Fatty Acid Composition and Bioactive Profiles in the Aerial Parts of Cannabis sativa
Abstract
:1. Introduction
2. Results
2.1. Fatty Acids Profile and Other Substances
2.2. Lipid Quality Indices
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemical Composition
- Dry matter
- Crude fat
4.3. Fatty Acids Profile and Cannabinoids
4.4. Lipid Quality Indices
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| THCA | Tetrahydrocannabinolic acid |
| CBDA | Cannabidiolic acid |
| CBGA | Cannabigerolic acid |
| CBD | Cannabidiol |
| PUFAs | Polyunsaturated fatty acids |
| MUFAs | Monounsaturated fatty acids |
| SFAs | Saturated fatty acids |
| AI | Atherogenic index |
| TI | Thrombogenic index |
| HPI | Health-promoting index |
| h/H | Hypocholesterolemic/hypercholesterolemic fatty acid ratio |
| H_IF | Hemp inflorescences |
| H_L | Hemp leaves |
| DM | Dry matter |
| CF | Crude fat |
| SD | Standard deviation |
| UFAs | Unsaturated fatty acids |
| FAs | Fatty acids |
| OS | Other substances |
| CBC | Cannabichromene |
| Δ8-THC | Δ8-Tetrahydrocannabinol |
| Δ9-THC | Δ9-Tetrahydrocannabinol |
| FAMEs | Fatty acid methyl esters |
| GCMS | Gas chromatography mass spectrometry |
| tR | Retention time |
| DFAs | Hypocholesterolemic fatty acids |
| OFAs | Hypercholesterolemic fatty acids |
References
- Warf, B. High Points: An Historical Geography of Cannabis. Geogr. Rev. 2014, 104, 414–438. [Google Scholar] [CrossRef]
- McPartland, J.M.; Hegman, W.; Long, T. Cannabis in Asia: Its center of origin and early cultivation, based on a synthesis of subfossil pollen and archaeobotanical studies. Veg. Hist. Archaeobot. 2019, 28, 691–702. [Google Scholar] [CrossRef]
- Wimalasiri, E.M.; Wijesekara Mudiyanselage, A.U.K.M.W.; Madhuwanthi, P.I.; Ranasinghe, P.; Jahanshiri, E. Uncovering the Potential and Handicaps of Non–drug Hemp Cultivation in South and Southeast Asia. Rev. Agric. Sci. 2023, 11, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Cherney, J.; Small, E. Industrial Hemp in North America: Production, Politics and Potential. Agronomy 2016, 6, 58. [Google Scholar] [CrossRef]
- Karche, T.; Singh, M.R. The application of hemp (Cannabis sativa L.) for a green economy: A review. Turk. J. Bot. 2019, 43, 710–723. [Google Scholar] [CrossRef]
- Touw, M. The religious and medicinal uses of Cannabis in China, India and Tibet. J. Psychoact. Drugs 1981, 13, 23–34. [Google Scholar] [CrossRef]
- Li, H.–L. An archaeological and historical account of cannabis in China. Econ. Bot. 1973, 28, 437–448. [Google Scholar] [CrossRef]
- Spitzer–Rimon, B.; Duchin, S.; Bernstein, N.; Kamenetsky, R. Architecture and florogenesis in female Cannabis sativa plants. Front. Plant Sci. 2019, 10, 350. [Google Scholar] [CrossRef]
- Jin, D.; Henry, P.; Shan, J.; Chen, J. Identification of Chemotypic Markers in Three Chemotype Categories of Cannabis Using Secondary Metabolites Profiled in Inflorescences, Leaves, Stem Bark, and Roots. Front. Plant Sci. 2021, 12, 699530. [Google Scholar] [CrossRef]
- Schilling, S.; Dowling, C.A.; Shi, J.; Ryan, L.; Hunt, D.; O’Reilly, E.; Perry, A.S.; Kinnane, O.; McCabe, P.F.; Melzer, R. The cream of the crop: Biology, breeding and applications of Cannabis sativa. Authorea 2020, 21–45. [Google Scholar] [CrossRef]
- Aizpurua–Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, É.; Monthony, A.S.; Torkamaneh, D. Genomics-based taxonomy to clarify cannabis classification. Genome 2023, 66, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Fiddes, K.; Yang, L. A narrative review on environmental impacts of cannabis cultivation. J. Cannabis Res. 2021, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.; Arathi, H.S. Bee diversity and abundance on flowers of industrial hemp (Cannabis sativa L.). Biomass Bioenergy 2019, 122, 331–335. [Google Scholar] [CrossRef]
- Flicker, N.R.; Poveda, K.; Grab, H. The Bee Community of Cannabis sativa and Corresponding Effects of Landscape Composition. Environ. Entomol. 2020, 49, 197–202. [Google Scholar] [CrossRef]
- Razmaitė, V.; Pileckas, V.; Bliznikas, S.; Šiukščius, A. Fatty acid composition of Cannabis sativa, Linum usitatissimum and Camelina sativa seeds harvested in Lithuania for food use. Foods 2021, 10, 1902. [Google Scholar] [CrossRef]
- Alonso-Esteban, J.I.; González-Fernández, M.J.; Fabrikov, D.; Torija-Isasa, E.; Sánchez-Mata, M.D.C.; Guil-Guerrero, J.L. Hemp (Cannabis sativa L.) Varieties: Fatty Acid Profiles and Upgrading of γ–Linolenic Acid–Containing Hemp Seed Oils. Eur. J. Lipid Sci. Technol. 2020, 122, 1900445. [Google Scholar] [CrossRef]
- Teleszko, M.; Zając, A.; Rusak, T. Hemp Seeds of the Polish ‘Białobrzeskie’ and ‘Henola’ Varieties (Cannabis sativa L. var. sativa) as Prospective Plant Sources for Food Production. Molecules 2022, 27, 1448. [Google Scholar]
- Gaundal, L.; Myhrstad, M.C.W.; Leder, L.; Byfuglien, M.G.; Gjovaag, T.; Rud, I.; Retterstøl, K.; Holven, K.B.; Ulven, S.M.; Telle–Hansen, V.H. Beneficial effect on serum cholesterol levels, but not glycaemic regulation, after replacing SFA with PUFA for 3 days: A randomised crossover trial. Br. J. Nutr. 2021, 125, 915–925. [Google Scholar] [CrossRef]
- Maki, K.C.; Dicklin, M.R.; Kirkpatrick, C.F. Saturated fats and cardiovascular health: Current evidence and controversies. J. Clin. Lipidol. 2021, 15, 765–772. [Google Scholar] [CrossRef]
- Dong, X.; Rawiwan, P.; Middleditch, M.; Guo, G.; Woo, M.W.; Quek, S.Y. Effects of protein variations by different extraction and dehydration approaches on hempseed protein isolate: Protein pattern, amino acid profiling and label–free proteomics. Food Chem. 2024, 460, 140426. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Tang, C.H.; Yang, X.Q.; Gao, W.R. Characterization, amino acid composition and in vitro digestibility of hemp (Cannabis sativa L.) proteins. Food Chem. 2008, 107, 11–18. [Google Scholar] [CrossRef]
- Burton, R.A.; Andres, M.; Cole, M.; Cowley, J.M.; Augustin, M.A. Industrial hemp seed: From the field to value–added food ingredients. J. Cannabis Res. 2022, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Ferreira–Santos, P.; Nobre, C.; Rodrigues, R.M.; Genisheva, Z.; Botelho, C.; Teixeira, J.A. Extraction of phenolic compounds from grape pomace using ohmic heating: Chemical composition, bioactivity and bioaccessibility. Food Chem. 2024, 436, 137780. [Google Scholar] [CrossRef]
- Valková, V.; Ďúranová, H.; Havrlentová, M.; Ivanišová, E.; Mezey, J.; Tóthová, Z.; Gabríny, L.; Kačániová, M. Selected physico–chemical, nutritional, antioxidant and sensory properties of wheat bread supplemented with apple pomace powder as a by–product from juice production. Plants 2022, 11, 1256. [Google Scholar] [CrossRef]
- Belović, M.M.; Gironés–Vilaplana, A.; Moreno, D.A.; Milovanović, I.L.J.; Novaković, A.R.; Karaman, M.A.; Ilić, N.M. Tomato (Solanum lycopersicum L.) processing main product (juice) and by–product (pomace) bioactivity potential measured as antioxidant activity and angiotensin–converting enzyme inhibition. J. Food Process. Preserv. 2016, 40, 1229–1237. [Google Scholar] [CrossRef]
- Bośko, P.; Biel, W.; Smetanska, I.; Witkowicz, R.; Piątkowska, E. Sea Buckthorn Leaves as a Potential Source of Antioxidant Substances. Appl. Sci. 2024, 14, 5038. [Google Scholar] [CrossRef]
- Jafari, S.; Alizadeh, A.; Imani, A.; Meng, G.Y.; Rajion, M.A.; Ebrahimi, M. In situ degradation of almond (Prunus dulcis L.) hulls, a potential feed material for ruminants. Turk. J. Vet. Anim. Sci. 2015, 39, 676–681. [Google Scholar] [CrossRef]
- Obeidat, B.S. Influence of corn–dried distiller’s grain with solubles on growth performance and blood metabolites of Awassi lambs offered a concentrate diet. Ital. J. Anim. Sci. 2018, 17, 636–642. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Bio–processing of agro–byproducts to animal feed. Crit. Rev. Biotechnol. 2012, 32, 382–400. [Google Scholar] [CrossRef]
- Obeidat, B.S. Olive Cake in Livestock Nutrition. Jordan J. Agric. Sci. 2021, 17, 187–197. [Google Scholar] [CrossRef]
- Garcia Gonzalez, M.N.; Björnsson, L. Life cycle assessment of the production of beet sugar and its by–products. J. Clean. Prod. 2022, 346, 131211. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Ali, H. Integration of Forage Sorghum and By–Products of Sugarcane and Sugar Beet Industries for Ruminant Nutrition: A Review. Glob. Vet. 2015, 14, 752–760. [Google Scholar]
- Radwan, M.M.; Chandra, S.; Gul, S.; Elsohly, M.A. Cannabinoids, phenolics, terpenes and alkaloids of cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef]
- Pagano, C.; Navarra, G.; Coppola, L.; Avilia, G.; Bifulco, M.; Laezza, C. Cannabinoids: Therapeutic Use in Clinical Practice. Int. J. Mol. Sci. 2022, 23, 3344. [Google Scholar] [CrossRef]
- Khalsa, J.H.; Bunt, G.; Blum, K.; Maggirwar, S.B.; Galanter, M.; Potenza, M.N. Review: Cannabinoids as Medicinals. Curr. Addict. Rep. 2022, 9, 630–646. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross–talk between apoptosis and autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef]
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupré, D.J. Anti–Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers 2020, 12, 1985. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Mariano, A.; Gullì, M.; Fraschetti, C.; Vitalone, A.; Filippi, A.; Mannina, L.; Scotto d’Abusco, A.; Di Sotto, A. Role of caryophyllane sesquiterpenes in the entourage effect of Felina 32 hemp inflorescence phytocomplex in triple–negative MDA–MB–468 breast cancer cells. Molecules 2021, 26, 6688. [Google Scholar] [CrossRef] [PubMed]
- Holcik, M.; Yeh, C.; Korneluk, R.G.; Chow, T. Translational upregulation of X-linked inhibitor of apoptosis (XIAP) increases resistance to radiation induced cell death. Oncogene 2000, 19, 4174–4177. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Jo, M.J.; Yun, H.K.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Park, S.H.; Na, Y.J.; Jeong, Y.A.; Kim, B.G. Cannabidiol promotes apoptosis via regulation of XIAP/Smac in gastric cancer. Cell Death Dis. 2019, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- Bautista, J.L.; Yu, S.; Tian, L. Flavonoids in Cannabis sativa: Biosynthesis, Bioactivities, and Biotechnology. ACS Omega 2021, 6, 5119–5123. [Google Scholar] [CrossRef]
- Jha, S.K.; Kumar, C.; Bharadwaj, S.; Chauhan, P.; Doshi, R.; Lohiya, G. Synthesis, in–silico design and spectral characterization, elucidation of Cannabis sativa L. Cannabaceae containing phytoconstituents demonstrating novel therapeutic efficacy against epilepsy. World J. Adv. Res. Rev. 2023, 18, 1280–1293. [Google Scholar]
- Das, B. Antibacterial analysis of crude extracts from the leaves of Tagetes erecta and Cannabis sativa. Int. J. Environ. Sci. 2012, 2, 1605–1608. [Google Scholar] [CrossRef]
- Hayes, J.; Benson, G. What the latest evidence tells us about fat and cardiovascular health. Diabetes Spectr. 2016, 29, 171–175. [Google Scholar] [CrossRef]
- Primer, K.R.; Psaltis, P.J.; Tan, J.T.M.; Bursill, C.A. The role of high–density lipoproteins in endothelial cell metabolism and diabetes–impaired angiogenesis. Int. J. Mol. Sci. 2020, 21, 3633. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: A systematic review and meta–analysis of randomized controlled trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini–Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Stefler, D.; Brett, D.; Sarkadi–Nagy, E.; Kopczynska, E.; Detchev, S.; Bati, A.; Scrob, M.; Koenker, D.; Aleksov, B.; Douarin, E. Traditional Eastern European diet and mortality: Prospective evidence from the HAPIEE study. Eur. J. Nutr. 2021, 60, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Gaidai, O.; Cao, Y.; Loginov, S. Global Cardiovascular Diseases Death Rate Prediction. Curr. Probl. Cardiol. 2023, 48, 101622. [Google Scholar] [CrossRef]
- Stasiłowicz–Krzemień, A.; Sip, S.; Szulc, P.; Cielecka–Piontek, J. Determining Antioxidant Activity of Cannabis Leaves Extracts from Different Varieties—Unveiling Nature’s Treasure Trove. Antioxidants 2023, 12, 1390. [Google Scholar] [CrossRef] [PubMed]
- Cicaloni, V.; Salvini, L.; Vitalini, S.; Garzoli, S. Chemical Profiling and Characterization of Different Cultivars of Cannabis sativa L. Inflorescences by SPME–GC–MS and UPLC–MS. Separations 2022, 9, 90. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef]
- Nagy, D.U.; Cianfaglione, K.; Maggi, F.; Sut, S.; Dall’Acqua, S. Chemical Characterization of Leaves, Male and Female Flowers from Spontaneous Cannabis sativa L. Growing in Hungary. Chem. Biodivers. 2019, 16, e1800562. [Google Scholar] [CrossRef]
- Lee, S.; Kim, E.J.; Kwon, E.; Oh, S.J.; Cho, M.; Kim, C.M.; Lee, W.; Hong, J. Identification of terpene compositions in the leaves and inflorescences of hybrid Cannabis species using headspace–gas chromatography/mass spectrometry. Molecules 2023, 28, 8082. [Google Scholar] [CrossRef]
- Spano, M.; Di Matteo, G.; Ingallina, C.; Botta, B.; Quaglio, D.; Ghirga, F.; Balducci, S.; Cammarone, S.; Campiglia, E.; Giusti, A.M. A multimethodological characterization of Cannabis sativa L. inflorescences from seven dioecious cultivars grown in Italy: The effect of different harvesting stages. Molecules 2021, 26, 2912. [Google Scholar] [CrossRef]
- Kotecka–Majchrzak, K.; Kasałka–Czarna, N.; Montowska, M.; Spychaj, A.; Mikołajczak, B. The effect of hemp cake (Cannabis sativa L.) on the characteristics of meatballs stored in refrigerated conditions. Molecules 2021, 26, 5284. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, F.; Nikolai, A.; Marchart, R.; Sosa, S.; Tubaro, A.; Novak, J. Residues of herbal hemp leaf teas—How much of the cannabinoids remain? Food Control 2021, 127, 108146. [Google Scholar] [CrossRef]
- Pecyna, A.; Buczaj, A.; Różyło, R.; Kobus, Z. Physical and Antioxidant Properties of Innovative Gluten–Free Bread with the Addition of Hemp Inflorescence. Appl. Sci. 2023, 13, 4889. [Google Scholar] [CrossRef]
- Jozinović, A.; Ačkar, Đ.; Jokić, S.; Babić, J.; Balentić, J.P.; Banožić, M.; Šubarić, D. Optimisation of extrusion variables for the production of corn snack products enriched with defatted hemp cake. Czech J. Food Sci. 2017, 35, 6. [Google Scholar] [CrossRef]
- Banskota, A.H.; Jones, A.; Hui, J.P.M.; Stefanova, R. Triacylglycerols and Other Lipids Profiling of Hemp By–Products. Molecules 2022, 27, 2339. [Google Scholar] [CrossRef] [PubMed]
- Pexová Kalinová, J.; Vrchotová, N.; Tříska, J.; Hellerová, Š. Industrial hemp (Cannabis sativa L.) as a possible source of cannabidiol. J. Cent. Eur. Agric. 2021, 22, 110–118. [Google Scholar] [CrossRef]
- Yang, R.; Berthold, E.C.; McCurdy, C.R.; da Silva Benevenute, S.; Brym, Z.T.; Freeman, J.H. Development of cannabinoids in flowers of industrial hemp (Cannabis sativa L.): A pilot study. J. Agric. Food Chem. 2020, 68, 6058–6064. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Decorti, D.; Natolino, A. Potential oil yield, fatty acid composition, and oxidation stability of the hempseed oil from four Cannabis sativa L. cultivars. J. Diet. Suppl. 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Piovesana, S.; Aita, S.E.; Cannazza, G.; Capriotti, A.L.; Cavaliere, C.; Cerrato, A.; Guarnaccia, P.; Montone, C.M.; Laganà, A. In–depth cannabis fatty acid profiling by ultra–high performance liquid chromatography coupled to high–resolution mass spectrometry. Talanta 2021, 228, 122249. [Google Scholar] [CrossRef]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The seed of industrial hemp (Cannabis sativa L.): Nutritional quality and potential functionality for human health and nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef]
- Sheashea, M.; Xiao, J.; Farag, M.A. MUFA in metabolic syndrome and associated risk factors: Is MUFA the opposite side of the PUFA coin? Food Funct. 2021, 12, 12221–12234. [Google Scholar] [CrossRef]
- Rahimi, V.; Tavanai, E.; Falahzadeh, S.; Ranjbar, A.R.; Farahani, S. Omega–3 fatty acids and health of auditory and vestibular systems: A comprehensive review. Eur. J. Nutr. 2024, 63, 1453–1469. [Google Scholar] [CrossRef]
- Siol, M.; Chołuj, N.; Mańko-Jurkowska, D.; Bryś, J. Assessment of the stability and nutritional quality of hemp oil and pumpkin seed oil blends. Foods 2024, 13, 3813. [Google Scholar] [CrossRef]
- Claro–Cala, C.M.; Grao–Cruces, E.; Toscano, R.; Millan–Linares, M.C.; Montserrat–de la Paz, S.; Martin, M.E. Acyclic diterpene phytol from hemp seed oil (Cannabis sativa L.) exerts anti–inflammatory activity on primary human monocytes–macrophages. Foods 2022, 11, 2366. [Google Scholar] [CrossRef]
- Ding, L.L.; Matsumura, M.; Obitsu, T.; Sugino, T. Phytol supplementation alters plasma concentrations of formate, amino acids, and lipid metabolites in sheep. Animal 2021, 15, 100174. [Google Scholar] [CrossRef] [PubMed]
- Saranya, C.; Madhusudhanan, M.; John, M.S.; Sanjay, B.M.; Suraj, S.V.; Ranjith, D.; Nair, S.N.; Ajithkumar, K.G.; Nisha, A.R.; Ravindranand Sanis, J. Effect of phytol following single–dose oral and intravenous administration in healthy rats. Pharma Innov. J. 2022, 881, 881–886. [Google Scholar]
- Hoseini, S.M.; Gharavi, B.; Taheri Mirghaed, A.; Hoseinifar, S.H.; Van Doan, H. Effects of dietary phytol supplementation on growth performance, immunological parameters, antioxidant, and stress responses to ammonia exposure in common carp, Cyprinus carpio (Linnaeus, 1758). Aquaculture 2021, 545, 737151. [Google Scholar] [CrossRef]
- Judžentienė, A.; Garjonytė, R.; Būdienė, J. Phytochemical Composition and Antioxidant Activity of Various Extracts of Fibre Hemp (Cannabis sativa L.) Cultivated in Lithuania. Molecules 2023, 28, 4928. [Google Scholar] [CrossRef] [PubMed]
- Rukaiyat, M.; Garba, S.; Labaran, S. Antimicrobial activities of hexacosane isolated from Sanseveria liberica (Gerome and Labroy) plant. Adv. Med. Plant Res. 2015, 3, 120–125. [Google Scholar]
- Kalsoom, R.; Haider, M.S.; Chohan, S. Phytochemical analysis and antifungal activity of some medicinal plants against Alternaria species isolated from onion. J. Anim. Plant Sci. 2020, 30, 454–460. [Google Scholar]
- Austrich–Olivares, A.; García–Gutiérrez, M.S.; Illescas, L.; Gasparyan, A.; Manzanares, J. Cannabinoid CB1 Receptor Involvement in the Actions of CBD on Anxiety and Coping Behaviors in Mice. Pharmaceuticals 2022, 15, 473. [Google Scholar] [CrossRef]
- Cherkasova, V.; Wang, B.; Gerasymchuk, M.; Fiselier, A.; Kovalchuk, O.; Kovalchuk, I. Use of Cannabis and Cannabinoids for Treatment of Cancer. Cancers 2022, 14, 5142. [Google Scholar] [CrossRef] [PubMed]
- Miranda–Cortés, A.; Mota–Rojas, D.; Crosignani–Outeda, N.; Casas–Alvarado, A.; Martínez–Burnes, J.; Olmos–Hernández, A.; Mora–Medina, P.; Verduzco–Mendoza, A.; Hernández–Ávalos, I. The role of cannabinoids in pain modulation in companion animals. Front. Vet. Sci. 2023, 9, 1050884. [Google Scholar] [CrossRef]
- Rodziewicz, P.; Loroch, S.; Marczak, Ł.; Sickmann, A.; Kayser, O. Cannabinoid synthases and osmoprotective metabolites accumulate in the exudates of Cannabis sativa L. glandular trichomes. Plant Sci. 2019, 284, 108–116. [Google Scholar] [CrossRef]
- Happyana, N.; Agnolet, S.; Muntendam, R.; Van Dam, A.; Schneider, B.; Kayser, O. Analysis of cannabinoids in laser–microdissected trichomes of medicinal Cannabis sativa using LCMS and cryogenic NMR. Phytochemistry 2013, 87, 51–59. [Google Scholar] [CrossRef]
- Livingston, S.J.; Quilichini, T.D.; Booth, J.K.; Wong, D.C.J.; Rensing, K.H.; Laflamme–Yonkman, J.; Castellarin, S.D.; Bohlmann, J.; Page, J.E.; Samuels, A.L. Cannabis glandular trichomes alter morphology and metabolite content during flower maturation. Plant J. 2020, 101, 37–56. [Google Scholar] [CrossRef]
- Smart, R.; Caulkins, J.P.; Kilmer, B.; Davenport, S.; Midgette, G. Variation in cannabis potency and prices in a newly legal market: Evidence from 30 million cannabis sales in Washington state. Addiction 2017, 112, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Romano, B.; Borrelli, F.; Fasolino, I.; Capasso, R.; Piscitelli, F.; Cascio, M.G.; Pertwee, R.G.; Coppola, D.; Vassallo, L.; Orlando, P. The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. Br. J. Pharmacol. 2013, 169, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Bartho, L.; Nordtveit, E.; Szombati, V.; Benko, R. Purinoceptor–mediated, capsaicin–resistant excitatory effect of allyl isothiocyanate on neurons of the guinea–pig small intestine. Basic Clin. Pharmacol. Toxicol. 2013, 113, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A.; Capasso, R.; Aviello, G.; Borrelli, F.; Romano, B.; Piscitelli, F.; Gallo, L.; Capasso, F.; Orlando, P.; Di Marzo, V. Inhibitory effect of cannabichromene, a major non–psychotropic cannabinoid extracted from Cannabis sativa, on inflammation–induced hypermotility in mice. Br. J. Pharmacol. 2012, 166, 1444–1460. [Google Scholar] [CrossRef]
- Charles, A.P.R.; Gu, Z.; Archer, R.; Auwarter, C.; Hatterman–Valenti, H.; Rao, J.; Chen, B. Effect of High–Tunnel and Open–Field Production on the Yield, Cannabinoids, and Volatile Profiles in Industrial Hemp (Cannabis sativa L.) Inflorescence. J. Agric. Food Chem. 2024, 72, 12975–12987. [Google Scholar] [CrossRef]
- Mohamed, A.A.I.; Kehail, M.A.A.; Hilmi, Z.A.; Homida, A.E.; Abdelrahim, Y.M.; Mohamed, A.I.A. Evaluation of bio–insecticidal capacity of Cannabis sativa L. plants using GC–MS and phytochemical techniques. Int. J. Phytol. Res. 2022, 2, 38–41. [Google Scholar]
- Hollister, L.E.; Gillespie, H.K. Delta-8- and delta-9-tetrahydrocannabinol; Comparison in man by oral and intravenous administration. Clin. Pharmacol. Ther. 1973, 14, 353–357. [Google Scholar] [CrossRef]
- Vanegas, S.O.; Reck, A.M.; Rodriguez, C.E.; Marusich, J.A.; Yassin, O.; Sotzing, G.; Wiley, J.L.; Kinsey, S.G. Assessment of dependence potential and abuse liability of Δ8–tetrahydrocannabinol in mice. Drug Alcohol Depend. 2022, 240, 109640. [Google Scholar] [CrossRef]
- Nusantara, G.B.; Rahmania, T. Identification of Δ9–tetrahydrocannabinol compounds in Cannabis sativa using gas chromatography–mass spectrometry. Pharm. Educ. 2024, 24, 43–49. [Google Scholar] [CrossRef]
- Reber, J.D.; Karschner, E.L.; Seither, J.Z.; Knittel, J.L.; Dozier, K.V.; Walterscheid, J.P. An enhanced LC–MS-MS technique for distinguishing Δ8- and Δ9-tetrahydrocannabinol isomers in blood and urine specimens. J. Anal. Toxicol. 2022, 46, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Gorman, M.; McSweeney, M.B. How do consumers describe cannabis? Using a sorting task to create a lexicon to describe cannabis. J. Sens. Stud. 2024, 39, e12919. [Google Scholar]
- Sarma, N.D.; Waye, A.; Elsohly, M.A.; Brown, P.N.; Elzinga, S.; Johnson, H.E.; Marles, R.J.; Melanson, J.E.; Russo, E.; Deyton, L. Cannabis Inflorescence for Medical Purposes: USP Considerations for Quality Attributes. J. Nat. Prod. 2020, 83, 1334–1351. [Google Scholar] [CrossRef] [PubMed]
- Steel, L.; Welling, M.; Ristevski, N.; Johnson, K.; Gendall, A. Comparative genomics of flowering behavior in Cannabis sativa. Front. Plant Sci. 2023, 14, 1227898. [Google Scholar] [CrossRef]
- Hazekamp, A.; Tejkalová, K.; Papadimitriou, S. Cannabis: From Cultivar to Chemovar II—A Metabolomics Approach to Cannabis Classification. Cannabis Cannabinoid Res. 2016, 1, 202–215. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Kwon, D.B.; Injamum-Ul-Hoque, M.; Rahman, M.M.; Yeam, I.; Choi, H.W. De novo regeneration of Cannabis sativa cv. Cheungsam and evaluation of secondary metabolites of its callus. Horticulturae 2024, 10, 1331. [Google Scholar] [CrossRef]
- Tagen, M.; Klumpers, L.E. Review of delta–8–tetrahydrocannabinol (Δ8–THC): Comparative pharmacology with Δ9–THC. Br. J. Pharmacol. 2022, 179, 3915–3933. [Google Scholar] [CrossRef]
- Komarnytsky, S.; Rathinasabapathy, T.; Wagner, C.; Metzger, B.; Carlisle, C.; Panda, C.; Le Brun–Blashka, S.; Troup, J.P.; Varadharaj, S. Endocannabinoid system and its regulation by polyunsaturated fatty acids and full spectrum hemp oils. Int. J. Mol. Sci. 2021, 22, 5479. [Google Scholar] [CrossRef]
- Balachandran, A.; Choi, S.B.; Beata, M.M.; Małgorzata, J.; Froemming, G.R.A.; Lavilla, C.A.; Billacura, M.P.; Siyumbwa, S.N.; Okechukwu, P.N. Antioxidant, Wound Healing Potential and In Silico Assessment of Naringin, Eicosane, and Octacosane. Molecules 2023, 28, 1043. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 21st ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Ecker, J.; Scherer, M.; Schmitz, G.; Liebisch, G. A rapid GC–MS method for quantification of positional and geometric isomers of fatty acid methyl esters. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 897, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Acay, A.; Ulu, M.S.; Ahsen, A.; Ozkececi, G.; Demir, K.; Ozuguz, U.; Yuksel, S.; Acarturk, G. Atherogenic index as a predictor of atherosclerosis in subjects with familial Mediterranean fever. Medicina 2014, 50, 329–333. [Google Scholar] [CrossRef]
- Garaffo, M.A.; Vassallo–Agius, R.; Nengas, Y.; Lembo, E.; Rando, R.; Maisano, R.; Dugo, G.; Giuffrida, D. Fatty Acids Profile, Atherogenic (IA) and Thrombogenic (IT) Health Lipid Indices, of Raw Roe of Blue Fin Tuna (Thunnus thynnus L.) and Their Salted Product “Bottarga”. Food Nutr. Sci. 2011, 2, 736–743. [Google Scholar]
- Chen, S.; Bobe, G.; Zimmerman, S.; Hammond, E.G.; Luhman, C.M.; Boylston, T.D.; Terri, D.; Freeman, A.E.; Beitz, D.C. Physical and sensory properties of dairy products from cows with various milk fatty acid compositions. J. Agric. Food Chem. 2004, 52, 3422–3428. [Google Scholar] [CrossRef]
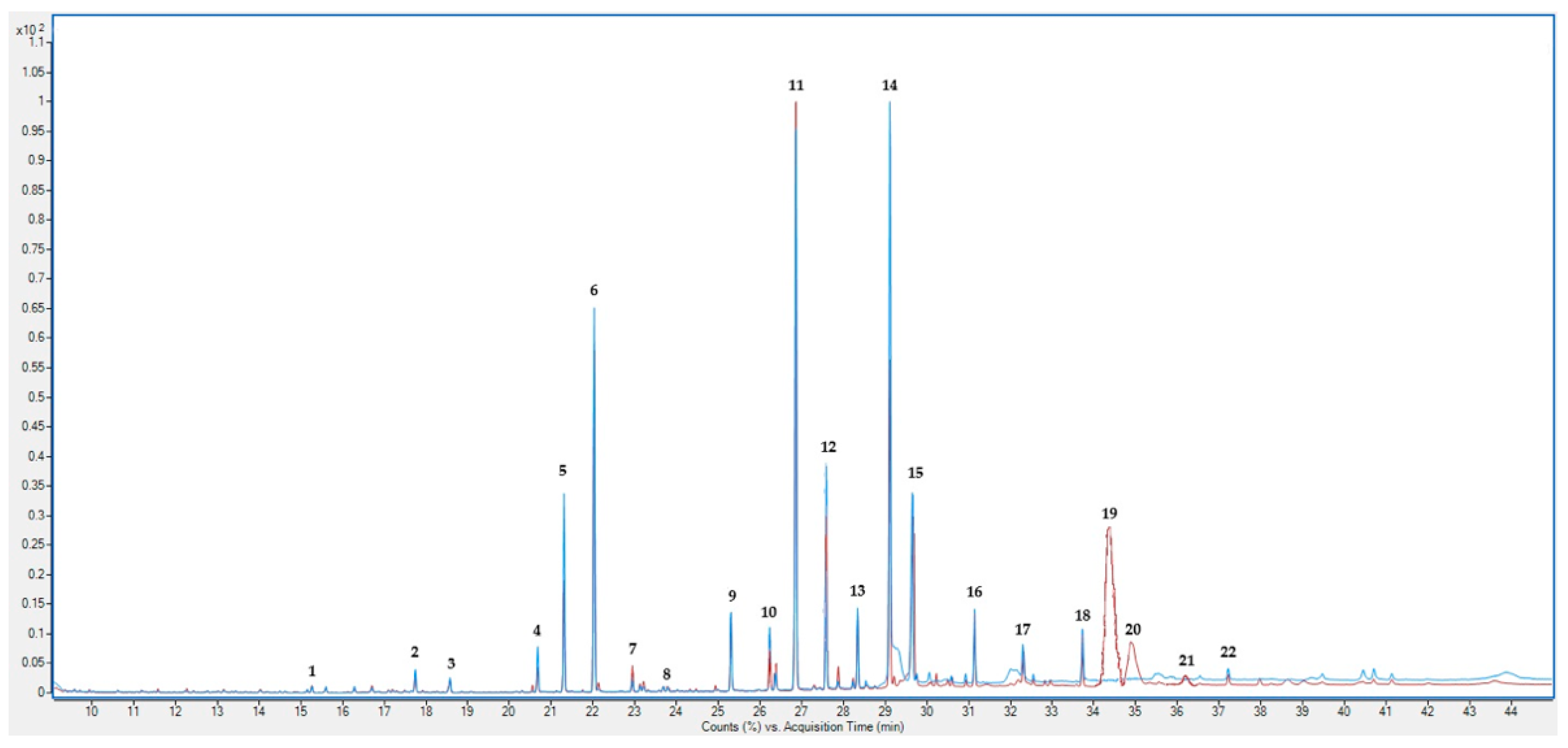
| Item | H_IF | H_L |
|---|---|---|
| Mean (±SD) | ||
| Dry matter (DM) [g/100 g] | 89.68 (±0.05) a | 89.11 (±0.03) b |
| Crude fat (CF) [g/100 g DM] | 12.63 (±0.14) a | 7.35 (±0.22) b |
| Fatty Acid | H_IF | H_L |
|---|---|---|
| % * | ||
| Mean (±SD) | ||
| C12:0 | 0.27 a (±0.05) | 0.31 b (±0.00) |
| C14:0 | 0.63 a (±0.03) | 0.92 b (±0.01) |
| C16:0 | 11.60 a (±0.31) | 18.95 b (±0.42) |
| C17:0 | 0.74 a (±0.02) | 0.23 b (±0.00) |
| C18:0 | 2.63 a (±0.08) | 4.08 b (±0.12) |
| C20:0 | 2.53 a (±0.06) | 4.03 b (±0.22) |
| C22:0 | 4.80 a (±0.40) | 3.79 b (±0.03) |
| C24:0 | 3.27 a (±0.19) | 3.00 b (±0.18) |
| C16:1 n-7 | 0.39 a (±0.01) | 1.29 b (±0.01) |
| C18:1 n-9 | 2.23 a (±0.02) | 1.99 b (±0.11) |
| C18:2 n-6 | 8.13 a (±0.07) | 7.00 b (±0.22) |
| C18:3 n-3 | 26.39 a (±0.53) | 29.48 b (±0.16) |
| Item | H_IF | H_L |
|---|---|---|
| % * | ||
| Mean (±SD) | ||
| SFAs | 26.48 a (±0.21) | 35.32 b (±0.24) |
| MUFAs | 2.62 a (±0.54) | 3.28 b (±0.10) |
| PUFAs | 34.52 a (±0.54) | 36.49 b (±0.18) |
| UFAs | 37.14 a (±0.53) | 39.77 b (±0.25) |
| n-3 | 26.39 a (±0.53) | 29.48 b (±0.16) |
| n-6 | 8.13 a (±0.07) | 7.00 b (±0.22) |
| n-6/n-3 | 0.31 a (±0.01) | 0.24 b (±0.01) |
| Σ FAs | 63.62 | 75.09 |
| Σ OS | 36.38 | 24.91 |
| Item | H_IF | H_L | H_IF | H_L | |
|---|---|---|---|---|---|
| % * | %/100 g DM | ||||
| Mean (±SD) | Mean (±SD) | ||||
| 2-Norpinanol 3,6,6-trimethyl | C10H18O | 1.55 a (±0.03) | 0.48 b (±0.00) | 0.20 a (±0.00) | 0.03 b (±0.00) |
| Phytyl, 2-methylbuanoate | C25H48O2 | 2.92 a (±0.03) | 0.65 b (±0.03) | 0.37 a (±0.01) | 0.05 b (±0.00) |
| Phytol | C20H40O | 3.90 a (±0.07) | 9.55 b (±0.21) | 0.49 a (±0.01) | 0.70 b (±0.01) |
| Hexacosane | C26H55 | 10.33 a (±0.11) | 12.21 b (±0.23) | 1.30 a (±0.03) | 0.90 b (±0.04) |
| Octacosane | C28H30O2 | 1.19 a (±0.00) | 1.69 b (±0.02) | 0.15 a (±0.00) | 0.12 b (±0.00) |
| Cannabidiol | C21H30O2 | 2.03 a (±0.05) | 0.00 b (±0.00) | 0.26 a (±0.01) | 0.00 b (±0.00) |
| Cannabichromene | C21H30O2 | 5.78 a (±0.25) | 0.00 b (±0.00) | 0.73 a (±0.04) | 0.00 b (±0.00) |
| Δ8-Tetrahydrocannabinol | C21H30O2 | 7.83 a (±0.04) | 0.00 b (±0.00) | 0.99 a (±0.01) | 0.00 b (±0.00) |
| Hexacosanoic acid, methyl ester | C27H54O2 | 0.86 a (±0.03) | 0.34 b (±0.04) | 0.11 a (±0.00) | 0.02 b (±0.00) |
| H_IF | H_L | |
|---|---|---|
| Index | Mean (±SD) | |
| AI | 0.39 a (±0.01) | 0.58 b (±0.01) |
| AI (+C18:0) | 3.02 a (±0.09) | 4.66 b (±0.11) |
| TI | 0.17 a (±0.00) | 0.24 b (±0.00) |
| h/H | 2.94 a (±0.05) | 1.91 b (±0.05) |
| HPI | 2.58 a (±0.05) | 1.73 b (±0.04) |
| Index | Equation | |
|---|---|---|
| Atherogenic Index (AI) | [C12:0 + (4 × C14:0) + C16:0]/ΣUFA | (2) |
| Corrected Atherogenic Index (AI) | [C12:0 + (4 × C14:0) + C16:0]/ΣUFA + C18:0 | (3) |
| Thrombogenic Index (TI) | (C14:0 + C16:0 + C18:0)/((0.5 × C18:1) + (0.5 × other MUFA) + (0.5 × Σn-6 PUFA) + (3 × Σn-3 PUFA) + Σn-3 PUFA/Σn-6 PUFA) | (4) |
| Hypocholesterolemic/Hypercholesterolemic Ratio (h/H) | (C18:1 + ΣPUFA)/(C12:0 + C14:0 + C16:0) | (5) |
| Health-Promoting Index (HPI) | ΣUFA/[C12:0 + (4 × C14:0) + C16:0] | (6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacuńska, W.; Biel, W.; Tokarczyk, G.; Biernacka, P.; Bienkiewicz, G.; Janda-Milczarek, K. Fatty Acid Composition and Bioactive Profiles in the Aerial Parts of Cannabis sativa. Molecules 2025, 30, 1947. https://doi.org/10.3390/molecules30091947
Jacuńska W, Biel W, Tokarczyk G, Biernacka P, Bienkiewicz G, Janda-Milczarek K. Fatty Acid Composition and Bioactive Profiles in the Aerial Parts of Cannabis sativa. Molecules. 2025; 30(9):1947. https://doi.org/10.3390/molecules30091947
Chicago/Turabian StyleJacuńska, Weronika, Wioletta Biel, Grzegorz Tokarczyk, Patrycja Biernacka, Grzegorz Bienkiewicz, and Katarzyna Janda-Milczarek. 2025. "Fatty Acid Composition and Bioactive Profiles in the Aerial Parts of Cannabis sativa" Molecules 30, no. 9: 1947. https://doi.org/10.3390/molecules30091947
APA StyleJacuńska, W., Biel, W., Tokarczyk, G., Biernacka, P., Bienkiewicz, G., & Janda-Milczarek, K. (2025). Fatty Acid Composition and Bioactive Profiles in the Aerial Parts of Cannabis sativa. Molecules, 30(9), 1947. https://doi.org/10.3390/molecules30091947








