brain 2011, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Q.; Zhang, Y.; Xu, H. Proteolytic Processing of Alzheimer’s Β-amyloid Precursor Protein. J. Neurochem. 2012, 120, 9–21. [Google Scholar] [CrossRef]
- Pfundstein, G.; Nikonenko, A.G.; Sytnyk, V. Amyloid Precursor Protein (APP) and Amyloid β (Aβ) Interact with Cell Adhesion Molecules: Implications in Alzheimer’s Disease and Normal Physiology. Front. Cell Dev. Biol. 2022, 10, 969547. [Google Scholar] [CrossRef]
- Coburger, I.; Dahms, S.O.; Roeser, D.; Gührs, K.-H.; Hortschansky, P.; Than, M.E. Analysis of the Overall Structure of the Multi-Domain Amyloid Precursor Protein (APP). PLoS ONE 2013, 8, e81926. [Google Scholar] [CrossRef]
- Buoso, E.; Lanni, C.; Schettini, G.; Govoni, S.; Racchi, M. β-Amyloid Precursor Protein Metabolism: Focus on the Functions and Degradation of Its Intracellular Domain. Pharmacol. Res. 2010, 62, 308–317. [Google Scholar] [CrossRef]
- Ring, S.; Weyer, S.W.; Kilian, S.B.; Waldron, E.; Pietrzik, C.U.; Filippov, M.A.; Herms, J.; Buchholz, C.; Eckman, C.B.; Korte, M.; et al. The Secreted β-Amyloid Precursor Protein Ectodomain APPsα Is Sufficient to Rescue the Anatomical, Behavioral, and Electrophysiological Abnormalities of APP-Deficient Mice. J. Neurosci. 2007, 27, 7817–7826. [Google Scholar] [CrossRef]
- Orobets, K.S.; Karamyshev, A.L. Amyloid Precursor Protein and Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 14794. [Google Scholar] [CrossRef] [PubMed]
- Li, N.-M.; Liu, K.-F.; Qiu, Y.-J.; Zhang, H.-H.; Nakanishi, H.; Qing, H. Mutations of Beta-Amyloid Precursor Protein Alter the Consequence of Alzheimer’s Disease Pathogenesis. Neural Regen. Res. 2019, 14, 658. [Google Scholar] [CrossRef] [PubMed]
- Holsinger, R.M.D.; McLean, C.A.; Beyreuther, K.; Masters, C.L.; Evin, G. Increased Expression of the Amyloid Precursor Β-secretase in Alzheimer’s Disease. Ann. Neurol. 2002, 51, 783–786. [Google Scholar] [CrossRef]
- TCW, J.; Goate, A.M. Genetics of β-Amyloid Precursor Protein in Alzheimer’s Disease. Cold Spring Harb. Perspect. Med. 2017, 7, a024539. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Koo, E.H. Biology and Pathophysiology of the Amyloid Precursor Protein. Mol. Neurodegener. 2011, 6, 27. [Google Scholar] [CrossRef]
- Kuhn, P.-H.; Wang, H.; Dislich, B.; Colombo, A.; Zeitschel, U.; Ellwart, J.W.; Kremmer, E.; Roßner, S.; Lichtenthaler, S.F. ADAM10 Is the Physiologically Relevant, Constitutive α-Secretase of the Amyloid Precursor Protein in Primary Neurons. EMBO J. 2010, 29, 3020–3032. [Google Scholar] [CrossRef]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. β-Secretase Cleavage of Alzheimer’s Amyloid Precursor Protein by the Transmembrane Aspartic Protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef]
- Ling, Y.; Morgan, K.; Kalsheker, N. Amyloid Precursor Protein (APP) and the Biology of Proteolytic Processing: Relevance to Alzheimer’s Disease. Int. J. Biochem. Cell Biol. 2003, 35, 1505–1535. [Google Scholar] [CrossRef]
- Olsen, O.; Kallop, D.Y.; McLaughlin, T.; Huntwork-Rodriguez, S.; Wu, Z.; Duggan, C.D.; Simon, D.J.; Lu, Y.; Easley-Neal, C.; Takeda, K.; et al. Genetic Analysis Reveals That Amyloid Precursor Protein and Death Receptor 6 Function in the Same Pathway to Control Axonal Pruning Independent of β-Secretase. J. Neurosci. 2014, 34, 6438–6447. [Google Scholar] [CrossRef]
- Tiwari, M.K.; Kepp, K.P. Modeling the Aggregation Propensity and Toxicity of Amyloid-β Variants. J. Alzheimer’s Dis. 2015, 47, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.; Gokce, O.; Luthi-Carter, R.; Lashuel, H.A. The Ratio of Monomeric to Aggregated Forms of Aβ40 and Aβ42 Is an Important Determinant of Amyloid-β Aggregation, Fibrillogenesis, and Toxicity. J. Biol. Chem. 2008, 283, 28176–28189. [Google Scholar] [CrossRef] [PubMed]
- García-González, L.; Pilat, D.; Baranger, K.; Rivera, S. Emerging Alternative Proteinases in APP Metabolism and Alzheimer’s Disease Pathogenesis: A Focus on MT1-MMP and MT5-MMP. Front. Aging Neurosci. 2019, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Rivera, S.; García-González, L.; Khrestchatisky, M.; Baranger, K. Metalloproteinases and Their Tissue Inhibitors in Alzheimer’s Disease and Other Neurodegenerative Disorders. Cell. Mol. Life Sci. 2019, 76, 3167–3191. [Google Scholar] [CrossRef]
- Baranger, K.; Marchalant, Y.; Bonnet, A.E.; Crouzin, N.; Carrete, A.; Paumier, J.-M.; Py, N.A.; Bernard, A.; Bauer, C.; Charrat, E.; et al. MT5-MMP Is a New pro-Amyloidogenic Proteinase That Promotes Amyloid Pathology and Cognitive Decline in a Transgenic Mouse Model of Alzheimer’s Disease. Cell. Mol. Life Sci. 2016, 73, 217–236. [Google Scholar] [CrossRef]
- Paumier, J.-M.; Py, N.A.; García-González, L.; Bernard, A.; Stephan, D.; Louis, L.; Checler, F.; Khrestchatisky, M.; Baranger, K.; Rivera, S. Proamyloidogenic Effects of Membrane Type 1 Matrix Metalloproteinase Involve MMP-2 and BACE-1 Activities, and the Modulation of APP Trafficking. FASEB J. 2019, 33, 2910–2927. [Google Scholar] [CrossRef]
- Pilat, D.; Paumier, J.-M.; García-González, L.; Louis, L.; Stephan, D.; Manrique, C.; Khrestchatisky, M.; Di Pasquale, E.; Baranger, K.; Rivera, S. MT5-MMP Promotes Neuroinflammation, Neuronal Excitability and Aβ Production in Primary Neuron/Astrocyte Cultures from the 5xFAD Mouse Model of Alzheimer’s Disease. J. Neuroinflamm. 2022, 19, 65. [Google Scholar] [CrossRef]
- Becker-Pauly, C.; Pietrzik, C.U. The Metalloprotease Meprin β Is an Alternative β-Secretase of APP. Front. Mol. Neurosci. 2017, 9, 159. [Google Scholar] [CrossRef]
- Bien, J.; Jefferson, T.; Čaušević, M.; Jumpertz, T.; Munter, L.; Multhaup, G.; Weggen, S.; Becker-Pauly, C.; Pietrzik, C.U. The Metalloprotease Meprin β Generates Amino Terminal-Truncated Amyloid β Peptide Species. J. Biol. Chem. 2012, 287, 33304–33313. [Google Scholar] [CrossRef]
- Menon, P.K.; Koistinen, N.A.; Iverfeldt, K.; Ström, A.-L. Phosphorylation of the Amyloid Precursor Protein (APP) at Ser-675 Promotes APP Processing Involving Meprin β. J. Biol. Chem. 2019, 294, 17768–17776. [Google Scholar] [CrossRef]
- Werny, L.; Grogro, A.; Bickenbach, K.; Bülck, C.; Armbrust, F.; Koudelka, T.; Pathak, K.; Scharfenberg, F.; Sammel, M.; Sheikhouny, F.; et al. MT1-MMPand ADAM10 /17 Exhibit a Remarkable Overlap of Shedding Properties. FEBS J. 2023, 290, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.; Čaušević, M.; auf dem Keller, U.; Schilling, O.; Isbert, S.; Geyer, R.; Maier, W.; Tschickardt, S.; Jumpertz, T.; Weggen, S.; et al. Metalloprotease Meprin β Generates Nontoxic N-Terminal Amyloid Precursor Protein Fragments in Vivo. J. Biol. Chem. 2011, 286, 27741–27750. [Google Scholar] [CrossRef] [PubMed]
- Marengo, L.; Armbrust, F.; Schoenherr, C.; Storck, S.E.; Schmitt, U.; Zampar, S.; Wirths, O.; Altmeppen, H.; Glatzel, M.; Kaether, C.; et al. Meprin β Knockout Reduces Brain Aβ Levels and Rescues Learning and Memory Impairments in the APP/Lon Mouse Model for Alzheimer’s Disease. Cell. Mol. Life Sci. 2022, 79, 168. [Google Scholar] [CrossRef]
- Liu, X.-H.; Liu, X.-T.; Wu, Y.; Li, S.-A.; Ren, K.-D.; Cheng, M.; Huang, B.; Yang, Y.; Liu, P.-P. Broadening Horizons: Exploring the Cathepsin Family as Therapeutic Targets for Alzheimer’s Disease. Aging Dis. 2024, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Embury, C.M.; Dyavarshetty, B.; Lu, Y.; Wiederin, J.L.; Ciborowski, P.; Gendelman, H.E.; Kiyota, T. Cathepsin B Improves SS-Amyloidosis and Learning and Memory in Models of Alzheimer’s Disease. J. Neuroimmune Pharmacol. 2017, 12, 340–352. [Google Scholar] [CrossRef]
- Oberstein, T.J.; Utz, J.; Spitzer, P.; Klafki, H.W.; Wiltfang, J.; Lewczuk, P.; Kornhuber, J.; Maler, J.M. The Role of Cathepsin B in the Degradation of Aβ and in the Production of Aβ Peptides Starting With Ala2 in Cultured Astrocytes. Front. Mol. Neurosci. 2021, 13, 615740. [Google Scholar] [CrossRef]
- Zhao, J.; Lang, M. New Insight into Protein Glycosylation in the Development of Alzheimer’s Disease. Cell Death Discov. 2023, 9, 314. [Google Scholar] [CrossRef]
- Liu, F.; Xu, K.; Xu, Z.; de las Rivas, M.; Wang, C.; Li, X.; Lu, J.; Zhou, Y.; Delso, I.; Merino, P.; et al. The Small Molecule Luteolin Inhibits N-Acetyl-α-Galactosaminyltransferases and Reduces Mucin-Type O-Glycosylation of Amyloid Precursor Protein. J. Biol. Chem. 2017, 292, 21304–21319. [Google Scholar] [CrossRef]
- Haukedal, H.; Freude, K.K. Implications of Glycosylation in Alzheimer’s Disease. Front. Neurosci. 2021, 14, 625348. [Google Scholar] [CrossRef]
- Goettig, P. Effects of Glycosylation on the Enzymatic Activity and Mechanisms of Proteases. Int. J. Mol. Sci. 2016, 17, 1969. [Google Scholar] [CrossRef]
- Goth, C.K.; Vakhrushev, S.Y.; Joshi, H.J.; Clausen, H.; Schjoldager, K.T. Fine-Tuning Limited Proteolysis: A Major Role for Regulated Site-Specific O-Glycosylation. Trends Biochem. Sci. 2018, 43, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Zhang, Q.; Xu, S.; Yu, Y. The Alteration and Role of Glycoconjugates in Alzheimer’s Disease. Front. Aging Neurosci. 2024, 16, 1398641. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Kirino, Y.; Suzuki, T. Cleavage of Alzheimer’s Amyloid Precursor Protein (APP) by Secretases Occurs after O-Glycosylation of APP in the Protein Secretory Pathway. J. Biol. Chem. 1998, 273, 6277–6284. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Kurosaka, A. Mucin-Type Glycosylation as a Regulatory Factor of Amyloid Precursor Protein Processing. J. Biochem. 2019, 165, 205–208. [Google Scholar] [CrossRef]
- Akasaka-Manya, K.; Kawamura, M.; Tsumoto, H.; Saito, Y.; Tachida, Y.; Kitazume, S.; Hatsuta, H.; Miura, Y.; Hisanaga, S.; Murayama, S.; et al. Excess APP O. -Glycosylation by GalNAc-T6 Decreases Aβ Production. J. Biochem. 2017, 161, 99–111. [Google Scholar] [CrossRef]
- Yang, K.; Yang, Z.; Chen, X.; Li, W. The Significance of Sialylation on the Pathogenesis of Alzheimer’s Disease. Brain Res. Bull. 2021, 173, 116–123. [Google Scholar] [CrossRef]
- Fastenau, C.; Bunce, M.; Keating, M.; Wickline, J.; Hopp, S.C.; Bieniek, K.F. Distinct Patterns of Plaque and Microglia Glycosylation in Alzheimer’s Disease. Brain Pathol. 2024, 34, e13267. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, J.; Wang, B.; Sun, M.; Yang, H. Microglia in the Neuroinflammatory Pathogenesis of Alzheimer’s Disease and Related Therapeutic Targets. Front. Immunol. 2022, 13, 856376. [Google Scholar] [CrossRef]
- Wang, R.; Lan, C.; Benlagha, K.; Camara, N.O.S.; Miller, H.; Kubo, M.; Heegaard, S.; Lee, P.; Yang, L.; Forsman, H.; et al. The Interaction of Innate Immune and Adaptive Immune System. MedComm 2024, 5, e714. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An Introduction to Immunology and Immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef]
- Ousman, S.S.; Kubes, P. Immune Surveillance in the Central Nervous System. Nat. Neurosci. 2012, 15, 1096–1101. [Google Scholar] [CrossRef]
- Morimoto, K.; Nakajima, K. Role of the Immune System in the Development of the Central Nervous System. Front. Neurosci. 2019, 13, e714. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Ma, L.; Kaarela, T.; Li, Z. Neuroimmune Crosstalk in the Central Nervous System and Its Significance for Neurological Diseases. J. Neuroinflamm. 2012, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. A Polarizing Question: Do M1 and M2 Microglia Exist? Nat. Neurosci. 2016, 19, 987–991. [Google Scholar] [CrossRef]
- Bisht, K.; Sharma, K.P.; Lecours, C.; Gabriela Sánchez, M.; El Hajj, H.; Milior, G.; Olmos-Alonso, A.; Gómez-Nicola, D.; Luheshi, G.; Vallières, L.; et al. Dark Microglia: A New Phenotype Predominantly Associated with Pathological States. Glia 2016, 64, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zong, S.; Cui, X.; Wang, X.; Wu, S.; Wang, L.; Liu, Y.; Lu, Z. The Effects of Microglia-Associated Neuroinflammation on Alzheimer’s Disease. Front. Immunol. 2023, 14, 1117172. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, G.; Luo, Y.; Jiang, L.; Chi, H.; Tian, G. Neuroinflammation in Alzheimer’s Disease: Insights from Peripheral Immune Cells. Immun. Ageing 2024, 21, 38. [Google Scholar] [CrossRef]
- Pan, X.; Zhu, Y.; Lin, N.; Zhang, J.; Ye, Q.; Huang, H.; Chen, X. Microglial Phagocytosis Induced by Fibrillar β-Amyloid Is Attenuated by Oligomeric β-Amyloid: Implications for Alzheimer’s Disease. Mol. Neurodegener. 2011, 6, 45. [Google Scholar] [CrossRef]
- Yang, T.; Li, S.; Xu, H.; Walsh, D.M.; Selkoe, D.J. Large Soluble Oligomers of Amyloid β-Protein from Alzheimer Brain Are Far Less Neuroactive Than the Smaller Oligomers to Which They Dissociate. J. Neurosci. 2017, 37, 152–163. [Google Scholar] [CrossRef]
- d’Errico, P.; Ziegler-Waldkirch, S.; Aires, V.; Hoffmann, P.; Mezö, C.; Erny, D.; Monasor, L.S.; Liebscher, S.; Ravi, V.M.; Joseph, K.; et al. Microglia Contribute to the Propagation of Aβ into Unaffected Brain Tissue. Nat. Neurosci. 2022, 25, 20–25. [Google Scholar] [CrossRef]
- Walker, L.C.; Schelle, J.; Jucker, M. The Prion-Like Properties of Amyloid-β Assemblies: Implications for Alzheimer’s Disease. Cold Spring Harb. Perspect. Med. 2016, 6, a024398. [Google Scholar] [CrossRef] [PubMed]
- Morales, I.; Jiménez, J.M.; Mancilla, M.; Maccioni, R.B. Tau Oligomers and Fibrils Induce Activation of Microglial Cells. J. Alzheimer’s Dis. 2013, 37, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Long, H.-Z.; Zhou, Z.-W.; Cheng, Y.; Luo, H.-Y.; Li, F.-J.; Xu, S.-G.; Gao, L.-C. The Role of Microglia in Alzheimer’s Disease From the Perspective of Immune Inflammation and Iron Metabolism. Front. Aging Neurosci. 2022, 14, 888989. [Google Scholar] [CrossRef]
- Lee, S.-H.; Meilandt, W.J.; Xie, L.; Gandham, V.D.; Ngu, H.; Barck, K.H.; Rezzonico, M.G.; Imperio, J.; Lalehzadeh, G.; Huntley, M.A.; et al. Trem2 Restrains the Enhancement of Tau Accumulation and Neurodegeneration by β-Amyloid Pathology. Neuron 2021, 109, 1283–1301.e6. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Salih, D. TREM2-Mediated Activation of Microglia Breaks Link between Amyloid and Tau. Lancet Neurol. 2021, 20, 416–417. [Google Scholar] [CrossRef]
- Garner, O.B.; Baum, L.G. Galectin–Glycan Lattices Regulate Cell-Surface Glycoprotein Organization and Signalling. Biochem. Soc. Trans. 2008, 36, 1472–1477. [Google Scholar] [CrossRef]
- Pinho, S.S.; Alves, I.; Gaifem, J.; Rabinovich, G.A. Immune Regulatory Networks Coordinated by Glycans and Glycan-Binding Proteins in Autoimmunity and Infection. Cell. Mol. Immunol. 2023, 20, 1101–1113. [Google Scholar] [CrossRef]
- Teichberg, V.I.; Silman, I.; Beitsch, D.D.; Resheff, G. A Beta-D-Galactoside Binding Protein from Electric Organ Tissue of Electrophorus Electricus. Proc. Natl. Acad. Sci. USA 1975, 72, 1383–1387. [Google Scholar] [CrossRef]
- Liu, F.-T.; Stowell, S.R. The Role of Galectins in Immunity and Infection. Nat. Rev. Immunol. 2023, 23, 479–494. [Google Scholar] [CrossRef]
- Liu, F.-T.; Rabinovich, G.A. Galectins as Modulators of Tumour Progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef]
- Di Lella, S.; Sundblad, V.; Cerliani, J.P.; Guardia, C.M.; Estrin, D.A.; Vasta, G.R.; Rabinovich, G.A. When Galectins Recognize Glycans: From Biochemistry to Physiology and Back Again. Biochemistry 2011, 50, 7842–7857. [Google Scholar] [CrossRef] [PubMed]
- Tazhitdinova, R.; Timoshenko, A.V. The Emerging Role of Galectins and O-GlcNAc Homeostasis in Processes of Cellular Differentiation. Cells 2020, 9, 1792. [Google Scholar] [CrossRef]
- Troncoso, M.F.; Elola, M.T.; Blidner, A.G.; Sarrias, L.; Espelt, M.V.; Rabinovich, G.A. The Universe of Galectin-Binding Partners and Their Functions in Health and Disease. J. Biol. Chem. 2023, 299, 105400. [Google Scholar] [CrossRef]
- Nielsen, M.I.; Stegmayr, J.; Grant, O.C.; Yang, Z.; Nilsson, U.J.; Boos, I.; Carlsson, M.C.; Woods, R.J.; Unverzagt, C.; Leffler, H.; et al. Galectin Binding to Cells and Glycoproteins with Genetically Modified Glycosylation Reveals Galectin–Glycan Specificities in a Natural Context. J. Biol. Chem. 2018, 293, 20249–20262. [Google Scholar] [CrossRef] [PubMed]
- Dimitroff, C.J. Galectin-Binding O-Glycosylations as Regulators of Malignancy. Cancer Res. 2015, 75, 3195–3202. [Google Scholar] [CrossRef]
- Nguyen, J.T.; Evans, D.P.; Galvan, M.; Pace, K.E.; Leitenberg, D.; Bui, T.N.; Baum, L.G. CD45 Modulates Galectin-1-Induced T Cell Death: Regulation by Expression of Core 2 O-Glycans. J. Immunol. 2001, 167, 5697–5707. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.C.; Pang, M.; Hsu, D.K.; Liu, F.-T.; de Vos, S.; Gascoyne, R.D.; Said, J.; Baum, L.G. Galectin-3 Binds to CD45 on Diffuse Large B-Cell Lymphoma Cells to Regulate Susceptibility to Cell Death. Blood 2012, 120, 4635–4644. [Google Scholar] [CrossRef]
- Ilarregui, J.M.; Croci, D.O.; Bianco, G.A.; Toscano, M.A.; Salatino, M.; Vermeulen, M.E.; Geffner, J.R.; Rabinovich, G.A. Tolerogenic Signals Delivered by Dendritic Cells to T Cells through a Galectin-1-Driven Immunoregulatory Circuit Involving Interleukin 27 and Interleukin 10. Nat. Immunol. 2009, 10, 981–991. [Google Scholar] [CrossRef]
- Siew, J.J.; Chern, Y. Microglial Lectins in Health and Neurological Diseases. Front. Mol. Neurosci. 2018, 11, 158. [Google Scholar] [CrossRef]
- Boza-Serrano, A.; Vrillon, A.; Minta, K.; Paulus, A.; Camprubí-Ferrer, L.; Garcia, M.; Andreasson, U.; Antonell, A.; Wennström, M.; Gouras, G.; et al. Galectin-3 Is Elevated in CSF and Is Associated with Aβ Deposits and Tau Aggregates in Brain Tissue in Alzheimer’s Disease. Acta Neuropathol. 2022, 144, 843–859. [Google Scholar] [CrossRef]
- Tao, C.-C.; Cheng, K.-M.; Ma, Y.-L.; Hsu, W.-L.; Chen, Y.-C.; Fuh, J.-L.; Lee, W.-J.; Chao, C.-C.; Lee, E.H.Y. Galectin-3 Promotes Aβ Oligomerization and Aβ Toxicity in a Mouse Model of Alzheimer’s Disease. Cell Death Differ. 2020, 27, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Siew, J.J.; Chen, H.-M.; Chiu, F.-L.; Lee, C.-W.; Chang, Y.-M.; Chen, H.-L.; Nguyen, T.N.A.; Liao, H.-T.; Liu, M.; Hagar, H.-T.; et al. Galectin-3 Aggravates Microglial Activation and Tau Transmission in Tauopathy. J. Clin. Investig. 2024, 134, e165523. [Google Scholar] [CrossRef]
- Angata, T.; Varki, A. Discovery, Classification, Evolution and Diversity of Siglecs. Mol. Aspects Med. 2023, 90, 101117. [Google Scholar] [CrossRef] [PubMed]
- Ayyalasomayajula, R.; Cudic, M. Targeting Siglec–Sialylated MUC1 Immune Axis in Cancer. Cancers 2024, 16, 1334. [Google Scholar] [CrossRef]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and Their Roles in the Immune System. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, T.K.; Nath, D.; Ziltener, H.J.; Vestweber, D.; Fukuda, M.; van Die, I.; Crocker, P.R. Cutting Edge: CD43 Functions as a T Cell Counterreceptor for the Macrophage Adhesion Receptor Sialoadhesin (Siglec-1). J. Immunol. 2001, 166, 3637–3640. [Google Scholar] [CrossRef]
- Stewart, N.; Daly, J.; Drummond-Guy, O.; Krishnamoorthy, V.; Stark, J.C.; Riley, N.M.; Williams, K.C.; Bertozzi, C.R.; Wisnovsky, S. The Glycoimmune Checkpoint Receptor Siglec-7 Interacts with T-Cell Ligands and Regulates T-Cell Activation. J. Biol. Chem. 2024, 300, 105579. [Google Scholar] [CrossRef]
- Takamiya, R.; Ohtsubo, K.; Takamatsu, S.; Taniguchi, N.; Angata, T. The Interaction between Siglec-15 and Tumor-Associated Sialyl-Tn Antigen Enhances TGF- Secretion from Monocytes/Macrophages through the DAP12-Syk Pathway. Glycobiology 2013, 23, 178–187. [Google Scholar] [CrossRef]
- Büll, C.; Nason, R.; Sun, L.; Van Coillie, J.; Madriz Sørensen, D.; Moons, S.J.; Yang, Z.; Arbitman, S.; Fernandes, S.M.; Furukawa, S.; et al. Probing the Binding Specificities of Human Siglecs by Cell-Based Glycan Arrays. Proc. Natl. Acad. Sci. USA 2021, 118, e2026102118. [Google Scholar] [CrossRef]
- Siew, J.J.; Chern, Y.; Khoo, K.-H.; Angata, T. Roles of Siglecs in Neurodegenerative Diseases. Mol. Aspects Med. 2023, 90, 101141. [Google Scholar] [CrossRef]
- Eskandari-Sedighi, G.; Jung, J.; Macauley, M.S. CD33 Isoforms in Microglia and Alzheimer’s Disease: Friend and Foe. Mol. Aspects Med. 2023, 90, 101111. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.-L.; Sun, P.-Y.; Fan, D.-Y.; Wang, J.; Sun, H.-L.; Cheng, Y.; Zeng, G.-H.; Chen, D.-W.; Li, H.-Y.; Yi, X.; et al. Associations of Plasma Soluble CD22 Levels with Brain Amyloid Burden and Cognitive Decline in Alzheimer’s Disease. Sci. Adv. 2022, 8, eabm5667. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, S.; Yamazaki, T.; Makioka, K.; Fujita, Y.; Ikeda, M.; Takatama, M.; Okamoto, K.; Yokoo, H.; Ikeda, Y. Hypersialylation Is a Common Feature of Neurofibrillary Tangles and Granulovacuolar Degenerations in Alzheimer’s Disease and Tauopathy Brains. Neuropathology 2016, 36, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Zelensky, A.N.; Gready, J.E. The C-type Lectin-like Domain Superfamily. FEBS J. 2005, 272, 6179–6217. [Google Scholar] [CrossRef]
- Fischer, S.; Stegmann, F.; Gnanapragassam, V.S.; Lepenies, B. From Structure to Function—Ligand Recognition by Myeloid C-Type Lectin Receptors. Comput. Struct. Biotechnol. J. 2022, 20, 5790–5812. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. A New Receptor for β-Glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef]
- Reis e Sousa, C.; Yamasaki, S.; Brown, G.D. Myeloid C-Type Lectin Receptors in Innate Immune Recognition. Immunity 2024, 57, 700–717. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; van Kooyk, Y.; Cobb, B.A. Glycobiology of Immune Responses. Ann. N. Y Acad. Sci. 2012, 1253, 1–15. [Google Scholar] [CrossRef]
- van Vliet, S.J.; Gringhuis, S.I.; Geijtenbeek, T.B.H.; van Kooyk, Y. Regulation of Effector T Cells by Antigen-Presenting Cells via Interaction of the C-Type Lectin MGL with CD45. Nat. Immunol. 2006, 7, 1200–1208. [Google Scholar] [CrossRef]
- Wang, X.; Liu, G.; Gao, Q.; Li, N.; Wang, R. C-type Lectin-like Receptor 2 and Zonulin Are Associated with Mild Cognitive Impairment and Alzheimer’s Disease. Acta Neurol. Scand. 2020, 141, 250–255. [Google Scholar] [CrossRef]
- Meng, D.; Ma, X.; Li, H.; Wu, X.; Cao, Y.; Miao, Z.; Zhang, X. A Role of the Podoplanin-CLEC-2 Axis in Promoting Inflammatory Response After Ischemic Stroke in Mice. Neurotox. Res. 2021, 39, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Szczykutowicz, J. Ligand Recognition by the Macrophage Galactose-Type C-Type Lectin: Self or Non-Self?—A Way to Trick the Host’s Immune System. Int. J. Mol. Sci. 2023, 24, 17078. [Google Scholar] [CrossRef] [PubMed]
- Tumoglu, B.; Keelaghan, A.; Avci, F.Y. Tn Antigen Interactions of Macrophage Galactose-Type Lectin (MGL) in Immune Function and Disease. Glycobiology 2023, 33, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Gibadullin, R.; Farnsworth, D.W.; Barchi, J.J.; Gildersleeve, J.C. GalNAc-Tyrosine Is a Ligand of Plant Lectins, Antibodies, and Human and Murine Macrophage Galactose-Type Lectins. ACS Chem. Biol. 2017, 12, 2172–2182. [Google Scholar] [CrossRef]
- Marcelo, F.; Supekar, N.; Corzana, F.; van der Horst, J.C.; Vuist, I.M.; Live, D.; Boons, G.-J.P.H.; Smith, D.F.; van Vliet, S.J. Identification of a Secondary Binding Site in Human Macrophage Galactose-Type Lectin by Microarray Studies: Implications for the Molecular Recognition of Its Ligands. J. Biol. Chem. 2019, 294, 1300–1311. [Google Scholar] [CrossRef]
- Gabba, A.; Bogucka, A.; Luz, J.G.; Diniz, A.; Coelho, H.; Corzana, F.; Cañada, F.J.; Marcelo, F.; Murphy, P.V.; Birrane, G. Crystal Structure of the Carbohydrate Recognition Domain of the Human Macrophage Galactose C-Type Lectin Bound to GalNAc and the Tumor-Associated Tn Antigen. Biochemistry 2021, 60, 1327–1336. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vela Navarro, N.; De Nadai Mundim, G.; Cudic, M. Implications of Mucin-Type O-Glycosylation in Alzheimer’s Disease. Molecules 2025, 30, 1895. https://doi.org/10.3390/molecules30091895
Vela Navarro N, De Nadai Mundim G, Cudic M. Implications of Mucin-Type O-Glycosylation in Alzheimer’s Disease. Molecules. 2025; 30(9):1895. https://doi.org/10.3390/molecules30091895
Chicago/Turabian StyleVela Navarro, Nancy, Gustavo De Nadai Mundim, and Maré Cudic. 2025. "Implications of Mucin-Type O-Glycosylation in Alzheimer’s Disease" Molecules 30, no. 9: 1895. https://doi.org/10.3390/molecules30091895
APA StyleVela Navarro, N., De Nadai Mundim, G., & Cudic, M. (2025). Implications of Mucin-Type O-Glycosylation in Alzheimer’s Disease. Molecules, 30(9), 1895. https://doi.org/10.3390/molecules30091895