Evaluation of Endothelial Dysfunction and Autophagy in Fibromyalgia-Related Vascular and Cerebral Cortical Changes and the Ameliorative Effect of Fisetin
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Animals
2.2. Induction of Fibromyalgia
2.3. Sampling
2.4. Histological Study
2.5. Morphometric Study
- The carotid intima-media thickness (IMT) from the innermost endothelial boundary to the outermost tunica media, which is difficult to differentiate between the intima and media.
- The mean area percentage of collagen was measured inside a standard measuring frame of a known area: the blue area was masked by the collagen fibers and was then measured and expressed as an area percent to the area of the standard measuring frame.
- The mean area percentage of immune reaction for eNOS.
2.6. Molecular Study
2.7. Biochemical Investigations
2.8. Statistical Analysis
3. Results
3.1. Histological Results
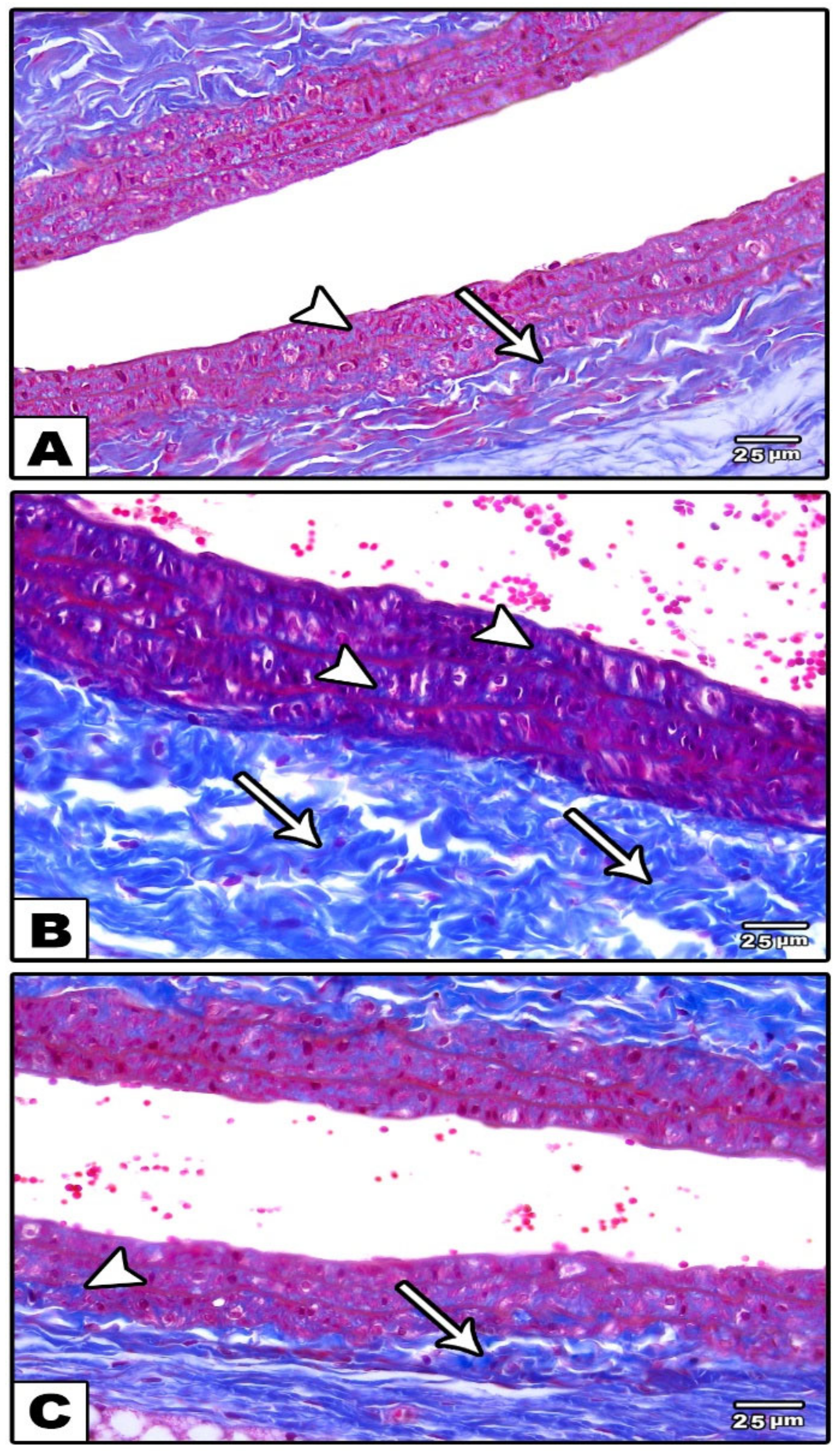
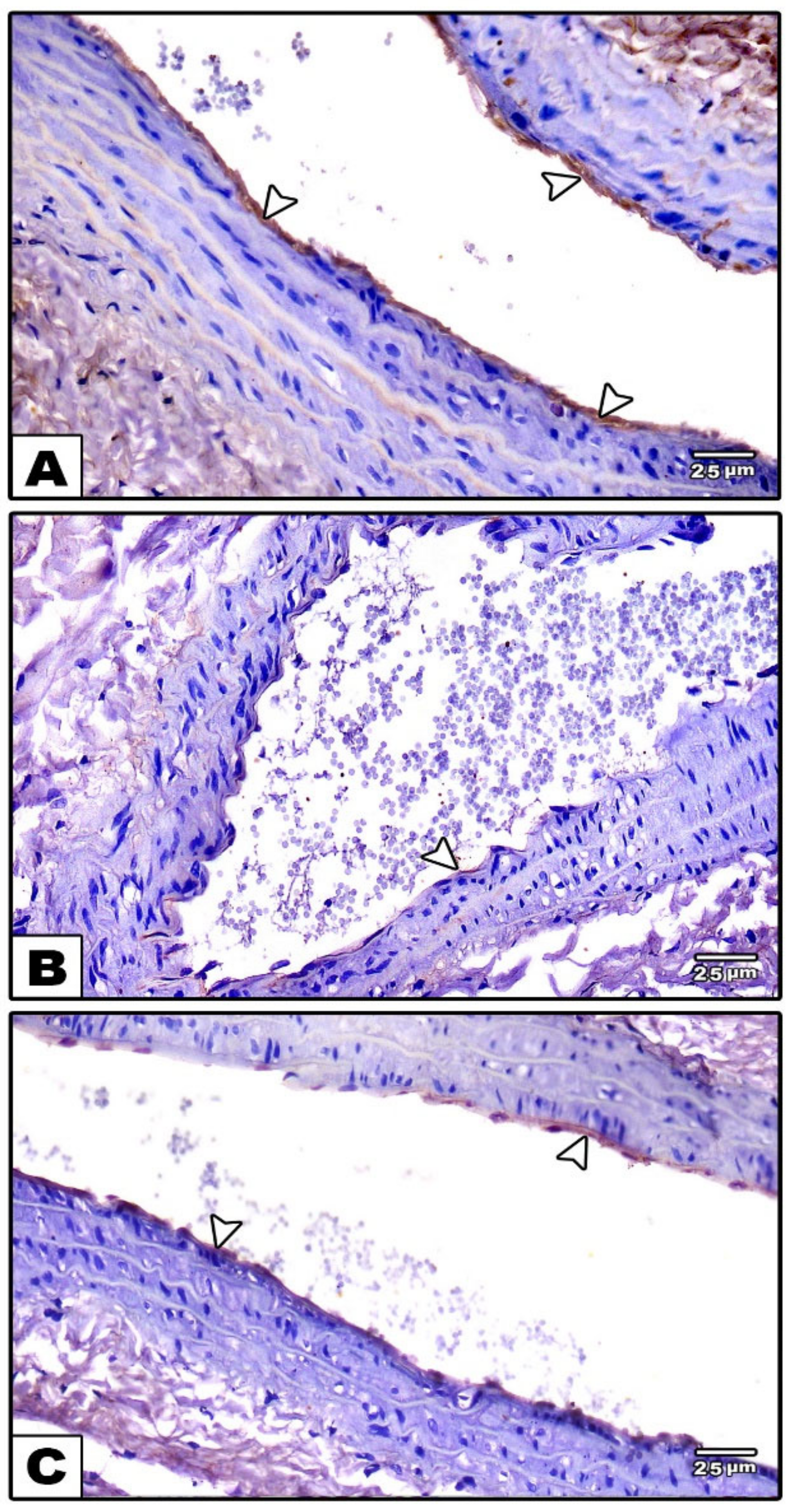
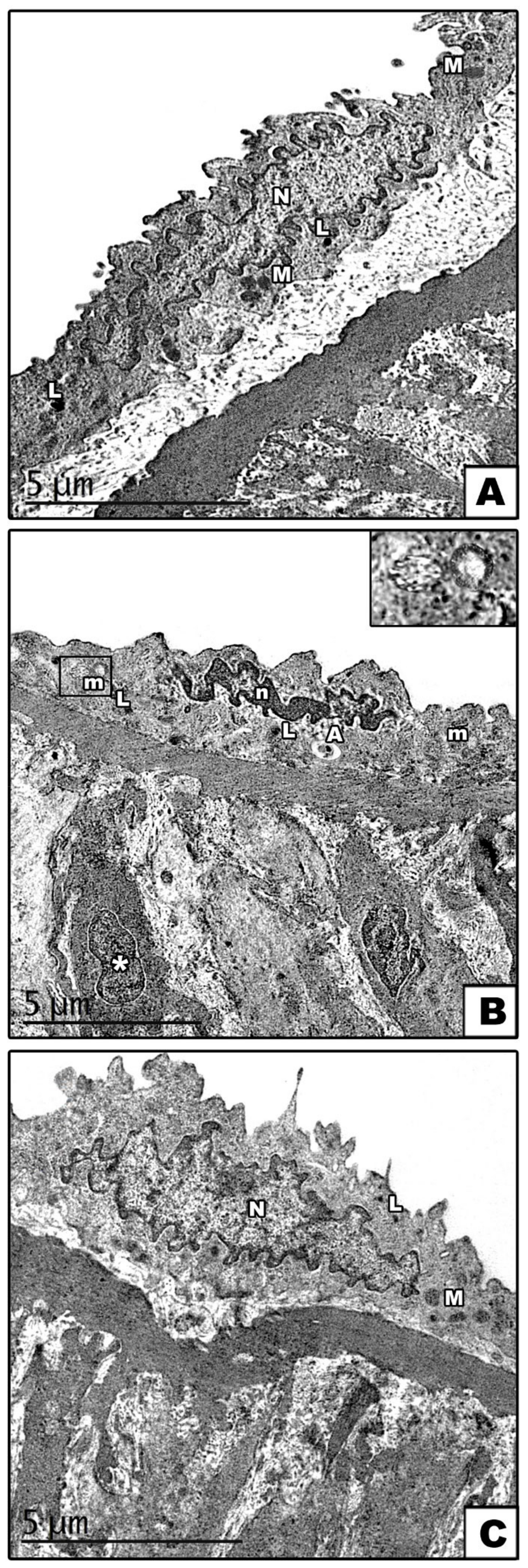
3.1.1. Carotid Artery
Light Microscopic Results
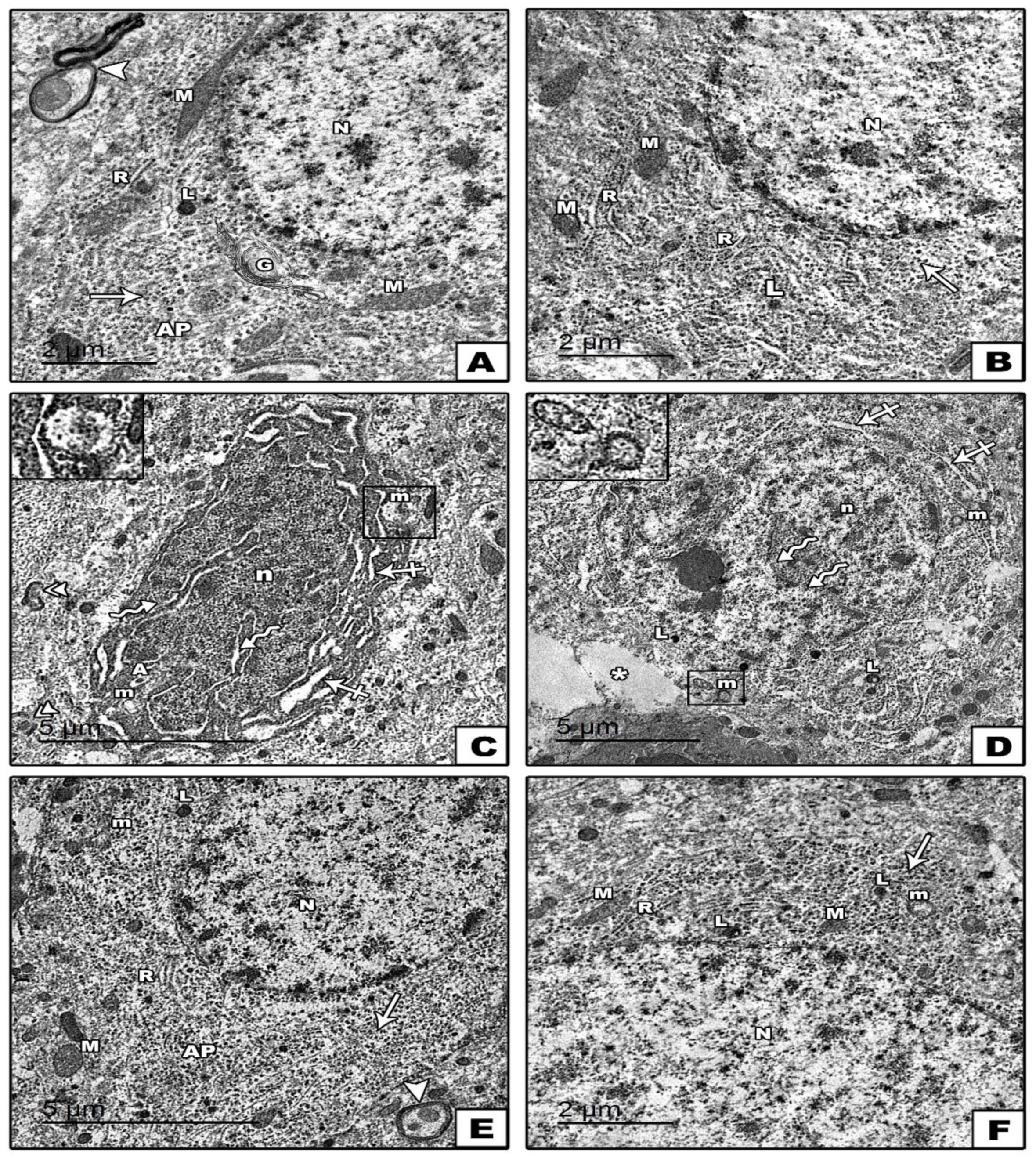
Electron Microscopic Results
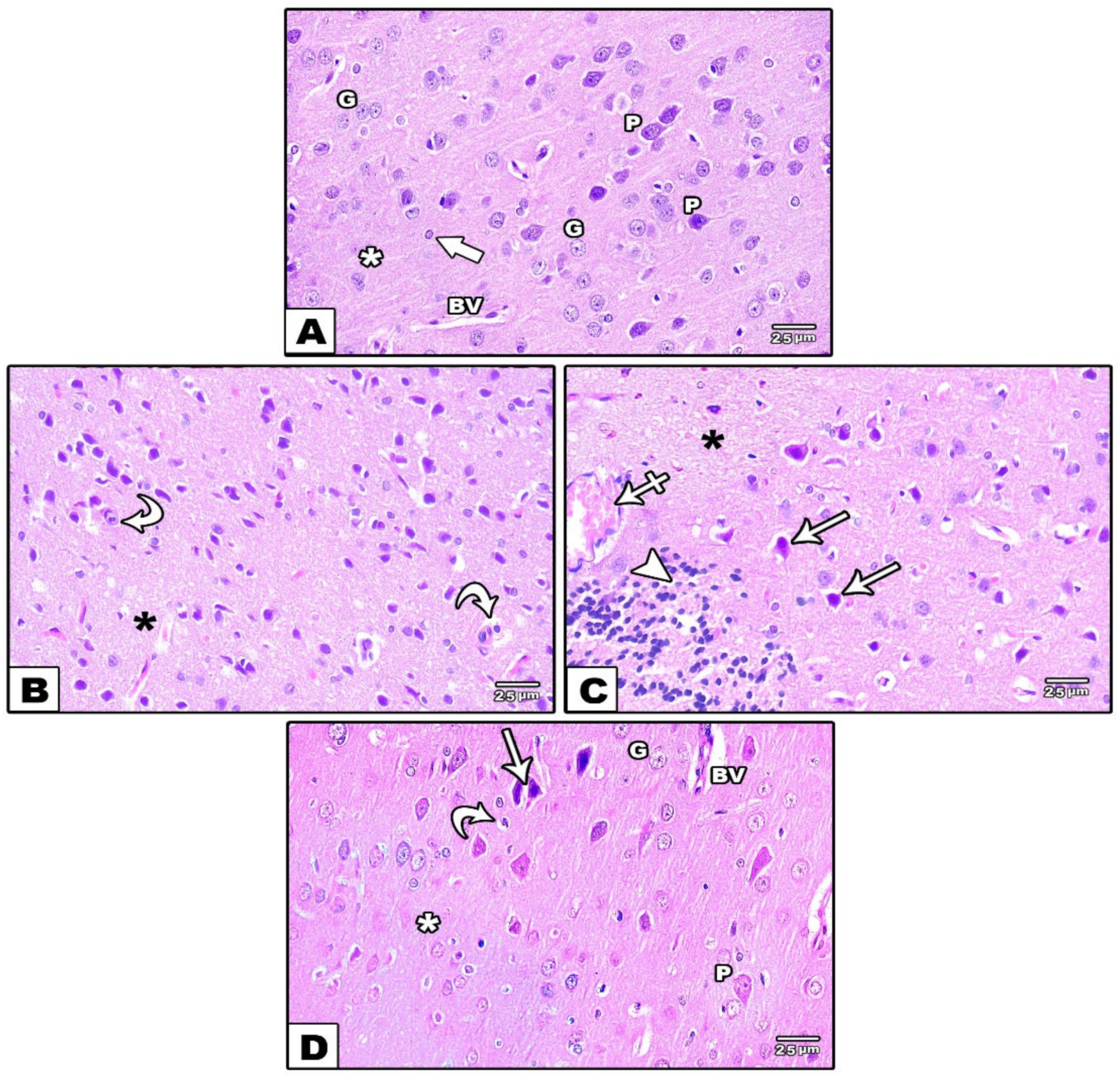
3.1.2. Cerebral Cortex
Light Microscopic Results
Electron Microscopic Results
3.2. Biochemical Study Results
3.3. Morphometric, Molecular and Statistical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, G.T.; Atzeni, F.; Beasley, M.; Flu, B.E.; Sarzi-Puttini, P.; Macfarlane, G.J. The prevalence of fibromyalgia in the general population: A comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis Rheumatol. 2015, 67, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Kim, H.S. Quantitative analysis of nailfold capillary morphology in patients with fibromyalgia. Korean J. Intern. Med. 2015, 30, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Son, C.N.; Kim, S.H.; Chang, H.W.; Kim, J.M. A neurometabolite study of chronic daily headache in patients with systemic lupus erythematosus using magnetic resonance spectroscopy: Comparison with fibromyalgia patients and healthy controls. Korean J. Intern. Med. 2016, 31, 1171–1177. [Google Scholar] [CrossRef][Green Version]
- Bradley, L.A. Pathophysiology of fibromyalgia. Am. J. Med. 2009, 122, S22–S30. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, N.; Szklarski, M.; Hartwig, J.; Sotzny, F.; Lorenz, S.; Meyer, A.; Grabowski, P.; Doehner, W.; Scheibenbogen, C. Peripheral endothelial dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome. ESC Heart Fail. 2020, 7, 1064–1071. [Google Scholar] [CrossRef]
- Mertoglu, C.; Gunay, M.; Yerligok, O. Could Endocan, a Marker of Inflammation and Endothelial Dysfunction, be a New Diagnostic Marker for Fibromyalgia? Clin. Lab. 2018, 64, 405–410. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 2010, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.M. Autophagy: In sickness and in health. Trends Cell Biol. 2004, 14, 70–77. [Google Scholar] [CrossRef]
- Martínez-Lara, A.; Moreno-Fernández, A.M.; Jiménez-Guerrero, M.; Díaz-López, C.; De-Miguel, M.; Cotán, D.; Sánchez-Alcázar, J.A. Mitochondrial Imbalance as a New Approach to the Study of Fibromyalgia. Open Access Rheumatol. 2020, 12, 175–185. [Google Scholar] [CrossRef]
- La Torre, F.; Nicolai, A.P. Clinical use of micronized purified flavonoid fraction for treatment of symptoms after hemorrhoidectomy: Results of a randomized, controlled, clinical trial. Dis. Colon Rectum 2004, 47, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Maher, P.; Dargusch, R.; Bodai, L.; Gerard, P.E.; Purcell, J.M.; Marsh, J.L. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington’s disease. Hum. Mol. Genet. 2011, 20, 261–270. [Google Scholar] [CrossRef]
- Yao, X.; Li, L.; Kandhare, A.D.; Mukherjee-Kandhare, A.A.; Bodhankar, S.L. Attenuation of reserpine-induced fibromyalgia via ROS and serotonergic pathway modulation by fisetin, a plant flavonoid polyphenol. Exp. Ther. Med. 2020, 19, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Nagakura, Y.; Takahashi, M.; Noto, T.; Sekizawa, T.; Oe, T.; Yoshimi, E.; Tamaki, K.; Shimizu, Y. Different pathophysiology underlying animal models of fibromyalgia and neuropathic pain: Comparison of reserpine-induced myalgia and chronic constriction injury rats. Behav. Brain Res. 2012, 226, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Kandhare, A.D.; Raygude, K.S.; Ghosh, P.; Ghule, A.E.; Bodhankar, S.L. Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia 2012, 83, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Gamble, M. The Hematoxylins and Eosin. In Theory and Practice of Histological Techniques; Elsevier: Amsterdam, The Netherlands, 2008; pp. 121–134. [Google Scholar] [CrossRef]
- Woods, A.E.; Stirling, J.W. Electron microscopy. In Theory and Practice of Histological Techniques, 6th ed.; Bancroft, J.D., Gamble, M., Eds.; Churchill Livingstone Elsevier: Edinburgh, UK, 2008; pp. 601–636. [Google Scholar]
- Cemek, M.; Kağa, S.; Şimşek, N.; Büyükokuroğlu, M.E.; Konuk, M. Antihyperglycemic and antioxidative potential of Matricaria chamomilla L. in streptozotocin-induced diabetic rats. J. Nat. Med. 2008, 62, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, J.; Fan, G.; Qi, S.; Li, B. Reperfusion promotes mitochondrial biogenesis following focal cerebral ischemia in rats. PLoS ONE 2014, 9, e92443. [Google Scholar] [CrossRef]
- Stavenuiter, A.W.; Arcidiacono, M.V.; Ferrantelli, E.; Keuning, E.D.; Vila Cuenca, M.; ter Wee, P.M.; Beelen, R.H.J.; Vervloet, M.G.; Dusso, A.S. A novel rat model of vitamin D deficiency: Safe and rapid induction of vitamin D and calcitriol deficiency without hyperparathyroidism. BioMed Res. Int. 2015, 2015, 604275. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sun, J.; Huang, X. Expression of caspase-3, Bax and Bcl-2 in hippocampus of rats with diabetes and subarachnoid hemorrhage. Exp. Ther. Med. 2018, 15, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Gong, Y.; Yan, J.Z.; Zhang, L.L. Autophagy inhibitor 3-methyladenine regulates the expression of LC3, Beclin-1 and ZnTs in rat cerebral cortex following recurrent neonatal seizures. World J. Emerg. Med. 2010, 1, 216–223. [Google Scholar] [PubMed]
- Abo El-Khair, S.M.; Ghoneim, F.M.; Shabaan, D.A.; Elsamanoudy, A.Z. Molecular and ultrastructure study of endoplasmic reticulum stress in hepatic steatosis: Role of hepatocyte nuclear factor 4α and inflammatory mediators. Histochem. Cell Biol. 2020, 153, 49–62. [Google Scholar] [CrossRef]
- Draper, H.H.; Squires, E.J.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M.A. Comparative evaluation of thiobarbituric acid methods for the determination of malondialdehydes in biological materials. Free. Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Nagakura, Y.; Oe, T.; Aoki, T.; Matsuoka, N. Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: A putative animal model of fibromyalgia. Pain 2009, 146, 26–33. [Google Scholar] [CrossRef] [PubMed]
- DeSantana, J.M.; da Cruz, K.M.; Sluka, K.A. Animal models of fibromyalgia. Arthritis Res. Ther. 2013, 15, 222. [Google Scholar] [CrossRef] [PubMed]
- Staud, R. Peripheral and central mechanisms of fatigue in inflammatory and noninflammatory rheumatic diseases. Curr. Rheumatol. Rep. 2012, 14, 539–548. [Google Scholar] [CrossRef]
- Kadetoff, D.; Lampa, J.; Westman, M.; Andersson, M.; Kosek, E. Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J. Neuroimmunol. 2012, 242, 33–38. [Google Scholar] [CrossRef]
- Montoro, C.I.; Duschek, S.; Schuepbach, D.; Gandarillas, M.A.; Reyes Del Paso, G.A. Cerebral blood flow variability in fi-bromyalgia syndrome: Relationships with emotional, clinical and functional variables. PLoS ONE 2018, 13, e0204267. [Google Scholar] [CrossRef]
- Giske, L.; Vøllestad, N.K.; Mengshoel, A.M.; Jensen, J.; Knardahl, S.; Røe, C. Attenuated adrenergic responses to exercise in women with fibromyalgia: A controlled study. Eur. J. Pain 2008, 12, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, K.I.; Kim, S.M.; Lee, H.G.; Kim, T.I. Arterial stiffness in female patients with fibromyalgia and its relationship to chronic emotional and physical stress. Korean Circ. J. 2011, 41, 596–602. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harris, K.F.; Matthews, K.A.; Sutton-Tyrrell, K.; Kuller, L.H. Associations between psychological traits and endothelial function in postmenopausal women. Psychosom. Med. 2003, 65, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Zaroff, J.G.; Rordorf, G.A.; Titus, J.S.; Newell, J.B.; Nowak, N.J.; Torchiana, D.F.; Aretz, H.T.; Picard, M.H.; Macdonald, R.L. Regional myocardial perfusion after experimental subarachnoid hemorrhage. Stroke 2000, 31, 1136–1143. [Google Scholar] [CrossRef]
- Nah, S.S.; Lee, H.; Hong, Y.; Im, J.; Won, H.; Chang, S.H.; Kim, H.K.; Kwon, J.T.; Kim, H.J. Association between endothelin 1 and fibromyalgia syndrome. Mol. Med. Rep. 2017, 16, 6234–6239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kohn, J.C.; Lampi, M.C.; Reinhart-King, C.A. Age-related vascular stiffening causes and consequences. Front. Genet. 2015, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, I.B.; Qasem, A.; McEniery, C.M.; Webb, D.J.; Avolio, A.P.; Cockcroft, J.R. Nitric oxide regulates local arterial distensibility in vivo. Circulation 2002, 105, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Domínguez, B.; Bullón, P.; Román-Malo, L.; Marín-Aguilar, F.; Alcocer-Gómez, E.; Carrión, A.M.; Sánchez-Alcazar, J.A.; Cordero, M.D. Oxidative stress, mitochondrial dysfunction and, inflammation common events in skin of patients with Fibromyalgia. Mitochondrion 2015, 21, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Kumar, D.S.; Ali, M.A.; Agarwal, S.; Khandpur, S. Nitric oxide, lipid peroxidation products, and antioxidants in primary fibromyalgia and correlation with disease severity. J. Med. Biochem. 2020, 39, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Sendur, O.F.; Turan, Y.; Tastaban, E.; Yenisey, C.; Serter, M. Serum antioxidants and nitric oxide levels in fibromyalgia: A controlled study. Rheumatol. Int. 2009, 29, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Porreca, F.; Cuzzocrea, S.; Galen, K.; Lightfoot, R.; Masini, E.; Muscoli, C.; Mollace, V.; Ndengele, M.; Ischirop-oulos, H.; et al. A Newly Identified Role for Superoxide in Inflammatory Pain. J. Pharmacol. Exp. Ther. 2004, 309, 869. [Google Scholar] [CrossRef]
- Cimen, O.B.; Cimen, M.Y.; Yapici, Y.; Camdeviren, H. Arginase, NOS activities, and clinical features in fibromyalgia patients. Pain Med. 2009, 10, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Suwa, M.; Nakano, H.; Radak, Z.; Kumagai, S. Effects of Nitric Oxide Synthase Inhibition on Fiber-Type Composition, Mitochondrial Biogenesis, and SIRT1 Expression in Rat Skeletal Muscle. J. Sports Sci. Med. 2015, 14, 548–555. [Google Scholar]
- Ozgocmen, S.; Ozyurt, H.; Sogut, S.; Akyol, O.; Ardicoglu, O.; Yildizhan, H. Antioxidant status, lipid peroxidation and nitric oxide in fibromyalgia: Etiologic and therapeutic concerns. Rheumatol. Int. 2006, 26, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Dolcino, M.; Tinazzi, E.; Puccetti, A.; Lunardi, C. Gene Expression Profiling in Fibromyalgia Indicates an Autoimmune Origin of the Disease and Opens New Avenues for Targeted Therapy. J. Clin. Med. 2020, 9, 1814. [Google Scholar] [CrossRef]
- Yüksel, E.; Nazıroğlu, M.; Şahin, M.; Çiğ, B. Involvement of TRPM2 and TRPV1 channels on hyperalgesia, apoptosis and oxidative stress in rat fibromyalgia model: Protective role of selenium. Sci. Rep. 2017, 7, 17543. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; De Miguel, M.; Moreno Fernández, A.M.; Carmona López, I.M.; Garrido Maraver, J.; Cotán, D.; Gómez Izquierdo, L.; Bonal, P.; Campa, F.; Bullon, P.; et al. Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: Implications in the pathogenesis of the disease. Arthritis Res. Ther. 2010, 12, R17. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Yang, S.; Yin, J. Blocking the REDD1/TXNIP axis ameliorates LPS-induced vascular endothelial cell injury through repressing oxidative stress and apoptosis. Am. J. Physiol. Cell Physiol. 2019, 316, C104–C110. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I.; Bawazeer, M. Mast Cells, Neuroinflammation and Pain in Fibromyalgia Syndrome. Front. Cell Neurosci. 2019, 13, 353. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Oezel, L.; Then, H.; Jung, A.L.; Jabari, S.; Bonaterra, G.A.; Wissniowski, T.T.; Önel, S.F.; Ocker, M.; Thieme, K.; Kinscherf, R.; et al. Fibromyalgia syndrome: Metabolic and autophagic processes in intermittent cold stress mice. Pharmacol. Res. Perspect. 2016, 4, e00248. [Google Scholar] [CrossRef]
- Tolkovsky, A.M. Mitophagy. Biochim. Biophys. Acta 2009, 1793, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Auer, R.N.; Sutherland, G.R. Hypoxia and related conditions. Greenfield’s Neuropathol. 2002, 1, 233–280. [Google Scholar]
- Coimbra-Costa, D.; Alva, N.; Duran, M.; Carbonell, T.; Rama, R. Oxidative stress and apoptosis after acute respiratory hy-poxia and reoxygenation in rat brain. Redox Biol. 2017, 12, 216–225. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yuan, M.; Guo, Y.S.; Shen, X.Y.; Gao, Z.K.; Bi, X. Mechanism of Endoplasmic Reticulum Stress in Cerebral Ischemia. Front. Cell. Neurosci. 2021, 15, 704334. [Google Scholar] [CrossRef]
- Hetz, C.; Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 2017, 13, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.T.; Liao, Y.L.; Zheng, J.; Li, W.N. Icariin protects neurons from endoplasmic reticulum stress-induced apoptosis after OGD/R injury via suppressing IRE1α-XBP1 signaling pathway. Life Sci. 2020, 255, 117847. [Google Scholar] [CrossRef]
- Hetz, C.; Papa, F.R. The unfolded protein response and cell fate control. Mol. Cell. 2018, 69, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gu, Y.; Wang, W.; Chen, W. Astragalin alleviates ischemia/reperfusion-induced brain injury via suppression of endoplasmic reticulum stress. Mol. Med. Rep. 2020, 22, 4070–4078. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Liu, R.; Wu, Y.; Huang, S. Astragaloside IV reduces cerebral ischemia/reperfusion-induced blood-brain barrier permeability in rats by inhibiting ER stress-mediated apoptosis. Evid. Based Complement. Altern. Med. 2020, 2020, 9087873. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Chen, L. Endoplasmic Reticulum Stress and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, M.; Ramachandran, R. Fisetin protects against rotenone-induced neurotoxicity through signaling pathway. Front. Biosci. (Elite Ed.) 2019, 11, 20–28. [Google Scholar] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Adegbola, P.; Aderibigbe, I.; Hammed, W.; Omotayo, T. Antioxidant and anti-inflammatory medicinal plants have potential role in the treatment of cardiovascular disease: A review. Am. J. Cardiovasc. Dis. 2017, 7, 19–32. [Google Scholar] [PubMed]
- Rodius, S.; de Klein, N.; Jeanty, C.; Sánchez-Iranzo, H.; Crespo, I.; Ibberson, M.; Xenarios, I.; Dittmar, G.; Mercader, N.; Niclou, S.P.; et al. Fisetin protects against cardiac cell death through reduction of ROS production and caspases activity. Sci. Rep. 2020, 10, 2896. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qin, H.; Zhang, H.; Feng, X.; Yang, L.; Hou, D.X.; Chen, J. Fisetin inhibits inflammation and induces autophagy by mediating PI3K/AKT/mTOR signaling in LPS-induced RAW264.7 cells. Food Nutr. Res. 2021, 25, 65. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Lall, R.K.; Chamcheu, J.C.; Haidar, O.; Mukhtar, H. Involvement of ER stress and activation of apoptotic pathways in fisetin induced cytotoxicity in human melanoma. Arch. Biochem. Biophys. 2014, 563, 108–117. [Google Scholar] [CrossRef]


| Group I (-ve Control DMSO) | Group II (+ve Control Fisetin) | Group III (Reserpine Treated Group) | Group IV (Reserpine + Fisetin Treated Group) | F | p | |
|---|---|---|---|---|---|---|
| Behavioral tests | ||||||
| Randall-Selitto paw pressure test (grams) | 220.40 ± 6.50 | 221.70 ± 6.34 | 83.90 ± 6.54 AB | 172.90 ± 5.45 ABC | 179.713 | <0.001 |
| Hargreaves test (seconds) | 12.60 ± 3.50 | 13.90 ± 3.60 | 4.50 ± 1.58 AB | 11.30 ± 1.89 C | 23.836 | <0.001 |
| Tail suspension test (TST) (seconds) | 7.20 ± 1.81 | 7.90 ± 2.28 | 48 ± 2.36 AB | 17.40 ± 4.50 ABC | 227.691 | <0.001 |
| Serum oxidative stress parameters | ||||||
| MDA (μmol MDA/L) | 14.41 ± 1.75 | 15.11 ± 1.26 | 36.08 ± 4.63 AB | 21.37 ± 3.54 ABC | 111.835 | <0.001 |
| TAC (mmol/L) | 862.82 ± 138.28 | 873.45 ± 144.99 | 406.74 ± 56.76 AB | 815.39 ± 70 C | 39.942 | <0.001 |
| Serum NO (μmol/L) | 24.82 ± 2.47 | 23.97 ± 2.78 | 13.83 ± 5.18 AB | 28.46 ± 8.85 C | 54.166 | <0.001 |
| Group I (-ve Control DMSO) | Group II (+ve Control Fisetin) | Group III (Reserpine Treated Group) | Group IV (Reserpine + Fisetin Treated Group) | F | p | |
|---|---|---|---|---|---|---|
| IMT | 32.94 ± 6.53 | 33.07 ± 5.09 | 44.02 ± 11.94 AB | 36.03 ± 7.76 C | 5.979 | 0.001 |
| Area % of collagen | 8.92 ± 2.20 | 8.34 ± 1.83 | 14.30 ± 3.59 AB | 9.38 ± 3.72 C | 12.915 | <0.001 |
| Total number of mitochondria | 6.07 ± 1.33 | 5.47 ± 0.99 | 14.87 ± 1.30 AB | 6.46 ± 1.06 BC | 22.620 | <0.001 |
| Area % of eNOS | 2.41 ± 0.13 | 2.13 ± 0.15 | 1.63 ± 0.75 AB | 2.11 ± 0.08 C | 13.549 | <0.001 |
| eNOS gene expression (2−∆∆CT) | 1 ± 0.02 | 1.02 ± 0.03 | 0.47 ± 0.11 AB | 0.90 ± 0.13 BC | 84.120 | <0.001 |
| Bcl2 gene expression (2−∆∆CT) | 1 ± 0.03 | 1.04 ± 0.03 | 0.60 ± 0.18 AB | 1.19 ± 0.19 AC | 37.860 | <0.001 |
| Caspase-3 gene expression (2−∆∆CT) | 1 ± 0.02 | 1.02 ± 0.06 | 2.33 ± 0.36 AB | 1.41 ± 0.19 ABC | 91.075 | <0.001 |
| LC-3 gene expression (2−∆∆CT) | 1 ± 0.03 | 1.01 ± 0.02 | 2.28 ± 0.22 AB | 1.60 ± 0.21 ABC | 152.775 | <0.001 |
| BECN-1 gene expression (2−∆∆CT) | 1.01 ± 0.02 | 1.03 ± 0.02 | 2.24 ± 0.29 AB | 1.46 ± 0.17 ABC | 116.248 | <0.001 |
| CHOP gene expression (2−∆∆CT) | 1.05 ± 0.07 | 1.02 ± 0.04 | 1.36 ± 0.17 AB | 1.21 ± 0.12 BC | 15.130 | <0.001 |
| TNF-α gene expression (2−∆∆CT) | 1.01 ± 0.03 | 1.02 ± 0.04 | 2.28 ± 0.19 AB | 1.47 ± 0.29 ABC | 201.37 | <0.001 |
| Group I (-ve Control DMSO) | Group II (+ve Control Fisetin) | Group III (Reserpine Treated Group) | Group IV (Reserpine + Fisetin Treated Group) | F | p | |
|---|---|---|---|---|---|---|
| Total number of mitochondria | 9.20 ± 1.37 | 10.07 ± 0.88 | 21.07 ± 1.33 | 11 ± 1.19 | 48.419 | <0.001 |
| Area % of eNOS | 1.90 ± 0.18 | 1.81 ± 0.17 | 0.92 ± 0.33 AB | 1.55 ± 0.28 ABC | 43.306 | <0.001 |
| eNOS gene expression (2−∆∆CT) | 1 ± 0.02 | 1.04 ± 0.03 | 0.57 ± 0.14 AB | 0.91 ± 0.13 BC | 49.354 | <0.001 |
| Bcl2 gene expression (2−∆∆CT) | 1.01 ± 0.03 | 1.04 ± 0.03 | 0.62 ± 0.16 AB | 1.12 ± 0.13 C | 42.636 | <0.001 |
| Caspase-3 gene expression (2−∆∆CT) | 1.01 ± 0.02 | 1.03 ± 0.03 | 2.36 ± 0.24 AB | 1.52 ± 0.14 ABC | 209.567 | <0.001 |
| LC-3 gene expression (2−∆∆CT) | 1 ± 0.02 | 1.01 ± 0.02 | 1.74 ± 0.23 AB | 1.18 ± 0.11 ABC | 73.270 | <0.001 |
| BECN-1 gene expression (2−∆∆CT) | 1.01 ± 0.02 | 1.01 ± 0.02 | 2.39 ± 0.23 AB | 1.44 ± 0.14 ABC | 222.45 | <0.001 |
| CHOP gene expression (2−∆∆CT) | 1.01 ± 0.07 | 1.03 ± 0.04 | 2.03 ± 0.21 AB | 1.20 ± 0.19 BC | 24.611 | <0.001 |
| TNF-α gene expression (2−∆∆CT) | 1.01 ± 0.03 | 1.04 ± 0.02 | 2.18 ± 0.19 AB | 1.33 ± 0.19 ABC | 213.39 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghoneim, F.M.; Abo-Elkhair, S.M.; Elsamanoudy, A.Z.; Shabaan, D.A. Evaluation of Endothelial Dysfunction and Autophagy in Fibromyalgia-Related Vascular and Cerebral Cortical Changes and the Ameliorative Effect of Fisetin. Cells 2022, 11, 48. https://doi.org/10.3390/cells11010048
Ghoneim FM, Abo-Elkhair SM, Elsamanoudy AZ, Shabaan DA. Evaluation of Endothelial Dysfunction and Autophagy in Fibromyalgia-Related Vascular and Cerebral Cortical Changes and the Ameliorative Effect of Fisetin. Cells. 2022; 11(1):48. https://doi.org/10.3390/cells11010048
Chicago/Turabian StyleGhoneim, Fatma Mohamed, Salwa Mohamed Abo-Elkhair, Ayman Zaky Elsamanoudy, and Dalia A. Shabaan. 2022. "Evaluation of Endothelial Dysfunction and Autophagy in Fibromyalgia-Related Vascular and Cerebral Cortical Changes and the Ameliorative Effect of Fisetin" Cells 11, no. 1: 48. https://doi.org/10.3390/cells11010048
APA StyleGhoneim, F. M., Abo-Elkhair, S. M., Elsamanoudy, A. Z., & Shabaan, D. A. (2022). Evaluation of Endothelial Dysfunction and Autophagy in Fibromyalgia-Related Vascular and Cerebral Cortical Changes and the Ameliorative Effect of Fisetin. Cells, 11(1), 48. https://doi.org/10.3390/cells11010048






