Abstract
Drosophila melanogaster has been utilized to model human brain diseases. In most of these invertebrate transgenic models, some aspects of human disease are reproduced. Although investigation of rodent models has been of significant impact, invertebrate models offer a wide variety of experimental tools that can potentially address some of the outstanding questions underlying neurological disease. This review considers what has been gleaned from invertebrate models of neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, metabolic diseases such as Leigh disease, Niemann-Pick disease and ceroid lipofuscinoses, tumor syndromes such as neurofibromatosis and tuberous sclerosis, epilepsy as well as CNS injury. It is to be expected that genetic tools in Drosophila will reveal new pathways and interactions, which hopefully will result in molecular based therapy approaches.
1. Introduction
1.1. Drosophila as a brain disease model
Modelling human brain diseases in Drosophila melanogaster offers several advantages for investigation of molecular and cellular mechanisms underlying human disease. Short life span, large number of offspring, many genetic techniques, a well known anatomical situation and a wide variety of mutants are convenient characteristics of Drosophila as a model organism. Time and tissue specific inducible promoters are available [1–3]. Anatomic divergence between the fruit fly and humans is apparent, which may be not sufficient to recapitulate some morphological features of neurological disease but fundamental molecular pathways are highly conserved [4]. Functional analysis of human disease genes including high–throughput pharmacological screens as well as behavioral assays have become available in Drosophila.
Sequencing of the Drosophila genome revealed about 13.600 genes [5], being less than the human genome [6,7] and comparison of the genomes of humans and Drosophila reveals further differences concerning a higher microsatellite mutation rate in humans, whereas nucleotide diversity is more prominent in Drosophila [8] but this does not compromise Drosophila as a disease model.
One obvious disadvantage of using fly models is the risk that important pathogenetic factors are vertebrate-specific and may be ignored in invertebrate models. For example, immunological diseases such as multiple sclerosis cannot be modelled convincingly in Drosophila melanogaster. Furthermore, brain infarcts and brain hemorrhage cannot be analyzed in Drosophila because vessels are lacking and blood cells are mainly restricted to primitive hemocytes. However, most Drosophila models do reproduce some aspects of human diseases although one has to bear in mind that the differences between mammals and invertebrates represent potential drawbacks in modelling brain diseases.
1.2. Genetic tools in Drosophila
In Drosophila a wide armamentarium of genetic tools is available. In a reverse genetic approach a candidate gene is tested for its potential functional role. One of the most important genetic systems used in reverse genetic approaches is the GAL4/UAS-system (see Figure 1.). Human proteins or Drosophila proteins can be expressed in a tissue and time dependent manner in the fly [9]. In most Drosophila disease models a human disease related transgene is inserted downstream of a UAS (upstream activating sequence) and can be expressed under the control of the yeast transcriptional activator GAL4. Absence of GAL4 results in inactivity of the transgene. After crossing flies carrying the transgene to flies expressing GAL4 under control of a cell- or tissue specific promoter, human protein expression is subsequently restricted to GAL4-expressing tissues. A large number of GAL4 driver lines including the glial promoter repo (reversed polarity), the pan-neuronal promoter elav (embryonal lethal, abnormal vision) and the eye-specific promoter GMR (Glass Multimer Reporter) are available in Drosophila.
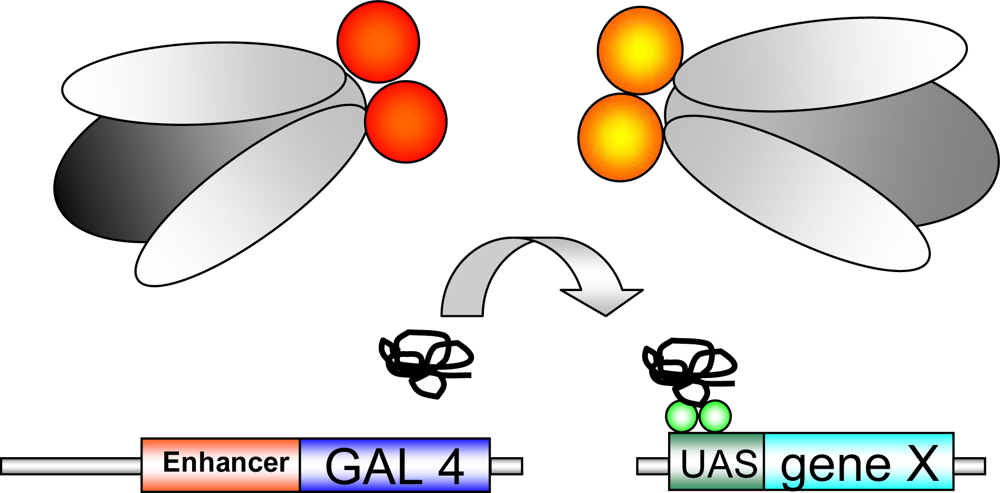
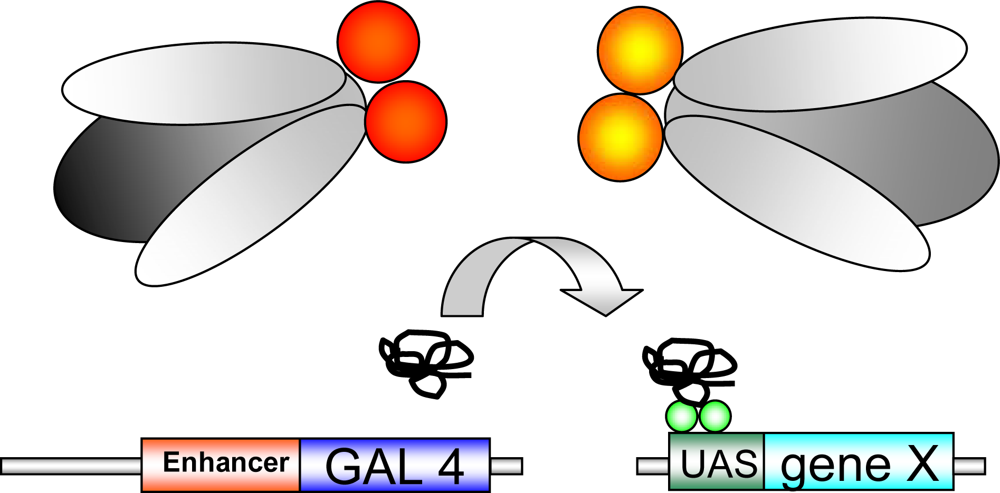
Figure 1.
GAL4-UAS system: Transgenic flies expressing GAL4, a yeast transcriptional activator, are crossed against UAS-transgenic flies, carrying a gene of interest (“gene X”), inserted downstream of the UAS (upstream activating sequence;green balls). “Gene X” is activated in the offspring by crossing the two transgenic lines. The transgene is expressed in a time-/tissue dependent manner dependent on the selected GAL4-driver line.
Compared to reverse genetics the forward genetic approach is an unbiased method – as to the function of a gene - for identification of genes based on phenotype. These approaches are usually conducted in one of two ways. On the one hand, a screen for mutations (chemical mutagenesis or insertional mutagenesis techniques (Enhancer-Promoter (EP)-elements and RNAi lines) (see Figure 2.) that reduce life-span, exhibit behavioral abnormalities or induce neuronal degeneration can be carried out. Several genes have been detected using these screens, among those the mutant bubblegum [10–12]. In principle, this approach can be useful to understand diseases whose genetic basis is undetermined yet [13].
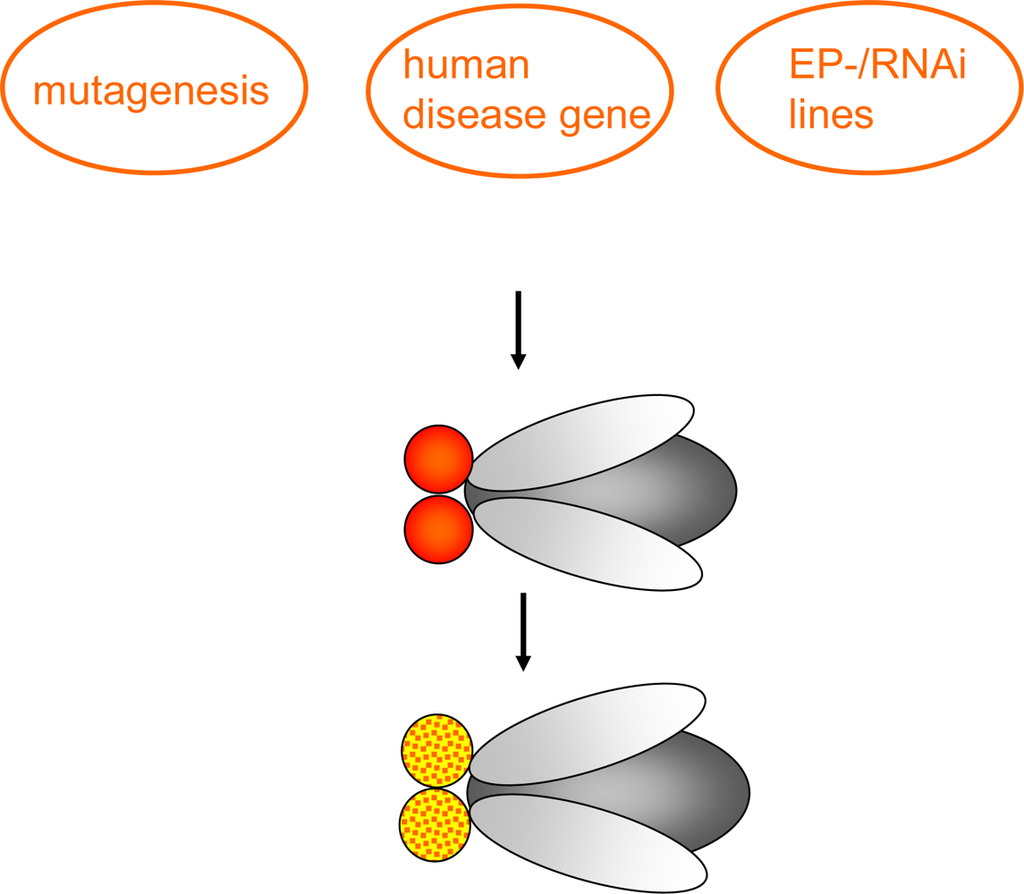
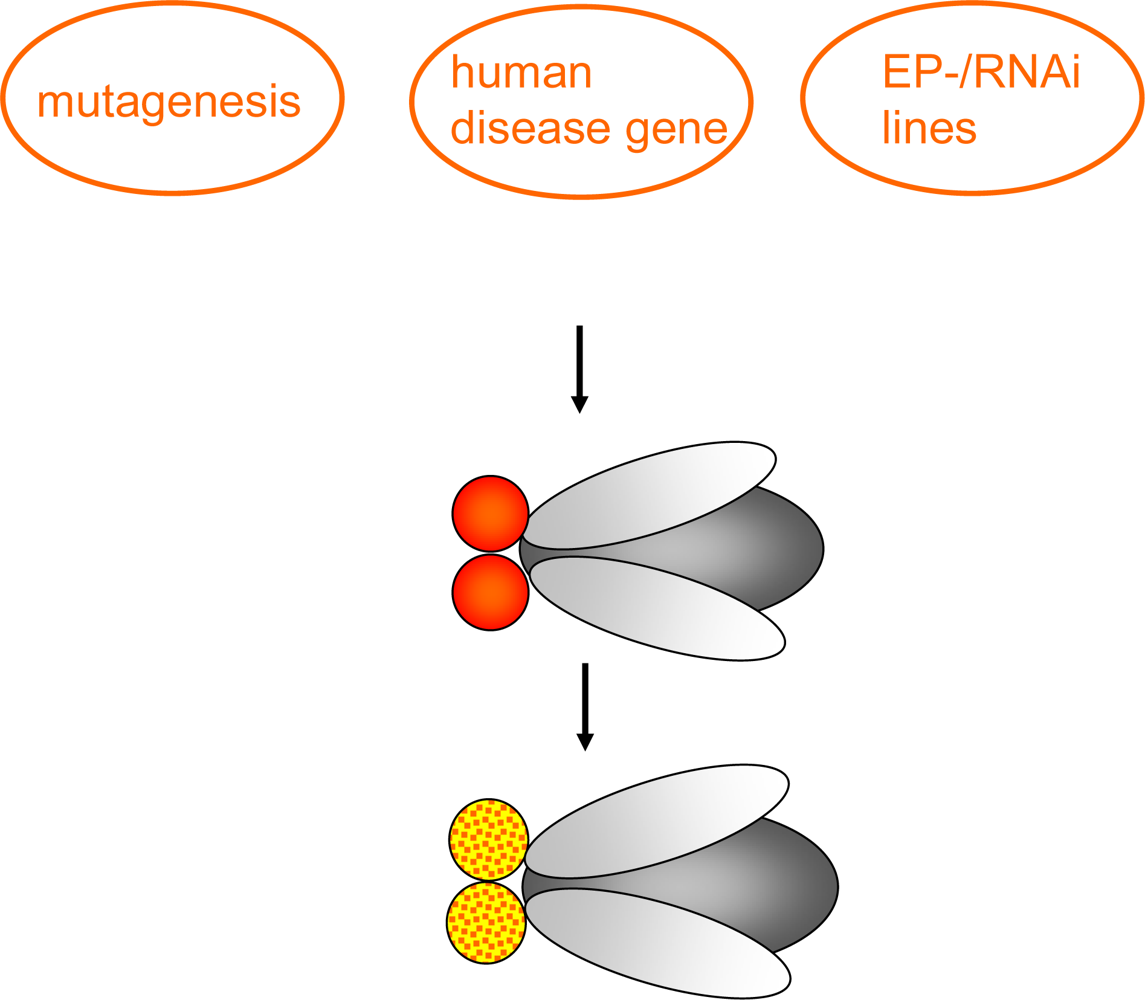
Figure 2.
Forward genetic screen: Chemical mutagenesis (the chemical mutagen ethyl methane sulphonate (EMS) is common) or insertional mutagenesis techniques, having a disease-causing human gene, Enhancer-Promoter (EP)-element or RNAi construct placed under control of GAL4-responsive UAS sites are used to investigate the effect in specific tissues or the whole organism e.g. change of eye color (as indicated), reduced life-span, behavioral abnormalities or neuronal degeneration.
On the other hand, modifier screens can be used in order to find modifiers (suppressors/enhancers) of the disease phenotype in a mutant background (see Figure 3). Modifier genes are assumed to encode proteins that are related to pathways involved in the disease phenotype.
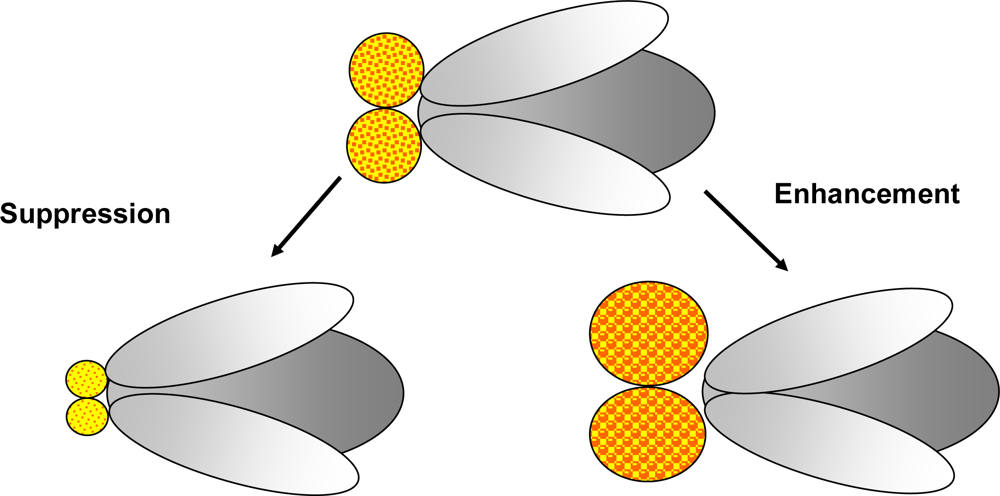
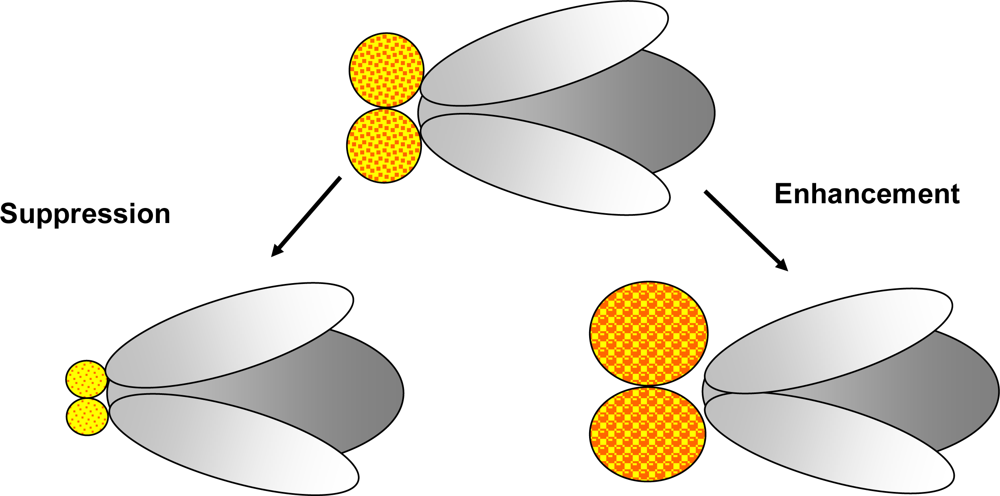
Figure 3.
Modifier screens: A modifier screen is conducted in order to find genes that play a role in a process of interest. Random mutations created by mutagenesis or selected mutants already suspected to be involved in the pathway investigated as well as collections of Enhancer-Promoter (EP)-elements and RNAi stocks may be used to identify genes able to modify (enhance or suppress) the phenotype. In this figure suppression and enhancement of an eye phenotype is illustrated.
1.3. Drosophila CNS development
Drosophila melanogaster is an insect undergoing metamorphosis, showing different developmental stages: embryo, larva, pupal stages and the adult fly. The central nervous system of the Drosophila embryo is composed of neurons and glial cells (Figure 4). The neurons build commissures in a close association with midline glial cells [14,15]. Glial cells in the Drosophila CNS can be classified either as midline glia or as lateral glia [16]. Surface glial cells form a continuous covering of the CNS and peripheral nerves. They comprise peripheral and exit glial cells, subperineurial glial cells, which enclose the CNS, and channel glial cells lining the channels lancing the ventral nerve cord [17].
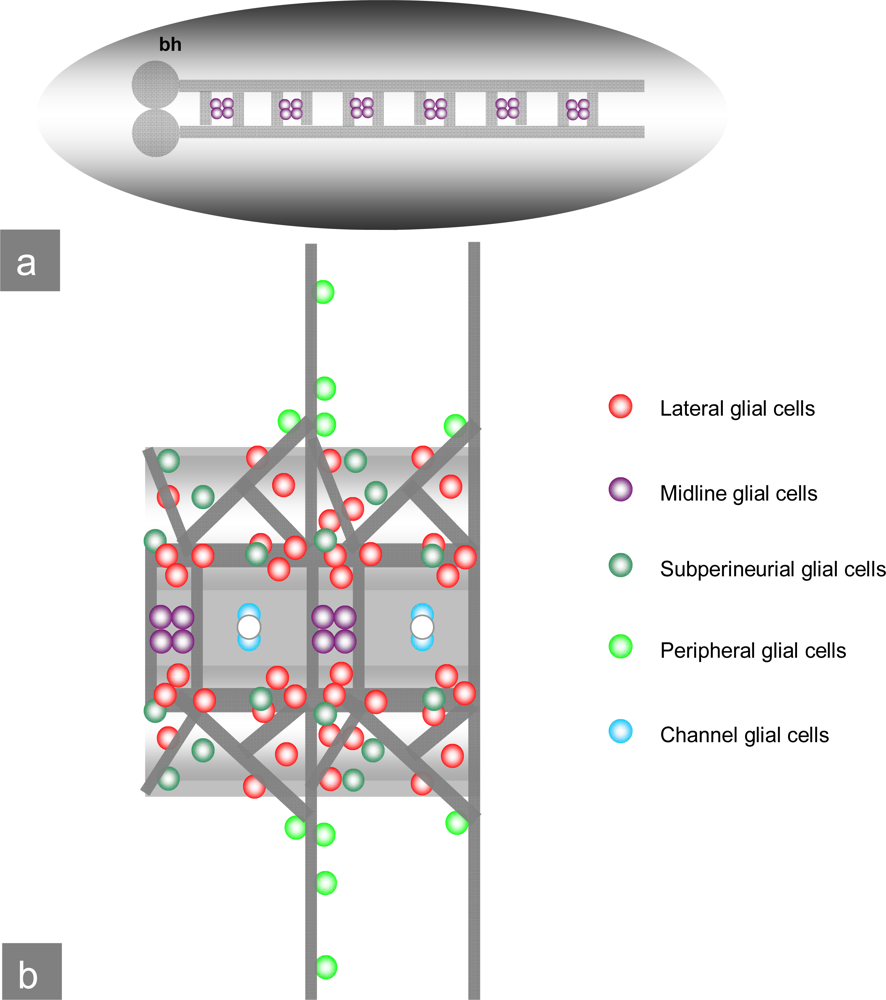
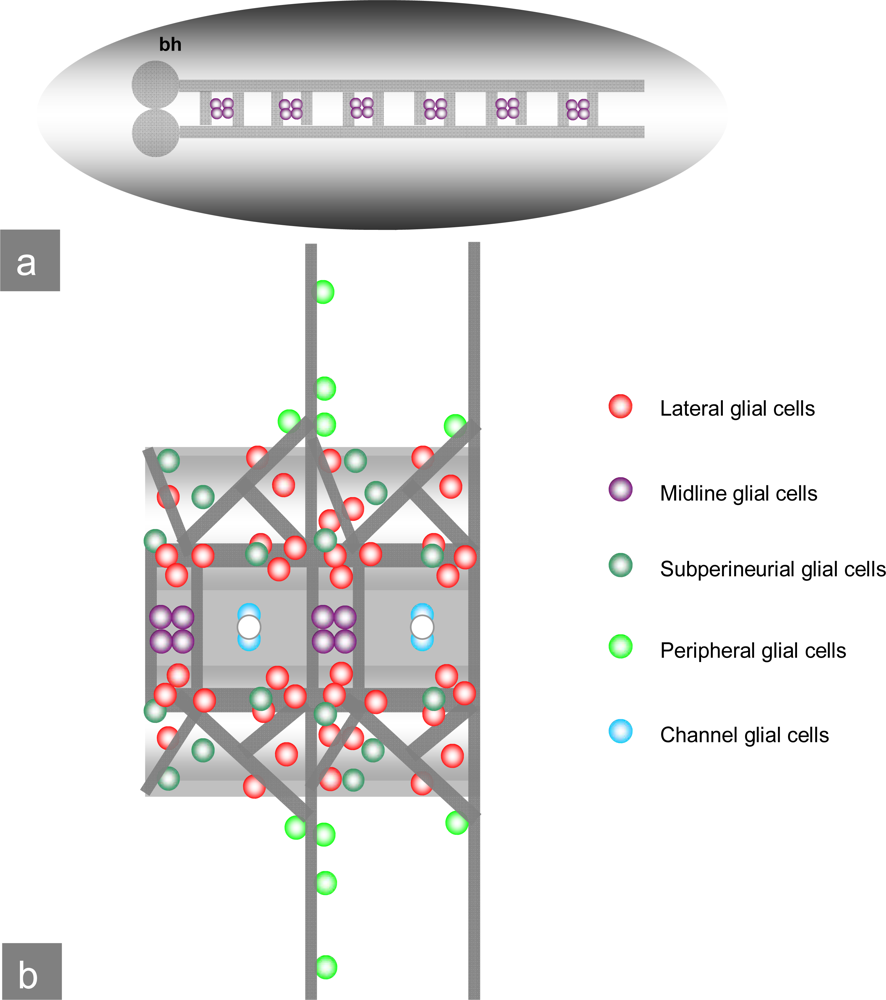
Figure 4.
Drosophila embryonal CNS: a) Schematic view of the Drosophila CNS and ventral nerve cord with brain hemispheres (bh), midline glial cells and commissures. b) Ventral view of the ventral nerve cord: Commissures, midline glial cells as well as subperineurial, peripheral and channel glial cells. (pictures modified after V. Hartenstein.)
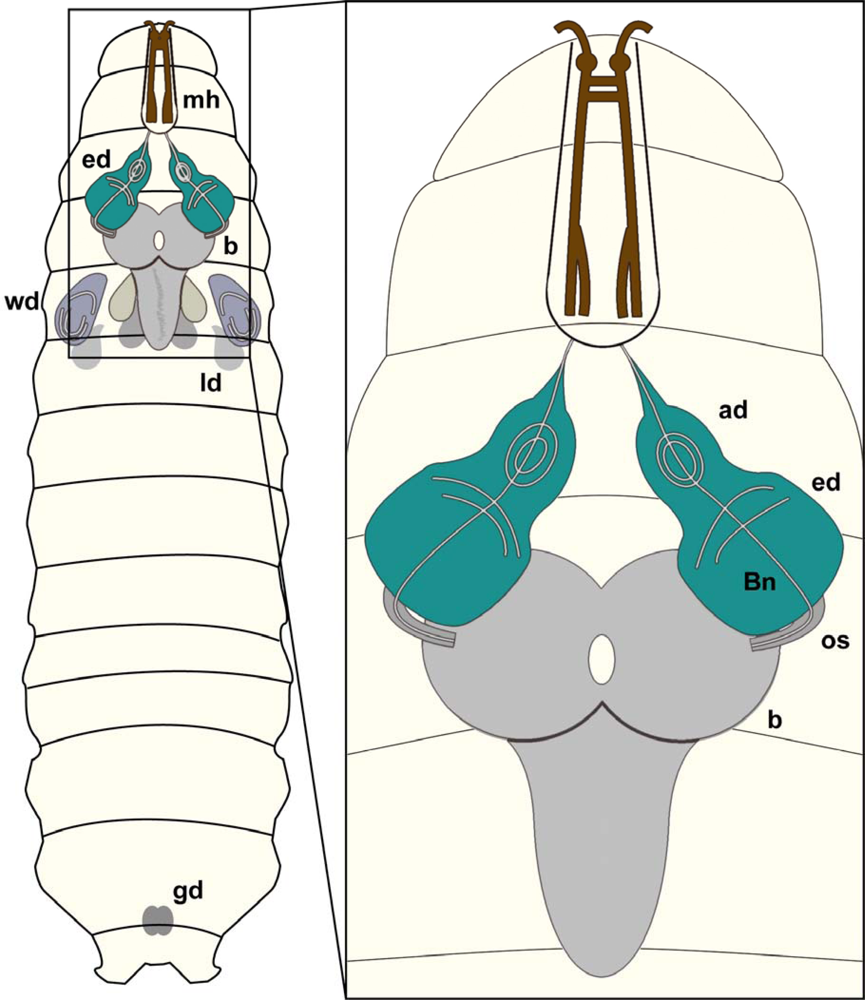
In Drosophila, various organs and anatomical structures arise from ten pairs of imaginal discs and the genital disc. The brain of the Drosophila larva is composed of two hemispheres and the subesophageal ganglion (Figure 5).
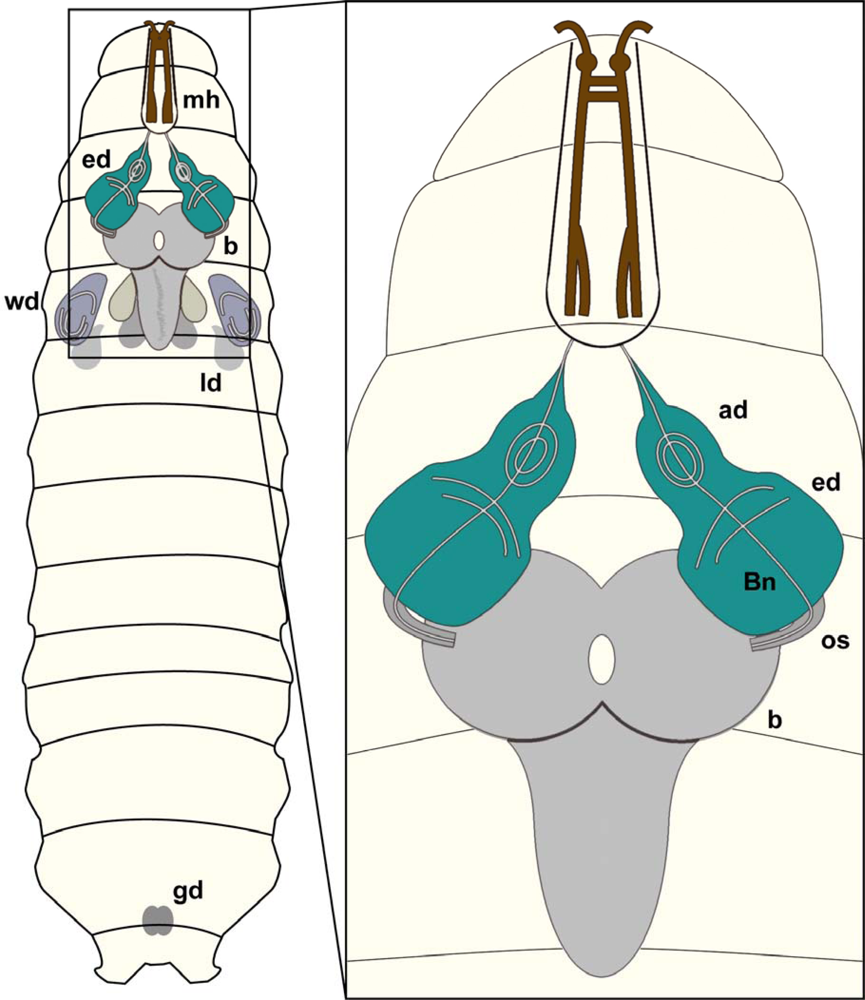
Figure 5.
Drosophila larval CNS:Schematic overview of a Drosophila larva showing brain (b), eye imaginal discs (ed), wing discs (wd), leg discs (ld), mouth hooks (mh) and gonads (gd).Magnification: Schematic view of brain hemispheres (b), eye imaginal discs (ed) as well as antennal discs (ad). The eye imaginal disc will form the adult compound eye whereas the antennal disc will develop into the antenna, the adult olfactory organ. The optic stalk (os) connects the brain hemispheres with the eye imaginal discs, whereas Bolwig nerve (Bn) constitutes the link of the larval brain to the larval photo receptors.
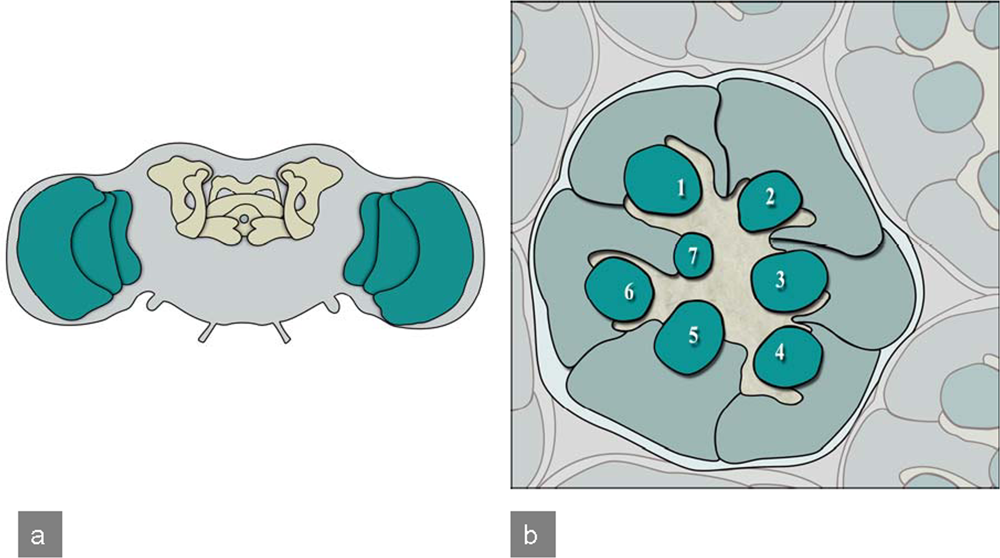
In brain disease models, brain and the complex eye of adult Drosophila are often used. Especially the mushroom bodies (Figure 6), association areas necessary for olfactory learning and memory [18], composed of about 2,500 Kenyon cells, neurons with small, densely packed cell bodies [19], are relevant in brain disease models. Additionally, the fly visual system is also often used as a model system in brain research [20,21] as it is less complex than the brain, formed by neurons which develop in a very stereotyped manner and can be conveniently investigated. In particular a “rough eye” phenotype caused by pathological processes can be easily studied, enabling screening for genes related to the process of interest. Eye development always begins with the differentiation of R8 photoreceptor neurons at uniformly spaced positions. These neurons signal to neighboring cells to develop ommatidia (unit eyes). Sequential differentiation of the other seven photoreceptor types (R1-R7) follows afterwards [22]. Each R8 neuron recruits one cell of each type, such that seven photoreceptors cluster around each R8 neuron [23] (Figure 6).
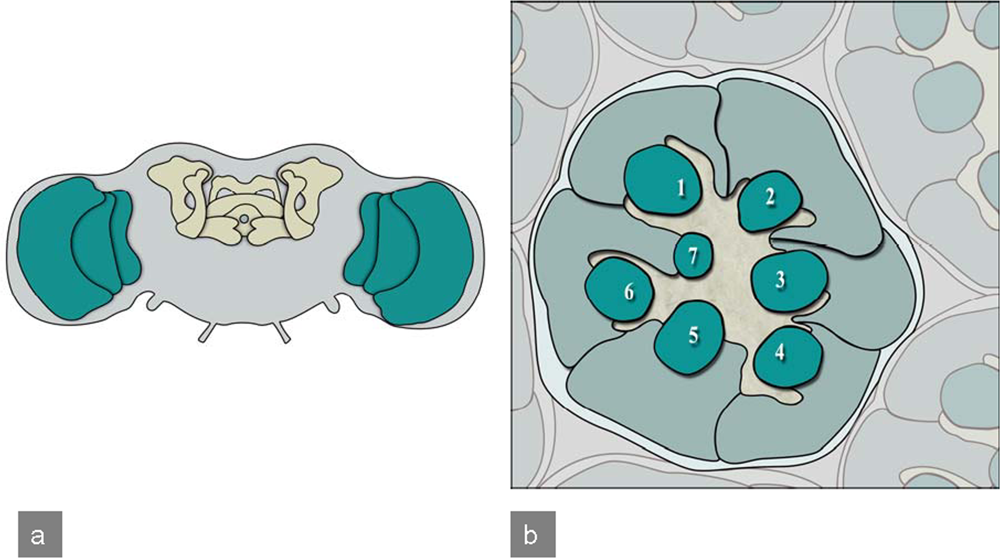
Figure 6.
Drosophila adult CNS and compound eye: a) Frontal view of the adult Drosophila brain. Highlighted are mushroom bodies and the central complex (yellow) as well as the optic lobes (green).b) Tangential section of a Drosophila compound eye reveals the highly stereotyped arrangement of ommatidia. Here the organization of photoreceptors for a single ommatidium is illustrated. Numbers 1–7 indicate the light-sensing organelles of the photoreceptors, called rhabdomeres. Photoreceptors R1-R6 have a longer rhabdomere and represent the outer photoreceptors, as they surround the inner rhabdomeres R7 and R8. In this section only R7 is visible as R8 sits directly underneath.
2. Neurodegeneration
2.1. Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common neurodegenerative disease characterized by extracellular accumulation of Aß peptide in senile plaques and intracellular accumulation of hyperphosphorylated tau as neurofibrillary tangles and neuropil threads [24,25]. Familial AD, which makes up only less than 1% of cases, is caused by mutations of genes encoding amyloid precursor protein (APP), presenilin 1 and presenilin 2 [26].
Because not all components of APP proteolytic processing are conserved, modelling AD in Drosophila is quite challenging. The Drosophila APP homolog (dAPPL) does not contain the Aß domain and cannot be cleaved in flies [27,28]. Studies in Drosophila overexpressing wildtype human APP (hAPP695), hAPP695 with pathogenic mutations (hAPP-Swedish), full length hAPP with N-terminal myc tag as well as a construct comprising a signal peptide, ßA4 region and a C-terminal myc tag demonstrated γ-secretase activity in flies [29]. BACE activity is not present in Drosophila, whereas γ-secretase, presenilins and nicastrin are conserved [30–32].
Expression of human Aß40 and Aß42 in the fly brain caused age-dependent learning defects but only Aß42 led to formation of diffuse amyloid deposits in the Kenyon cell region positive in the thioflavin S staining but in transmission electron microscopy without clear amyloid fibril structure, neurodegeneration, and decline of locomotor functions [33] Expression of Aß42 in the CNS was found to cause short-term memory impairment [34]. Expression of human Aß42 in the eye caused degeneration of the retina, which could be suppressed by neprilysin overexpression [35]. In another approach human BACE and hAPP were expressed singly or in combination in fly eyes using gmr-GAL4. Expression of hAPP alone or in combination with BACE resulted in degeneration of retinal photoreceptors and age-dependent plaque formation, which could be enhanced by coexpression of the Drosophila presenilin (UAS-DPsn) containing a point mutation corresponding to the FAD mutant L235P. Amyloid deposits were positive for thioflavin S, ß-amyloid as well as Aß42 detecting antibodies in flies expressing hAPP and DPsnL235P or hAPP, BACE and DPsnL235P. Electron microscopy revealed “star-like” deposits in the retina, neuropil regions and optic ganglia [36]. Taken together in this study a sequence of neuronal degeneration followed by plaque formation is described, which does not recapitulate the chronological sequence of AD in humans. Secondly gender differences could be detected with respect to plaque formation being more severe in male than in female fliese, a fact which is not seen in human AD. On the other hand, age dependent progression of neurodegeneration and plaque formation is detected, immunohistochemical staining profile and EM ultrastructure with star-like formation resembles human AD plaques [37].
Further studies using deletion and overexpression of dAPPL, expression of hAPP695 and of hAPP mutants (hAPP-London and hAPP-Swedish) in larval motor neurons found accumulation of organelles along axonal tracts [38], suiting well to axonal transport disturbances seen in transgenic mouse models [39]. The neurofibrillary, tau associated pathology is lacking in Drosophila Alzheimer models, a fact that has also to be considered in the majority of mouse Alzheimer models [40].
Taken together, the fly can be used to study some molecular aspects of Alzheimer disease although the disease models remain more artificial than rodent models.
2.2. Tauopathies
Neurofibrillary tangles (NFT), neuritic plaques and neuropil threads composed of hyperphosphorylated tau forming intraneuronal inclusions are characteristic of AD, but tau inclusions are also associated with other neurodegenerative diseases such as Pick’s disease, corticobasal degeneration, argyrophilic grain disease, progressive supranuclear palsy and fronto-temporal lobar degeneration (FTLD) [41] characterized by diverse clinico-pathological phenotypes from aphasia to dementia syndromes [42].
Drosophila has one tau gene which is expressed in neurons and localized in axonal processes [43]. By expressing human wildtype tau and FTLD-linked mutant forms of tau (R406W, V337M) in cholinergic neurons a fly model of tauopathies was established showing adult-onset progressive neurodegeneration with vacuolization and reduced life span, particularly in R406W transgenic flies. Abnormal tau protein accumulated but NFT could not be detected [44]. When wildtype human tau (4R isoform) was coexpressed with the fly homolog of GSK-3β (shaggy), presumably involved in hyperphosphorylation of tau, neurodegeneration was enhanced and insoluble intraneuronal NFT formation, positive for AT100 antibody was observed. Ultrastructurally filamentous structures, including paired helical filaments with a characteristic periodicity as well as straight filaments were detected [45]. Antibody AT100 stains both intracellular NFT and extracellular NFT [46]. As a consequence of neuronal expression in this Drosophila model extracellular NFTs cannot be dectected in these flies. Ultrastructural findings in these flies are similar to paired helical filaments and straight tubules found in a wide range tauopathies [47] as well as detergent-resistance, another characteric of tau accumulations [42]. Toxicity of wildtype dtau and htau under control of gmrGAL4 was dosage dependent, and coexpression of these genes with known modifiers of tau differed in approximately half of the investigated Drosophila lines. Comparing dtau and htau in modifier screens in Drosophila model may clarify the degree of functional homology [48].
Antiapoptotic genes (p35, DIAP1 and DIAP2) were shown to reduce tau toxicity [45]. Overexpression of wildtype human tau in motor neurons disrupted axonal transport leading to locomotor phenotypes. Again, these could be enhanced by coexpression of GSK-3β and reversed by GSK-3β inhibitors lithium and AR-A014418 [49]. Modifier screens revealed a number of kinases, among those par-1 [48,50,51] to be involved in tau related neurodegeneration.
Thus, Drosophila tau models replicate several important features of human tauopathies, including tau hyperphosphorylation, accumulation, NFT formation and neuronal degeneration.
2.3. Parkinson’s Disease
Parkinson’s disease (PD) is a movement disorder showing resting tremor, rigidity, akinesia and postural instability, which mostly occurs sporadically, while hereditary cases represent less than 10% of patients [52]. Familial PD cases have been related with mutations, duplications and triplications in SNCA (α-synuclein)/PARK1, parkin/PARK2, UCHL-1 (ubiquitin carboxy-terminal hydrolase L1)/PARK5, DJ-1/PARK7, PINK-1 (PTEN-induced putative kinase)/PARK6, LRRK2/PARK8, ATP13A2 (p-type ATPase)/PARK9, and HTRA2 (HtrA serine peptidase 2)/PARK13. Other PARK loci have been identified, but the mutated gene is unknown [52]. Neuropathological hallmarks are loss of dopaminergic nigrostriatal neurons and typically accumulation of α-synuclein in cytoplasmic inclusions called Lewy bodies (LB) and Lewy neurites [52].
To create a fly model of PD, wildtype human α-synuclein and two familial mutant forms (A30P and A53T) were expressed in dopaminergic neurons. Expression of α-synuclein led to age-related loss of dopaminergic neurons, LB-like accumulations and behavioral deficits. In flies expressing mutant α-synuclein in a pan-neuronal pattern α-synuclein inclusions were also found in non-dopaminergic neuronal cell bodies like in human PD brains [53]. LB-like structures in the fly stained positive for α-synuclein and were ultrastructurally composed of filaments with granular material similar to human Lewy bodies. [53,54]. l-DOPA, a drug used to treat PD patients, could suppress behavioral defects [55].
Mutations of PARK2 encoding parkin are linked to autosomal recessive, juvenile onset parkinsonism [52]. Loss of function mutations of Drosophila parkin increased sensitivity to oxidative stress [56,57] and showed pathological mitochondrial structure although no dopaminergic neurodegeneration could be observed. Other studies suggested parkin protection against α-synuclein damage to dopaminergic neurons [58]. In another model expression of two mutant forms of human parkin, Q311X and T249R in dopaminergic and serotoninergic neurons under control of the ddc-GAL4 driver, selectively caused degeneration of dopaminergic neurons and progressive locomotor dysfunction [59].
In humans mutations in PINK-1 lead to early-onset autosomal PD [52]. Loss-of-function mutations of the Drosophila PINK1 homolog show dopaminergic neuronal degeneration, flight muscle degeneration, locomotor defects and mitochondrial defects. Interestingly, expression of parkin could ameliorate PINK1 phenotypes [60].
Mutations of DJ-1 lead to an early onset autosomal recessive variant of PD [52]. Loss of the two DJ-1 homologs in Drosophila DJ-1α and DJ-1β was investigated and flies with deletions of DJ-1 β and DJ-1β were viable, fertile and showed normal lifespan. Interestingly these flies were selectively sensitive to toxins like paraquat and rotenone, linked to sporadic PD in humans [61]. Loss of function of DJ-1β led to locomotor deficits without loss of dopaminergic neurons [62].
Mutations of LRRK2 cause a late onset autosomal-dominant form of PD [52]. In flies expressing wildtype fly LRRK and the Arg1069Cys mutation corresponding to pathogenic Arg1441Cys mutation in LRRK2 associated with PD [63] under control of various GAL4 driver lines inducing whole body, muscle, eye and dopaminergic neuron specific expression did not show defects, but LRRK loss-of-function mutants showed decreased locomotor activity and reduction of dopaminergic neurons [64]. A second gain-of-function LRRK2-PD model was established using human LRRK2 and LRRK2-G2019S, another mutation associated with PD [65,66]. Expression of wildtype and mutant human LRRK2 in photoreceptor cells by gmr-GAL4 led to neuronal degeneration and expression in dopaminergic neurons resulted in selective loss of those neurons, locomotor dysfunction and reduction of life span [67].
Rotenone treatment of wildtype flies led to loss of dopaminergic neurons and locomotor defects [68]. Treatment of wildtype flies with paraquat led to impaired climbing capability and decreased survival which could be restored by cannabinoid receptor agonists (CP55,940) and a specific inhibitor of stress responsive Jun-N-terminal kinase signalling (SP600125) to different extent. [69].
Taken together, several genes involved in PD as well as PD models utilizing toxins have been investigated in the fly. To a remarkable extent neuropathological hallmarks could be modeled in Drosophila. Interdependence of different genes can be suitably investigated in this organism and is expected to influence understanding of the disease.
2.4. Prion Diseases
Prion diseases are rare fatal neurological diseases of genetic or infectious origin, but most often occur sporadically. In humans, sporadic (sCJD), familial (fCJD) and variant (vCJD) Creutzfeldt-Jakob disease, Gerstmann-Sträussler-Scheinker disease (GSS), fatal familial insomnia (FFI) and kuru are known [70]. Prion diseases are induced by misfolding of prion protein PrPC into one of several pathogenic isoforms [70,71].
GSS disease, one of the inherited prion diseases can be caused by some mutations of the prion protein gene, but most commonly by a point mutation at codon 102 and methionine at position 129 [72]. Histologically in GSS, widespread, large, multi-centric amyloid plaques with a dense core encircled by satellite globules located predominantly in the cerebral cortex and cerebellum prevail, positively stained by PrP antibodies, accompanied by white matter degeneration and neuronal loss, spongiform changes, gliosis and NFTs. Ultrastructurally amyloid plaques consist of radiating bundles of curvilinear filaments without definite periodicity [73].
The first effort to model a prion disease in Drosophila, an organism which does not have a prion gene, involved the expression of Syrian hamster prion protein under control of a HSP70 promoter [74]. Transgenic flies produced full-length prion protein on heatshock. No phenotypes were observed and the expressed prion protein never appeared to achieve the pathological protease-resistant form [74]. In the only prion fly model so far, a mouse prion protein with a proline to leucine mutation (P101L), homologous to a human mutation (P102L) causing GSS was used [75], leading to locomotor dysfunction, reduced life span, progressive vacuolar pathology and PrP inclusion bodies. Increase in proteinase K resistance (a specific feature of PrPSc diseases) was not seen, but biochemical analysis suggested that PrP is aberrantly folded. Transmissibility to other flies and ultrastructure of inclusions were not investigated [75].
This model can be useful with regard to understanding the pathobiology of P102L. GSS is a transmissible disease characterized mainly by proteinase K-resistant PrP positive plaques, amyloidosis and spongiform degeneration whereas the fly model shows vacuolation and inclusions rather than plaque formation, but not proteinase K resistance. Although neuropathology and biochemistry appear to be different in these two species, modifier screens may be of interest to better understand selected molecular pathogenetic pathways.
2.5. Polyglutamine Disorders
Several hereditary neurodegenerative diseases known as polyQ diseases result from CAG repeat expansion within the respective disease genes [76]. These include Huntington disease, X-linked spinobulbar muscular atrophy (SBMA, Kennedy disease) and the spinocerebellar ataxias SCA1, SCA2, SCA3 (also known as Machado-Joseph disease), SCA6, SCA7 and SCA17 as well as dentatorubral pallidoluysian atrophy (DRPLA).
2.5.1. Huntington’s disease
Huntington’s disease (HD) is an autosomal dominant illness with psychiatric, cognitive and motor symptoms, in particular chorea, caused by unstable expansion of CAG repeats within the coding region of the gene IT15 on 4p16.3. The disease occurs when more than 37 polyQ repeats are present [76]. In the majority of cases atrophy of frontal lobes and bilateral atrophy of the striatum are seen. In the caudate nucleus neuronal loss and reactive astrocytosis are detected. Ubiquinated, neuronal nuclear inclusions can be found [76].
Directed expression of exon 1 of the human IT15 gene containing 2, 75 or 120 polyglutamine repeats in Drosophila causes late-onset progressive neurodegeneration dependent on repeat-length as it is typical of human HD. Huntingtin protein accumulates in the nucleus and could be stained with anti-huntingtin antibodies but does not form HD-specific inclusions. Nuclei were found to have spherical particles indistinguishable from virus-like particles induced by a transposable element found in several fly strains [77].
Knock-down of the Drosophila homolog of IT15 gene huntingtin (htt) causes axonal transport defects, showing a phenotype similar to overexpression of the human HD gene [78], indicating that Drosophila htt is required for normal axonal transport.
In a recent study repeat instability of an HD Httexon1Q93 transgene could be demonstrated, a key aspect of polyQ diseases [79]. Modifier screens comparing a Drosophila Huntington model and SCA1 revealed a number of genes related to both diseases but others even showed opposite effects in the different disease models [80]. The question of how polyglutamine expansion mediates toxicity was addressed using a yeast two-hybrid screen in a Drosophila SCA1 model using several human ATXN1 constructs with wildtype (30Q) or expanded (82 Q) polyglutamine tracts and varying phosphorylation status at serine 776 (S776). The screen showed that polyQ expansion favors formation of a protein complex containing RBM17 (RNA-binding motif protein 17) and attenuates formation and function of a protein complex containing the HMG-box protein capicua (CIC), providing insight into molecular pathogenesis of SCA presumably representative for other polyglutamine diseases [81].
2.5.2. Spinocerebellar ataxia type 3 (Machado-Joseph disease; SCA3)
SCA3 patients present with cerebellar ataxia, pyramidal signs, extrapyramidal symptoms, peripheral neuropathy, nystagmus, eyelid retraction and facial fasciculation [82]. This dominantly inherited disorder is linked to an unstable CAG repeat ataxin-3 gene on chromosome 14q32.1 [82]. Pathologically, brain atrophy, atrophy of the brain stem and spinal cord, as well as depigmentation of substantia nigra are found. Anterior horns show severe neuronal loss which leads to atrophy of anterior spinal roots and skeletal muscles. Nuclear inclusions are found in almost all brain regions but Purkinje cells are spared. Inclusions are positive for ubiquitin and ultrastructurally are non-membrane bound, containing a mixture of granular and filamentous structures.
The first model of a polyQ disease in Drosophila was made by expressing the C-terminally truncated domain of the pathogenic human protein (SCA3tr-Q78) and the control protein (SCA3tr-Q27). Phenotypes were only observed in fly strains expressing the longer polyglutamine repeat [83]. Expression of SCA3tr-Q78 in the eye using gmr-GAL4 caused loss of pigmentation, destruction of the retina and nuclear inclusions. The fly SCA3 model is promising because several key features of human disease are present, including neuronal degeneration, nuclear inclusions and trinucleotide repeat instability.
Partial rescue of fly pathology could be achieved by co-expression of the antiapoptotic protein p35 [83] or HSP 70 [84]. Furthermore, suppression by HSP70 can be synergistically enhanced by co-expression of DnaJ-like-1 (DnaJ-1), a homolog of the HSP40 chaperone protein [85]. Pathogenicity of the truncated ataxin-3 protein is more severe than that of the full-length protein, due at least in part to the protective nature of functional domains of the normal protein [86]. A recent modifier screen for SCA3trQ78 toxicity yielded 17 suppressor and one enhancer gene which belonged mainly to chaperones and ubiquitin-pathway components and were considered to some extent to play a role in protein misfolding in general [87]. Coexpression of a the Drosophila homolog of ATAXN2, using a UAS-Atx2 construct under control of gmr-GAL4 together with the pathogenetic human Atx3 UAS-SCA3trQ78 strongly enhanced eye degeneration and increased inclusion formation [88]. Understanding of the interdependence of different SCA related genes and RNA-based trinucleotide repeat expansion diseases may be crucial with respect to future therapeutic advances.
As one important factor seen in polyQ expansion diseases is trinucleotide repeat instability, this was investigated in flies. In a SCA3 model using SCA3tr-Q78 instability was shown to be enhanced by reduction of cAMP response element-binding protein (CREB)-binding protein (CBP), a key regulator in dna repair, whereas treatment with histone deacetylase (HDAC) inhibitors seemed to protect against repeat instability [79].
2.5.3. X-linked spinobulbar muscular atrophy (SBMA; Kennedy Disease)
SBMA is a rare X-linked progressive motor neuronopathy caused by CAG repeat expansion in the first exon of the androgen receptor (AR) gene on Xq13–21 [82]. It is characterized by muscle cramps, proximal muscle weakness, atrophy and fasciculations as well as endocrine abnormalities like gynecomastia and testicular atrophy [82].
Pathologically, reduced numbers of motor neurons in the spinal anterior horns, facial and hypoglossal nuclei prevail and intranuclear inclusions consisting of granular dense aggregates of AR-positive materials are detected in the remaining motor neurons as well as in the skin, testis and other organs [82].
A SBMA fly model expressing mutant hAR (polyQ 52), a pathogenic form of the androgen receptor gene under control of gmr-GAL4 showed ligand-dependent neurodegeneration, encompassing marked disruption of the eye, reduced ommatidia numbers and loss of pigmentation with thinned retinas [89]. Although neuronal loss is represented in this model, other features of the human disease are not encountered, such as inclusions and involvement of other organs like skin and testis. Nuclear localization of the mutant protein was an obligate requirement for toxicity [89]. In a modifier screen hoi-polloi (hoip) gene, involved in small nucleolar RNA-protein (snoRNP) complexes which play a role in ribosomal RNA processing was identified as an enhancer of neurodegeneration, linking polyQ toxicity to dysregulation of translational activity [90].
2.5.4. Non coding trinucleotide repeat diseases
Non coding trinucleotide repeat diseases characterized by expansion of trinucleotide repeats comprising CGG, CTG, CAG, GCC and GAA within the 5’ or 3’ untranslated region (UTR) or introns of a gene [91] cause diseases like spinal muscular atrophy, SCA 8 and SCA12. Repeat expansion within the noncoding region is made responsible for loss of function of the disease gene or gain of function of the disease-associated mRNA or both, leading to neuronal degeneration.
2.5.5. Spinocerebellar ataxia type 8 (SCA8)
SCA8 is characterized by progressive gait and limb ataxia, dysarthria and nystagmus at variable ages of onset [82] caused by expansion of a CTG repeat in the 3’ UTR of the SCA8 gene on chromosome 13q21 [92]. Histologically, severe loss of Purkinje cells is a predominant finding but neuronal loss may also occur in the inferior olivary nucleus and substantia nigra. Surviving Purkinje cells are atrophic and show somatic sprouts. Intranuclear inclusions positive for polyglutamine and ubiquitin positive inclusions are found in Purkinje, dentate and medullary neurons [82].
A Drosophila model using human SCA8 cDNA placed under control of a UAS element using a wildtype (SCA8[CTG9]) and CTG expanded (SCA8[CTG112]) form has been established [93]. Expression of both constructs in the eye under gmr-GAL4 control causes a rough eye phenotype and progressive degeneration of photoreceptor cells. Inclusions were not found. In a modifier screen mRNA binding proteins (staufen (stau), muscleblind (mbl) and split ends (spen)) have been identified but their functional role in RNA induced toxicity is still unclear [93]. Although fly neuropathology does not convincingly recapitulate human disease, the model may lead to better understanding of fundamental aspects of RNA induced toxicity.
2.5.6. Spinal muscular atrophy (SMA)
Spinal muscular atrophies (SMAs) are genetically heterogeneous inherited diseases with progressive muscle degeneration caused by loss of spinal motorneurons. [94]. Nearly all SMA patients show alterations of the survival of motor neuron gene 1 (SMN1) on 5q13, which leads to loss of its protein product SMN [95]. The SMN protein is found in the nucleus and cytoplasm of all cells, but most abundantly in motorneurons. Histologically, symmetric loss of motor neurons as well as neurogenic atrophy of muscles is characteristic [94].
Ectopic expression of the human SMN1 in Drosophila leads to pupal lethality [96]. Abnormal larval locomotion in homozygous mutants for the Drosophila survival motor neuron (Smn) homolog [97] could be explained by defect larval neuro-muscular junctions which showed disorganized and enlarged synaptic boutons as it is seen in SMA patients. No obvious muscular or neuronal defects were seen immunohistochemically. In a genetic screen using an SMN allele encoding a point mutation seen in SMA patients 27 modifiers of Smn lethality have been found, including some (wishful thinking (wit) and Fmr1) that have been shown previously to function at the NMJ and others which were not associated with NMJ function before [98]. Synapses at the neuromuscular junction are glutamatergic, providing at least some similarities to the spinal cord synapse which is affected in SMA.
2.6. Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is characterized by upper and lower motor neuron defects including brisk reflexes, spasticity and pathological reflexes, fasciculations and weakness [99]. More than 90% of ALS cases are sporadic, but the disease can be inherited as an autosomal or X-linked familial condition. 15% of familial cases are caused by mutations in the gene encoding the enzyme superoxide dismutase-1 (SOD1) [100]. Histologically, ALS is characterized by selective loss of motor neurons in the brain and spinal cord. ALS is characterized by ubiquitin-positive inclusions (“skein-like”, “Lewy body-like” or a combination of the two), small eosinophilic inclusions in spinal motor neurons (“Bunina bodies”), hyaline inclusions [101] as well as TDP-43 positive filamentous inclusions [102].
Expression of hSOD1 (WT and A4V and G85R mutants) in motor neurons using the D42 motor neuron driver led to progressive motor dysfunction, whereas expression of Drosophila Superoxide dismutase (Sod) using the same driver line did not show an effect [103]. Loss of climbing ability was not restricted to mutant forms of hSOD1 but was also observed in wildtype hSOD1. Hypothetically hSOD1 may be recognized as toxic mutant form of SOD1 in Drosophila and therefore led to this effect. Interestingly neither loss of motor neurons nor retinal degeneration was observed. HSOD1 forms intracellular inclusions but solubility was not altered. [103].
A recent publication of a Drosophila model of ALS8 [104], established by using the respective Drosophila mutation (dVAP33A) of the human disease causing dominant mutation of VABP (vesicle-associated membrane protein (VAMP)-associated membrane protein B) showed that the the mutant protein aggregates, is ubiquitinated and recruits wild type protein into aggregates. Furthermore a new mechanism underlying ALS pathogenesis at the neuromuscular junction involving BMP signaling pathways could be identified [104]. In Drosophila synapses at the neuromuscular junction are glutamatergic, which provides similarities to the spinal cord synapse being of central interest in ALS in humans [104].
Earlier attempts to model ALS yielded confusing results as expression of hSOD1 transgene and human mutated Gly41-to-Ser SOD in motorneurons by D42-GAL4 led to increased longevity and could rescue the lifespan of a dSod null mutant with shortened lifespan [105,106].
Taken together, publications about ALS in Drosophila did not show motor neuron loss, the observed intracellular inclusions did not reflect characteristics of human ALS inclusions, and investigations focussed on the neuromuscular junction. But despite of apparent discrepancies, these results will constitute the basis of modifier screens and are expected to contribute to further understanding of ALS pathogenesis.
3. Metabolic disorders
Up to now in Drosophila a number of metabolic brain disease models for lysosomal storage diseases, mitochondrial diseases and peroxisomal diseases exist. Three of them will be presented.
3.1. Leigh Disease
Leigh disease is a progressive mitochondrial encephalopathy characterized by psychomotor delay starting during the first months of life. Life expectancy is 1–4 years. Optic atrophy is also frequently observed [107]. The disease is caused by inherited mutations in both nuclear- and mitochondrial-encoded genes involved in energy metabolism, including mitochondrial respiratory chain complexes I, II, III, IV, and V, crucial to electron transport [108].
Macroscopically, symmetric necrotizing lesions in subcortical areas of the CNS (brain stem, cerebellum, diencephalon and corpus striatum), which are associated with spongiosis, demyelination and astrocytosis, are observed [109]. Ultrastructurally mitochondrial inclusions are found in the neurons of the brain, as well as increase of mitochondrial antigens in neurons, vessels, plexus and astrocytes [110].
In the fly photoreceptor cells mutant for Succinate dehydrogenase (Sdh) develop completely normally and innervate the appropriate synaptic partners. After some time receptor cells degenerate, progressively losing expression of synaptic markers, and undergoing extensive morphological changes [111]. Thus this model may capture some elements of the human disease.
Another model of Leigh disease was established using downregulation of Surfeit1 (Surf1) showing a number of behavioral and electrophysiological abnormalities including reduced photoresponsiveness, reduced locomotor speed and impaired optomotor response as well as abnormal electroretinograms with different driver lines [112]. Surf1 downregulation driven by an actin-GAL4 line showed an underdeveloped CNS, while CNS wide silencing of Surf1 driven by elav-GAL4 led to prolonged lifespan, normal CNS development, slight impairment of locomotor activity and photobehavior; histochemical reaction to COX was reduced in the optic lobes.
Ultrastructural investigation of the body wall muscle fibers in actin-GAL4 driven flies showed larger mitochondria, different distribution and morphological alterations [112], while other key features of human brain pathology were not observed.
Remarkably, mutant flies exhibited increased longevity which does not reflect the course of human disease.
3.2. Nieman-Pick-Disease
Niemann-Pick disease represents a group of lysosomal lipid storage disorders. Niemann-Pick type C (NPC), an autosomal-recessive disease, shows a wide spectrum of phenotypes with variable begin from perinatal period to adult age. Major neurological symptoms include cerebellar ataxia, dysarthria, dysphagia, seizures and progressive dementia [113]. NPC is characterized by accumulation of cholesterol, glycospingolipids and other lipids.
A defect of organelle trafficking and a failure of lipid homeostasis are prominent [114], caused by mutations of NPC1 or NPC2 [115,116]. Histologically, NPC is characterized by progressive loss of neurons, particularly Purkinje cells in the cerebellum, lipid storage, formation of meganeurites and ectopic dendrites as well as the presence of neurofibrillary tangles [117].
NPC1a null alleles in Drosophila die at an early larval stage, but feeding NPC1a mutants the steroid hormone (molting hormone) 20-hydroxyecdysone (20E) extends lifespan, suggesting that reduced ecdysone synthesis results from NPC1a loss. Feeding with excess cholesterol compounds extends lifespan further till adult stages. [118].
In another Drosophila dnpc1a model flies showed sterol accumulation as in human disease. By 7-dehydrocholesterol treatment life expectancy of dnpc1a mutants could be extended till adulthood. Brain morphology was unremarkable without any neurodegenerative changes [119].
In another study using the same dnpc1a mutants more extensive brain investigation revealed neuronal cholesterol deposits, accumulation of multilamellar bodies and age-dependent vacuolization. Age-dependent neurodegeneration, early lethality and movement disorders could all be completely rescued by neuronal and partially rescued by glial expression of wild-type dNPC1a transgene [120]. Npc2a mutants displayed a shorter life span, but did not show any brain vacuolization. TUNEL staining revealed neurons undergoing apoptosis [121].
Taken together, NPC can be modelled suitably in Drosophila, as cholesterol storage and neurodegenerative aspects of the disease are represented. On the other hand, one has to keep in mind two potential drawbacks of the invertebrate model when interpreting the results. Firstly Drosophila and other insects have redundant npc1 and npc2 genes (npc1a, npc1b, npc2a, npc2b), whereas mammals including humans only possess one NPC1 and one NPC2 gene. Functions of the other npc genes are not understood yet. Secondly, steroid actions in flies and humans are certainly different as flies cannot synthesize sterol, in particular the molting hormone 20E.
3.3. Ceroid lipofuscinoses
Ceroid lipofuscinoses are characterized by variable but mainly pediatric onset, vision loss, motor dysfunction, seizures and decline of intellectual capacities. [122]. Several causative genes, including CLN2-3, 5–8, 10, PPT1 and MFSD8 were identified [123,124]. Infantile neuronal ceroid lipofuscinosis (INCL), caused by loss of palmitoyl-protein thioesterase 1 (PPT1) on 1p32, shows brain atrophy, neuronal swelling, sudanophilic changes, granular osmiophilic deposits, lysosomal accumulation positive for acidic phophatase in neuronal and astrocytic cells, and rarefaction and shrinking of corticabasal and bulbar neurons [122].
Palmitoyl-protein thioesterase 1 (Ppt1) mutant flies have reduced life span and CNS-specific accumulation of autofluorescent storage material, which unlike human granular osmiophilic deposits were homogeneous in structure and composed of concentric layers of material [125]. The deposits may also be biochemically different as they could not be detected with lipophilic stains [125].
Targeted overexpression of Ppt1 in the Drosophila visual system results in apoptotic neuronal cell loss, leading to misorganized ommatidia [126]. Performance of a gain-of-function modifier screen using enhancer-promoter lines could connect Ppt1 function to synaptic vesicle cycling, endolysosomal trafficking and synaptic plasticity [127]. Observations in Drosophila have to be analyzed carefully as downregulation of Ppt1 leads to accumulation of storage material, different from human GRODs without neurodegeneration und thus Ppt1 in Drosophila might have a different function than PPT1 in humans.
Although human disease is caused by loss of PPT1 and not overexpression, data from Drosophila studies reveal that misregulation of PPT1 may lead to neuronal cell loss and thus the correct titration of enzyme activity may be of importance. Nevertheless the possibility of modifier screens may reveal the physiological role of Ppt1 in Drosophila and this may promote understanding human disease.
4. Tumors
4.1. Neurofibromatosis 1
Neurofibromatosis type 1 (NF1) is an autosomal dominantly inherited neurocutaneous disorder due to mutations in NF1 on 17q11.2 [128]. NF1 is a common disease that mainly affects peripheral and central nervous system (neurofibromas, optic gliomas, astrocytomas, malignant peripheral nerve sheath tumors), the skin (café au lait spots, axillary and inguinal freckling), and may show further neuroendocrine/neuroectodermal tumors, hematopoietic tumors, osseous lesions, iris hamartomas and intellectual handicap [128].
Histologically dermal neurofibromas, well-circumscribed benign tumors composed of Schwann cells, as well as plexiform neurofibromas, producing diffuse enlargement of nerve trunks prevail [129]. Malignant peripheral nerve sheath tumors, highly aggressive tumors characterized by a herringbone pattern of cell growth, are localized within nerve fascicles but invade the adjacent soft tissues [129]. Gliomas are most often pilocytic astrocytomas within the optic nerve and bilateral growth is not uncommon in NF1 patients [129].
Manipulation of NF1 gene was investigated during the last ten years in Drosophila showing that neurofibromin gene plays a role in tissue growth in all developmental stages [130], learning [131], circadian rest-activity rhythm [132], lifespan determination [133], Ras and cAMP interactions [133,134] and stress resistance [133]. Whether these findings will gain therapeutic consequences, for example by using antioxidative drugs, remains controversial. The main point that neurofibromatosis is characterized by appearance of multiple tumors in humans is not represented in all studies yet, thus modelling this disease in Drosophila remains disappointing.
4.2. Neurofibromatosis 2
Neurofibromatosis type 2 (NF2) primarily affects the nervous system, bilateral vestibular schwannomas being prominent. Schwannomas of other cranial nerves, meningiomas, ocular abnormalities, meningioangiomatosis, glial hamartomas and neurofibromas may also be present [128]. NF2 is an autosomal dominantly inherited neurocutaneous disorder due to mutations in NF2 on 22q12.2 [128].
In Drosophila, Merlin mutations have been investigated and were shown to regulate cell growth and cell cycle [135]. It could be shown that Merlin is part of the hippo pathway [135]. Tumor formation in Drosophila could not be established by using Merlin mutations [136]. How these results could influence possible therapeutic options in NF2 patients remains to be elucidated.
4.3. Tuberous sclerosis
Tuberous sclerosis (TSC) is an autosomal dominant disease due to heterozygous mutations in TSC1 on chromosome 9q34 or TSC2 on chromosome 16p13 [137]. TSC is a neurocutaneous disorder characterized by brain abnormalities (cortical tubers, subependymal giant cell astrocytomas (SEGAs), subependymal glial nodules, seizures, mental retardation, autism and attention deficit-hyperactive disorders), kidney pathologies (angiomyolipomas, cysts and renal tumors) as well as rhabdomyomas of the heart [138].
Cortical tubers are strongly associated with the development of epilepsy, especially infantile spasms. They consist of giant cells, dysmorphic neurons, disrupted cortical lamination, gliosis and calcifications [139]. SEGAs are well-circumscribed, often calcified tumors with a mixed glioneuronal phenotype.
In Drosophila, gigas (gig) was identified as homolog of TSC2, leading to increase of cell size and imaginal discs as well as abnormal cell cycle progression [140]. While human SEGAs contain giant cells, corresponding Drosophila tissues in gigas mutated flies show hypertrophic changes but no distinct brain tumors.
4.4. Neuronal/neuroblastic tumors
Furthermore, several fly mutants interfering with asymmetric cell division of neuroblasts exhibit neuronal/neuroblastic tumors which are referred to as “hyperplastic” in case of preserved architecture such as malignant brain tumor (l(3)mbt) [141], or “neoplastic” with loss of architecture and invasion such as brain tumor (brat), raps, numb, pros and mira, the latter four genes encoding for epithelial polarity proteins [142]. Results from these experiments show that maintenance of properly organized apical and basolateral domains is essential to prevent movement of cells out of an epithelium. Mutation of these genes may therefore contribute to metastasis. Different approaches have been pursued in Drosophila to model metastatic spread. Transplantation experiments with brat, dlg and lgl mutated brains into abdomens of adult flies showed that serial transplantations may lead to selection of more aggressive cell clones [143].
Modifier screens have been carried out and Semaphorin-5c and apontic were found to inhibit metastasis in a lgl mutant [144]. These kinds of screens will provide new insights into pathways involved in metastatic events.
Also reverse genetics can be applied to study invasion events as Matrix metalloproteinase 1 (Mmp1) known to promote invasion in humans was upregulated in metastasis models using scribbled (scrib) LOF mutations. Loss of Mmp1 function by RNAi suppressed invasiveness [145].
Although some important anatomical features like vessels are lacking in Drosophila and polarity genes will be more important for carcinoma formation than for endogenous brain tumors, these models may help to understand a variety of basic cell biological features underlying tumor biology and may lead to new concepts in human tumor therapy. Remarkably, fly models of gliomas and medulloblastomas, the most common human brain tumors in adults and childhood, respectively, have not been published yet.
5. Epilepsy
Epilepsy is a neurological disorder characterized by bursts of abnormal electrical brain activity which can be classified into generalized seizures, focal (partial) seizures with or without secondary generalization and can be related to epilepsy syndromes which begin at a specific age and are associated with characteristic EEG patterns [146]. Epilepsies are classified into idiopathic forms that have no known cause except hereditary factors and symptomatic forms caused by brain lesions, such as malformations, tumors or asphyxia) [146]. Idiopathic epilepsy is predominantly associated with ion channel defects, including mutations in potassium-/sodium or calcium channel genes. Mutations of non-ion channel genes like leucine-rich, glioma inactivated 1 gene (LGI1) or Aristaless related homeobox gene (ARX) may also cause epilepsy syndromes [147,148]. Hippocampal sclerosis is the commonest neuropathological lesion identified in epilepsy patients. It is considered to represent both cause and consequence of seizures and can be classified according to varying degrees and localizations of neuronal cell loss [149].
Interestingly, electrical discharge patterns in flies resemble those described in kindling or after discharge stimulation protocols used in rodents [150,151]. By identifying the minimal voltage, which has to be applied to the fly brain in order to evoke a pattern of high-frequency neuronal firing followed by refractory inactivity, seizure susceptibility mutations have been detected. Among those were ethanolamine kinase, mitochondrial ribosomal proteins [152] as well as a number of channelopathy mutants such as seizure, slowpoke, shaker and ether-a-go-go [153,154]. Anticonvulsant drug screenings were performed and phenytoin as well as gabapentin were identified to be effective [155,156]. In mutant backgrounds several genetic modifiers of seizure activity could be detected [157–160], such as top1, which reduced seizure susceptibility. Pharmaceutical inhibition of topoisomerase I protein (top1) enzymatic activity was shown to reduce seizure sensitivity [161], which may lead to the discovery of new substances in human epilepsy therapy. Neuropathological investigations of seizure sensitive fly mutants revealed neuronal loss of varying degree but these could not be deduced to seizure activity alone but also resulted from metabolic defects of the respective mutations [162].
Future perspectives of epilepsy fly models could address additional human hereditary diseases in order to better understand the underlying genetic basis and to develop new therapy approaches by using large scale pharmacological screens.
6. Trauma
Traumatic CNS injury is common, comprising brain and spinal cord hemorrhage, contusion and diffuse axonal injury (DAI) leading to life long disability [163,164]. The injured adult central nervous system (CNS) inhibits axonal outgrowth thus limiting recovery from traumatic injury [163].
Histologically axonal injury is characterized by peri-wound sprouting without significant axonal growth beyond the lesion edge [165] and by presence of axonal bulbs (“retraction balls”) in the white matter, corpus callosum and brain stem [164]. These are caused by axonal perturbation with impairment of axoplasmic transport and swelling of the axon while the myelin sheath remains intact [164]. ß-Amyloid precursor-protein (ßAPP) undergoes fast axonal transport and therefore accumulates where axonal transport is impaired [164].
A Drosophila model for axonal injury and regeneration in the adult brain was established using microdissection trauma in a subpopulation of neurons in the adult brain, the small lateral neurons ventral (sLNv) [166]. After traumatic injury, wildtype sLNv proximal axonal stumps developed bulbar enlargements but failed to regenerate in a long-term, whole-brain explant culture. Regeneration could be enhanced by adult-specific overexpression of protein kinase A specifically in these neurons by PD-F-Gal4 driver line [166,167].
This is a very promising model as post traumatic changes resemble those seen in mammals including fragmentation of the distal stump and forming of retraction bulbs in the proximal stump [168], which is not due to culture conditions as morphology and function remain intact. Additionally cell-type specific screening may reveal molecules and genes involved in CNS axonal regeneration.
7. Conclusions
Taken together, as a wide variety of Drosophila models for neurodegenerative and metabolic brain diseases as well as epilepsy, tumors and trauma exist, it will now be necessary to compare similarities and differences of invertebrate and rodent models and human disease. Genetic tools will allow large modifier screens to reveal new pathways and interactions which could bring light into disease processes, which are not understood yet. Detection of genes modulating disease processes in the brain in Drosophila screens will have to be confirmed in higher model organisms to reach the goal of potential new medications for human diseases.
References
- Matthews, KA; Kaufman, TC; Gelbart, WM. Research resources for Drosophila: The expanding universe. Nat Rev Genet 2005, 6(3), 179–193. [Google Scholar]
- Venken, KJ; Bellen, HJ. Emerging technologies for gene manipulation in Drosophila melanogaster. Nat Rev Genet 2005, 6(3), 167–178. [Google Scholar]
- Dietzl, G; Chen, D; Schnorrer, F; Su, KC; Barinova, Y; Fellner, M; Gasser, B; Kinsey, K; Oppel, S; Scheiblauer, S; Couto, A; Marra, V; Keleman, K; Dickson, BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 2007, 448(7150), 151–156. [Google Scholar]
- Rubin, GM; Lewis, EB. A brief history of Drosophila’s contributions to genome research. Science 2000, 287(5461), 2216–2218. [Google Scholar]
- Adams, MD; Celniker, SE; Holt, RA; Evans, CA; Gocayne, JD; Amanatides, PG; Scherer, SE; Li, PW; Hoskins, RA; Galle, RF; George, RA; Lewis, SE; Richards, S; Ashburner, M; Henderson, SN; Sutton, GG; Wortman, JR; Yandell, MD; Zhang, Q; Chen, LX; Brandon, RC; Rogers, YH; Blazej, RG; Champe, M; Pfeiffer, BD; Wan, KH; Doyle, C; Baxter, EG; Helt, G; Nelson, CR; Gabor, GL; Abril, JF; Agbayani, A; An, HJ; Andrews-Pfannkoch, C; Baldwin, D; Ballew, RM; Basu, A; Baxendale, J; Bayraktaroglu, L; Beasley, EM; Beeson, KY; Benos, PV; Berman, BP; Bhandari, D; Bolshakov, S; Borkova, D; Botchan, MR; Bouck, J; Brokstein, P; Brottier, P; Burtis, KC; Busam, DA; Butler, H; Cadieu, E; Center, A; Chandra, I; Cherry, JM; Cawley, S; Dahlke, C; Davenport, LB; Davies, P; de Pablos, B; Delcher, A; Deng, Z; Mays, AD; Dew, I; Dietz, SM; Dodson, K; Doup, LE; Downes, M; Dugan-Rocha, S; Dunkov, BC; Dunn, P; Durbin, KJ; Evangelista, CC; Ferraz, C; Ferriera, S; Fleischmann, W; Fosler, C; Gabrielian, AE; Garg, NS; Gelbart, WM; Glasser, K; Glodek, A; Gong, F; Gorrell, JH; Gu, Z; Guan, P; Harris, M; Harris, NL; Harvey, D; Heiman, TJ; Hernandez, JR; Houck, J; Hostin, D; Houston, KA; Howland, TJ; Wei, MH; Ibegwam, C; Jalali, M; Kalush, F; Karpen, GH; Ke, Z; Kennison, JA; Ketchum, KA; Kimmel, BE; Kodira, CD; Kraft, C; Kravitz, S; Kulp, D; Lai, Z; Lasko, P; Lei, Y; Levitsky, AA; Li, J; Li, Z; Liang, Y; Lin, X; Liu, X; Mattei, B; McIntosh, TC; McLeod, MP; McPherson, D; Merkulov, G; Milshina, NV; Mobarry, C; Morris, J; Moshrefi, A; Mount, SM; Moy, M; Murphy, B; Murphy, L; Muzny, DM; Nelson, DL; Nelson, DR; Nelson, KA; Nixon, K; Nusskern, DR; Pacleb, JM; Palazzolo, M; Pittman, GS; Pan, S; Pollard, J; Puri, V; Reese, MG; Reinert, K; Remington, K; Saunders, RD; Scheeler, F; Shen, H; Shue, BC; Siden-Kiamos, I; Simpson, M; Skupski, MP; Smith, T; Spier, E; Spradling, AC; Stapleton, M; Strong, R; Sun, E; Svirskas, R; Tector, C; Turner, R; Venter, E; Wang, AH; Wang, X; Wang, ZY; Wassarman, DA; Weinstock, GM; Weissenbach, J; Williams, SM; Woodage, T; Worley, KC; Wu, D; Yang, S; Yao, QA; Ye, J; Yeh, RF; Zaveri, JS; Zhan, M; Zhang, G; Zhao, Q; Zheng, L; Zheng, XH; Zhong, FN; Zhong, W; Zhou, X; Zhu, S; Zhu, X; Smith, HO; Gibbs, RA; Myers, EW; Rubin, GM; Venter, JC. The genome sequence of Drosophila melanogaster. Science 2000, 287(5461), 2185–2195. [Google Scholar]
- Lander, ES; Linton, LM; Birren, B; Nusbaum, C; Zody, MC; Baldwin, J; Devon, K; Dewar, K; Doyle, M; FitzHugh, W; Funke, R; Gage, D; Harris, K; Heaford, A; Howland, J; Kann, L; Lehoczky, J; LeVine, R; McEwan, P; McKernan, K; Meldrim, J; Mesirov, JP; Miranda, C; Morris, W; Naylor, J; Raymond, C; Rosetti, M; Santos, R; Sheridan, A; Sougnez, C; Stange-Thomann, N; Stojanovic, N; Subramanian, A; Wyman, D; Rogers, J; Sulston, J; Ainscough, R; Beck, S; Bentley, D; Burton, J; Clee, C; Carter, N; Coulson, A; Deadman, R; Deloukas, P; Dunham, A; Dunham, I; Durbin, R; French, L; Grafham, D; Gregory, S; Hubbard, T; Humphray, S; Hunt, A; Jones, M; Lloyd, C; McMurray, A; Matthews, L; Mercer, S; Milne, S; Mullikin, JC; Mungall, A; Plumb, R; Ross, M; Shownkeen, R; Sims, S; Waterston, RH; Wilson, RK; Hillier, LW; McPherson, JD; Marra, MA; Mardis, ER; Fulton, LA; Chinwalla, AT; Pepin, KH; Gish, WR; Chissoe, SL; Wendl, MC; Delehaunty, KD; Miner, TL; Delehaunty, A; Kramer, JB; Cook, LL; Fulton, RS; Johnson, DL; Minx, PJ; Clifton, SW; Hawkins, T; Branscomb, E; Predki, P; Richardson, P; Wenning, S; Slezak, T; Doggett, N; Cheng, JF; Olsen, A; Lucas, S; Elkin, C; Uberbacher, E; Frazier, M; Gibbs, RA; Muzny, DM; Scherer, SE; Bouck, JB; Sodergren, EJ; Worley, KC; Rives, CM; Gorrell, JH; Metzker, ML; Naylor, SL; Kucherlapati, RS; Nelson, DL; Weinstock, GM; Sakaki, Y; Fujiyama, A; Hattori, M; Yada, T; Toyoda, A; Itoh, T; Kawagoe, C; Watanabe, H; Totoki, Y; Taylor, T; Weissenbach, J; Heilig, R; Saurin, W; Artiguenave, F; Brottier, P; Bruls, T; Pelletier, E; Robert, C; Wincker, P; Smith, DR; Doucette-Stamm, L; Rubenfield, M; Weinstock, K; Lee, HM; Dubois, J; Rosenthal, A; Platzer, M; Nyakatura, G; Taudien, S; Rump, A; Yang, H; Yu, J; Wang, J; Huang, G; Gu, J; Hood, L; Rowen, L; Madan, A; Qin, S; Davis, RW; Federspiel, NA; Abola, AP; Proctor, MJ; Myers, RM; Schmutz, J; Dickson, M; Grimwood, J; Cox, DR; Olson, MV; Kaul, R; Shimizu, N; Kawasaki, K; Minoshima, S; Evans, GA; Athanasiou, M; Schultz, R; Roe, BA; Chen, F; Pan, H; Ramser, J; Lehrach, H; Reinhardt, R; McCombie, WR; de la Bastide, M; Dedhia, N; Blocker, H; Hornischer, K; Nordsiek, G; Agarwala, R; Aravind, L; Bailey, JA; Bateman, A; Batzoglou, S; Birney, E; Bork, P; Brown, DG; Burge, CB; Cerutti, L; Chen, HC; Church, D; Clamp, M; Copley, RR; Doerks, T; Eddy, SR; Eichler, EE; Furey, TS; Galagan, J; Gilbert, JG; Harmon, C; Hayashizaki, Y; Haussler, D; Hermjakob, H; Hokamp, K; Jang, W; Johnson, LS; Jones, TA; Kasif, S; Kaspryzk, A; Kennedy, S; Kent, WJ; Kitts, P; Koonin, EV; Korf, I; Kulp, D; Lancet, D; Lowe, TM; McLysaght, A; Mikkelsen, T; Moran, JV; Mulder, N; Pollara, VJ; Ponting, CP; Schuler, G; Schultz, J; Slater, G; Smit, AF; Stupka, E; Szustakowski, J; Thierry-Mieg, D; Thierry-Mieg, J; Wagner, L; Wallis, J; Wheeler, R; Williams, A; Wolf, YI; Wolfe, KH; Yang, SP; Yeh, RF; Collins, F; Guyer, MS; Peterson, J; Felsenfeld, A; Wetterstrand, KA; Patrinos, A; Morgan, MJ; de Jong, P; Catanese, JJ; Osoegawa, K; Shizuya, H; Choi, S; Chen, YJ. Initial sequencing and analysis of the human genome. Nature 2001, 409(6822), 860–921. [Google Scholar]
- Venter, JC; Adams, MD; Myers, EW; Li, PW; Mural, RJ; Sutton, GG; Smith, HO; Yandell, M; Evans, CA; Holt, RA; Gocayne, JD; Amanatides, P; Ballew, RM; Huson, DH; Wortman, JR; Zhang, Q; Kodira, CD; Zheng, XH; Chen, L; Skupski, M; Subramanian, G; Thomas, PD; Zhang, J; Gabor Miklos, GL; Nelson, C; Broder, S; Clark, AG; Nadeau, J; McKusick, VA; Zinder, N; Levine, AJ; Roberts, RJ; Simon, M; Slayman, C; Hunkapiller, M; Bolanos, R; Delcher, A; Dew, I; Fasulo, D; Flanigan, M; Florea, L; Halpern, A; Hannenhalli, S; Kravitz, S; Levy, S; Mobarry, C; Reinert, K; Remington, K; Abu-Threideh, J; Beasley, E; Biddick, K; Bonazzi, V; Brandon, R; Cargill, M; Chandramouliswaran, I; Charlab, R; Chaturvedi, K; Deng, Z; Di Francesco, V; Dunn, P; Eilbeck, K; Evangelista, C; Gabrielian, AE; Gan, W; Ge, W; Gong, F; Gu, Z; Guan, P; Heiman, TJ; Higgins, ME; Ji, RR; Ke, Z; Ketchum, KA; Lai, Z; Lei, Y; Li, Z; Li, J; Liang, Y; Lin, X; Lu, F; Merkulov, GV; Milshina, N; Moore, HM; Naik, AK; Narayan, VA; Neelam, B; Nusskern, D; Rusch, DB; Salzberg, S; Shao, W; Shue, B; Sun, J; Wang, Z; Wang, A; Wang, X; Wang, J; Wei, M; Wides, R; Xiao, C; Yan, C; Yao, A; Ye, J; Zhan, M; Zhang, W; Zhang, H; Zhao, Q; Zheng, L; Zhong, F; Zhong, W; Zhu, S; Zhao, S; Gilbert, D; Baumhueter, S; Spier, G; Carter, C; Cravchik, A; Woodage, T; Ali, F; An, H; Awe, A; Baldwin, D; Baden, H; Barnstead, M; Barrow, I; Beeson, K; Busam, D; Carver, A; Center, A; Cheng, ML; Curry, L; Danaher, S; Davenport, L; Desilets, R; Dietz, S; Dodson, K; Doup, L; Ferriera, S; Garg, N; Gluecksmann, A; Hart, B; Haynes, J; Haynes, C; Heiner, C; Hladun, S; Hostin, D; Houck, J; Howland, T; Ibegwam, C; Johnson, J; Kalush, F; Kline, L; Koduru, S; Love, A; Mann, F; May, D; McCawley, S; McIntosh, T; McMullen, I; Moy, M; Moy, L; Murphy, B; Nelson, K; Pfannkoch, C; Pratts, E; Puri, V; Qureshi, H; Reardon, M; Rodriguez, R; Rogers, YH; Romblad, D; Ruhfel, B; Scott, R; Sitter, C; Smallwood, M; Stewart, E; Strong, R; Suh, E; Thomas, R; Tint, NN; Tse, S; Vech, C; Wang, G; Wetter, J; Williams, S; Williams, M; Windsor, S; Winn-Deen, E; Wolfe, K; Zaveri, J; Zaveri, K; Abril, JF; Guigo, R; Campbell, MJ; Sjolander, KV; Karlak, B; Kejariwal, A; Mi, H; Lazareva, B; Hatton, T; Narechania, A; Diemer, K; Muruganujan, A; Guo, N; Sato, S; Bafna, V; Istrail, S; Lippert, R; Schwartz, R; Walenz, B; Yooseph, S; Allen, D; Basu, A; Baxendale, J; Blick, L; Caminha, M; Carnes-Stine, J; Caulk, P; Chiang, YH; Coyne, M; Dahlke, C; Mays, A; Dombroski, M; Donnelly, M; Ely, D; Esparham, S; Fosler, C; Gire, H; Glanowski, S; Glasser, K; Glodek, A; Gorokhov, M; Graham, K; Gropman, B; Harris, M; Heil, J; Henderson, S; Hoover, J; Jennings, D; Jordan, C; Jordan, J; Kasha, J; Kagan, L; Kraft, C; Levitsky, A; Lewis, M; Liu, X; Lopez, J; Ma, D; Majoros, W; McDaniel, J; Murphy, S; Newman, M; Nguyen, T; Nguyen, N; Nodell, M; Pan, S; Peck, J; Peterson, M; Rowe, W; Sanders, R; Scott, J; Simpson, M; Smith, T; Sprague, A; Stockwell, T; Turner, R; Venter, E; Wang, M; Wen, M; Wu, D; Wu, M; Xia, A; Zandieh, A; Zhu, X. The sequence of the human genome. Science 2001, 291(5507), 1304–1351. [Google Scholar]
- Aquadro, CF; Bauer DuMont, V; Reed, FA. Genome-wide variation in the human and fruitfly: A comparison. Curr Opin Genet Dev 2001, 11(6), 627–634. [Google Scholar]
- Brand, AH; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118(2), 401–415. [Google Scholar]
- Min, KT; Benzer, S. Spongecake and eggroll: Two hereditary diseases in Drosophila resemble patterns of human brain degeneration. Curr Biol 1997, 7(11), 885–888. [Google Scholar]
- Kretzschmar, D; Hasan, G; Sharma, S; Heisenberg, M; Benzer, S. The swiss cheese mutant causes glial hyperwrapping and brain degeneration in Drosophila. J Neurosci 1997, 17(19), 7425–7432. [Google Scholar]
- Min, KT; Benzer, S. Preventing neurodegeneration in the Drosophila mutant bubblegum. Science 1999, 284(5422), 1985–1988. [Google Scholar]
- Cauchi, RJ; van den Heuvel, M. The fly as a model for neurodegenerative diseases: Is it worth the jump. Neurodegener Dis 2006, 3(6), 338–356. [Google Scholar]
- Mitchell, KJ; Doyle, JL; Serafini, T; Kennedy, TE; Tessier-Lavigne, M; Goodman, CS; Dickson, BJ. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron 1996, 17(2), 203–215. [Google Scholar]
- Hummel, T; Schimmelpfeng, K; Klambt, C. Commissure formation in the embryonic CNS of Drosophila. Development 1999, 126(4), 771–779. [Google Scholar]
- Klambt, C; Hummel, T; Granderath, S; Schimmelpfeng, K. Glial cell development in Drosophila. Int J Dev Neurosci 2001, 19(4), 373–378. [Google Scholar]
- Hartenstein, V; Nassif, C; Lekven, A. Embryonic development of the Drosophila brain. II. Pattern of glial cells. J Comp Neurol 1998, 402(1), 32–47. [Google Scholar]
- Heisenberg, M. Mushroom body memoir: from maps to models. Nat Rev Neurosci 2003, 4(4), 266–275. [Google Scholar]
- Gu, H; O’Dowd, DK. Cholinergic synaptic transmission in adult Drosophila Kenyon cells in situ. J. Neurosci 2006, 26(1), 265–272. [Google Scholar]
- Marsh, JL; Thompson, LM. Drosophila in the study of neurodegenerative disease. Neuron 2006, 52(1), 169–178. [Google Scholar]
- Clandinin, TR; Lee, CH; Herman, T; Lee, RC; Yang, AY; Ovasapyan, S; Zipursky, SL. Drosophila LAR regulates R1–R6 and R7 target specificity in the visual system. Neuron 2001, 32(2), 237–248. [Google Scholar]
- Tomlinson, A. Cellular interactions in the developing Drosophila eye. Development 1988, 104(2), 183–193. [Google Scholar]
- Morante, J; Desplan, C; Celik, A. Generating patterned arrays of photoreceptors. Curr Opin Genet Dev 2007, 17(4), 314–319. [Google Scholar]
- Ball, MJ; Murdoch, GH. Neuropathological criteria for the diagnosis of Alzheimer’s disease: Are we really ready yet? Neurobiol Aging 1997, 18(4 Suppl), S3–12. [Google Scholar]
- Braak, H; Alafuzoff, I; Arzberger, T; Kretzschmar, H; Del Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006, 112(4), 389–404. [Google Scholar]
- van der Zee, J; Sleegers, K; Van Broeckhoven, C. Invited article: The Alzheimer disease-frontotemporal lobar degeneration spectrum. Neurology 2008, 71(15), 1191–1197. [Google Scholar]
- Martin-Morris, LE; White, K. The Drosophila transcript encoded by the beta-amyloid protein precursor-like gene is restricted to the nervous system. Development 1990, 110(1), 185–195. [Google Scholar]
- Rosen, DR; Martin-Morris, L; Luo, LQ; White, K. A Drosophila gene encoding a protein resembling the human beta-amyloid protein precursor. Proc Natl Acad Sci USA 1989, 86(7), 2478–2482. [Google Scholar]
- Fossgreen, A; Bruckner, B; Czech, C; Masters, CL; Beyreuther, K; Paro, R. Transgenic Drosophila expressing human amyloid precursor protein show gamma-secretase activity and a blistered-wing phenotype. Proc Natl Acad Sci USA 1998, 95(23), 13703–13708. [Google Scholar]
- Bonini, NM; Fortini, ME. Human neurodegenerative disease modeling using Drosophila. Annu. Rev. Neurosci 2003, 26, 627–656. [Google Scholar]
- Driscoll, M; Gerstbrein, B. Dying for a cause: Invertebrate genetics takes on human neurodegeneration. Nat Rev Genet 2003, 4(3), 181–194. [Google Scholar]
- Kopan, R; Goate, A. Aph-2/Nicastrin: An essential component of gamma-secretase and regulator of Notch signaling and Presenilin localization. Neuron 2002, 33(3), 321–324. [Google Scholar]
- Iijima, K; Liu, HP; Chiang, AS; Hearn, SA; Konsolaki, M; Zhong, Y. Dissecting the pathological effects of human Abeta40 and Abeta42 in Drosophila: a potential model for Alzheimer’s disease. Proc Natl Acad Sci USA 2004, 101(17), 6623–6628. [Google Scholar]
- Iijima, K; Iijima-Ando, K. Drosophila Models of Alzheimer’s Amyloidosis: The Challenge of Dissecting the Complex Mechanisms of Toxicity of Amyloid-beta 42. J Alzheimers Dis 2008, 15(4), 523–540. [Google Scholar]
- Finelli, A; Kelkar, A; Song, HJ; Yang, H; Konsolaki, M. A model for studying Alzheimer’s Abeta42-induced toxicity in Drosophila melanogaster. Mol Cell Neurosci 2004, 26(3), 365–375. [Google Scholar]
- Greeve, I; Kretzschmar, D; Tschape, JA; Beyn, A; Brellinger, C; Schweizer, M; Nitsch, RM; Reifegerste, R. Age-dependent neurodegeneration and Alzheimer-amyloid plaque formation in transgenic Drosophila. J Neurosci 2004, 24(16), 3899–3906. [Google Scholar]
- Yamaguchi, H; Yamazaki, T; Ishiguro, K; Shoji, M; Nakazato, Y; Hirai, S. Ultrastructural localization of Alzheimer amyloid beta/A4 protein precursor in the cytoplasm of neurons and senile plaque-associated astrocytes. Acta Neuropathol 1992, 85(1), 15–22. [Google Scholar]
- Gunawardena, S; Goldstein, LS. Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron 2001, 32(3), 389–401. [Google Scholar]
- Fiala, JC. Mechanisms of amyloid plaque pathogenesis. Acta Neuropathol 2007, 114(6), 551–571. [Google Scholar]
- Duyckaerts, C; Potier, MC; Delatour, B. Alzheimer disease models and human neuropathology: Similarities and differences. Acta Neuropathol 2008, 115(1), 5–38. [Google Scholar]
- Cairns, NJ; Bigio, EH; Mackenzie, IR; Neumann, M; Lee, VM; Hatanpaa, KJ; White, CL, 3rd; Schneider, JA; Grinberg, LT; Halliday, G; Duyckaerts, C; Lowe, JS; Holm, IE; Tolnay, M; Okamoto, K; Yokoo, H; Murayama, S; Woulfe, J; Munoz, DG; Dickson, DW; Ince, PG; Trojanowski, JQ; Mann, DM. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 2007, 114(1), 5–22. [Google Scholar]
- Frank, S; Clavaguera, F; Tolnay, M. Tauopathy models and human neuropathology: similarities and differences. Acta Neuropathol 2008, 115(1), 39–53. [Google Scholar]
- Heidary, G; Fortini, ME. Identification and characterization of the Drosophila tau homolog. Mech Dev 2001, 108. [Google Scholar]
- Wittmann, CW; Wszolek, MF; Shulman, JM; Salvaterra, PM; Lewis, J; Hutton, M; Feany, MB. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 2001, 293(5530), 711–714. [Google Scholar]
- Jackson, GR; Wiedau-Pazos, M; Sang, TK; Wagle, N; Brown, CA; Massachi, S; Geschwind, DH. Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron 2002, 34(4), 509–519. [Google Scholar]
- Augustinack, JC; Schneider, A; Mandelkow, EM; Hyman, BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol 2002, 103(1), 26–35. [Google Scholar]
- Iwatsubo, T; Hasegawa, M; Ihara, Y. Neuronal and glial tau-positive inclusions in diverse neurologic diseases share common phosphorylation characteristics. Acta Neuropathol 1994, 88(2), 129–136. [Google Scholar]
- Chen, X; Li, Y; Huang, J; Cao, D; Yang, G; Liu, W; Lu, H; Guo, A. Study of tauopathies by comparing Drosophila and human tau in Drosophila. Cell Tissue Res 2007, 329(1), 169–178. [Google Scholar]
- Mudher, A; Shepherd, D; Newman, TA; Mildren, P; Jukes, JP; Squire, A; Mears, A; Drummond, JA; Berg, S; MacKay, D; Asuni, AA; Bhat, R; Lovestone, S. GSK-3beta inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Mol Psychiatry 2004, 9(5), 522–530. [Google Scholar]
- Shulman, JM; Shulman, LM; Weiner, WJ; Feany, MB. From fruit fly to bedside: Translating lessons from Drosophila models of neurodegenerative disease. Curr Opin Neurol 2003, 16(4), 443–449. [Google Scholar]
- Nishimura, I; Yang, Y; Lu, B. PAR-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in Drosophila. Cell 2004, 116(5), 671–682. [Google Scholar]
- Meredith, GE; Sonsalla, PK; Chesselet, MF. Animal models of Parkinson’s disease progression. Acta Neuropathol 2008, 115(4), 385–398. [Google Scholar]
- Kuzuhara, S; Mori, H; Izumiyama, N; Yoshimura, M; Ihara, Y. Lewy bodies are ubiquitinated. A light and electron microscopic immunocytochemical study. Acta Neuropathol 1988, 75(4), 345–353. [Google Scholar]
- Feany, MB; Bender, WW. A Drosophila model of Parkinson’s disease. Nature 2000, 404(6776), 394–8. [Google Scholar]
- Pendleton, RG; Parvez, F; Sayed, M; Hillman, R. Effects of pharmacological agents upon a transgenic model of Parkinson’s disease in Drosophila melanogaster. J Pharmacol Exp Ther 2002, 300(1), 91–96. [Google Scholar]
- Greene, JC; Whitworth, AJ; Kuo, I; Andrews, LA; Feany, MB; Pallanck, LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci USA 2003, 100(7), 4078–4083. [Google Scholar]
- Pesah, Y; Pham, T; Burgess, H; Middlebrooks, B; Verstreken, P; Zhou, Y; Harding, M; Bellen, H; Mardon, G. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development 2004, 131(9), 2183–2194. [Google Scholar]
- Yang, Y; Nishimura, I; Imai, Y; Takahashi, R; Lu, B. Parkin suppresses dopaminergic neuron-selective neurotoxicity induced by Pael-R in Drosophila. Neuron 2003, 37(6), 911–924. [Google Scholar]
- Sang, TK; Chang, HY; Lawless, GM; Ratnaparkhi, A; Mee, L; Ackerson, LC; Maidment, NT; Krantz, DE; Jackson, GR. A Drosophila model of mutant human parkin-induced toxicity demonstrates selective loss of dopaminergic neurons and dependence on cellular dopamine. J Neurosci 2007, 27(5), 981–992. [Google Scholar]
- Park, J; Lee, SB; Lee, S; Kim, Y; Song, S; Kim, S; Bae, E; Kim, J; Shong, M; Kim, JM; Chung, J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 2006, 441(7097), 1157–1161. [Google Scholar]
- Meulener, M; Whitworth, AJ; Armstrong-Gold, CE; Rizzu, P; Heutink, P; Wes, PD; Pallanck, LJ; Bonini, NM. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson’s disease. Curr Biol 2005, 15(17), 1572–1577. [Google Scholar]
- Park, J; Kim, SY; Cha, GH; Lee, SB; Kim, S; Chung, J. Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene 2005, 361, 133–139. [Google Scholar]
- Zimprich, A; Biskup, S; Leitner, P; Lichtner, P; Farrer, M; Lincoln, S; Kachergus, J; Hulihan, M; Uitti, RJ; Calne, DB; Stoessl, AJ; Pfeiffer, RF; Patenge, N; Carbajal, IC; Vieregge, P; Asmus, F; Muller-Myhsok, B; Dickson, DW; Meitinger, T; Strom, TM; Wszolek, ZK; Gasser, T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004, 44(4), 601–607. [Google Scholar]
- Lee, SB; Kim, W; Lee, S; Chung, J. Loss of LRRK2/PARK8 induces degeneration of dopaminergic neurons in Drosophila. Biochem Biophys Res Commun 2007, 358(2), 534–539. [Google Scholar]
- Nichols, WC; Pankratz, N; Hernandez, D; Paisan-Ruiz, C; Jain, S; Halter, CA; Michaels, VE; Reed, T; Rudolph, A; Shults, CW; Singleton, A; Foroud, T. Genetic screening for a single common LRRK2 mutation in familial Parkinson’s disease. Lancet 2005, 365(9457), 410–412. [Google Scholar]
- Gilks, WP; Abou-Sleiman, PM; Gandhi, S; Jain, S; Singleton, A; Lees, AJ; Shaw, K; Bhatia, KP; Bonifati, V; Quinn, NP; Lynch, J; Healy, DG; Holton, JL; Revesz, T; Wood, NW. A common LRRK2 mutation in idiopathic Parkinson’s disease. Lancet 2005, 365(9457), 415–416. [Google Scholar]
- Liu, Z; Wang, X; Yu, Y; Li, X; Wang, T; Jiang, H; Ren, Q; Jiao, Y; Sawa, A; Moran, T; Ross, CA; Montell, C; Smith, WW. A Drosophila model for LRRK2-linked parkinsonism. Proc Natl Acad Sci USA 2008, 105(7), 2693–2698. [Google Scholar]
- Coulom, H; Birman, S. Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J Neurosci 2004, 24(48), 10993–10998. [Google Scholar]
- Jimenez-Del-Rio, M; Daza-Restrepo, A; Velez-Pardo, C. The cannabinoid CP55,940 prolongs survival and improves locomotor activity in Drosophila melanogaster against paraquat: Implications in Parkinson’s disease. Neurosci Res 2008, 61(4), 404–411. [Google Scholar]
- Unterberger, U; Voigtlander, T; Budka, H. Pathogenesis of prion diseases. Acta Neuropathol 2005, 109(1), 32–48. [Google Scholar]
- Prusiner, SB. Prions. Proc Natl Acad Sci USA 1998, 95(23), 13363–13383. [Google Scholar]
- Ghetti, B; Tagliavini, F; Takao, M; Bugiani, O; Piccardo, P. Hereditary prion protein amyloidoses. Clin Lab Med 2003, 23(1). [Google Scholar]
- Collins, S; McLean, CA; Masters, CL. Gerstmann-Straussler-Scheinker syndrome, fatal familial insomnia, and kuru: A review of these less common human transmissible spongiform encephalopathies. J Clin Neurosci 2001, 8(5), 387–397. [Google Scholar]
- Raeber, AJ; Muramoto, T; Kornberg, TB; Prusiner, SB. Expression and targeting of Syrian hamster prion protein induced by heat shock in transgenic Drosophila melanogaster. Mech Dev 1995, 51. [Google Scholar]
- Gavin, BA; Dolph, MJ; Deleault, NR; Geoghegan, JC; Khurana, V; Feany, MB; Dolph, PJ; Supattapone, S. Accelerated accumulation of misfolded prion protein and spongiform degeneration in a Drosophila model of Gerstmann-Straussler-Scheinker syndrome. J Neurosci 2006, 26(48), 12408–12414. [Google Scholar]
- Vonsattel, JP. Huntington disease models and human neuropathology: similarities and differences. Acta Neuropathol 2008, 115(1), 55–69. [Google Scholar]
- Jackson, GR; Salecker, I; Dong, X; Yao, X; Arnheim, N; Faber, PW; MacDonald, ME; Zipursky, SL. Polyglutamine-expanded human huntingtin transgenes induce degeneration of Drosophila photoreceptor neurons. Neuron 1998, 21(3), 633–642. [Google Scholar]
- Gunawardena, S; Her, LS; Brusch, RG; Laymon, RA; Niesman, IR; Gordesky-Gold, B; Sintasath, L; Bonini, NM; Goldstein, LS. Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron 2003, 40(1), 25–40. [Google Scholar]
- Jung, J; Bonini, N. CREB-binding protein modulates repeat instability in a Drosophila model for polyQ disease. Science 2007, 315(5820), 1857–1859. [Google Scholar]
- Branco, J; Al-Ramahi, I; Ukani, L; Perez, AM; Fernandez-Funez, P; Rincon-Limas, D; Botas, J. Comparative analysis of genetic modifiers in Drosophila points to common and distinct mechanisms of pathogenesis among polyglutamine diseases. Hum Mol Genet 2008, 17(3), 376–390. [Google Scholar]
- Lim, J; Crespo-Barreto, J; Jafar-Nejad, P; Bowman, AB; Richman, R; Hill, DE; Orr, HT; Zoghbi, HY. Opposing effects of polyglutamine expansion on native protein complexes contribute to SCA1. Nature 2008, 452(7188), 713–718. [Google Scholar]
- Yamada, M; Sato, T; Tsuji, S; Takahashi, H. CAG repeat disorder models and human neuropathology: Similarities and differences. Acta Neuropathol 2008, 115(1), 71–86. [Google Scholar]
- Warrick, JM; Paulson, HL; Gray-Board, GL; Bui, QT; Fischbeck, KH; Pittman, RN; Bonini, NM. Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila. Cell 1998, 93(6), 939–949. [Google Scholar]
- Warrick, JM; Chan, HY; Gray-Board, GL; Chai, Y; Paulson, HL; Bonini, NM. Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat Genet 1999, 23(4), 425–428. [Google Scholar]
- Chan, HY; Warrick, JM; Gray-Board, GL; Paulson, HL; Bonini, NM. Mechanisms of chaperone suppression of polyglutamine disease: Selectivity, synergy and modulation of protein solubility in Drosophila. Hum Mol Genet 2000, 9(19), 2811–2820. [Google Scholar]
- Warrick, JM; Morabito, LM; Bilen, J; Gordesky-Gold, B; Faust, LZ; Paulson, HL; Bonini, NM. Ataxin-3 suppresses polyglutamine neurodegeneration in Drosophila by a ubiquitin-associated mechanism. Mol Cell 2005, 18(1), 37–48. [Google Scholar]
- Bilen, J; Bonini, NM. Genome-wide screen for modifiers of ataxin-3 neurodegeneration in Drosophila. PLoS Genet 2007, 3(10), 1950–1964. [Google Scholar]
- Lessing, D; Bonini, NM. Polyglutamine genes interact to modulate the severity and progression of neurodegeneration in Drosophila. PLoS Biol 2008, 6(2), e29. [Google Scholar]
- Takeyama, K; Ito, S; Yamamoto, A; Tanimoto, H; Furutani, T; Kanuka, H; Miura, M; Tabata, T; Kato, S. Androgen-dependent neurodegeneration by polyglutamine-expanded human androgen receptor in Drosophila. Neuron 2002, 35(5), 855–864. [Google Scholar]
- Murata, T; Suzuki, E; Ito, S; Sawatsubashi, S; Zhao, Y; Yamagata, K; Tanabe, M; Fujiyama, S; Kimura, S; Ueda, T; Matsukawa, H; Kouzmenko, A; Furutani, T; Kuranaga, E; Miura, M; Takeyama, K; Kato, S. RNA-binding protein hoip accelerates polyQ-induced neurodegeneration in Drosophila. Biosci Biotechnol Biochem 2008, 72(9), 2255–2261. [Google Scholar]
- Ranum, LP; Day, JW. Pathogenic RNA repeats: an expanding role in genetic disease. Trends Genet 2004, 20(10), 506–512. [Google Scholar]
- Nemes, JP; Benzow, KA; Moseley, ML; Ranum, LP; Koob, MD. The SCA8 transcript is an antisense RNA to a brain-specific transcript encoding a novel actin-binding protein (KLHL1). Hum Mol Genet 2000, 9(10), 1543–1551. [Google Scholar]
- Mutsuddi, M; Marshall, CM; Benzow, KA; Koob, MD; Rebay, I. The spinocerebellar ataxia 8 noncoding RNA causes neurodegeneration and associates with staufen in Drosophila. Curr Biol 2004, 14(4), 302–308. [Google Scholar]
- Simic, G; Mladinov, M; Seso Simic, D; Jovanov Milosevic, N; Islam, A; Pajtak, A; Barisic, N; Sertic, J; Lucassen, PJ; Hof, PR; Kruslin, B. Abnormal motoneuron migration, differentiation, and axon outgrowth in spinal muscular atrophy. Acta Neuropathol 2008, 115(3), 313–326. [Google Scholar]
- Lefebvre, S; Burglen, L; Reboullet, S; Clermont, O; Burlet, P; Viollet, L; Benichou, B; Cruaud, C; Millasseau, P; Zeviani, M; et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80(1), 155–165. [Google Scholar]
- Miguel-Aliaga, I; Chan, YB; Davies, KE; van den Heuvel, M. Disruption of SMN function by ectopic expression of the human SMN gene in Drosophila. FEBS Lett 2000, 486(2), 99–102. [Google Scholar]
- Chan, YB; Miguel-Aliaga, I; Franks, C; Thomas, N; Trulzsch, B; Sattelle, DB; Davies, KE; van den Heuvel, M. Neuromuscular defects in a Drosophila survival motor neuron gene mutant. Hum Mol Genet 2003, 12(12), 1367–1376. [Google Scholar]
- Chang, HC; Dimlich, DN; Yokokura, T; Mukherjee, A; Kankel, MW; Sen, A; Sridhar, V; Fulga, TA; Hart, AC; Van Vactor, D; Artavanis-Tsakonas, S. Modeling spinal muscular atrophy in Drosophila. PLoS ONE 2008, 3(9), e3209. [Google Scholar]
- Lomen-Hoerth, C. Amyotrophic lateral sclerosis from bench to bedside. Semin Neurol 2008, 28(2), 205–211. [Google Scholar]
- Rosen, DR; Siddique, T; Patterson, D; Figlewicz, DA; Sapp, P; Hentati, A; Donaldson, D; Goto, J; O’Regan, JP; Deng, HX; et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993, 362(6415), 59–62. [Google Scholar]
- Kato, S. Amyotrophic lateral sclerosis models and human neuropathology: similarities and differences. Acta Neuropathol 2008, 115(1), 97–114. [Google Scholar]
- Lin, WL; Dickson, DW. Ultrastructural localization of TDP-43 in filamentous neuronal inclusions in various neurodegenerative diseases. Acta Neuropathol 2008, 116(2), 205–213. [Google Scholar]
- Watson, MR; Lagow, RD; Xu, K; Zhang, B; Bonini, NM. A drosophila model for amyotrophic lateral sclerosis reveals motor neuron damage by human SOD1. J Biol Chem 2008, 283(36), 24972–24981. [Google Scholar]
- Ratnaparkhi, A; Lawless, GM; Schweizer, FE; Golshani, P; Jackson, GR. A Drosophila model of ALS: Human ALS-associated mutation in VAP33A suggests a dominant negative mechanism. PLoS ONE 2008, 3(6), e2334. [Google Scholar]
- Parkes, TL; Elia, AJ; Dickinson, D; Hilliker, AJ; Phillips, JP; Boulianne, GL. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat Genet 1998, 19(2), 171–4. [Google Scholar]
- Elia, AJ; Parkes, TL; Kirby, K; St George-Hyslop, P; Boulianne, GL; Phillips, JP; Hilliker, AJ. Expression of human FALS SOD in motorneurons of Drosophila. Free Radic Biol Med 1999, 26. [Google Scholar]
- Birch-Machin, MA; Taylor, RW; Cochran, B; Ackrell, BA; Turnbull, DM. Late-onset optic atrophy, ataxia, and myopathy associated with a mutation of a complex II gene. Ann Neurol 2000, 48(3), 330–335. [Google Scholar]
- Horvath, R; Abicht, A; Holinski-Feder, E; Laner, A; Gempel, K; Prokisch, H; Lochmuller, H; Klopstock, T; Jaksch, M. Leigh syndrome caused by mutations in the flavoprotein (Fp) subunit of succinate dehydrogenase (SDHA). J Neurol Neurosurg Psychiatry 2006, 77(1), 74–76. [Google Scholar]
- Leigh, D. Subacute necrotizing encephalomyelopathy in an infant. J Neurol Neurosurg Psychiatry 1951, 14(3), 216–221. [Google Scholar]
- Paulus, W; Peiffer, J. Intracerebral distribution of mitochondrial abnormalities in 21 cases of infantile spongy dystrophy. J Neurol Sci 1990, 95(1), 49–62. [Google Scholar]
- Mast, JD; Tomalty, KM; Vogel, H; Clandinin, TR. Reactive oxygen species act remotely to cause synapse loss in a Drosophila model of developmental mitochondrial encephalopathy. Development 2008, 135(15), 2669–2679. [Google Scholar]
- Zordan, MA; Cisotto, P; Benna, C; Agostino, A; Rizzo, G; Piccin, A; Pegoraro, M; Sandrelli, F; Perini, G; Tognon, G; De Caro, R; Peron, S; Kronnie, TT; Megighian, A; Reggiani, C; Zeviani, M; Costa, R. Post-transcriptional silencing and functional characterization of the Drosophila melanogaster homolog of human Surf1. Genetics 2006, 172(1), 229–241. [Google Scholar]
- Vanier, MT; Millat, G. Niemann-Pick disease type C. Clin Genet 2003, 64(4), 269–281. [Google Scholar]
- Liscum, L; Sturley, SL. Intracellular trafficking of Niemann-Pick C proteins 1 and 2: Obligate components of subcellular lipid transport. Biochim Biophys Acta 2004, 1685. [Google Scholar]
- Carstea, ED; Morris, JA; Coleman, KG; Loftus, SK; Zhang, D; Cummings, C; Gu, J; Rosenfeld, MA; Pavan, WJ; Krizman, DB; Nagle, J; Polymeropoulos, MH; Sturley, SL; Ioannou, YA; Higgins, ME; Comly, M; Cooney, A; Brown, A; Kaneski, CR; Blanchette-Mackie, EJ; Dwyer, NK; Neufeld, EB; Chang, TY; Liscum, L; Strauss, JF, 3rd; Ohno, K; Zeigler, M; Carmi, R; Sokol, J; Markie, D; O’Neill, RR; van Diggelen, OP; Elleder, M; Patterson, MC; Brady, RO; Vanier, MT; Pentchev, PG; Tagle, DA. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science 1997, 277(5323), 228–231. [Google Scholar]
- Naureckiene, S; Sleat, DE; Lackland, H; Fensom, A; Vanier, MT; Wattiaux, R; Jadot, M; Lobel, P. Identification of HE1 as the second gene of Niemann-Pick C disease. Science 2000, 290(5500), 2298–2301. [Google Scholar]
- Vance, JE. Lipid imbalance in the neurological disorder, Niemann-Pick C disease. FEBS Lett 2006, 580(23), 5518–5524. [Google Scholar]
- Fluegel, ML; Parker, TJ; Pallanck, LJ. Mutations of a Drosophila NPC1 gene confer sterol and ecdysone metabolic defects. Genetics 2006, 172(1), 185–196. [Google Scholar]
- Huang, X; Suyama, K; Buchanan, J; Zhu, AJ; Scott, MP. A Drosophila model of the Niemann-Pick type C lysosome storage disease: dnpc1a is required for molting and sterol homeostasis. Development 2005, 132(22), 5115–5124. [Google Scholar]
- Phillips, SE; Woodruff, EA, 3rd; Liang, P; Patten, M; Broadie, K. Neuronal loss of Drosophila NPC1a causes cholesterol aggregation and age-progressive neurodegeneration. J Neurosci 2008, 28(26), 6569–6582. [Google Scholar]
- Huang, X; Warren, JT; Buchanan, J; Gilbert, LI; Scott, MP. Drosophila Niemann-Pick type C-2 genes control sterol homeostasis and steroid biosynthesis: A model of human neurodegenerative disease. Development 2007, 134(20), 3733–3742. [Google Scholar]
- Wisniewski, KE; Kida, E; Golabek, AA; Kaczmarski, W; Connell, F; Zhong, N. Neuronal ceroid lipofuscinoses: Classification and diagnosis. Adv. Genet 2001, 45, 1–34. [Google Scholar]
- Siintola, E; Lehesjoki, AE; Mole, SE. Molecular genetics of the NCLs — status and perspectives. Biochim Biophys Acta 2006, 1762(10), 857–864. [Google Scholar]
- Stogmann, E; El Tawil, S; Wagenstaller, J; Gaber, A; Edris, S; Abdelhady, A; Assem-Hilger, E; Leutmezer, F; Bonelli, S; Baumgartner, C; Zimprich, F; Strom, TM; Zimprich, A. A novel mutation in the MFSD8 gene in late infantile neuronal ceroid lipofuscinosis. Neurogenetics 2008. [Google Scholar]
- Hickey, AJ; Chotkowski, HL; Singh, N; Ault, JG; Korey, CA; MacDonald, ME; Glaser, RL. Palmitoyl-protein thioesterase 1 deficiency in Drosophila melanogaster causes accumulation of abnormal storage material and reduced life span. Genetics 2006, 172(4), 2379–2390. [Google Scholar]
- Korey, CA; MacDonald, ME. An over-expression system for characterizing Ppt1 function in Drosophila. BMC Neurosci 2003, 4, 30. [Google Scholar]
- Buff, H; Smith, AC; Korey, CA. Genetic modifiers of Drosophila palmitoyl-protein thioesterase 1-induced degeneration. Genetics 2007, 176(1), 209–220. [Google Scholar]
- Ferner, RE. Neurofibromatosis 1 and neurofibromatosis 2: A twenty first century perspective. Lancet Neurol 2007, 6(4), 340–351. [Google Scholar]
- Louis, DN; Ohgaki, H; Wiestler, OD; Cavenee, WK; Burger, PC; Jouvet, A; Scheithauer, BW; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007, 114(2), 97–109. [Google Scholar]
- The, I; Hannigan, GE; Cowley, GS; Reginald, S; Zhong, Y; Gusella, JF; Hariharan, IK; Bernards, A. Rescue of a Drosophila NF1 mutant phenotype by protein kinase A. Science 1997, 276(5313), 791–794. [Google Scholar]
- Guo, HF; Tong, J; Hannan, F; Luo, L; Zhong, Y. A neurofibromatosis-1-regulated pathway is required for learning in Drosophila. Nature 2000, 403(6772), 895–898. [Google Scholar]
- Williams, JA; Su, HS; Bernards, A; Field, J; Sehgal, A. A circadian output in Drosophila mediated by neurofibromatosis-1 and Ras/MAPK. Science 2001, 293(5538), 2251–2256. [Google Scholar]
- Tong, JJ; Schriner, SE; McCleary, D; Day, BJ; Wallace, DC. Life extension through neurofibromin mitochondrial regulation and antioxidant therapy for neurofibromatosis-1 in Drosophila melanogaster. Nat Genet 2007, 39(4), 476–485. [Google Scholar]
- Walker, JA; Bernards, A. Drosophila melanogaster neurofibromatosis-1: ROS, not Ras? Nat Genet 2007, 39(4), 443–445. [Google Scholar]
- Pellock, BJ; Buff, E; White, K; Hariharan, IK. The Drosophila tumor suppressors Expanded and Merlin differentially regulate cell cycle exit, apoptosis, and Wingless signaling. Dev Biol 2007, 304(1), 102–115. [Google Scholar]
- Hamaratoglu, F; Willecke, M; Kango-Singh, M; Nolo, R; Hyun, E; Tao, C; Jafar-Nejad, H; Halder, G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol 2006, 8(1), 27–36. [Google Scholar]
- Povey, S; Burley, MW; Attwood, J; Benham, F; Hunt, D; Jeremiah, SJ; Franklin, D; Gillett, G; Malas, S; Robson, EB; et al. Two loci for tuberous sclerosis: one on 9q34 and one on 16p13. Ann Hum Genet 1994, 58, 107–127. [Google Scholar]
- Crino, PB; Nathanson, KL; Henske, EP. The tuberous sclerosis complex. N Engl J Med 2006, 355(13), 1345–1356. [Google Scholar]
- Huttenlocher, PR; Heydemann, PT. Fine structure of cortical tubers in tuberous sclerosis: A Golgi study. Ann Neurol 1984, 16(5), 595–602. [Google Scholar]
- Tapon, N; Ito, N; Dickson, BJ; Treisman, JE; Hariharan, IK. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell 2001, 105(3), 345–355. [Google Scholar]
- Brumby, AM; Richardson, HE. Using Drosophila melanogaster to map human cancer pathways. Nat Rev Cancer 2005, 5(8), 626–639. [Google Scholar]
- Betschinger, J; Mechtler, K; Knoblich, JA. Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell 2006, 124(6), 1241–1253. [Google Scholar]
- Beaucher, M; Goodliffe, J; Hersperger, E; Trunova, S; Frydman, H; Shearn, A. Drosophila brain tumor metastases express both neuronal and glial cell type markers. Dev Biol 2007, 301(1), 287–297. [Google Scholar]
- Woodhouse, EC; Fisher, A; Bandle, RW; Bryant-Greenwood, B; Charboneau, L; Petricoin, EF, 3rd; Liotta, LA. Drosophila screening model for metastasis: Semaphorin 5c is required for l(2)gl cancer phenotype. Proc Natl Acad Sci USA 2003, 100(20), 11463–11468. [Google Scholar]
- Uhlirova, M; Bohmann, D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J 2006, 25(22), 5294–5304. [Google Scholar]
- Scheffer, IE; Berkovic, SF. The genetics of human epilepsy. Trends Pharmacol Sci 2003, 24(8), 428–433. [Google Scholar]
- Kalachikov, S; Evgrafov, O; Ross, B; Winawer, M; Barker-Cummings, C; Martinelli Boneschi, F; Choi, C; Morozov, P; Das, K; Teplitskaya, E; Yu, A; Cayanis, E; Penchaszadeh, G; Kottmann, AH; Pedley, TA; Hauser, WA; Ottman, R; Gilliam, TC. Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat Genet 2002, 30(3), 335–341. [Google Scholar]
- Stromme, P; Mangelsdorf, ME; Shaw, MA; Lower, KM; Lewis, SM; Bruyere, H; Lutcherath, V; Gedeon, AK; Wallace, RH; Scheffer, IE; Turner, G; Partington, M; Frints, SG; Fryns, JP; Sutherland, GR; Mulley, JC; Gecz, J. Mutations in the human ortholog of Aristaless cause X-linked mental retardation and epilepsy. Nat Genet 2002, 30(4), 441–445. [Google Scholar]
- Blumcke, I; Pauli, E; Clusmann, H; Schramm, J; Becker, A; Elger, C; Merschhemke, M; Meencke, HJ; Lehmann, T; von Deimling, A; Scheiwe, C; Zentner, J; Volk, B; Romstock, J; Stefan, H; Hildebrandt, M. A new clinico-pathological classification system for mesial temporal sclerosis. Acta Neuropathol 2007, 113(3), 235–244. [Google Scholar]
- Pinel, JP; Chorover, SL. Inhibition by arousal of epilepsy induced by chlorambucil in rats. Nature 1972, 236(5344), 232–234. [Google Scholar]
- Racine, R. Kindling: The first decade. Neurosurgery 1978, 3(2), 234–252. [Google Scholar]
- Pavlidis, P; Tanouye, MA. Seizures and failures in the giant fiber pathway of Drosophila bang-sensitive paralytic mutants. J Neurosci 1995, 15(8), 5810–5819. [Google Scholar]
- Ganetzky, B. Genetic analysis of ion channel dysfunction in Drosophila. Kidney Int 2000, 57(3), 766–771. [Google Scholar]
- Titus, SA; Warmke, JW; Ganetzky, B. The Drosophila erg K+ channel polypeptide is encoded by the seizure locus. J Neurosci 1997, 17(3), 875–881. [Google Scholar]
- Reynolds, ER; Stauffer, EA; Feeney, L; Rojahn, E; Jacobs, B; McKeever, C. Treatment with the antiepileptic drugs phenytoin and gabapentin ameliorates seizure and paralysis of Drosophila bang-sensitive mutants. J Neurobiol 2004, 58(4), 503–513. [Google Scholar]
- Stilwell, GE; Saraswati, S; Littleton, JT; Chouinard, SW. Development of a Drosophila seizure model for in vivo high-throughput drug screening. Eur J Neurosci 2006, 24(8), 2211–2222. [Google Scholar]
- Song, J; Tanouye, MA. From bench to drug: Human seizure modeling using Drosophila. Prog Neurobiol 2008, 84(2), 182–191. [Google Scholar]
- Kuebler, D; Tanouye, MA. Modifications of seizure susceptibility in Drosophila. J Neurophysiol 2000, 83(2), 998–1009. [Google Scholar]
- Kuebler, D; Zhang, H; Ren, X; Tanouye, MA. Genetic suppression of seizure susceptibility in Drosophila. J Neurophysiol 2001, 86(3), 1211–1225. [Google Scholar]
- Song, J; Hu, J; Tanouye, M. Seizure suppression by top1 mutations in Drosophila. J Neurosci 2007, 27(11), 2927–2937. [Google Scholar]
- Song, J; Parker, L; Hormozi, L; Tanouye, MA. DNA topoisomerase I inhibitors ameliorate seizure-like behaviors and paralysis in a Drosophila model of epilepsy. Neuroscience 2008, 156(3), 722–728. [Google Scholar]
- Fergestad, T; Olson, L; Patel, KP; Miller, R; Palladino, MJ; Ganetzky, B. Neuropathology in Drosophila mutants with increased seizure susceptibility. Genetics 2008, 178(2), 947–956. [Google Scholar]
- Profyris, C; Cheema, SS; Zang, D; Azari, MF; Boyle, K; Petratos, S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis 2004, 15(3), 415–436. [Google Scholar]
- Niess, C; Grauel, U; Toennes, SW; Bratzke, H. Incidence of axonal injury in human brain tissue. Acta Neuropathol 2002, 104(1), 79–84. [Google Scholar]
- Batchelor, PE; Wills, TE; Hewa, AP; Porritt, MJ; Howells, DW. Stimulation of axonal sprouting by trophic factors immobilized within the wound core. Brain Res 2008, 1209, 49–56. [Google Scholar]
- Ayaz, D; Leyssen, M; Koch, M; Yan, J; Srahna, M; Sheeba, V; Fogle, KJ; Holmes, TC; Hassan, BA. Axonal injury and regeneration in the adult brain of Drosophila. J Neurosci 2008, 28(23), 6010–6021. [Google Scholar]
- Kaneko, M; Hall, JC. Neuroanatomy of cells expressing clock genes in Drosophila: Transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J Comp Neurol 2000, 422(1), 66–94. [Google Scholar]
- Silver, J; Miller, JH. Regeneration beyond the glial scar. Nat Rev Neurosci 2004, 5(2), 146–156. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).