Abstract
4,5-disubstituted-thizolyl amides, derivatives of 4-hydroxy-piperidine and of 4-N-methyl piperazine, were synthesized and tested as anti-inflammatory agents. Log P values were theoretically calculated and experimentally determined. These compounds were tested for antioxidant activity, as hydroxyl radical scavengers and for their ability to interact with stable 1,1-diphenyl-2-picryl hydrazyl free radical (DPPH). The effect of the synthesized compounds on inflammation, using the carrageenin induced mice paw edema model was studied. Both anti-inflammatory and antioxidant activities depended on some structural characteristics of the synthesized compounds.
Introduction
Various non-steroidal anti-inflammatory drugs (NSAIDs) are in widespread clinical use for the treatment of inflammation diseases. However, despite their great number, their mechanism of action as well as their precise therapeutic activities are still under investigation. Furthermore, almost all of them present a number of unwanted, often serious, side effects as consequence of interference with the arachidonic cascade [1]. It is well known that free radicals play an important role in the inflammatory process [2]. Superoxide anion radicals, hydrogen peroxide and hydroxyl radicals, produced by activation of phagocytes, are considered to be involved in inflammation and tissue destruction. Free radicals are also involved in the biosynthesis of prostaglandins, important mediators of inflammation [2]. Compounds with antioxidant properties are generally expected to protect against inflammation.
Several substituted thiazolyl derivatives [3,4] are reported to possess anti-inflammatory and/or antioxidant activities. This paper is an extension of previous work on the synthesis of thiazole derivatives with structures justifying anti-inflammatory activity.
Results and Discussion
The structures of the synthesized compounds are given in Figure 1 and the general method used to prepare the final compounds is shown in Figure 2.
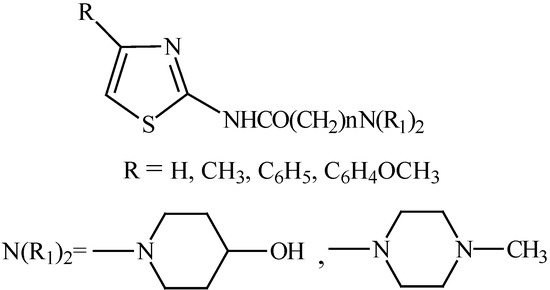
Figure 1.
Structures of synthesized compounds
Figure 1.
Structures of synthesized compounds
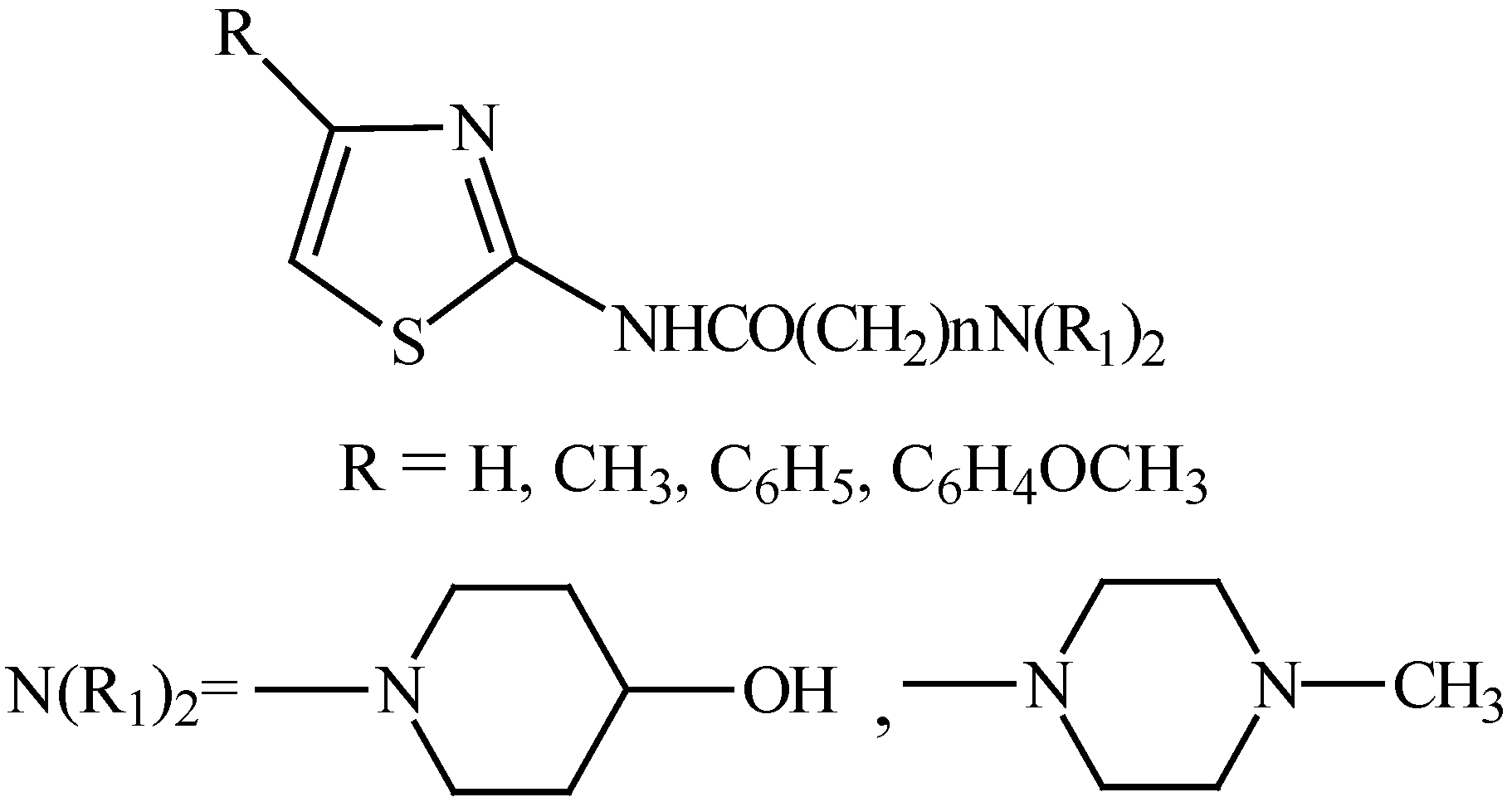
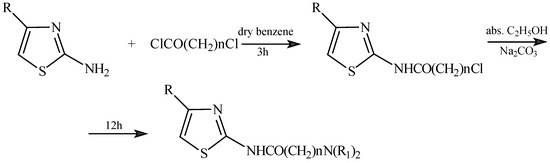
Figure 2.
Synthetic procedure
Figure 2.
Synthetic procedure
Overall the reactions proceeded smoothly in good yields. The structure of the synthesized compounds and their physicochemical properties are shown in Table 1. The amides were identified both by elemental analyses as well as by their spectroscopic analyses.

Table 1.
Characterization data of the synthesized amides
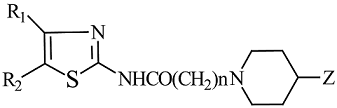
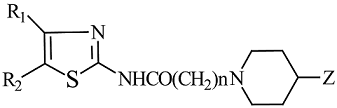
| N | R1 | R2 | Z | n | m.p. oC | ms | Mol. Structure b |
|---|---|---|---|---|---|---|---|
| 1 | H | H | OH | 1 | 85-86 | 241 | C12H14N3O2S |
| 2 | CH3 | H | OH | 1 | 89-92 | 254 | C11H16N3O2S |
| 3 | Ph | H | OH | 1 | 89.5-92 | 316 | C16H18N3O2S |
| 4 | Ph | CH3(CH2)13 | OH | 1 | 79-80 | 512 | C30H46N3O2S |
| 5 | Ph-OCH3 | H | OH | 1 | 115-117 | 346 | C17H20N3O4S |
| 6 | CH2COOEt | H | OH | 1 | 79-82c | 326 | C14H20N3O4S |
| 7 | H | H | OH | 2 | semisolid | 255 | C11H16N3O2S |
| 8 | CH3 | H | OH | 2 | 148-150 | 269 | C12H19N3O2S |
| 9 | Ph | H | OH | 2 | 227-8 | 331 | C17H21N3O2S |
| 10 | Ph-OCH3 | H | OH | 2 | 193-5 | 360 | C18H22N3O2S |
| 11 | Ph | CH3(CH2)13 | OH | 2 | semisolid | 526 | C31H48N3O2S |
| 12 | CH2COOEt | H | OH | 2 | 116-8 | 343 | C15H23N3O4S |
| 13a | H | H | CH3 | 1 | 225-228 | 240 | C13H16N3OS |
| 14a | Ph | H | CH3 | 1 | semisolid | 316 | C17H20N3OS |
| 15a | CH2COOEt | H | CH3 | 1 | semisolid | 326 | C15H22N3O3S |
| 16a | Ph-OCH3 | H | CH3 | 2 | 248-50 | 382.5 | C15H22N3O3S |
Notes: a Piperazinyl – ring; b Elemental analyses for molecular formula; c m.p for hydrochloride salt
Physicochemical parameters, e.g. log P have been calculated theoretically, as an expression of lipophilicity (Table 2). The in vivo antiinflammatory activity of the synthesized compound was determined using carrageenin induced mice paw edema (26.1-64.3 %) (see Table 2). The compounds have also been screened for their in vitro reducing activity towards the free stable radical DPPH (15.6-26.6 %) and for their hydroxyl free radical scavenging activity (44.6-100 %) (Table 3). For the 4-OH-piperidinyl amides the inhibition on the soybean LOX has been performed (77.5-100 %). The preliminary results reveal that the tested compounds exhibit in generally good biological activity. The results are discussed in terms of structural characteristics. The presence of a R1 = phenyl group seems to be crucial for high activity in vivo. The length of the chain also plays an important role. The derivatives with n = 1 are stronger inhibitors. The nature of the alicyclic amine is an important feature, since the 4-N-methyl piperazinyl derivatives possess higher in vivo results.

Table 2.
Lipophilicity values a) experimentally performed RM values; b) theoretically calculated lipophilicity values using: i) Suzuki-Kudo’s method ii) the clog P program from Biobyte. In vivo carrageenin rat paw edema % inhibition after 3.5 h (CPE %).
| N | RM | logPsk | Clog P | CPE% |
|---|---|---|---|---|
| 1 | -0.494± 0.03 | -4.287 | -0.34 | 52.3 |
| 2 | nt | -1.878 | 0.159 | 59 |
| 3 | -0.578± 0.031 | -0.434 | 1.758 | 62.6 |
| 4 | 0.911± 0.037 | 6.322 | 6.322 | 44.2 |
| 5 | -0.490± 0.031 | -1.062 | 1.777 | 45 |
| 6 | nt | -2.473 | -0.133 | 33.4 |
| 7 | -0.504± 0.021 | -1.751 | -0.143 | 26.1 |
| 8 | -0.534± 0.037 | -0.365 | 0.212 | 27.5 |
| 9 | -0.589± 0.039 | 1.081 | -1.0955 | 47.5 |
| 10 | -0.504± 0.024 | 0.453 | 1.974 | 57 |
| 11 | 0.894± 0.033 | 6.5 | 8.684 | 32.2 |
| 12 | nt | -0.958 | 0.014 | 59.6 |
| 13 | nt | -3.747 | 1.102 | 61 |
| 14 | nt | -1.969 | 3.2 | 64.3 |
| 15 | nt | -2.161 | 1.309 | 47.9 |
| 16 | nt | 0.97 | 3.219 | 38.5 |

Table 3.
Biological testing results. In vitro effects of the examined and reference drugs (in % at 0.1 mmol/L and 0.2 mmol/L) on reducing ability (DPPH), on hydroxyl radical scavenging activity (. OH) and on soybean lipoxygenase activity (LOX).
| N | DPPH (0.1 mmol/L) 20min | DPPH (0.1 mmol/L) 1hr | DPPH (0.2 mol/L) 1 hr | . OH | LOX |
|---|---|---|---|---|---|
| 1 | 25.4 | 24.6 | 23.5 | 97.2 | no |
| 2 | 13.5 | 17.3 | 18.1 | 100 | 100 |
| 3 | nt | nt | nt | 75.3 | 100 |
| 4 | 15.6 | 29.8 | 17.5 | 78.4 | 100 |
| 5 | no | 27.6 | no | 44.6 | 100 |
| 6 | nt | nt | nt | no | 100 |
| 7 | nt | nt | nt | no | 100 |
| 8 | nt | nt | nt | nt | nt |
| 9 | 23.7 | 25.1 | 36 | no | 100 |
| 10 | 26.7 | 32 | 35.3 | 95.9 | 100 |
| 11 | 17.8 | 17.7 | 16.1 | 40 | 100 |
| 12 | 13.3 | 14 | 20.6 | 87 | 77.5 |
| ASA | 80.5 | – | – | – | – |
| NDGA | 94 | – | – | – | 91 |
Conclusions
We have presented a facile route for the formation of new 4,5-disubstituted thiazolyl amides, possessing anti-inflammatory activity. These compounds also present mild antioxidant activity. They highly compete with DMSO for hydroxyl radical and strongly inhibit LOX. A phenyl group in R1, the length of the chain and a value of n = 1 are all significant structural features for the activity observed.
Experimental
General
Melting points were obtained with a MELTEMP II capillary apparatus (LAB. Devices Holliston, MA, USA) and are reported without correction. Infrared spectra (Nujol mulls) were recorded on a Perkin Elmer 597 spectrophotometer. UV-Vis were determined on a Perkin Elmer 554 UV-Vis spectrophotometer. Proton NMR spectra were obtained on a Brucker AW 80 apparatus at 80 MHz and are reported in ppm downfield from tetramethylsilane (TMS). Analyses indicated by the symbols of elements were within 0.4 % of theoretical values. Mass spectra (MS) were determined on a VG-250 instrument (VG Labs. Tritech, England) with the ionization energy maintained at 70 eV. The reactions were monitored by TLC on silica gel 60 F254 (Merck). All reagents were obtained from commercial sources. 2-Aminothiazole, 2-amino-4-methylthiazole, 2-amino-4-phenylthiazole and 2-amino-4-(4-methoxyphenyl)thiazole were synthesized as described previously [5].
General synthetic procedure for the synthesis of the 2-chloroacetamido- and 3-chloropropionylamido thiazoles [5].
To a solution of 2-aminothiazole or suitable 4-substituted aminothiazole (0.02 mol) in dry benzene a cooled solution of chloroacetyl or 3-chloropropionyl chloride (0.033 mol) in dry benzene (7.5 mL) was added dropwise. The reaction mixture was refluxed in a water bath at 80 oC for 3 h. Benzene and excess 3-chloropropionyl chloride were removed by distillation. The residue was washed with aqueous sodium bicarbonate (5 % w/v) followed by cold water. The crude product was dried and crystallized from ethanol.
General synthetic procedure for the synthesis of the 2-(N-substituted aminoacetamido)/3-(N-substituted aminopropioamido) thiazoles [5].
A mixture of 2-chloroacetamido or 3-chloropropionamido thiazole (0.006mol), amine (4-OH-piperidine or N-methyl-piperazine, 0.7 mol), absolute ethanol (15 mL) and anhydrous sodium carbonate (1.48 g) was heated under reflux in a water bath for 12 h. The excess of amine and ethanol was removed by distillation and the residue was treated with 5 % sodium bicarbonate solution to remove acid impurities, filtered, washed with water and dried. It was crystallized from ethanol (95 %) to give white crystals.
Preparation of the hydrochlorides of 2-(N-substituted- aminoacetamido) / 3-(N-substituted-amino-propionamido) thiazoles.
A solution of the base in anhydrous ethanol was saturated with dry hydrogen chloride gas. The salt was filtered off, washed with dry ether and recrystallized from absolute ethanol to give white crystals.
The structure of all compounds was identified both by elemental analyses as well as by spectroscopic analysis (IR, 1H-NMR, MS).
Spectral Data: 1H-NMR (CDCl3, DMSO) for representative compounds ( δ):
Compound 1: 1.94-2.16 (d, 4H, piperidyl C3-H,), 2.157 (s, 2H, COCH2), 3.2 (s, 1H, piperidyl C4-H), 3.2-3.38 (d, 4H, piperidyl NCH2), 6.4 (d, 1H, thiazolyl C5-H), 6.86 (d, 1H, thiazolyl C4-H). Compound 9: 1.71-1.89 (d, 4H, piperidyl C3-H), 2.49-2.53 (m, 8H, NCH2, COCH2CH2 ), 7.42-7.45 (m, 3H, phenyl C3,4,5-H), 7.46-7.48 (m, 2H, phenyl C-2.6-H), 9.05 (b, 1H, NH). Compound 16: 2.78 (s, 3H, NCH3), 2.78-3.53 (m, 8H, piperidyl), 3.35 (s, 2H, COCH2), 3.71 (s, 3H, OCH3), 6.96-6.99 (d, 2H, phenyl C2,6-H), 7.51 (s, 1H, thiazolyl C5),7.8-7.89 (d, 2H, phenyl C3,5-H); IR cm-1: 3190-3230, 1680, 1620
Biological assays
- 1.
- Physicochemical Studies [4]
Lipophilicity is an important factor affecting the distribution and fate of drug molecules. Increased lipophilicity is correlated with increased biological activity and more rapid metabolism. Theoretical calculations of lipophilicity as clog P and Suzuki-Kudo’s method [10] were performed (Table 2). The program CLOG P [11], has been designed to calculate the lipophilicity of a molecule using the additivity method. Reversed phase TLC (RPTLC) was performed on silica gel plates impregnated with 55 (v/v) liquid paraffin in light petroleum ether. Mobile phase: methanol/water mixture (70/30, v/v) containing 2 % aqueous ammonia (27 %). The plates were developed in closed chromatography tanks saturated with the mobile phase at 24 oC. Spots were detected under UV light or by iodine vapours. RM values were determined from the corresponding Rf values (from ten individual measurements) using the equation RM = log [( 1/Rf ) -1 ]. For results see Table 2.
- 2.
- Inhibition of carrageenin induced paw oedema [4]
All tested compounds were suspended in water with few drops of Tween-80 and ground in a mortar before use. Groups of 5-6 AKR mice weighing 20-30 g were used. A single dose of 0.2 mmol/Kg or indomethacin (0.11 mmol/Kg) was administered intraperitoneally i.p., simultaneously to the administration of the phlogistic agent : carrageenin (2%, 0.05 ml was injected intradermally i.d. into the right foot pad, the left serving as control). Results are given in Table 2.
- 3.
- Competition of the tested compounds with DMSO for hydroxyl radicals [6]
The hydroxyl radicals generated by the Fe3+/ascorbic acid system were detected by the determination of formaldehyde produced from the oxidation of DMSO. The reaction mixture contained EDTA (0.1mM), Fe3+(167 μM, as a 1:2 mixture with EDTA) and DMSO (33mM) in phosphate buffer (50 mM, pH 7.4) and the tested compounds (final concentration 1mM - final volume of the samples 1 mL). Ascorbic acid (150 μL, 10 mM in phosphate buffer) was added at the end in order to initiate the reaction. The mixture was incubated at 37 oC for 30 min. The reaction was stopped by the addition of trichloroacetic acid (250 μL, 17.5 % w/v) and the formaldehyde formed was detected spectrophotometrically at 412 nm by the method of Nash [7].
Interaction of the synthesized compounds with DPPH [8]
To a solution of DPPH (0.1 mM) in absolute ethanol, an equal volume of the compounds dissolved in ethanol was added (0.1 mM). A control solution containing ethanol was also used. After 20 and 60 mins at room temperature, absorbance was recorded at 517 nm. For the results see Table 3.
- 4.
- Soybean lipoxygenase inhibition [9]
The conversion of sodium linoleate to 13-hydroxy-peroxylinoleic acid at 234 nm, was recorded and compared with appropriate standard, see Table 3.
Each in vitro experiment was performed at least in triplicate and the standard deviation in absorbance was less than ±10 %. Acetyl salicylic acid (ASA) and/or nor-dihydroguaeretic acid (NDGA) were used as standards.
Acknowledgements
This work was supported by INTAS (grant No. INTAS 00-0711). Thanks are also due to Drs. C. Hansch and A. Leo for providing the Mac Clog P software, to the Theagenium Anticancer Hospital for providing mice and to D. Rigas, Lab. of Organic Chemistry Dept., for providing accurate mass spectra.
References
- Shanbag, V.R.; Crider, M.A; Gohkale, R.; Harpalani, A.; Dick, R. M. Ester and amide prodrugs of obuprofen and naproxen: synthesis, anti-inflammatory activity, and gastrointestinal toxicity. J. Pharm. Sci. 1992, 81, 149–153. [Google Scholar]
- Lazer, E. S.; Wong, H.C.; Possanza, G.; Graham, A. G.; Farina, P. R. Anti-inflammatory 2,6-Di-tert-butyl-4-(2-arylethenyl)phenols. J. Med. Chem. 1989, 32, 100–104. [Google Scholar]
- Geronikaki, A.; Hadjipavlou-Litina, D. Synthesis of some new aryloxy/aroyl-2-amino-1,3-thiazole derivatives with anti-inflammatory. Arzneim. Forsc./Drug Res. 1996, 46, 1134–1138. [Google Scholar]
- Geronikaki, A.; Hadjipavlou-Litina, D. Lipophilicity and biological studies of some 2-(amino-acetylamino)-thiazole derivatives with anti-inflammatory. Pharmazie 1993, 48, 948–949. [Google Scholar]
- Geronikaki, A.; Theophilidis, G. Synthesis of 2-(aminoacetylamino)thiazole derivatives and comparison of their local anaesthetic activity by the method of action potential. Eur. J. Med. Chem. 1992, 27, 1–9. [Google Scholar]
- Klein, S. M.; Cohen, G.; Cederbaum, A. I. Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical generating systems. Biochemistry 1981, 20, 6006–6012. [Google Scholar]
- Nash, T. The colorimetric estimation of formaldehyde by means of the Hantzch reaction. Biochem. J. 1953, 55, 416–421. [Google Scholar]
- Blois, M.S. Antioxidant determination by the use of a stable free radical. Nature (London) 1958, 181, 1199–1200. [Google Scholar]
- Sircar, J.C.; Schwender, C.F.; Johnson, E.A. Synthesis and structure-activity relationships of anti-inflammatory 9,10-dihydro-9-oxo-2-acridine-alkanoic acids and 4-(2-carboxyphenyl)amino-benzenealkanoic acids. Prostaglandins 1983, 25, 393. [Google Scholar]
- Suzuki, T.; Kudo, Y. Automatic log p estimation based on combineed additive modelling methods. J. Comput.-Aided Mol. Design 1990, 4, 155–198. [Google Scholar]
- Clog P. Biobyte Corp. Claremont Ca 91711 USA.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
