A Green Synthesis of Controllable Shear-Assisted Catalytically Graphitized Biomass-Derived Carbon and Its Multi-Scale Reinforcement Mechanism in Natural Rubber
Abstract
1. Introduction
2. Results
2.1. Physicochemical Characterization of Fillers
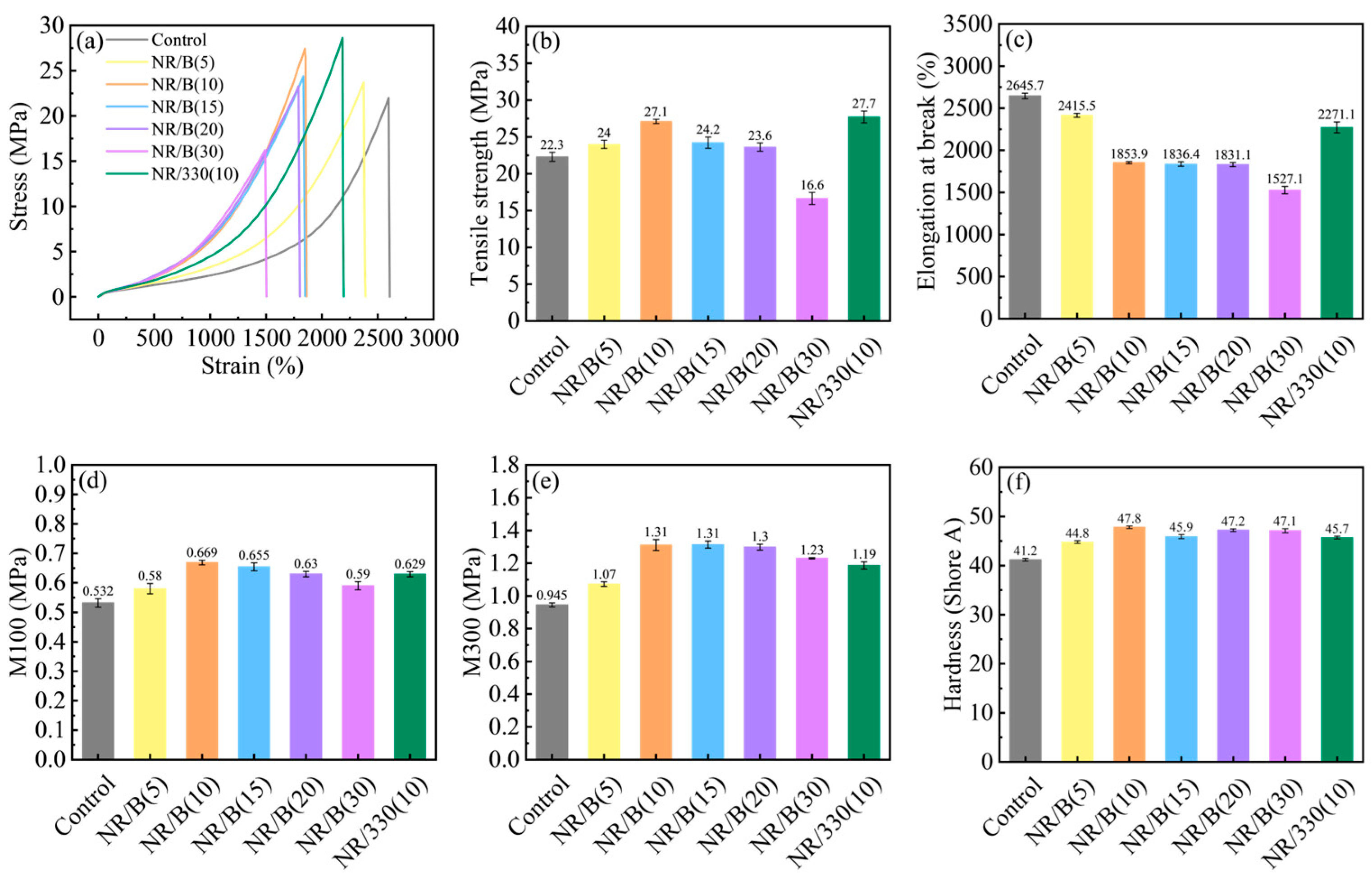
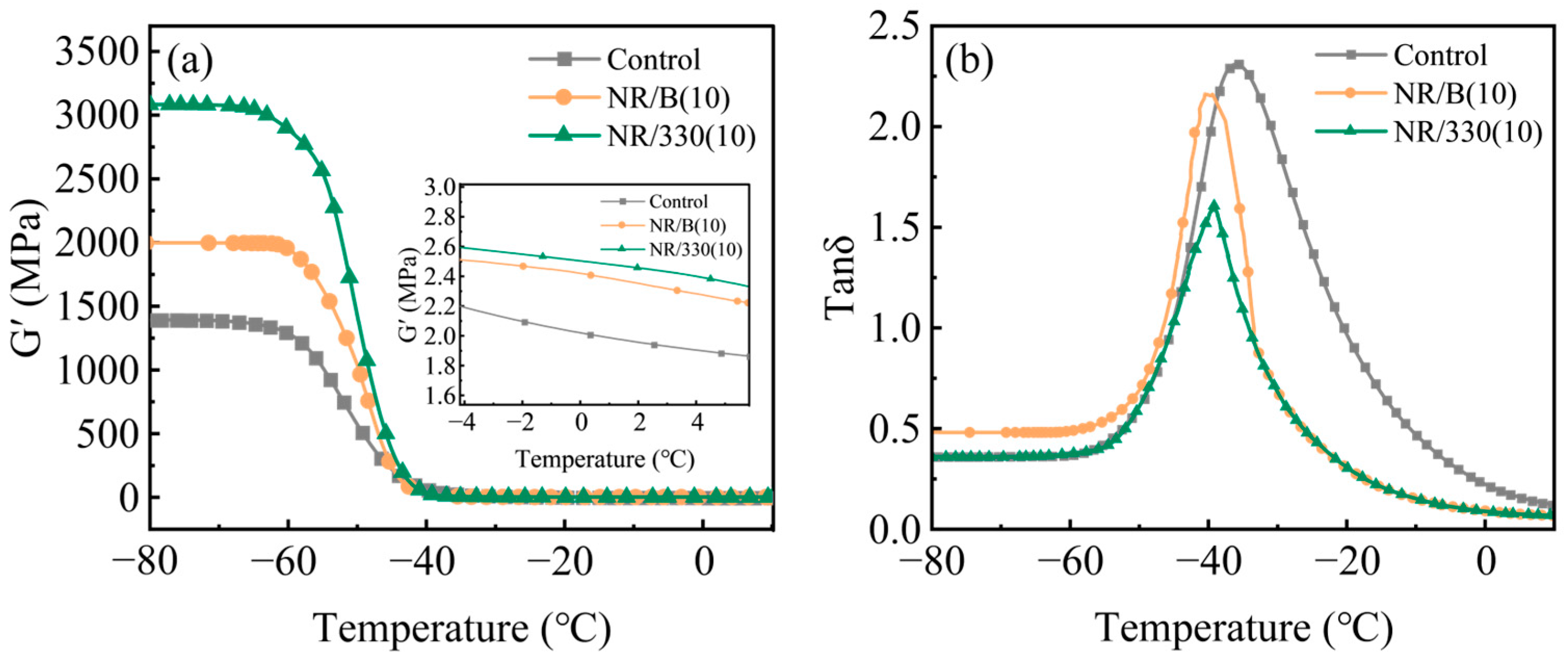
2.2. Properties of Rubber Composites
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Preparation of Ball-Milled Walnut Shell Carbon
4.3. Preparation of NR/WSB Vulcanizates
4.4. Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Hartomy, O.A.; Al-Solamy, F.; Al-Ghamdi, A.; Dishovsky, N.; Ivanov, M.; Mihaylov, M.; El-Tantawy, F. Influence of Carbon Black Structure and Specific Surface Area on the Mechanical and Dielectric Properties of Filled Rubber Composites. Int. J. Polym. Sci. 2011, 64, 33–39. [Google Scholar] [CrossRef]
- Ismail, H.; Omar, N.F.; Othman, N. Effect of carbon black loading on curing characteristics and mechanical properties of waste tyre dust/carbon black hybrid filler filled natural rubber compounds. J. Appl. Polym. Sci. 2011, 121, 1143–1150. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, P.; Zhang, Q.; Chen, B.; Zhu, Z. Study on the Properties of Rubber with Different Contents of Carbon Black. IOP Conf. Ser. Mater. Sci. Eng. 2019, 677, 022043. [Google Scholar] [CrossRef]
- Kang, H.; Tang, Y.; Yao, L.; Yang, F.; Fang, Q.; Hui, D. Fabrication of graphene/natural rubber nanocomposites with high dynamic properties through convenient mechanical mixing. Compos. Part B Eng. 2017, 112, 1–7. [Google Scholar] [CrossRef]
- Savetlana, S.; Sukmana, I.; Saputra, F.A. The effect of carbon black loading and structure on tensile property of natural rubber composite. IOP Conf. Ser. Mater. Sci. Eng. 2017, 223, 012009. [Google Scholar] [CrossRef]
- Abdelsalam, A.A.; Araby, S.; El-Sabbagh, S.H.; Abdelmoneim, A.; Hassan, M.A. Effect of carbon black loading on mechanical and rheological properties of natural rubber/styrene-butadiene rubber/nitrile butadiene rubber blends. J. Thermoplast. Compos. Mater. 2021, 34, 490–507. [Google Scholar] [CrossRef]
- Chang, B.P.; Gupta, A.; Muthuraj, R.; Mekonnen, T.H. Bioresourced fillers for rubber composite sustainability: Current development and future opportunities. Green Chem. 2021, 23, 5337–5378. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Greenough, S.; Dumont, M.J.; Prasher, S. The physicochemical properties of biochar and its applicability as a filler in rubber composites: A review. Mater. Today Commun. 2021, 29, 102912. [Google Scholar] [CrossRef]
- Bélanger, N.; Prasher, S.; Dumont, M.J. Tailoring biochar production for use as a reinforcing bio-based filler in rubber composites: A review. Polym. Plast. Technol. Mater. 2023, 62, 54–75. [Google Scholar] [CrossRef]
- Jong, L.; Peterson, S.C.; Jackson, M.A. Utilization of Porous Carbons Derived from Coconut Shell and Wood in Natural Rubber. J. Polym. Environ. 2014, 22, 289–297. [Google Scholar] [CrossRef]
- Lay, M.; Rusli, A.; Abdullah, M.K.; Hamid, Z.A.A.; Shuib, R.K. Converting dead leaf biomass into activated carbon as a potential replacement for carbon black filler in rubber composites. Compos. Part B Eng. 2020, 201, 108366. [Google Scholar] [CrossRef]
- Zainal Abidin, Z.; Mamauod, S.N.L.; Romli, A.Z.; Sarkawi, S.S.; Zainal, N.H. Synergistic effect of partial replacement of carbon black by palm kernel shell biochar in carboxylated nitrile butadiene rubber composites. Polymers 2023, 15, 943. [Google Scholar] [CrossRef]
- Mago, J.; Negi, A.; Pant, K.K.; Fatima, S. Development of natural rubber-bamboo biochar composites for vibration and noise control applications. J. Clean. Prod. 2022, 373, 133760. [Google Scholar]
- Dai, A.; Wu, Q.; Xu, C.; Xiong, J.; Fan, L.; Ke, L.; Zeng, Y.; Cobb, K.; Ruan, R.; Wang, Y. Walnut shell oil-bath torrefaction coupled with fast pyrolysis: Effect of torrefaction heating modes. Bioresource Technol. 2024, 406, 130984. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wei, Y.; Li, B.; Wang, H. Cleaner recycling of iron from waste copper slag by using walnut shell char as green reductant. J. Clean. Prod. 2019, 217, 423–431. [Google Scholar] [CrossRef]
- Chundawat, N.S.; Parmar, B.S.; Deuri, A.S.; Vaidya, D.; Sepehr, K.S.; Chauhan, N.P.S. Walnut shell ash as a sustainable material for compounding with bromobutyl rubber for tire inner liner applications. Polym. Compos. 2020, 41, 5317–5330. [Google Scholar] [CrossRef]
- Bokobza, L. Natural rubber nanocomposites: A review. Nanomaterials 2018, 9, 12. [Google Scholar] [CrossRef]
- Gusiatin, M.Z.; Rouhani, A. Application of selected methods to modify pyrolyzed biochar for the immobilization of metals in soil: A review. Materials 2023, 16, 7342. [Google Scholar] [CrossRef]
- Lyu, H.; Yu, Z.; Gao, B.; He, F.; Huang, J.; Tang, J.; Shen, B. Ball-milled biochar for alternative carbon electrode. Environ. Sci. Pollut. Res. 2019, 26, 14693–14702. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, N.; Hu, X. Effects of wet and dry ball milling on the physicochemical properties of sawdust derived-biochar. Instrum. Sci. Technol. 2020, 48, 287–300. [Google Scholar] [CrossRef]
- Wang, J.; Tan, Y.; Yang, H.; Zhan, L.; Sun, G.; Luo, L. On the adsorption characteristics and mechanism of methylene blue by ball mill modified biochar. Sci. Rep. 2023, 13, 21174. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Wang, X.; Sui, J.; Xu, D.; Zhu, Y.; Liu, X. A facile ball milling method to produce sustainable pyrolytic rice husk bio-filler for reinforcement of rubber mechanical property. Ind. Crops Prod. 2019, 141, 111791. [Google Scholar] [CrossRef]
- Jiang, C.; Bo, J.; Xiao, X.; Zhang, S.; Wang, Z.; Yan, G.; Wu, Y.; Wong, C.; He, H. Converting waste lignin into nano-biochar as a renewable substitute of carbon black for reinforcing styrene-butadiene rubber. Waste Manag. 2020, 102, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Rouissi, T.; Verma, M.; Surampalli, R.Y.; Valero, J.R. A green method for production of nanobiochar by ball milling- optimization and characterization. J. Clean. Prod. 2017, 164, 1394–1405. [Google Scholar] [CrossRef]
- Jeon, I.Y.; Bae, S.Y.; Seo, J.M.; Baek, J.B. Scalable Production of Edge-Functionalized Graphene Nanoplatelets via Mechanochemical Ball-Milling. Adv. Funct. Mater. 2015, 25, 6961–6975. [Google Scholar] [CrossRef]
- Barrera, C.S.; Cornish, K. Processing and mechanical properties of natural rubber/waste-derived nano filler composites compared to macro and micro filler composites. Ind. Crops Prod. 2017, 107, 217–231. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Zhu, X.; Li, K.; Zhang, L.; Wu, X.; Zhu, X. Comparative study on the evolution of physicochemical characteristics of biochar produced from bio-oil distillation residue under different induction atmosphere. Energy Convers. Manag. 2018, 157, 288–293. [Google Scholar] [CrossRef]
- Yuan, T.; He, W.; Yin, G.; Xu, S. Comparison of bio-chars formation derived from fast and slow pyrolysis of walnut shell. Fuel 2020, 261, 116450. [Google Scholar] [CrossRef]
- Yuan, R.; Dong, Y.; Hou, R.; Shang, L.; Zhang, J.; Zhang, S.; Chen, X.; Song, H. Structural transformation of porous and disordered carbon during ball-milling. Chem. Eng. J. 2023, 454, 140418. [Google Scholar] [CrossRef]
- Chang, D.W.; Choi, H.J.; Jeon, I.Y.; Seo, J.M.; Dai, L.; Baek, J.B. Solvent-free mechanochemical reduction of graphene oxide. Carbon 2014, 77, 501–507. [Google Scholar] [CrossRef]
- Li, T.; Zhang, L.; Dong, L.; Li, C.Z. Effects of gasification atmosphere and temperature on char structural evolution during the gasification of Collie sub-bituminous coal. Fuel 2014, 117, 1190–1195. [Google Scholar] [CrossRef]
- Chollakup, R.; Suethao, S.; Suwanruji, P.; Boonyarit, J.; Smitthipong, W. Mechanical properties and dissipation energy of carbon black/rubber composites. Compos. Adv. Mater. 2021, 30, 26349833211005476. [Google Scholar] [CrossRef]
- Fu, W.; Wang, L.; Huang, J.; Liu, C.; Peng, W.; Xiao, H.; Li, S. Mechanical Properties and Mullins Effect in Natural Rubber Reinforced by Grafted Carbon Black. Adv. Polym. Technol. 2019, 2019, 4523696. [Google Scholar] [CrossRef]
- Rattanasom, N.; Prasertsri, S.; Ruangritnumchai, T. Comparison of the mechanical properties at similar hardness level of natural rubber filled with various reinforcing-fillers. Polym. Test. 2009, 28, 8–12. [Google Scholar] [CrossRef]
- Li, Y.; Han, B.; Wen, S.; Lu, Y.; Yang, H.; Zhang, L.; Liu, L. Effect of the temperature on surface modification of silica and properties of modified silica filled rubber composites. Compos. Part A Appl. Sci. Manuf. 2014, 62, 52–59. [Google Scholar] [CrossRef]


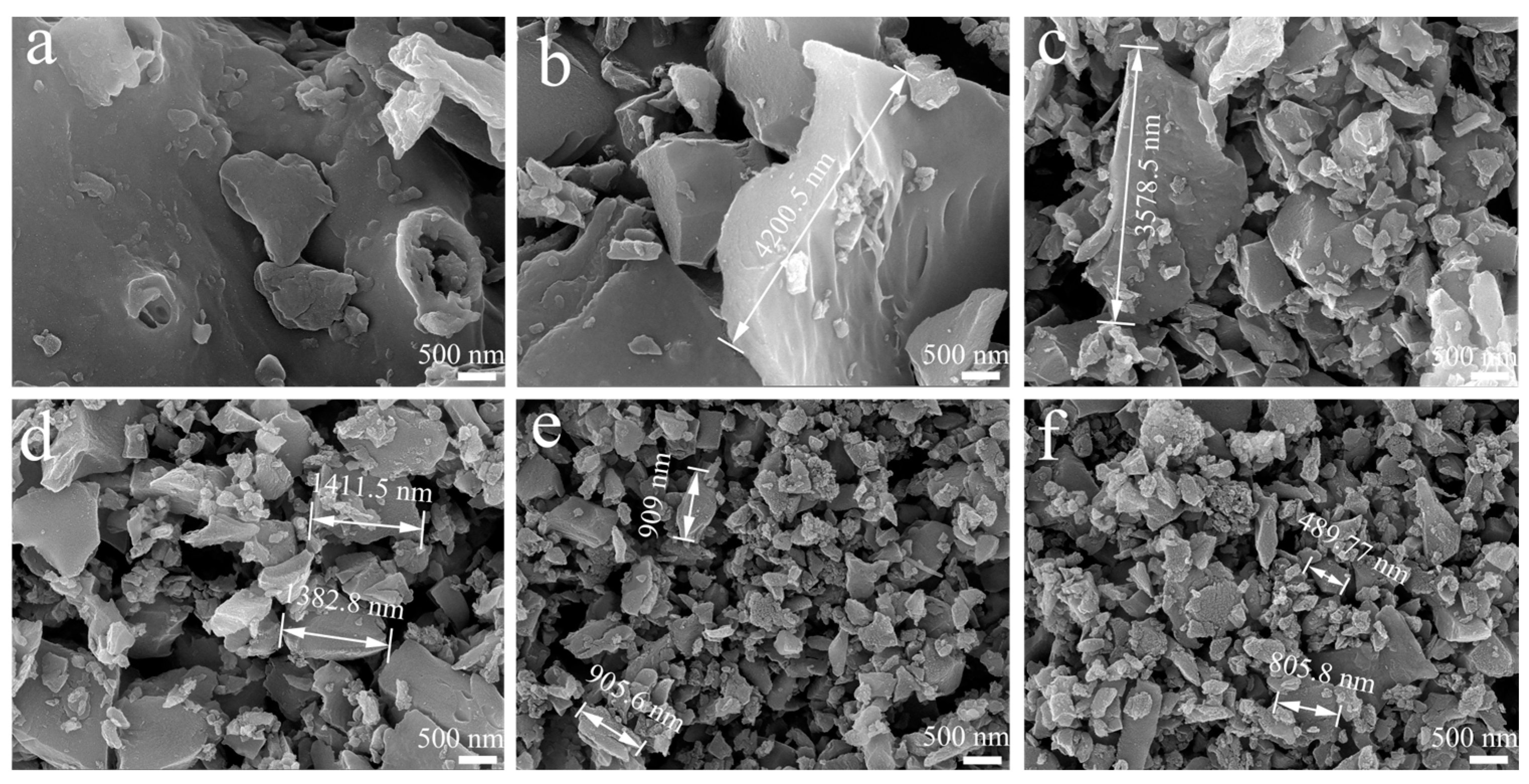
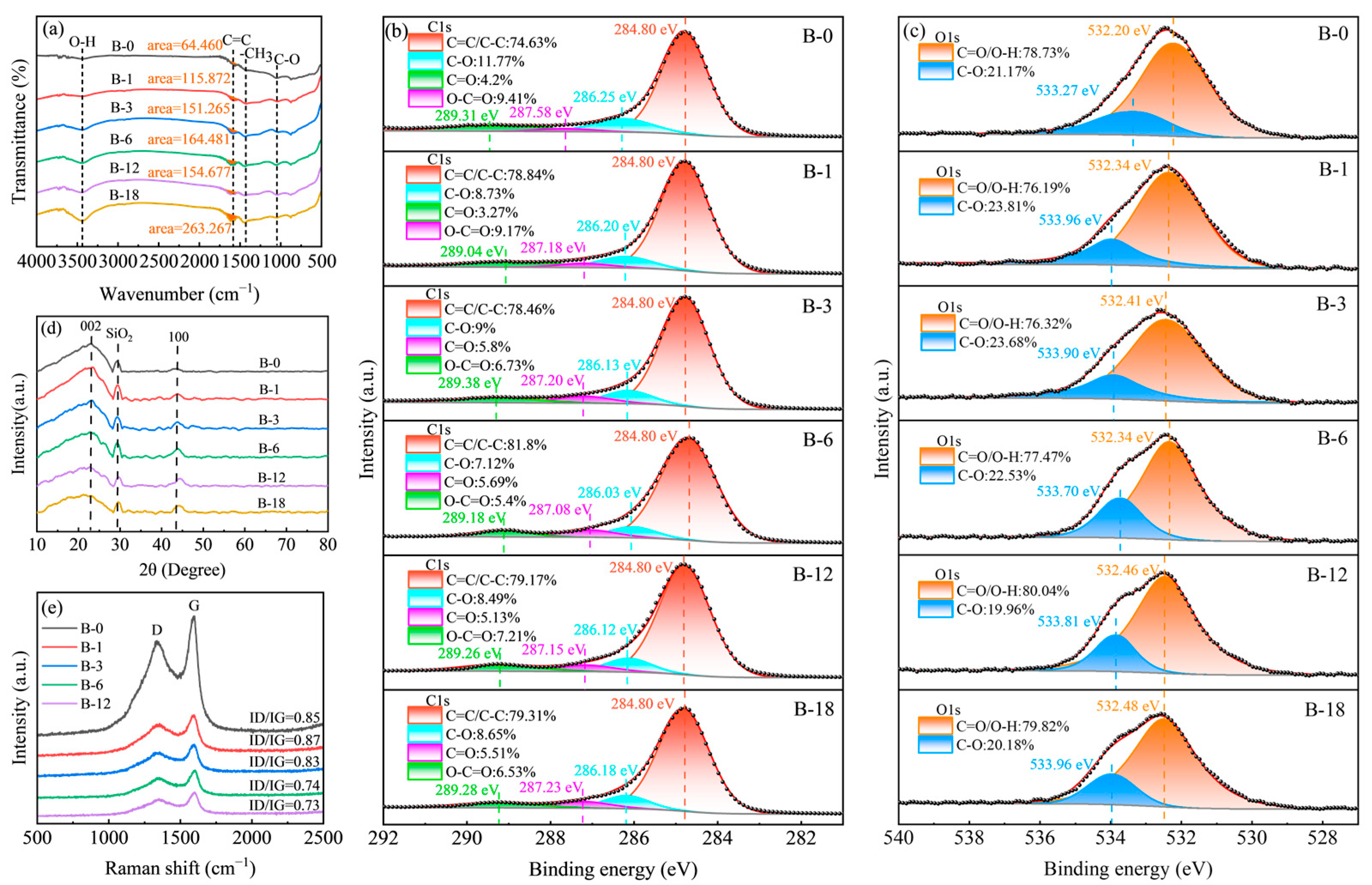
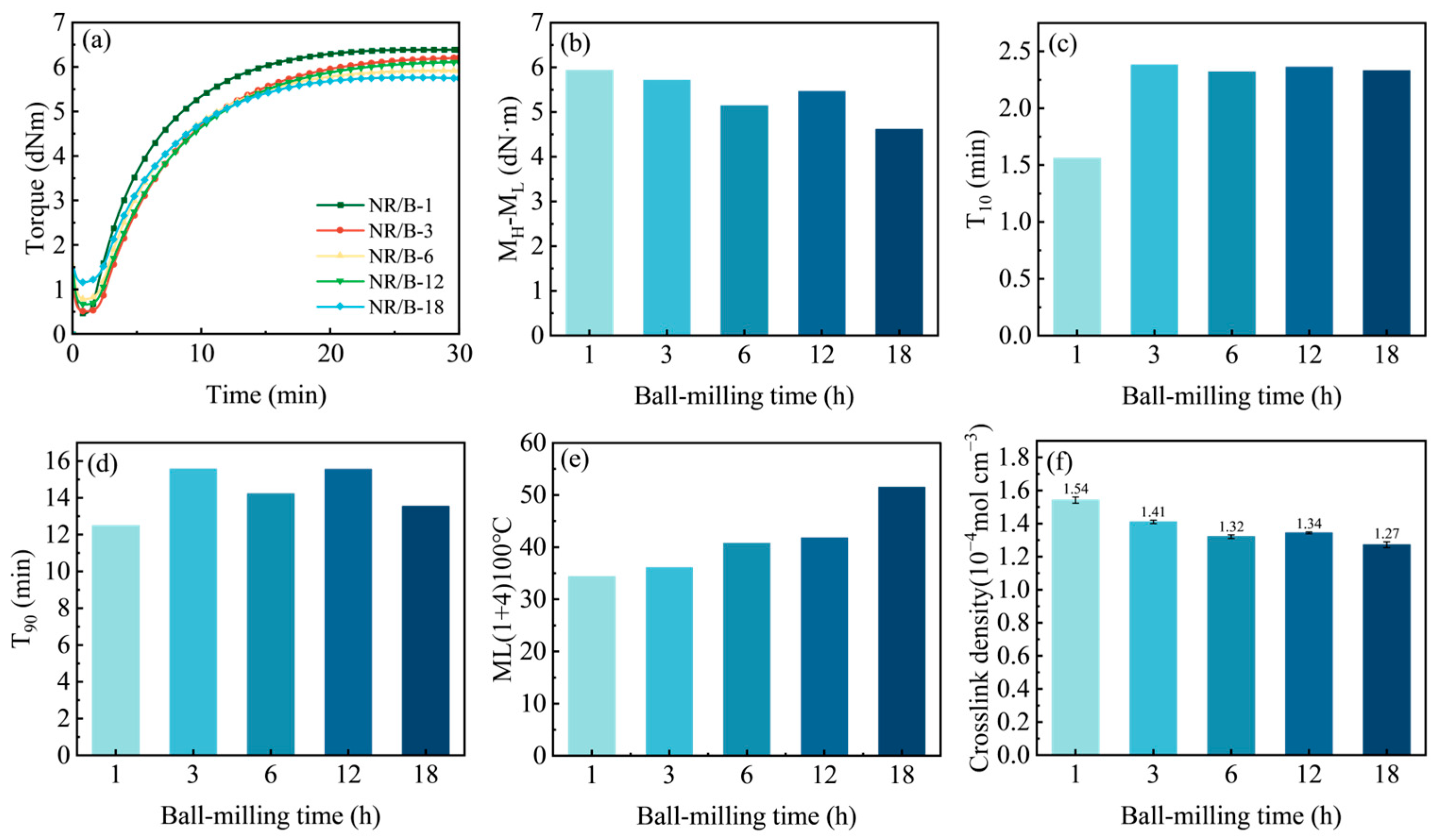

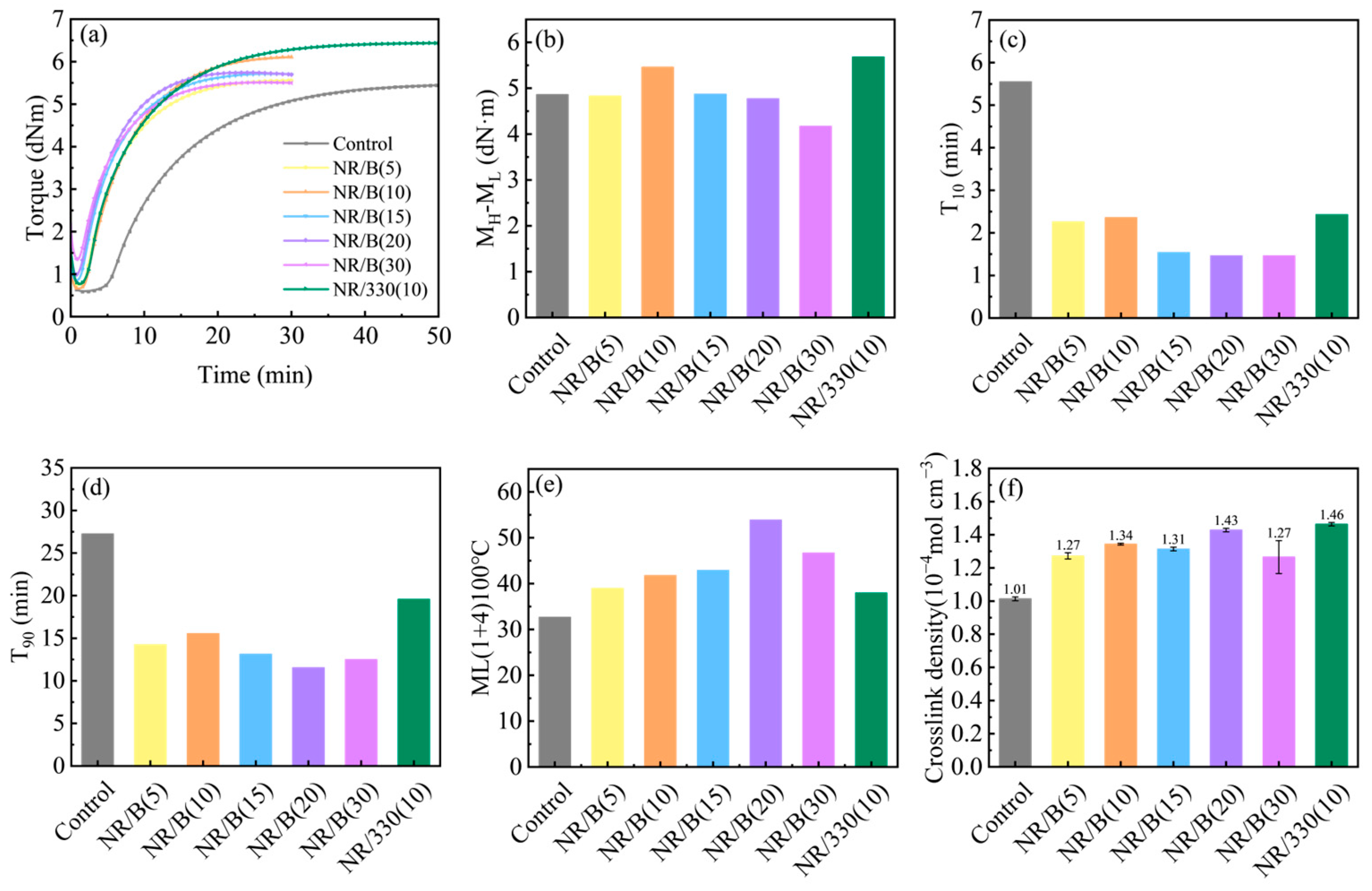
| Samples | Element Composition (At%) | |
|---|---|---|
| C1s | O1s | |
| B-0 | 90.26 | 8.34 |
| B-1 | 90.55 | 8.27 |
| B-3 | 92.17 | 7 |
| B-6 | 89.36 | 9.32 |
| B-12 | 90.05 | 8.97 |
| B-18 | 91.21 | 7.64 |
| Samples | Milling Time (h) |
|---|---|
| B-0 | 0 |
| B-1 | 1 |
| B-3 | 3 |
| B-6 | 6 |
| B-12 | 12 |
| B-18 | 18 |
| Component | Formula (1) (phr a) | Formula (2) (phr) | Formula (3) (phr) | Formula (4) (phr) | Formula (5) (phr) |
|---|---|---|---|---|---|
| Natural Rubber | 100 | 100 | 100 | 100 | 100 |
| B-1 | 10 | 0 | 0 | 0 | 0 |
| B-3 | 0 | 10 | 0 | 0 | 0 |
| B-6 | 0 | 0 | 10 | 0 | 0 |
| B-12 | 0 | 0 | 0 | 10 | 0 |
| B-18 | 0 | 0 | 0 | 0 | 10 |
| Zinc oxide | 5 | 5 | 5 | 5 | 5 |
| Stearic acid | 3 | 3 | 3 | 3 | 3 |
| Sulfur | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Promoter DM | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Component | Formula (1) (phr a) | Formula (2) (phr) | Formula (3) (phr) | Formula (4) (phr) | Formula (5) (phr) | Formula (6) (phr) | Formula (7) (phr) |
|---|---|---|---|---|---|---|---|
| Natural Rubber | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| B-12 | 0 | 5 | 10 | 15 | 20 | 30 | 0 |
| N330 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| Zinc oxide | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Stearic acid | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Sulfur | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Promoter DM | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Li, C.; Lin, X.; Hou, D.; Zheng, Y.; Yang, F.; Sun, H.; Liu, C. A Green Synthesis of Controllable Shear-Assisted Catalytically Graphitized Biomass-Derived Carbon and Its Multi-Scale Reinforcement Mechanism in Natural Rubber. Molecules 2025, 30, 1936. https://doi.org/10.3390/molecules30091936
Xu X, Li C, Lin X, Hou D, Zheng Y, Yang F, Sun H, Liu C. A Green Synthesis of Controllable Shear-Assisted Catalytically Graphitized Biomass-Derived Carbon and Its Multi-Scale Reinforcement Mechanism in Natural Rubber. Molecules. 2025; 30(9):1936. https://doi.org/10.3390/molecules30091936
Chicago/Turabian StyleXu, Xingxin, Chengjun Li, Xu Lin, Defa Hou, Yunwu Zheng, Fulin Yang, Hao Sun, and Can Liu. 2025. "A Green Synthesis of Controllable Shear-Assisted Catalytically Graphitized Biomass-Derived Carbon and Its Multi-Scale Reinforcement Mechanism in Natural Rubber" Molecules 30, no. 9: 1936. https://doi.org/10.3390/molecules30091936
APA StyleXu, X., Li, C., Lin, X., Hou, D., Zheng, Y., Yang, F., Sun, H., & Liu, C. (2025). A Green Synthesis of Controllable Shear-Assisted Catalytically Graphitized Biomass-Derived Carbon and Its Multi-Scale Reinforcement Mechanism in Natural Rubber. Molecules, 30(9), 1936. https://doi.org/10.3390/molecules30091936







