Chemical Composition and Toxicity of Achillea millefolium L. Essential Oil Against Acrobasis advenella (Lepidoptera, Pyralidae) Under Laboratory Conditions
Abstract
1. Introduction
2. Results
2.1. EOAM Chemical Composition
2.2. Settling Inhibitory Activity (SI)
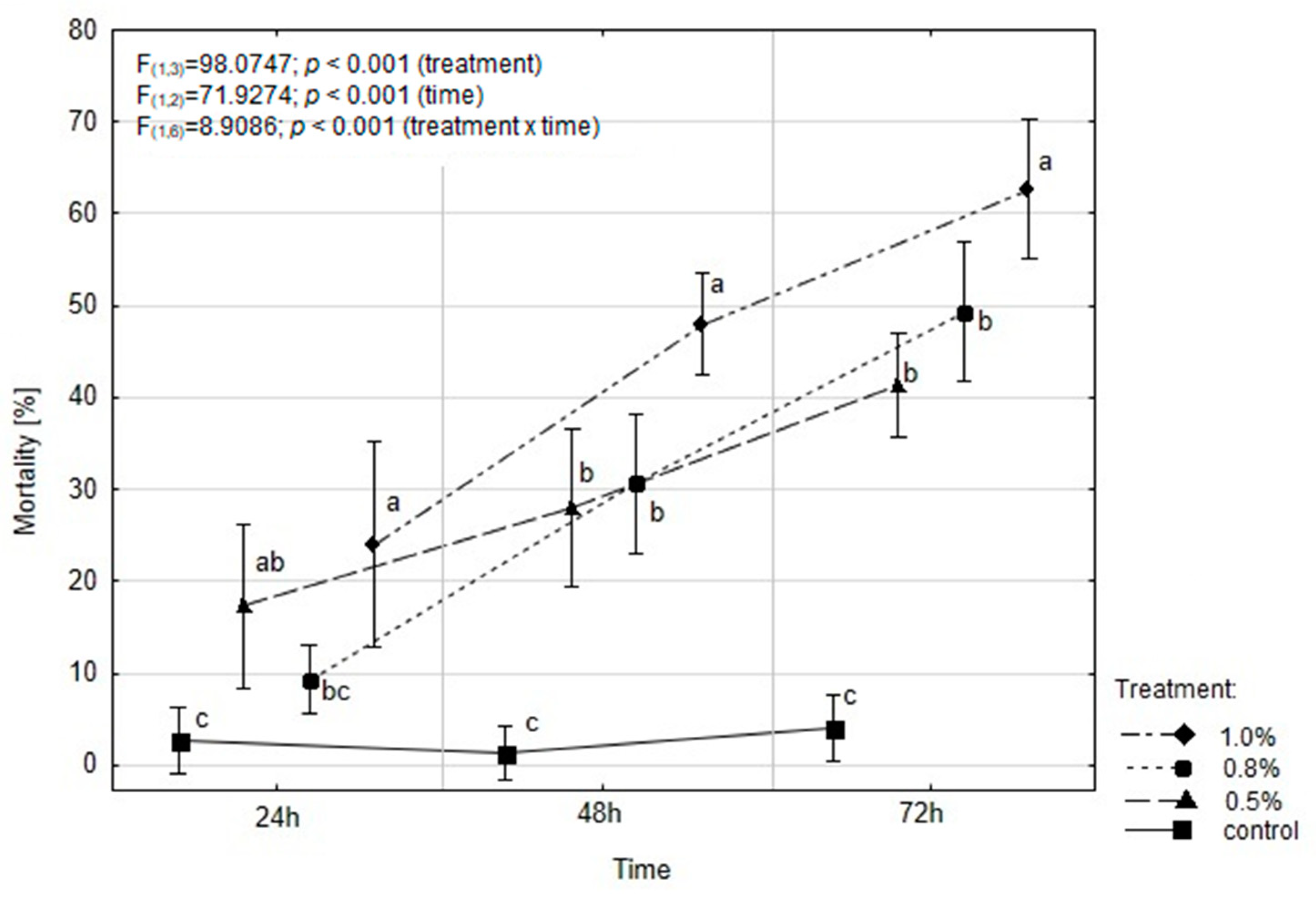
2.3. EOAM Effect on the Mortality of A. advenella larvae
2.4. EOAM Effects on A. advenella Demographic Parameters
2.5. EOAM Phytotoxicity
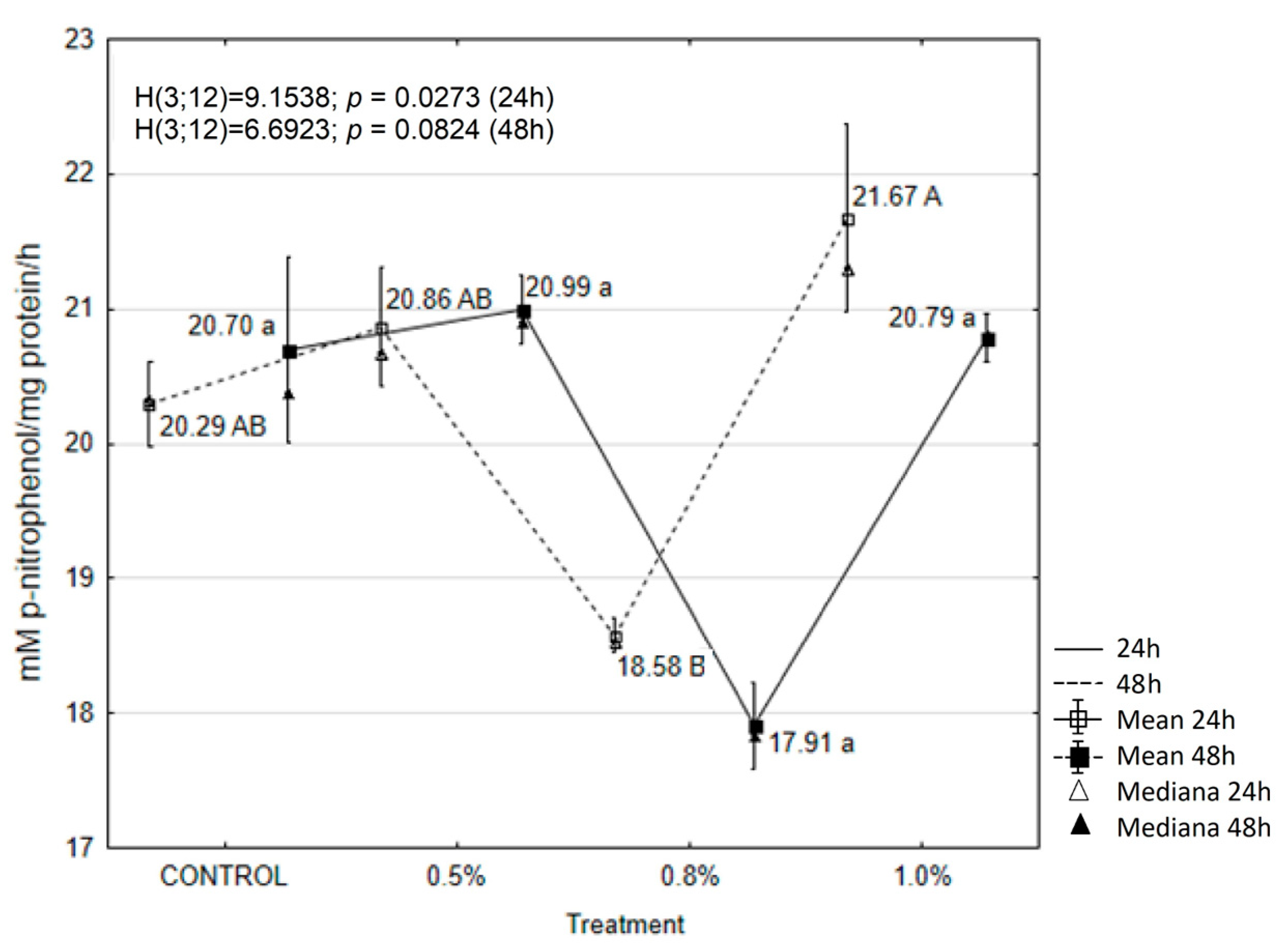
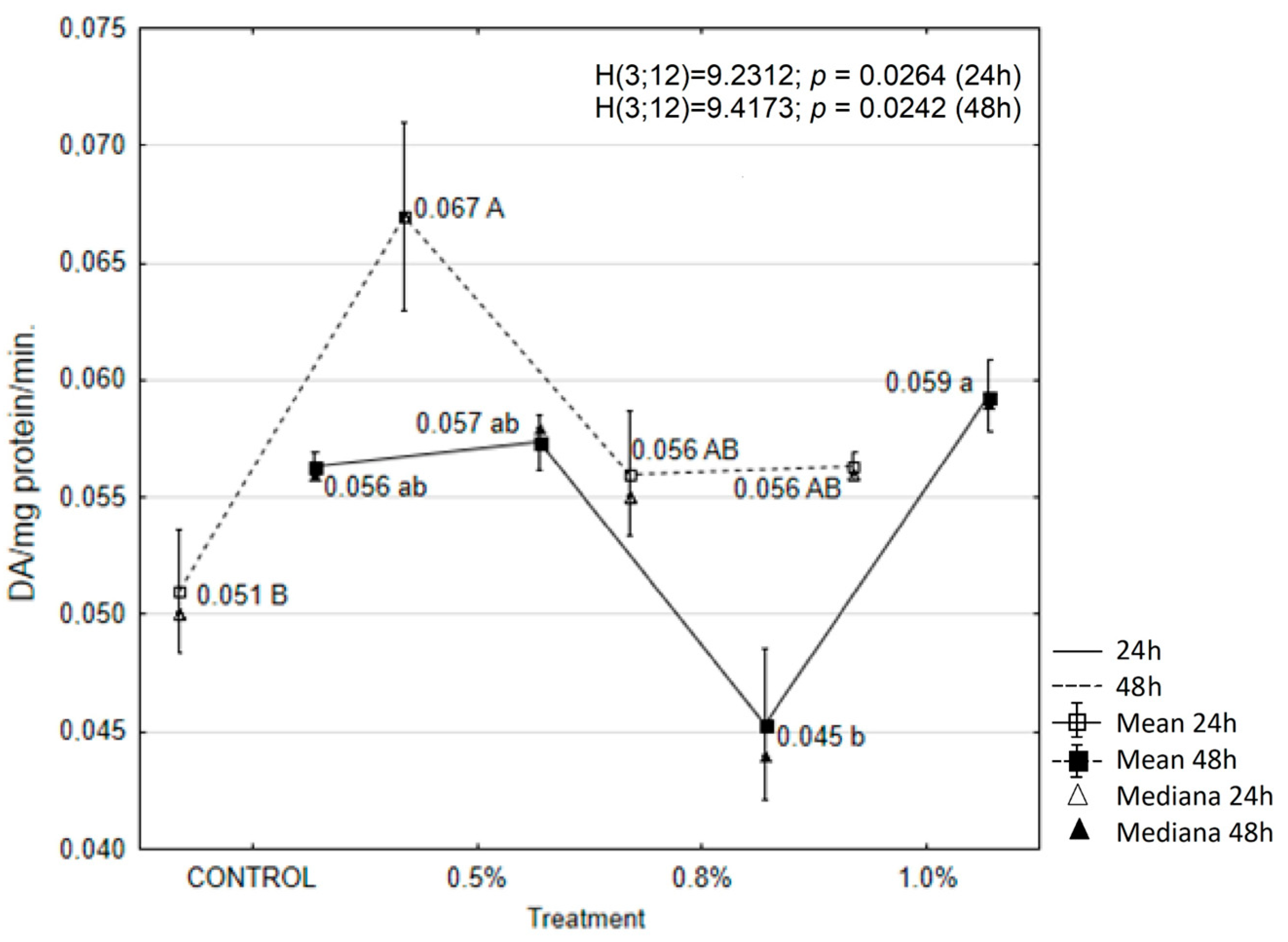
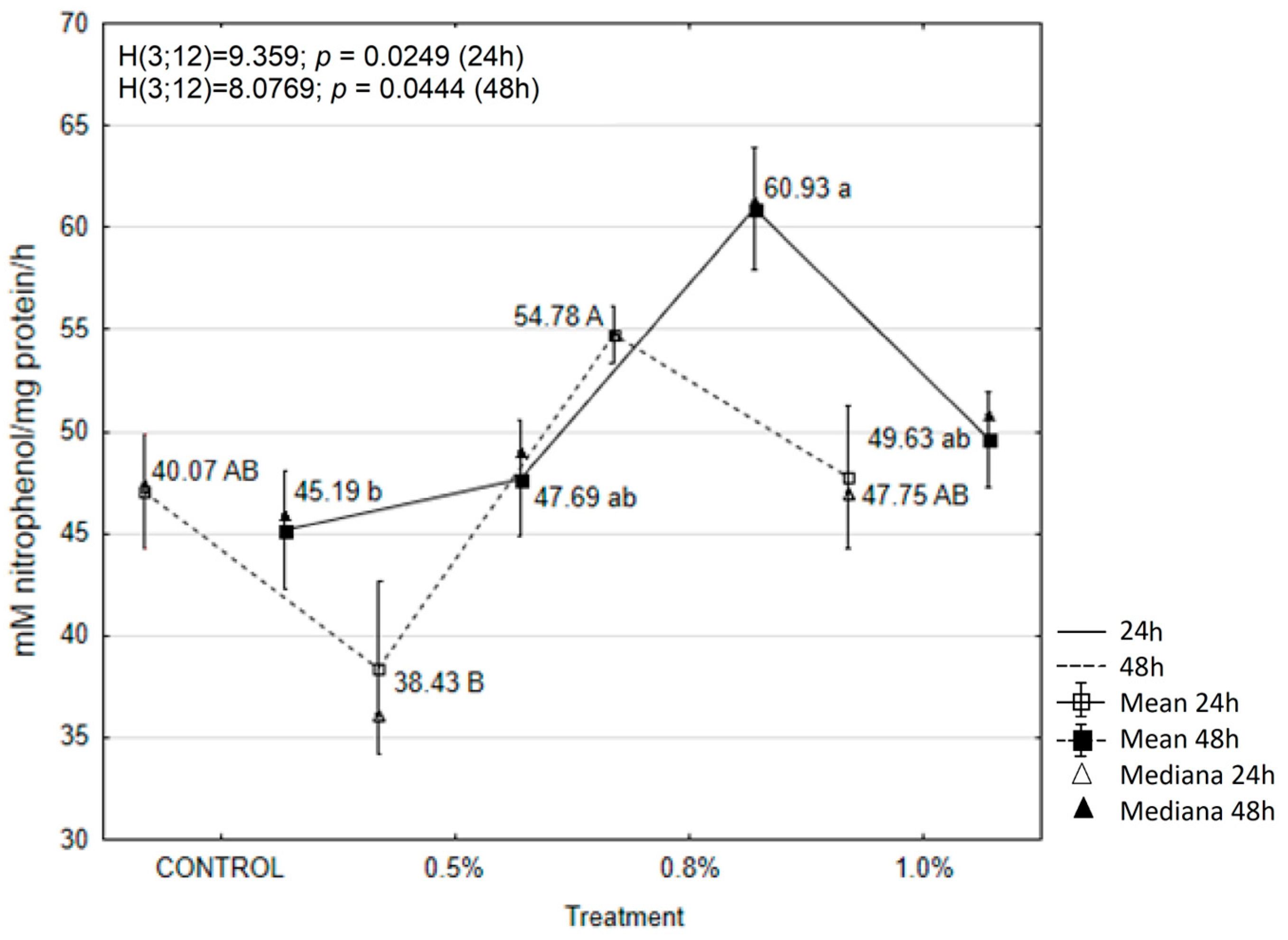
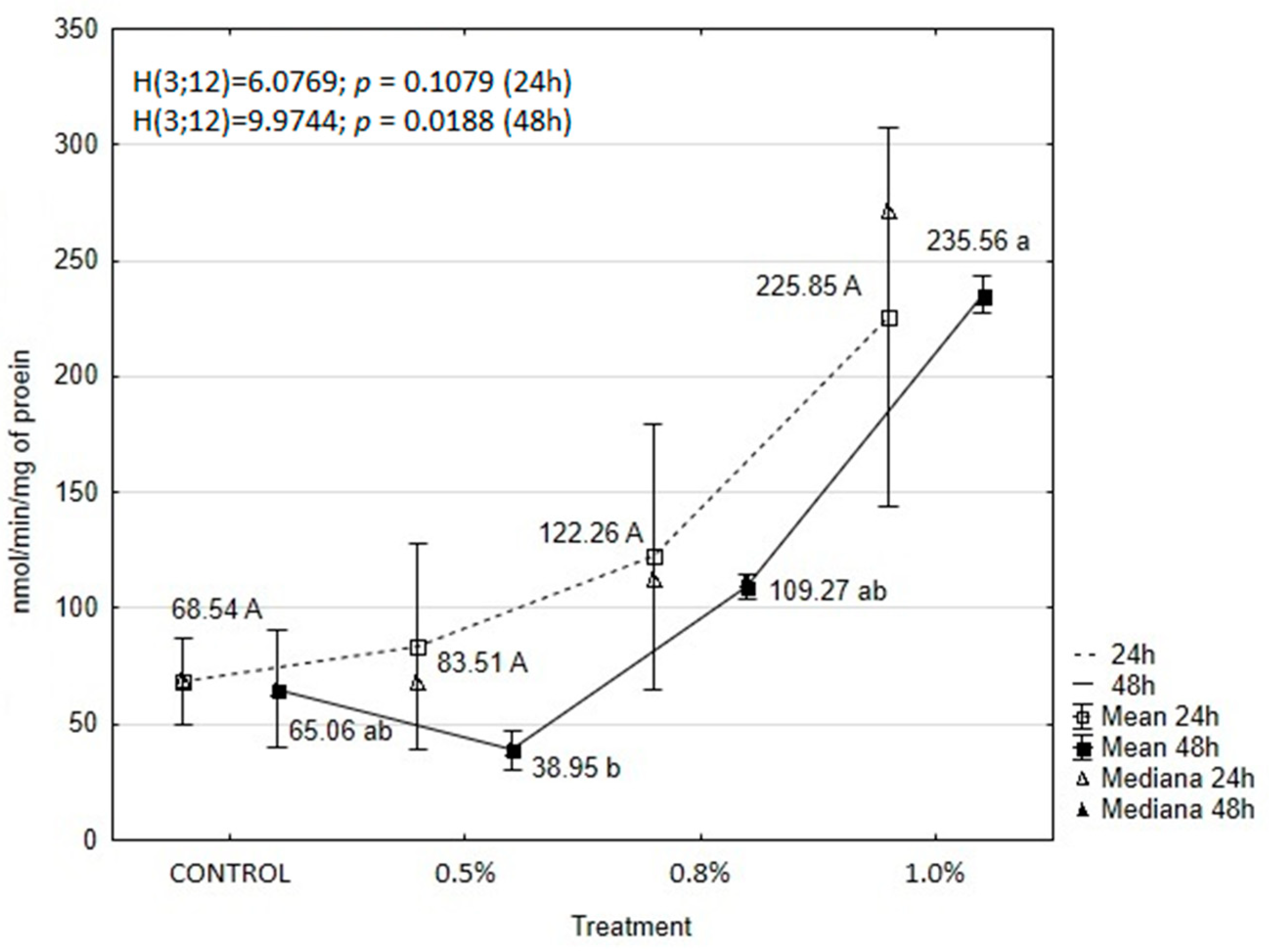
2.6. EOAM Effect on Larval Enzymes
3. Discussion
4. Materials and Methods
4.1. Insect Culture and Rearing Conditions
4.2. Plant Material and EOAM Extraction
4.3. Compound Identification and Estimation by Gas Chromatography–Mass Spectrophotometry
4.4. Laboratory Bioassays
4.4.1. Settling Inhibition Bioassays
4.4.2. EOAM Toxicity Test on Larvae
4.4.3. EOAM Influence on Demographic Parameters
4.5. EOAM Phytotoxicity Bioassay on A. melanocarpa
4.6. Enzymatic Analyses
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statistics Poland. Table 93. 2023. Available online: https://stat.gov.pl/en/topics/agriculture-forestry/agricultural-and-horticultural-crops/production-of-agricultural-and-horticultural-crops-in-2023,2,8.html (accessed on 23 July 2024).
- Wawer, I.; Egert, P.; Hołub, B. Aronia Superowoc; Wyd. Wektor: Warszawa, Poland, 2015; p. 221. [Google Scholar]
- Rusnak, J. Uprawa Aronii; Małopolski Ośrodek Doradztwa Rolniczego: Karnowice, Poland, 2013; p. 20. [Google Scholar]
- Górska-Drabik, E. Trachycera advenella (Zinck.) (Lepidoptera, Pyralidae)—A new pest on black chokeberry (Aronia melanocarpa). Prog. Plant Prot. 2009, 49, 531–534. [Google Scholar]
- Górska-Drabik, E. Occurrence of Acrobasis advenella (Zinck.) (Lepidoptera, Pyralidae, Phycitinae) on Black Chokeberry in Poland and Its Ciochemical Interaction with Host Plants; University of Life Science in Lublin: Lublin, Poland, 2013; Volume 382, p. 121. [Google Scholar]
- Slamka, F. Die Zünslerartigen (Pyraloidea) Mitteleuropas; Verlag nicht ermittelbar: Bratislava, Slovakia, 1997; p. 112. [Google Scholar]
- Górska-Drabik, E. Owady i roztocze zagrażające aronii czarnoowocowej. Insects and mites occurring in black chokeberry. In Proceedings of the 9th Fruit Conference ‘Trends in the Cultivation of Berry and Stone Fruit Species’, Kraśnik, Poland, 20–23 February 2013; pp. 30–32. [Google Scholar]
- Buler, Z.; Doruchowski, G.; Filipczak, J.; Głos, H.; Hołdaj, M.; Lisek, J.; Łabanowska, B.; Meszka, B.; Morgaś, H.; Poniatowska, A.; et al. Metodyka: Integrowana Produkcja Aronia; Instytut Ogrodnictwa: Skierniewice, Poland, 2016; p. 49. [Google Scholar]
- Chauhan, S.S.; Agrawal, S.; Srivastava, A. Effect of imidacloprid insecticide residue on biochemical parametersin potatoes and its estimation by HPLC. Asian J. Pharm. Clin. Res. 2013, 6, 114–117. [Google Scholar]
- Zhu, Q.; Yang, Y.; Zhong, Y.; Lao, Z.; O’Neill, P.; Hong, D.; Zhang, K.; Zhao, S. Synthesis, Insecticidal Activity, Resistance, Photodegradation and Toxicity of Pyrethroids (A Review). Chemosphere 2020, 254, 126779. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.F.; Miller, G.W.; Harvey, D.J.; Brander, S.M.; Geist, J.; Connon, R.E.; Lein, P.J. Bifenthrin causes transcriptomic alterations in mTOR and ryanodine receptor-dependent signaling and delayed hyperactivity in developing zebrafish (Danio rerio). Aquat. Toxicol. 2018, 200, 50–61. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, S.; Xiao, K.; Cai, M.; Liu, H. Occurrence, sources, and risk assessment of pyrethroid insecticides in surface water and tap water from Taihu Lake, China. J. Environ. Manag. 2023, 325, 116565. [Google Scholar] [CrossRef]
- Tang, T.; Wu, R.; Zhang, L.; Wang, Y.; Ling, J.; Du, W.; Shen, G.; Chen, Y.; Zhao, M. Distribution and partitioning of pyrethroid insecticides in agricultural lands: Critical influencing factors. Environ. Int. 2021, 156, 106736. [Google Scholar] [CrossRef] [PubMed]
- Broncel, M.; Koziróg, M.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Chojnowska-Jezierska, J. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med. Sci. Monit. 2010, 16, CR28–CR34. [Google Scholar]
- Ćujić, N.; Žugić, A.; Živković, J.; Zdunić, G. Preliminary safety estimate of cosmetic anti-age creams with chokeberry extract, using in vivo bioengineering techniques. Lek. Sirovine 2017, 37, 41–44. [Google Scholar] [CrossRef]
- Dey, D.; Gupta, M.K. Use of essential oils for insect pest management-a review. Innov. Farming. 2016, 1, 21–29. [Google Scholar]
- Chang, S.-T.; Chen, P.-F.; Chang, S.-C. Antibacterial Activity of Leaf Essential Oils and Their Constituents from Cinnamomum Osmophloeum. J. Ethnopharmacol. 2001, 77, 123–127. [Google Scholar] [CrossRef]
- Chen, F.; Chang, S.T.; Wu, H.H. Antimite activity of essential oils and their components from Cinnamomum osmophloeum. Quart. J. Chin. For. 2002, 35, 397–404. [Google Scholar]
- Cheng, S.; Chang, H.; Chang, S. Bioactivity of selected plant essential oils against the yellow fever mosquito Aedes aegypti larvae. Bioresour. Technol. 2003, 89, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Mssillou, I.; Agour, A.; Allali, A.; Saghrouchni, H.; Bourhia, M.; El Moussaoui, A.; Salamatullah, A.M.; Alzahrani, A.; Aboul-Soud, M.A.M.; Giesy, J.P.; et al. Antioxidant, Antimicrobial, and Insecticidal Properties of a Chemically Characterized Essential Oil from the Leaves of Dittrichia viscosa L. Molecules 2022, 27, 2282. [Google Scholar] [CrossRef]
- Benmeddour, T.; Messaoudi, K.; Flamini, G. First investigation of the chemical composition, antioxidant, antimicrobial and larvicidal activities of the essential oil of the subspecies Ononis angustissima Lam. subsp. filifolia Murb. Nat. Prod. Res. 2024, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C. The potential of botanical essential oils for insect pest control. Integr. Pest. Manag. Rev. 1997, 2, 25–34. [Google Scholar] [CrossRef]
- Sarwar, M.; Salman, M. Toxicity of oils formulation as a new useful tool in crop protection for insect pests control. Int. J. Chem. Biomol. Sci. 2015, 1, 297–302. [Google Scholar]
- Osman, S.E.I.; Swidan, M.H.; Kheirallah, D.A.; Nour, F.E. Histological effects of essential oils, their monoterpenoids and insect growth regulators on midgut, integument of larvae and ovaries of khapra beetle, Trogoderma granarium everts. J. Biol. Sci. 2016, 16, 93–101. [Google Scholar] [CrossRef]
- Ali, M.A.; Doaa, S.M.; El-Sayed, H.S.; Asmaa, M.E. Antifeedant activity and some biochemical effects of garlic and lemon essential oils on Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae). J. Entomol. Zool. 2017, 5, 1476–1482. [Google Scholar]
- Shaaya, E.; Rafaeli, A. Essential oils as biorational insecticides—Potency and mode of action. In Insecticide Design Using Advanced Technologies, 1st ed.; Ishaaya, I., Nauen, R., Horowitz, A.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 249–261. [Google Scholar]
- Koul, O.; Walia, S.; Dhaliwal, G.S. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Magierowicz, K.; Górska-Drabik, E.; Sempruch, C. The insecticidal activity of Satureja hortensis essential oil and its active ingredient carvacrol against Acrobasis advenella (Zinck.) (Lepidoptera, Pyralidae). Pestic. Biochem. Physiol. 2019, 153, 122–128. [Google Scholar] [CrossRef]
- Magierowicz, K.; Górska-Drabik, E.; Golan, K. Effects of plant extracts and essential oils on the behavior of Acrobasis advenella (Zinck.) caterpillars and females. J. Plant Dis. Prot. 2020, 127, 63–71. [Google Scholar] [CrossRef]
- Amini, S.; Khalaj, H.; Ahvazi, M.; Labbafi, M. Composition and biological effects of different populations of Cupressus to control adult weevil (Tribolium castaneum Herbst). J. Med. Plants 2023, 22, 113–126. [Google Scholar] [CrossRef]
- Zouirech, O.; El Moussaoui, A.; Saghrouchni, H.; Gaafar, A.R.Z.; Nafidi, H.A.; Bourhia, M.; Khallouki, F.; Lyoussi, B.; Derwich, E. Prefatory in silico studies and in vitro insecticidal effect of Nigella sativa (L.) essential oil and its active compound (carvacrol) against the Callosobruchus maculatus adults (Fab), a major pest of chickpea. Open Chem. 2023, 21, 20230133. [Google Scholar] [CrossRef]
- Mockute, D.; Judzentiene, A. Chemotypes of the essential oils of Achillea millefolium L. ssp. millefolium growing wild in Eastern Lithuania. Chemija 2002, 13, 168–173. [Google Scholar]
- Mazandarani, M.; Mirdeilami, S.Z.; Pessarakli, M. Essential oil composition and antibacterial activity of Achillea millefolium L. from different regions in North east of Iran. J. Med. Plant Res. 2013, 7, 1063–1069. [Google Scholar]
- Długokęcka, A. Zaprzyjaźnij się z ziołami. WMODR, Olsztyn, Poland. 2020, p. 17. Available online: https://wmodr.pl/files/bv1jQI81GI42ujDprjOGQmN6AQJ7FrzTGYW6tWPP.pdf (accessed on 20 July 2024).
- Perry, T.; Batterham, P.; Daborn, P.J. The biology of insecticidal activity and resistance. Insect Biochem. Mol. Biol. 2011, 41, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, M.; Wrzaszcz, W.; Sobierajewska, J.; Adamski, M. Development and Effects of Organic Farms in Poland, Taking into Account Their Location in Areas Facing Natural or Other Specific Constraints. Agriculture 2024, 14, 297. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef]
- Nemeth, E. Essential oil composition of species in the genus Achillea. J. Essent. Oil Res. 2005, 17, 501–512. [Google Scholar] [CrossRef]
- Nemeth, E.; Bernath, J. Biological activities of Yarrow Species (Achillea spp.). Curr. Pharm. Des. 2008, 14, 3151–3167. [Google Scholar] [CrossRef]
- Csupor-Löffler, B.; Hajdú, Z.; Zupkó, I.; Réthy, B.; Falkay, G.; Forgo, P.; Hohmann, J. Antiproliferative effect of flavonoids and sesquiterpenoids from Achillea millefolium s.l. on cultured human tumour cell lines. Phytother. Res. 2009, 23, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Barda, C.; Grafakou, M.-E.; Tomou, E.-M.; Skaltsa, H. Phytochemistry and evidence-based traditional uses of the genus Achillea L.: An Update (2011–2021). Sci. Pharm. 2021, 89, 50. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Chrzanowski, G.; Sprawka, I.; Sytykiewicz, H. Aphicidal activity of selected Asteraceae essential oils and their effect on enzyme activities of the green peach aphid, Myzus persicae (Sulzer). Pestic. Biochem. Phys. 2018, 145, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Nenaah, G.E. Chemical composition, toxicity and growth inhibitory activities of essential oils of three Achillea species and their nano-emulsions against Tribolium castaneum (Herbst). Ind. Crop. Prod. 2014, 53, 252–260. [Google Scholar] [CrossRef]
- Tampe, J.; Parra, L.; Huaiquil, K.; Mutis, A.; Quiroz, A. Repellent effect and metabolite volatile profile of the essential oil of Achillea millefolium against Aegorhinus nodipennis (Hope) (Coleoptera: Curculionide). Neotrop. Entomol. 2015, 44, 279–285. [Google Scholar] [CrossRef]
- Karahroodi, Z.R.; Moharramipour, S.; Rahbarpour, A. Investigated repellency effect of some essential oils of 17 native medicinal plants on adults Plodia interpunctella. Am.-Eurasian. J. Sustain. Agric. 2009, 3, 181–184. [Google Scholar]
- Naghizadeh, S.; Rafiee-Dastjerdi, H.; Golizadeh, A.; Esmaielpour, B.; Mahdavi, V. The effect of essential oils of Artemisia absinthum L., Achillea millefolium L. and Artemisia dracunculus L. against potato tuber moth, Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae). Jordan J. Agric. Sci. 2016, 12, 1115–1123. [Google Scholar] [CrossRef]
- Halbert, S.E.; Corsini, D.; Wiebe, M.; Vaughn, S.F. Plant-derived compounds and extracts with potential as aphid repellents. Ann. Appl. Biol. 2009, 154, 303–307. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Saber, M.; Bagheri, M.; Mahdavinia, G.R. Achillea millefolium essential oil and chitosan nanocapsules with enhanced activity against Tetranychus urticae. J. Pest. Sci. 2018, 91, 837–848. [Google Scholar] [CrossRef]
- Cetin, H.; Erler, F.; Yanikoglu, A. A comparative evaluation of Origanum onites essential oil and its four major components as larvicides against the pine processionary moth, Thaumetopoea wilkinsoni. Pest. Manag. Sci. 2007, 63, 830–833. [Google Scholar] [CrossRef]
- Akhtar, Y.; Pages, E.; Stevens, A.; Bradbury, R.; da Camara, C.A.G.; Isman, M.B. Effect of chemical complexity of essential oils onfeeding deterrence in larvae of the cabbage looper. Physiol. Entomol. 2012, 37, 81–89. [Google Scholar] [CrossRef]
- Felton, G.W.; Duffey, S.S. Protective action of midgut catalase in lepidopteran larvae against oxidative plant defenses. J. Chem. Ecol. 1991, 17, 1715–1732. [Google Scholar] [CrossRef]
- Kolawole, A.O.; Olajuyigbe, F.M.; Ajele, J.O.; Adedire, C.O. Activity of the antioxidant defense system in a typical bioinsecticide- and synthetic insecticide-treated bowpea storage beetle Callosobrochus maculatus F. (Coleoptera: Chrysomelidae). Int. J. Insect Sci. 2014, 6, IJIS-S19434. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Mardani-Talaee, M.; Rahimi, V.; Zibaee, A. Effects of host plants on digestive enzymatic activities and some components involved in intermediary metabolism of Chrysodeixis chalcites (Lepidoptera: Noctuidae). J. Entomol. Acarol. Res. 2014, 46, 96–101. [Google Scholar] [CrossRef]
- Popescu, I.E.; Gostin, I.N.; Blidar, C.F. An overview of the mechanisms of action and administration technologies of the essential oils used as green insecticides. Agri. Eng. 2024, 6, 1195–1217. [Google Scholar] [CrossRef]
- Magierowicz, K.; Górska-Drabik, E.; Sempruch, C. The effect of Tanacetum vulgare essential oil and its main components on some ecological and physiological parameters of Acrobasis advenella (Zinck.) (Lepidoptera: Pyralidae). Phestic. Biochem. Phys. 2020, 162, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Orav, A.; Arak, E.; Raal, A. Phytochemical analysis of the essential oil of Achillea millefolium L. from various European Countries. Nat. Prod. Res. 2006, 20, 1082–1088. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Kowalczyk, A. Chemical composition of the essential oils of Achillea millefolium L. isolated by different distillation methods. J. Essent. Oil Res. 2009, 21, 108–111. [Google Scholar] [CrossRef]
- Farajpour, M.; Ebrahimi, M.; Baghizadeh, A.; Aalifar, M. Phytochemical and yield variation among iranian Achillea millefolium accessions. Hort. Sci. 2017, 52, 827–830. [Google Scholar] [CrossRef]
- Aziz, E.E.; Badawy, E.M.; Zheljazkov, V.D.; Nicola, S.M.; Fouad, H. Yield and chemical composition of essential oil of Achillea millefolium L. as affected by harvest time. Egypt. J. Chem. 2019, 62, 533–540. [Google Scholar] [CrossRef]
- Judzentiene, A.; Mockute, D. Essential oil composition of two yarrow taxonomic forms. Cent. Europ. J. of Biol. 2010, 5, 346–352. [Google Scholar] [CrossRef]
- Naghizadeh, S.; Rafiee-Dastjerdi, H.; Naseri, B.; Golizadeh, A.; Esmaielpour, B. Insecticidal activity of essential oils from Artemisia absinthium L., Artemisia dracunculus L. and Achillea millefolium L. against Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae). J. Crop Prot. 2019, 8, 479–489. [Google Scholar]
- Zawiślak, G.; Nurzyńska-Wierdak, R. Rozwój roślin oraz skład chemiczny olejku eterycznego krwawnika pospolitego (Achillea millefolium L.) uprawianego w warunkach klimatu umiarkowanego. Ann. Hortic. 2017, 27, 31–38. [Google Scholar] [CrossRef]
- Bączek, K.; Kosakowska, O.; Przybył, L.J.; Kuźma, P.; Ejdys, M.; Obiedziński, M.; Węglarz, Z. Intraspecific variability of yarrow (Achillea millefolium L. s.l.) in respect of developmental and chemical traits. Herba Pol. 2015, 61, 37–52. [Google Scholar] [CrossRef]
- Rohloff, J.; Skagen, E.B.; Steen, A.H.; Iversen, T.H. Production of yarrow (Achillea millefolium L.) in Norway: Essential oil content and quality. J. Agric. Food Chem. 2000, 48, 6205–6209. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.; Barroso, J.G.; Pais, M.S.S.; Scheffer, J.J.C. Composition of the essential oils from leaves and flowers of Achillea millefolium L. ssp. millefolium. Flavour. Fragr. J. 1992, 7, 219–222. [Google Scholar] [CrossRef]
- Orav, A.; Kailas, T.; Ivask, K. Composition of the essential oil from Achillea millefolium L. from Estonia. J. Essent. Oil Res. 2001, 13, 290–294. [Google Scholar] [CrossRef]
- Mockute, D.; Judzentiene, A. Variability of the essential oils composition of Achillea millefolium L. ssp. millefolium growing wild in Lithuania. J. Biochem. Syst. Ecol. 2003, 31, 1033–1045. [Google Scholar] [CrossRef]
- Conti, B.; Canale, A.; Bertoli, A.; Gozzini, F.; Pistelli, L. Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2010, 107, 1455–1461. [Google Scholar] [CrossRef]
- Jaimand, K.; Rezaee, M.B. Investigation on the chemical constituents of essential oils from Achillea millefolium L. subsp. millefolium by distillation methods. Iranian J. Med. Aromat. Plant Res. 2004, 20, 181–190. [Google Scholar]
- Jaimand, K.; Rezaee, M.B.; Mozaffarian, V. Chemical constituents of the leaf and flower oils from Achillea millefolium ssp. elbursensis Hub.-Mor. from Iran rich in chamazulene. J. Essent. Oil Res. 2006, 18, 293–295. [Google Scholar] [CrossRef]
- Hwang, Y.-S.; Wu, K.-H.; Kumamoto, J.; Axelrod, H.; Mulla, M.S. Isolation and identification of mosquito repellents in Artemisia vulgaris. J. Chem. Ecol. 1985, 11, 1297–1306. [Google Scholar] [CrossRef]
- Rojht, H.; Meško, A.; Vidrih, M.; Trdan, S. Insecticidal activity of four different substances against larvae and adults of sycamore lace bug (Corythucha ciliata Say, Heteroptera, Tingidae). Acta Agric. Slov. 2009, 93, 31–36. [Google Scholar] [CrossRef]
- Szołyga, B.; Gniłka, R.; Szczepanik, M.; Szumny, A. Chemical composition and insecticidal activity of Thuja occidentalis and Tanacetum vulgare essential oils against larvae of the lesser mealworm, Alphitobius diaperinus. Entomol. Exp. Appl. 2014, 151, 1–10. [Google Scholar] [CrossRef]
- Krzyżanowski, R. Wpływ lotnych związków na zachowanie mszyc związane z żerowaniem. Kosmos 2017, 66, 413–420. [Google Scholar]
- Ebadollahi, A.; Ashouri, S. Toxicity of essential oils isolated from Achillea millefolium L., Artemisia dracunculus L. and Heracleum persicum Desf. against adults of Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) in Islamic Republic of Iran. Ecol. Balk. 2011, 3, 41–48. [Google Scholar]
- Stock, D.; Holloway, P.J. Possible mechanisms for surfactant-induced foliar uptake of agrochemicals. Pestic. Sci. 1993, 38, 165–177. [Google Scholar] [CrossRef]
- Karamaouna, F.; Kimbaris, A.; Michaelakis, A.; Papachristos, D.; Polissiou, M.; Papatsakona, P.; Tsora, E. Insecticidal activity of plant essential oils against the vine mealybug, Planococcus ficus. J. Insect Sci. 2013, 13, 142. [Google Scholar] [CrossRef]
- Fournier, D.; Bride, J.M.; Hoffmann, F.; Karch, F. Acetylcholiesterase. Two types of modifications confer resistance to insecticide. J. Biol. Chem. 1992, 267, 14270–14274. [Google Scholar] [CrossRef]
- Khanikor, B.; Parida, P.; Yadav, R.N.S.; Bora, D. Comparative mode of action of some terpene compounds agains octopamine receptor and acetyl cholinesterase of mosquito and human system by the help of homology modelling and docking studies. J. Appl. Pharm. Sci. 2013, 3, 6–12. [Google Scholar] [CrossRef]
- Seo, S.M.; Kim, J.; Kang, J.; Koh, S.A.; Ahn, Y.J.; Park, I.K. Fumigant toxicity and acetylcholinesterase inhibitory activity of 4 Asteraceae plant essential oils and their constituents against Japanese termite (Reticulitermes speratus Kolbe). Pestic. Biochem. Physiol. 2014, 113, 55–61. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Sytykiewicz, H.; Chrzanowski, G. The Effect of Essential Oils from Asteraceae Plants on Behavior and Selected Physiological Parameters of the Bird Cherry-Oat Aphid. Molecules 2024, 29, 1673. [Google Scholar] [CrossRef] [PubMed]
- Górska-Drabik, E.; Golan, K.; Kot, I.; Kmieć, K.; Poniewozik, M.; Dzida, K.; Bochniak, A. The Effect of pre-harvest treatments with Tanacetum vulgare L. and Satureja montana L. essential oils (EOs) on the yield and chemical composition of Aronia melanocarpa (Michx.) Elliot fruit. Agriculture 2024, 14, 12. [Google Scholar] [CrossRef]
- Angourani, H.R.; Zarei, A.; Moghadam, M.M.; Ramazani, A.; Mastinu, A. Investigation on the Essential Oils of the Achillea Species: From Chemical Analysis to the In Silico Uptake against SARS-CoV-2 Main Protease. Life 2023, 13, 378. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, R.; Wang, Q.; Xu, B. Programmed-temperature gaschromatographic retention index. J. Chromatogr. 1993, 657, 1–15. [Google Scholar] [CrossRef]
- Babushok, V.I.; Zenkevich, I.G. Retention Indices for Most Frequently Reported Essential Oil Compounds in GC. Chromatographia 2009, 69, 257–269. [Google Scholar] [CrossRef]
- Gutiérrez, C.; Fereres, A.; Reina, M.; Cabrera, R.; Gonzáles-Coloma, A. Behavioral and sublethal effects of structurally related lower terpenes on Myzus persicae, J. Chem.Ecol. 1997, 23, 1641–1650. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Aebi, H. Catalase In Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Ferman, H.; Dimond, A.E. Peroxidase activity and phytophthora resistance in different organs of the potato plant. Phytopathology 1967, 57, 69–72. [Google Scholar]
- Miles, P.W. Studies on the salivary physiology of plant bugs: Oxidase activity in the salivary apparatus and saliva. J. Insect Physiol. 1964, 10, 121–129. [Google Scholar] [CrossRef]
- Laurema, S.; Varis, A.L.; Miettinen, H. Studies on enzymes in the salivary glands of Lygus rugulipennis (Hemiptera, Miridae). Insect Biochem. 1985, 15, 211–224. [Google Scholar] [CrossRef]
- Katagiri, C. α-d-Glucosidase in the serum of the american cockroach, Periplaneta americana. Insect Biochem. 1979, 9, 199–204. [Google Scholar] [CrossRef]
- Chararas, C.; Chipoulet, J.M. Purification by chromatography and properties of a β-glucosidase from the larvae of Phoracantha semipunctata. Comp. Biochem. Physiol. Part B 1982, 72, 559–564. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Lowry, J.O.H.; Rosebrough, N.J.; Farr, A.L.; Randal, R.J. Protein measurement with the Folin phenol reagent. J. Biol.Chem. 1951, 193, 256–277. [Google Scholar] [CrossRef]



| No. | Compound | Retention Time [min] | CAS no. | Retention Index | Quantity [%] |
|---|---|---|---|---|---|
| 1. | α-Thujene | 4.971 | 2867-05-2 | 928 | 0.06 |
| 2. | α-Pinene | 5.213 | 7785-70-8 | 934 | 1.87 |
| 3. | Camphene | 5.507 | 79-92-5 | 948 | 0.33 |
| 4. | Sabinen | 6.065 | 3387-41-5 | 974 | 2.28 |
| 5. | β-Pinene | 6.177 | 127-91-3 | 979 | 12.84 |
| 6. | β-Myrcene | 6.700 | 123-35-3 | 994 | 0.22 |
| 7. | 2-Carene | 7.291 | 4497-92-1 | 1017 | 0.16 |
| 8. | β-Cymene | 7.485 | 535-77-3 | 1026 | 0.54 |
| 9. | Eucalyptol | 7.593 | 470-82-6 | 1031 | 9.15 |
| 10. | γ-Terpinene | 8.389 | 99-85-4 | 1059 | 1.29 |
| 11. | Linalool | 9.217 | 78-70-6 | 1102 | 0.40 |
| 12. | α-Campholenal | 9.668 | 4501-58-0 | 1130 | 0.30 |
| 13. | trans-Pinocarveol | 10.535 | 1674-08-4 | 1141 | 0.21 |
| 14. | Camphor | 10.632 | 76-22-2 | 1146 | 2.74 |
| 15. | Menthone | 10.989 | 14073-97-3 | 1154 | 0.34 |
| 16. | Isoborneol | 11.188 | 124-76-5 | 1156 | 0.44 |
| 17. | Pinocarvone | 11.288 | 30460-92-5 | 1165 | 2.88 |
| 18. | Terpinen-4-ol | 11.624 | 20126-76-5 | 1179 | 2.35 |
| 19. | α-Terpineol | 12.020 | 10482-56-1 | 1194 | 2.39 |
| 20. | trans-p-Menth-1-en-3-ol | 12.621 | 16721-39-4 | 1214 | 0.08 |
| 21. | Piperitone | 13.776 | 89-81-6 | 1259 | 0.20 |
| 22. | 4-Thujen-2-α-yl acetate | 14.370 | 95875-05-1 | 1274 | 0.08 |
| 23. | Bornyl acetate | 14.572 | 5655-61-8 | 1288 | 1.45 |
| 24. | Lavandulyl acetate | 14.869 | 25905-14-0 | 1292 | 0.12 |
| 25. | Thymol | 15.064 | 89-83-8 | 1299 | 0.06 |
| 26. | Eugenol | 15.252 | 97-53-0 | 1363 | 0.20 |
| 27. | Capric acid methyl ester | 15.777 | 110-42-9 | 1371 | 0.08 |
| 28. | α-Ylangene | 16.283 | 14912-44-8 | 1374 | 0.14 |
| 29. | Copaene | 16.930 | 3856-25-5 | 1377 | 0.31 |
| 30. | β-Bourbonene | 17.147 | 5208-59-3 | 1385 | 0.31 |
| 31. | β-Longipinene | 17.363 | 39703-25-8 | 1415 | 0.38 |
| 32. | β-Caryophyllene | 18.015 | 87-44-5 | 1422 | 7.26 |
| 33. | β-Copaene | 18.249 | 18252-44-3 | 1431 | 1.02 |
| 34. | Isogermacrene D | 18.633 | 317819-80-0 | 1483 | 0.45 |
| 35. | γ-Humulene | 18.837 | 6753-98-6 | 1484 | 1.39 |
| 36. | Isocaryophillene | 19.028 | 118-65-0 | 1485 | 0.31 |
| 37. | γ-Amorphene | 19.436 | 6980-46-7 | 1486 | 0.70 |
| 38. | γ-Curcumene | 19.541 | 28976-68-3 | 1487 | 0.58 |
| 39. | Ar-Curcumene | 19.625 | 644-30-4 | 1489 | 0.85 |
| 40. | epi-Cubebol | 19.925 | 38230-60-3 | 1498 | 0.71 |
| 41. | Guaia-6,9-diene | 20.016 | 36577-33-0 | 1442 | 0.29 |
| 42. | α-Muurolene | 20.333 | 10208-80-7 | 1503 | 0.71 |
| 43. | Δ-Cadinene | 20.561 | 483-76-1 | 1527 | 1.42 |
| 44. | Alloaromadendrene oxide-(2) | 20.885 | 85710-39-0 | 1536 | 0.23 |
| 45. | α-Calacorene | 21.013 | 21391-99-1 | 1548 | 0.12 |
| 46. | Unknown | 21.223 | − | 1554 | 0.28 |
| 47. | Unknown | 21.304 | − | 1562 | 0.16 |
| 48. | Nerolidol | 21.570 | 2306-78-7 | 1567 | 3.79 |
| 49. | Spathulenol | 21.821 | 77171-55-2 | 1584 | 1.23 |
| 50. | Caryophyllene oxide | 21.920 | 1139-30-6 | 1589 | 4.47 |
| 51. | Viridiflorol | 22.140 | 552-02-3 | 1598 | 1.54 |
| 52. | Humulene epoxide II | 22.426 | 19888-34-7 | 1615 | 0.76 |
| 53. | γ-Eudesmol | 22.517 | 473-15-4 | 1629 | 0.40 |
| 54. | Unknown | 22.617 | − | 1634 | 0.40 |
| 55. | Unknown | 22.991 | − | 1640 | 0.72 |
| 56. | Caryophylla-4(12),8(13)-dien-5α-ol | 23.150 | 19431-79-9 | 1643 | 0.73 |
| 57. | τ-Cadinol | 23.259 | 5937-11-1 | 1647 | 0.66 |
| 58. | α-Cadinol | 23.554 | 481-34-5 | 1662 | 2.23 |
| 59. | Unknown | 23.944 | − | 1679 | 1.68 |
| 60. | Aromadendrane-4,10-diol | 24.120 | 70051-38-6 | 1691 | 0.27 |
| 61. | Eudesma-4(15),7-dien-1β-ol | 24.255 | 119120-23-9 | 1711 | 1.32 |
| 62. | Unknown | 24.959 | − | 1736 | 0.30 |
| 63. | Chamazulene | 25.205 | 529-05-5 | 1741 | 9.05 |
| 64. | Bisabolone | 25.578 | 72441-71-5 | 1742 | 0.48 |
| 65. | α-Springene | 25.909 | 77898-97-6 | 1781 | 0.32 |
| 66. | Hexahydrofarnesyl acetone | 27.614 | 502-69-2 | 1830 | 1.58 |
| 67. | Hexadecanoic acid, methyl ester | 29.229 | 112-39-0 | 1927 | 1.97 |
| 68. | Phytol | 30.336 | 150-86-7 | 1951 | 0.22 |
| 69. | Unknown | 32.342 | − | 2057 | 1.82 |
| 70. | 9,12-Octadecadienoic acid, ethyl ester | 32.342 | 112-63-0 | 2078 | 1.25 |
| 71. | 18-Octadec-9-enolide | 32.697 | − | 2099 | 0.56 |
| 72. | Linolenic acid methyl ester | 33.148 | 463-40-1 | 2105 | 0.49 |
| 73. | Unknown | 33.665 | − | 2125 | 0.88 |
| 74. | Unknown | 34.609 | − | 2159 | 0.20 |
| Oil yield (%) | 0.31 | ||||
| Concentration (%) | No. of Larvae Per Inflorescence ± SD | p | SI (%) | |
|---|---|---|---|---|
| Treated | Control | |||
| 0.5 | 6.3 ± 0.577 | 5.6 ± 0.577 | 0.23 | 37.5 |
| 0.8 | 4.0 ± 1.0 | 7.33 ± 1.155 | 0.0194 | 45.3 |
| 1.0 | 4.33 ± 0.577 | 7.00 ± 1.00 | 0.016 | 38.6 |
| Exposure Time (In Hours) | LC50 a | 95% CL b | Regression Curve ± SE | χ2 | p |
|---|---|---|---|---|---|
| Slope | |||||
| 24 | 1.85 | 1.38–3.70 | 0.923 ± 0.288 | 19.11 | 0.385 |
| 48 | 1.22 | 1.07–1.49 | 1.507 ± 0.256 | 12.29 | 0.832 |
| 72 | 0.99 | 0.88–1.15 | 1.542 ± 0.222 | 10.74 | 0.905 |
| EOAM Concentration (%) | Pupa | Adults | Female Fertility | |||||
|---|---|---|---|---|---|---|---|---|
| Pupation (%) ± SD | Emergence (%) ± SD | Longevity (Days) | ||||||
| Min. | Max. | Mean ± SD | Min. | Max. | Mean ± SD | |||
| 0.5 | 35.56 ± 3.85 b | 75.57 ± 21.42 a | 5 | 11 | 8.20 ± 1.620 a | 87 | 149 | 118.30 ± 20.47 a |
| 0.8 | 22.22 ± 13.88 b | 86.67 ± 23.09 a | 5 | 10 | 7.50 ± 1.65 a | 65 | 156 | 115.60 ± 27.35 a |
| 1.0 | 15.55 ± 3.85 b | 88.90 ± 19.23 a | 5 | 11 | 9.00 ± 1.41 a | 16 | 153 | 107.10 ± 44.38 a |
| Control | 93.33± 6.67 a | 100.00 ± 0 a | 5 | 12 | 8.00 ± 1.83 a | 107 | 150 | 128.20 ± 13.55 a |
| F(1,3) = 56.5156 p = 0.000010 | F(1,3) = 0.8841 p = 0.489306 | F(1,3) = 0.4462 p = 0.721488 | F(1,3) = 0.9196 p = 0.441190 | |||||
| Exposure Time (In Hours) | Concentration of EOAM (% w/v) | ||
|---|---|---|---|
| 0.5 | 0.8 | 1.0 | |
| 48 | − | − | − |
| 72 | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górska-Drabik, E.; Golan, K.; Sempruch, C.; Chrzanowski, G.; Dybowski, M.P.; Poniewozik, M. Chemical Composition and Toxicity of Achillea millefolium L. Essential Oil Against Acrobasis advenella (Lepidoptera, Pyralidae) Under Laboratory Conditions. Molecules 2025, 30, 1927. https://doi.org/10.3390/molecules30091927
Górska-Drabik E, Golan K, Sempruch C, Chrzanowski G, Dybowski MP, Poniewozik M. Chemical Composition and Toxicity of Achillea millefolium L. Essential Oil Against Acrobasis advenella (Lepidoptera, Pyralidae) Under Laboratory Conditions. Molecules. 2025; 30(9):1927. https://doi.org/10.3390/molecules30091927
Chicago/Turabian StyleGórska-Drabik, Edyta, Katarzyna Golan, Cezary Sempruch, Grzegorz Chrzanowski, Michał P. Dybowski, and Monika Poniewozik. 2025. "Chemical Composition and Toxicity of Achillea millefolium L. Essential Oil Against Acrobasis advenella (Lepidoptera, Pyralidae) Under Laboratory Conditions" Molecules 30, no. 9: 1927. https://doi.org/10.3390/molecules30091927
APA StyleGórska-Drabik, E., Golan, K., Sempruch, C., Chrzanowski, G., Dybowski, M. P., & Poniewozik, M. (2025). Chemical Composition and Toxicity of Achillea millefolium L. Essential Oil Against Acrobasis advenella (Lepidoptera, Pyralidae) Under Laboratory Conditions. Molecules, 30(9), 1927. https://doi.org/10.3390/molecules30091927








