Purification, Characterization and Biological Activity of Polysaccharides from Dendrobium officinale
Abstract
:1. Introduction
2. Results
2.1. Extraction, Purification and Preliminary Characterization of DOPA Fractions
2.1.1. Extraction and Purification of DOPA Fractions
2.1.2. Molecular Weight and Chemical Composition of DOPA Fractions
2.1.3. Analysis of FT-IR Spectra of DOPA Fractions
2.1.4. Methylation and GC-MS Analysis
2.1.5. Analysis of the NMR Spectra of DOPA Fractions
2.2. Activation of RAW 264.7 Macrophages by D. officinale Polysaccharides in Vitro
2.2.1. Effect of D. officinale Polysaccharides on Macrophages Viability
2.2.2. Effects of D. officinale Polysaccharides on NO Production in Macrophages
2.3. Effects of D. officinale Polysaccharides on Activivation of Splenocytes
2.4. Antioxidant Activity Assay in Macrophages Treated with H2O2
2.4.1. Effect of H2O2 on the Viability of Macrophages
2.4.2. Effects of D. officinale Polysaccharides on the Viability of H2O2-Treated Macrophages
2.4.3. Effects of D. officinale Polysaccharides on The morphology of H2O2-Treated Macrophages
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Extraction and Isolation of Polysaccharides
4.2.1. Extraction Procedures
4.2.2. Isolation and Purification of the Polysaccharides
4.3. Preliminary Characterization of DOPA Fractions
4.3.1. Monosaccharide Composition Analysis
4.3.2. Molecular Weight Determination
4.3.4. Fourier Transform Infrared Spectroscopy Analysis
4.3.5. Methylation and GC-MS Analysis
4.3.6. Nuclear Magnetic Resonance Spectroscopy
4.4. Activation of RAW 264.7 Macrophages in Vitro
4.4.1. Cell Culture
4.4.2. Cell Stimulation Assay
4.4.3. Assay of the Nitric Oxide (NO) Production of Macrophages
4.5. Activation of Splenocytes in Vitro
4.6. Antioxidant Activity Assay in Macrophages Treated with H2O2
4.6.1. Assessment of Cell Viability
4.6.2. Morphological Observation
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Committee, C.P. Pharmacopoeia of China; Medical Science Press: Beijing, China, 2010; Volume I, pp. 265–266. [Google Scholar]
- Wu, Z.; Raven, P.H.; Hong, D. (Eds.) Flora of China; Science Press: Beijing, China, 1999; Volume 19, p. 67.
- Xing, X.H.; Cui, S.W.; Niec, S.P.; Phillips, G.O.; Goff, H.D.; Wang, Q. A review of isolation process, structural characteristics, and bioactivities of water-soluble polysaccharides from Dendrobium plants. Bioact. Carbohydr. Diet. Fibre 2013, 1, 131–147. [Google Scholar] [CrossRef]
- Fu, L.G. China Plant Red Data Book: Rare and Endangered Plants; Science Press: Beijing, China, 1992; pp. 492–493. [Google Scholar]
- Zhang, A.L.; Wei, T.; Si, J.P.; Jin, L.Y.; Mo, Y.N. Study on basic amino acid contents in Dendrobium officinale. J. Chin. Mater. Med. 2011, 19, 2632–2635. [Google Scholar]
- Chen, X.M.; Wang, F.F.; Wang, Y.Q.; Li, X.L.; Wang, A.R.; Wang, C.L.; Guo, S.X. Discrimination of the rare medicinal plant Dendrobium officinale based on naringenin, bibenzyl, and polysaccharides. Sci. China Life Sci. 2012, 55, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Y.; Li, C.; Luo, H.; Wang, L.; Qian, J.; Luo, X.; Xiang, L.; Song, J.; Sun, C.; et al. Analysis of the Dendrobium officinale transcriptome reveals putative alkaloid biosynthetic genes and genetic markers. Gene 2013, 527, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Z.; Xu, L. Simultaneous determination of phenols (bibenzyl, phenanthrene, and fluorenone) in dendrobium species by high-performance liquid chromatography with diode array detection. J. Chromatogr. A 2006, 1104, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.Y.; Lu, J.J.; Jin, C.S.; Xia, H. Chemical constituents from n-butanol extracts of Dendrobium officinale. Mod. Chin. Med. 2013, 23, 1042–1045. [Google Scholar]
- Hua, Y.F.; Zhang, M.; Fu, C.X.; Chen, Z.H.; Chan, G.Y. Structural characterization of a 2-O-acetylglucomannan from Dendrobium officinale stem. Carbohydr. Res. 2004, 339, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.Z.; Lv, G.P.; Hu, D.J.; Cheong, K.L.; Xie, J.; Zhao, J.; Li, S.P. Effects of polysaccharides from different species of Dendrobium (Shihu) on macrophage function. Molecules 2013, 18, 5779–5791. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.H.; Li, X.F.; Wang, M.N.; Zha, X.Q.; Yang, X.F.; Liu, Z.J.; Luo, Y.B.; Luo, J.P. Comparison of hypoglycemic and antioxidative effects of polysaccharides from four different Dendrobium species. Int. J. Biol. Macromol. 2014, 64, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Cao, Z.X.; Peng, C.; Li, X.H.; Xie, X.F.; Zhang, T.M.; Zhou, Q.M.; Yang, L.; Guo, L. Phenolic glucosides from Dendrobium aurantiacum var. Denneanum and their bioactivities. Molecules 2013, 18, 6153–6160. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.L.; Huang, X.J.; Nie, S.P.; Xie, M.Y.; Phillips, O.G.; Cui, S.W. Study on Dendrobium officinale O-acetyl-glucomannan (dendronan®): Part III. Immunomodulatory activity in vitro. Bioact. Carbohydr. Diet. Fibre 2015, 5, 99–105. [Google Scholar] [CrossRef]
- Huang, X.J.; Nie, S.P.; Cai, H.L.; Zhang, G.Y.; Cui, S.W.; Xie, M.Y.; Phillips, G.O. Study on Dendrobium officinale O-acetyl-glucomannan: Part VI. Protective effects against oxidative stress in immunosuppressed mice. Food Res. Int. 2015, 72, 168–173. [Google Scholar] [CrossRef]
- Huang, X.J.; Nie, S.P.; Cai, H.L.; Zhang, G.Y.; Cui, S.W.; Xie, M.Y.; Phillips, G.O. Study on Dendrobium officinale O-acetyl-glucomannan (dendronan®): Part IV. Immunomodulatory activity in vivo. J. Funct. Foods. 2015, 15, 525–532. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Nie, S.P.; Huang, X.J.; Hu, J.L.; Cui, S.W.; Xie, M.Y.; Phillips, G.O. Study on Dendrobium officinale O-acetyl-glucomannan (dendronan®). Part VII. Improving effects on colonic health of mice. J. Agric. Food Chem. 2015, 64, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, S.C.; Wang, Z.T.; Hu, Z.B. Structure analysis of polysaccharides from Dendrobium candidum. Chin. Pharm. J. 2004, 39, 254–256. [Google Scholar]
- Sheng, J.R. Extraction, Isolation, Purification, Structural Analysis of the Polysaccharide from Dendrobium Candidum; Guangxi Teachers Education University: Guangxi, China, 2009. [Google Scholar]
- Wang, S.L.; Zheng, G.Z.; He, J.B.; Yu, X.J.; Wu, Y. Studies on polysaccharides of Dendrobium candidum. Acta Bot. Yunnanica 1997, 10, 389–395. [Google Scholar]
- Bing, Y.B. Preliminary Study on Structure and Anti-Oxidation of the Polysaccharide from Dendrobium officinale; Beijing Forestry University: Beijing, China, 2014. [Google Scholar]
- Xia, L.J.; Liu, X.F.; Guo, H.Y.; Zhang, H.; Zhu, J.; Ren, F.Z. Partial characterization and immunomodulatory activity of polysaccharides from the stem of Dendrobium officinale (Tiepishihu) in vitro. J. Funct. Foods 2012, 4, 294–301. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Lv, G.Y.; He, W.Q.; Shi, L.G.; Pan, H.J.; Fan, L.F. Effects of extraction methods on the antioxidant activities of polysaccharides obtained from Flammulina velutipes. Carbohydr Polym. 2013, 98, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Thanzami, K.; Malsawmtluangi, C.; Lalhlenmawia, H.; Seelan, T.V.; Palanisamy, S.; Kandasamy, R.; Pachuau, L. Characterization and in vitro antioxidant activity of Albizia stipulata boiv. Gum exudates. Int. J. Biol. Macromol. 2015, 80, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Zheng, J.; Xia, X.J.; Kan, J.Q. Purification and structural identification of polysaccharides from bamboo shoots (Dendrocalamus latiflorus). Int. J. Mol. Sci. 2015, 16, 15560–15577. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.H.; Cui, S.W.; Nie, S.P.; Phillips, O.G.; Goff, D.H.; Wang, Q. Study on Dendrobium officinale O-acetyl-glucomannan (dendronan®): Part I. Extraction, purification, and partial structural characterization. Bioact. Carbohydr. Diet. Fibre 2014, 4, 74–85. [Google Scholar] [CrossRef]
- Wang, C.Z.; Zhang, H.Y.; Li, W.J.; Ye, J.Z. Chemical constituents and structural characterization of polysaccharides from four typical bamboo species leaves. Molecules 2015, 20, 4162–4179. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Y.; Yi, Y.; Zhang, L.F.; Zhang, R.F.; Zhang, Y.; Wei, Z.C.; Tang, X.J.; Zhang, M.W. Immunomodulatory activity and partial characterisation of polysaccharides from momordica charantia. Molecules 2014, 19, 13432–13447. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.J.; Lin, M.C.; Luo, A.S.; Chun, Z.; Luo, A.X. Characterization and antitumor activity of a polysaccharide from Sarcodia ceylonensis. Molecules 2014, 19, 10863–10876. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.H.; Cui, S.W.; Nie, S.P.; Phillips, G.O.; Goff, D.H.; Wang, Q. Study on Dendrobium officinale O-acetyl-glucomannan (dendronan®): Part V. Fractionation and structural heterogeneity of different fractions. Bioact. Carbohydr. Diet. Fibre 2015, 5, 106–115. [Google Scholar] [CrossRef]
- Xing, X.H.; Cui, S.W.; Nie, S.; Phillips, G.O.; Goff, H.D.; Wang, Q. Study on Dendrobium officinale O-acetyl-glucomannan (dendronan®): Part II. Fine structures of O-acetylated residues. Carbohydr. Polym. 2015, 117, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.Y.; Chien, C.; Liao, S.K.S.; Liao, S.F.; Hung, W.T.; Yang, W.B.; Lin, C.C.; Cheng, T.J.R.; Chang, C.C.; Fang, J.M.; et al. Structure and bioactivity of the polysaccharides in medicinal plant Dendrobium huoshanense. Bioorg. Med. Chem. 2008, 16, 6054–6068. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Luo, J.P.; Zha, X.Q. Structural features of a pectic polysaccharide from the stems of Dendrobium nobile lindl. Carbohydr. Polym. 2010, 81, 1–7. [Google Scholar] [CrossRef]
- Guo, Q.; Cui, S.W.; Wang, Q.; Hu, X.; Kang, J.; Yada, R.Y. Structural characterization of a low-molecular-weight heteropolysaccharide (glucomannan) isolated from Artemisia sphaerocephala krasch. Carbohydr. Res. 2012, 350, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Do, T.T.; Nguyen, T.D.; Pham, L.D.; Nguyen, V.D. Isolation and characteristics of polysaccharide from Amorphophallus corrugatus in Vietnam. Carbohydr. Polym. 2011, 84, 64–68. [Google Scholar] [CrossRef]
- Wang, J.H.; Zha, X.Q.; Luo, J.P.; Yang, X.F. An acetylated galactomannoglucan from the stems of Dendrobium nobile Lindl. Carbohydr. Res. 2010, 345, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Janeway, C. Innate immune recognition: Mechanisms and pathways. Immunol. Rev. 2000, 173, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Larghi, P.; Mancino, A.; Rubino, L.; Porta, C.; Totaro, M.G.; Rimoldi, M.; Biswas, S.K.; Allavena, P.; Mantovani, A. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008, 18, 349–355. [Google Scholar] [CrossRef] [PubMed]
- MacKay, R.J.; Russell, S.W. Protein changes associated with stages of activation of mouse macrophages for tumor cell killing. J. Immunol. 1986, 137, 1392–1398. [Google Scholar] [PubMed]
- Ali, R.; Kumar, S.; Naqvi, R.A.; Rao, D.N. B and T cell epitope mapping and study the humoral and cell mediated immune response to B-T constructs of YSCF antigen of Yersinia pestis. Comp. Immunol. Microb. 2013, 36, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, F.; Cordeiro-Da-Silva, A.; Gaspar-Marques, C.; Simoes, F.; Pinto, M.M.M.; Nascimento, M.S.J. Effect of abietane diterpenes from plectranthus grandidentatus on T- and B-lymphocyte proliferation. Bioorg. Med. Chem. 2004, 12, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, K.X.; Peng, J.Y.; Wang, C.Y.; Kang, L.; Chang, N.; Sun, H.J. Rhizoma Dioscoreae nipponicae polysaccharides protect HUVECs from H2O2-induced injury by regulating PPARγ factor and the NADPH oxidase/ROS-NF-κB signal pathway. Toxicol. Lett. 2015, 232, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Gille, J.J.P.; Joenje, H. Cell culture models for oxidative stress: Superoxide and hygen peroxide versus normobaric hyperoxia. Mutat. Res. 1992, 275, 405–414. [Google Scholar] [CrossRef]
- Wink, D.A.; Cook, J.A.; Pacelli, R.; DeGraff, W.; Gamson, J.; Liebmann, J.; Krishna, M.C.; Mitchell, J.B. The effect of various nitric oxide-donor agents on hydrogen peroxide-mediated toxicity: A direct correlation between nitric oxide formation and protection. Arch. Biochem. Biophys. 1996, 331, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, O.; Cattabeni, F.; Stocchi, V.; Meyn, R.E.; Cerutti, P.; Murray, D. Hydrogen peroxide insult in cultured mammalian cells: Relationships between DNA single-strand breakage, poly(ADP-ribose) metabolism and cell killing. BBA-Mol. Cell Res. 1989, 1014, 1–7. [Google Scholar] [CrossRef]
- Maeng, O.; Kim, Y.C.; Shin, H.J.; Lee, J.O.; Huh, T.L.; Kang, K.I.; Kim, Y.S.; Paik, S.G.; Lee, H. Cytosolic NADP+-dependent isocitrate dehydrogenase protects macrophages from LPS-induced nitric oxide and reactive oxygen species. Biochem. Biophys. Res. Commun. 2004, 317, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Thoeni, G.; Werner, E.R.; Werner-Felmayer, G. Tetrahydropteridines suppress gene expression and induce apoptosis of activated RAW264.7 cells via formation of hydrogen peroxide. Free Radic. Biol Med. 2004, 37, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Methacanon, P.; Madla, S.; Kirtikara, K.; Prasitsil, M. Structural elucidation of bioactive fungi-derived polymers. Carbohydr. Polym. 2005, 60, 199–203. [Google Scholar] [CrossRef]
- Wang, J.C.; Hu, S.H.; Su, C.H.; Lee, T.M. Antitumor and immunoenhancing activities of polysaccharide from culture broth of Hericium spp. Kaohsiung J. Med. Sci. 2001, 17, 461–467. [Google Scholar] [PubMed]
- Wang, Y.F.; Liu, Y.Y.; Mao, F.F.; Liu, Y.R.; Wei, X.L. Purification, characterization and biological activities in vitro of polysaccharides extracted from tea seeds. Int. J. Biol. Macromol. 2013, 62, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Q.H.; Sun, Y.P.; Yang, B.Y.; Wang, Z.B.; Chai, G.F.; Guan, Y.Z.; Zhu, W.G.; Shu, Z.P.; Lei, X.; et al. Purification, characterization and immunomodulatory effects of Plantago depressa polysaccharides. Carbohydr. Polym. 2014, 112, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Sun, S.S.; Li, R.; Shen, Z.P.; Wang, P.; Jiang, X.L. Antioxidant activity of polysaccharides produced by Hirsutella sp. and relation with their chemical characteristics. Carbohydr. Polym. 2015, 117, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.C.; Lu, T.J.; Hsieh, C.C.; Lin, W.C. Characterization and immunomodulatory activity of polysaccharides derived from Dendrobium tosaense. Carbohydr. Polym. 2014, 111, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Sevag, M.G.; Lackman, D.B.; Smolens, J. The isolation of the components of streptococcal nucleoproteins in serologically active form. J. Biol. Chem. 1938, 124, 425–436. [Google Scholar]
- Zhang, S.S.; Liu, X.Q.; Yan, L.H.; Zhang, Q.W.; Zhu, J.J.; Huang, N.; Wang, Z.M. Chemical compositions and antioxidant activities of polysaccharides from the sporophores and cultured products of Armillaria mellea. Molecules 2015, 20, 5680–5697. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, J.; Zhang, Y.Q.; Dai, B.N.; An, Y.; Yu, L.L. Structural, thermal, and anti-inflammatory properties of a novel pectic polysaccharide from Alfalfa (Medicago sativa L.) stem. J. Agric. Food Chem. 2015, 63, 3219–3228. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, W.W.; Lin, S.L.; Cheung, P.C.K. Cell wall structure of mushroom sclerotium (Pleurotus tuber regium): Part 1. Fractionation and characterization of soluble cell wall polysaccharides. Food Hydrocoll. 2014, 36, 189–195. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Sun, X.Y.; Liu, N.; Wu, Z.X.; Feng, Y.; Meng, X.J. Anti-tumor activity of a polysaccharide from blueberry. Molecules 2015, 20, 3841–3853. [Google Scholar] [CrossRef] [PubMed]
- Iribe, H.; Koga, T. Augmentation of the proliferative response of thymocytes to phytohemagglutinin by the muramyl dipeptide1. Cell. Immunol. 1984, 88, 9–15. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
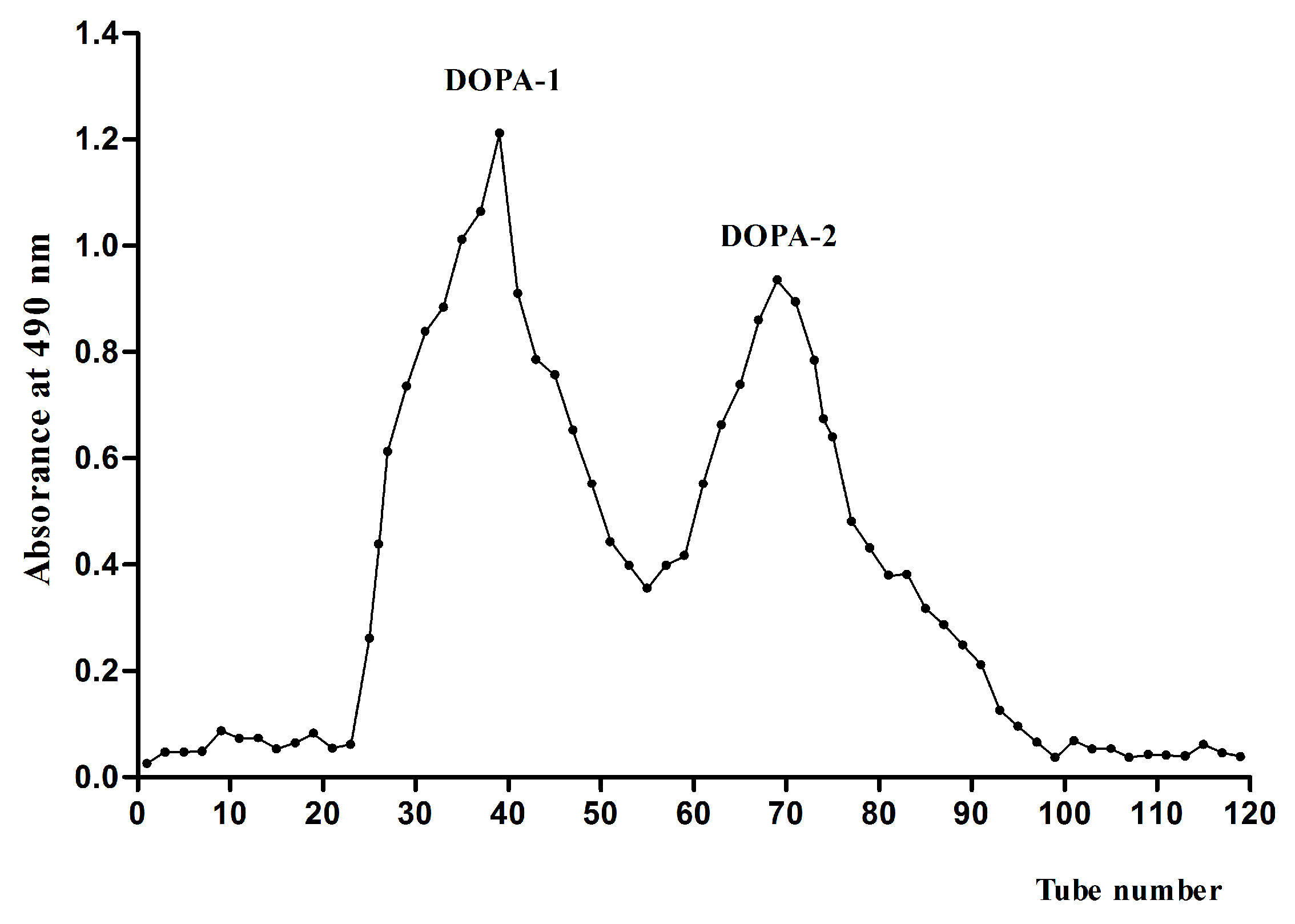






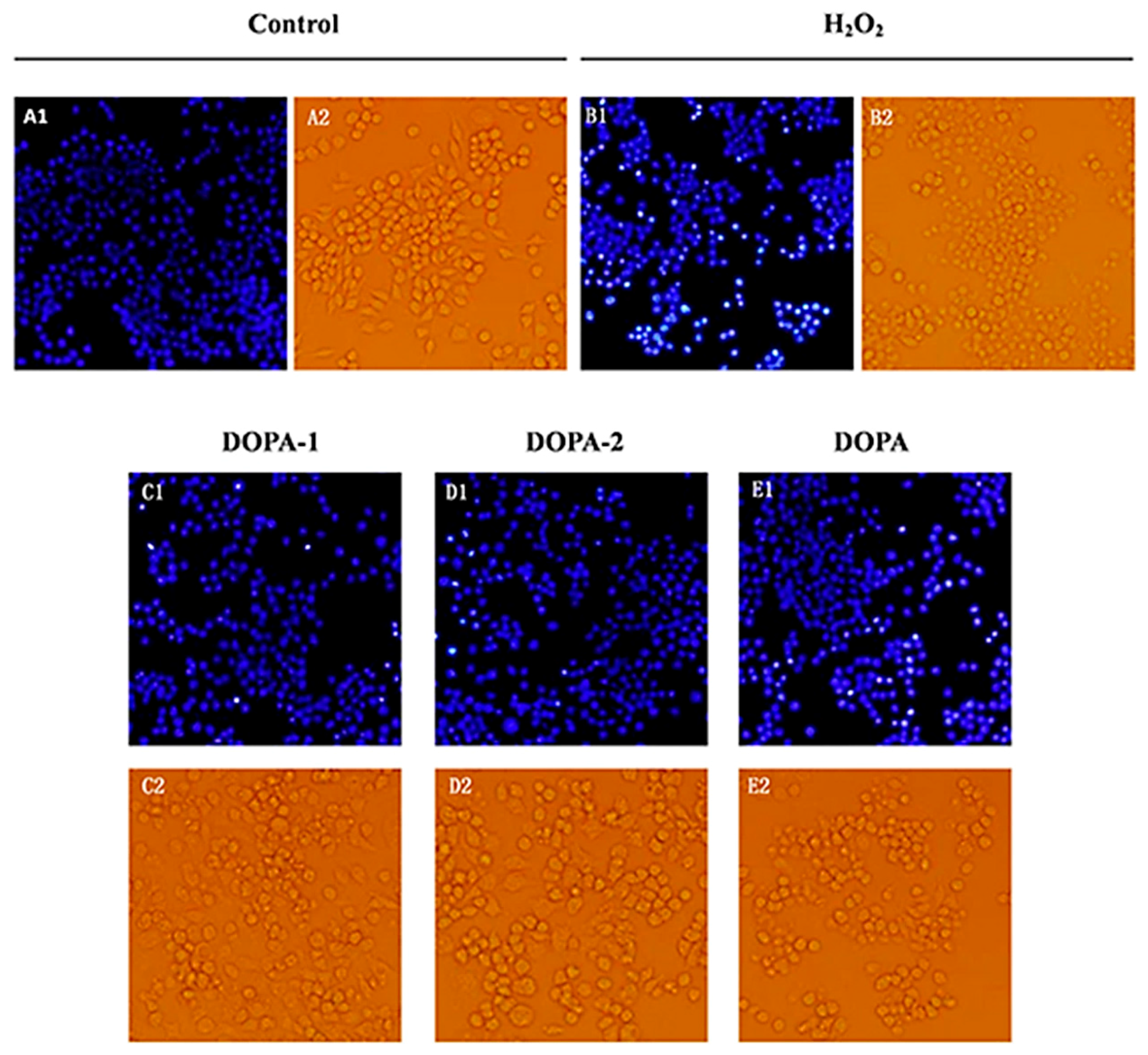
| Sample | Carbohydrate (%) | Molecular Weight (kDa) | Monosaccharide Composition (Molar Ratio) | |
|---|---|---|---|---|
| d-Mannose | d-Glucose | |||
| DOPA-1 | 93.80% | 394 | 5.8 | 1 |
| DOPA-2 | 91.60% | 362 | 4.5 | 1 |
| Retention Time (min) | Linkage Pattern | Major Mass Fragments (m/z) | Peak Area Percentage (%) | |
|---|---|---|---|---|
| DOPA-1 | DOPA-2 | |||
| 10.69 | T-Manp | 102, 117, 129, 145, 161, 205 | 4.08 | 2.43 |
| 12.44 | 1,4-linked Manp | 101, 113, 117, 129, 131, 143, 161, 173, 233 | 79.63 | 78.49 |
| 12.53 | 1,4-linked Glcp | 101, 113, 117, 129, 131, 143, 161, 173, 233 | 14.39 | 16.99 |
| 12.73 | 1,6-linked Manp | 101, 117, 129, 161, 189, 233 | - | 0.22 |
| 13.35 | 1,3,4-linked Manp | 118, 129, 160, 143, 185, 203, 231, 305 | 0.48 | 0.35 |
| 13.44 | 1,3,4-linked Glcp | 118, 129, 160, 143, 185, 203, 231, 305 | - | 0.15 |
| 13.71 | 1,2,4-linked Manp | 113, 130, 143, 172, 190, 231 | 0.51 | 0.57 |
| 14.05 | 1,4,6-linked Manp | 101, 117, 127, 142, 159, 201, 261 | 0.47 | 0.37 |
| 14.14 | 1,4,6-linked Glcp | 101, 117, 127, 142, 159, 201, 261 | 0.44 | 0.43 |
| A | Concentration (μg/mL) | NO Production (μM) | ||||
| Blank Control | LPS | DOPA-1 | DOPA-2 | DOPA | ||
| 0 | 2.38 ± 0.53 | |||||
| 0.2 | 20.28 ± 0.38 ** | |||||
| 6.25 | 3.59 ± 0.33 * | 4.12 ± 0.57 ** | 3.42 ± 0.36 * | |||
| 12.5 | 4.58 ± 0.74 ** | 4.86 ± 0.42 ** | 3.60 ± 0.73 ** | |||
| 25 | 5.11 ± 0.52 ** | 5.17 ± 0.54 ** | 3.89 ± 0.77 ** | |||
| 50 | 6.89 ± 0.48 ** | 7.43 ± 0.52 ** | 5.24 ± 0.78 ** | |||
| B | Time (h) | NO Production (μM) | ||||
| Blank Control | LPS | DOPA-1 | DOPA-2 | DOPA | ||
| 12 | 1.77 ± 0.04 | 3.59 ± 0.15 ** | 2.75 ± 0.15 ** | 2.98 ± 0.09 ** | 1.75 ± 0.05 | |
| 24 | 1.70 ± 0.58 | 13.11 ± 1.53 ** | 3.29 ± 0.46 * | 3.45 ± 0.34 ** | 2.25 ± 0.47 | |
| 36 | 1.94 ± 0.42 | 19.53 ± 0.43 ** | 4.23 ± 0.65 ** | 4.53 ± 0.73 ** | 3.26 ± 0.46 ** | |
| 48 | 2.12 ± 0.74 | 19.76 ± 0.45 ** | 6.13 ± 0.61 ** | 7.08 ± 0.67 ** | 4.97 ± 0.31 ** | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.; Li, Y.; Tao, S.; Wei, G.; Huang, Y.; Chen, D.; Wu, C. Purification, Characterization and Biological Activity of Polysaccharides from Dendrobium officinale. Molecules 2016, 21, 701. https://doi.org/10.3390/molecules21060701
Huang K, Li Y, Tao S, Wei G, Huang Y, Chen D, Wu C. Purification, Characterization and Biological Activity of Polysaccharides from Dendrobium officinale. Molecules. 2016; 21(6):701. https://doi.org/10.3390/molecules21060701
Chicago/Turabian StyleHuang, Kaiwei, Yunrong Li, Shengchang Tao, Gang Wei, Yuechun Huang, Dongfeng Chen, and Chengfeng Wu. 2016. "Purification, Characterization and Biological Activity of Polysaccharides from Dendrobium officinale" Molecules 21, no. 6: 701. https://doi.org/10.3390/molecules21060701
APA StyleHuang, K., Li, Y., Tao, S., Wei, G., Huang, Y., Chen, D., & Wu, C. (2016). Purification, Characterization and Biological Activity of Polysaccharides from Dendrobium officinale. Molecules, 21(6), 701. https://doi.org/10.3390/molecules21060701





