QSAR Analysis of 2-Amino or 2-Methyl-1-Substituted Benzimidazoles Against Pseudomonas aeruginosa
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Experimental
4.1. Synthesis of Compounds
4.2. Antibacterial Investigations
4.3. Molecular Modeling
4.4. Descriptor Generation
4.5. Statistical Methods
Acknowledgments
References and Notes
- Nguyen, PTM; Baldeck, JD; Olsson, J; Marquis, RE. Antimicrobial actions of benzimidazoles against oral streptococci. Oral Microbiol. Immunol 2005, 20, 93–99. [Google Scholar]
- Kazimierczuk, Z; Upcroft, JA; Upcroft, P; Gorska, A; Starosciak, B; Laudy, A. Synthesis, antiprotozoal and antibacterial activitiy of nitro- and halogeno-substituted benzimidazole derivatives. Acta Biochim. Polon 2002, 49, 185–195. [Google Scholar]
- Goker, H; Alp, M; Yildiz, S. Synthesis and potent antimicrobial activity of some novel N- (alkyl)-2-phenyl-1H-benzimidazole-5-carboxamidines. Molecules 2000, 10, 1377–1386. [Google Scholar]
- Podunavac-Kuzmanović, SO; Cvetković, DD. Biological activity of zinc(II) and nickel(II) complexes with some 2-aminobenzimidazole derivatives in microorganisms of environmental relevance synthesis. Centr. Eur. J. Occupat. Environ. Med 2006, 12, 55–60. [Google Scholar]
- Perišić-Janjić, NU; Podunavac-Kuzmanović, SO; Balaž, JS; Vlaović, Đ. Chromatographic behaviour and lipophilicity of some benzimidazole derivatives. J. Planar. Chromatogr 2000, 13, 123–129. [Google Scholar]
- Podunavac-Kuzmanović, SO; Leovac, VM; Perišić-Janjić, NU; Rogan, J; Balaž, J. Complexes cobalt(II), zinc(II) and copper(II) with some newly synthesized benzimidazole derivatives and their antibacterial activity. J. Serb. Chem. Soc 1999, 64, 381–388. [Google Scholar]
- Podunavac-Kuzmanović, SO; Markov, SL. Antimicrobial activity of copper(II) complexes with some benzimidazole derivatives against microorganisms widely distributed in the environment. Centr. Eur. J. Occupat. Environ. Med 2006, 12, 61–66. [Google Scholar]
- Podunavac-Kuzmanović, SO; Cvetković, D. Antibacterial evaluation of some benzimidazole derivatives and their zinc(II) complexes. J. Serb. Chem. Soc 2007, 75, 459–466. [Google Scholar]
- Ates-Alagoz, Z; Yildiz, S; Buyukbingol, E. Antimicrobial activities of some tetrahydronaphthalene-benzimidazole derivatives. Chemotherapy 2007, 53, 110–113. [Google Scholar]
- Ayhan-Kilcigil, G; Altanlar, N. Synthesis and antifungal properties of some benzimidazole derivatives. Turk. J. Chem 2006, 30, 223–228. [Google Scholar]
- Mohamed, BG; Hussein, MA; Abdel-Alim, AM; Hashem, M. Synthesis and antimicrobial activity of some new 1-alkyl-2-alkylthio-1,2,4-triazolobenzimidazole derivatives. Arch. Pharm. Res 2006, 29, 26–33. [Google Scholar]
- Kus, C; Ayhan-Kilcigil, G; Can-Eke, B; Iscan, M. Synthesis and antioxidant properties of some novel benzimidazole derivatives on lipid peroxidation in the rat liver. Arch. Pharm. Res 2004, 27, 156–163. [Google Scholar]
- Ayhan-Kilcigil, G; Kus, C; Coban, T; Can-Eke, B; Iscan, M. Synthesis, antioxidant and radical scavenging activities of novel benzimidazoles. J. Enz. Inhib. Med. Chem 2004, 19, 129–135. [Google Scholar]
- Nakano, H; Inoue, T; Kawasaki, N; Miyataka, H; Matsumoto, H; Taguchi, T; Inagaki, N; Nagai, H; Satoh, T. Synthesis of benzimidazole derivatives as antiallergic agents with 5- lipoxygenase inhibiting action. Chem. Pharm. Bull 1999, 47, 1573–1578. [Google Scholar]
- Fukuda, T; Saito, T; Tajima, S; Shimohara, K; Ito, K. Antiallergic Effect of 1-(2-ethoxyethyl)- 2-(4-methyl-1-homo-piperazinyl)-benzimidazole Difumarate (KB-2413). Arzneim. Forsch./Drug Res 1984, 34, 805–810. [Google Scholar]
- Valdez, J; Cedillo, R; Hernandez-Campos, A; Yepez, L; Hernandez-Luis, F; Navarrete- Vazquez, G; Tapia, A; Cortes, R; Hernandez, M; Castillo, R. Synthesis and antiparasitic activity of 1h-benzimidazole derivatives. Bioorg. Med. Chem. Lett 2002, 12, 2221–2224. [Google Scholar]
- Navarrete-Vazquez, G; Cedillo, R; Hernandez-Campos, A; Yepez, L; Hernandez-Luis, F; Valdez, J; Morales, R; Cortes, R; Hernandez, M; Castillo, R. Synthesys and antiparasitic activity of 5(6)-chloro-2-(trifluoromethyl) benzimidazole derivatives. Bioorg. Med. Chem. Lett 2001, 11, 187–190. [Google Scholar]
- Ward, C; Berthold, R; Koerwer, J; Tomlin, J; Manning, T. Synthesis and herbicidal activity of 1,2,3,4-tetrahydro-1,3,5-triazino[1,2-a]benzimidazoles. J. Agric. Food Chem 1986, 34, 1005– 1010. [Google Scholar]
- Lipkowitz, KB; McCracken, RO. molecular modeling: A tool for predicting anthelmintic activity in vivo. Parasitol. Res 1993, 79, 475–479. [Google Scholar]
- Campbell, WC. Benzimidazoles: Veterinary uses. Parasitol. Today 1990, 6, 130–133. [Google Scholar]
- Didier, ES; Stovall, ME; Green, LC; Brindley, PJ; Sestak, K; Didier, PJ. Epidemiology of microsporidiosis: sources and modes of transmission. Vet. Parasitol 2004, 126, 145–166. [Google Scholar]
- Zulu, I; Veitch, A; Sianongo, S; McPhail, G; Feakins, R; Farthing, MJG; Kelly, P. Albendazole chemotherapy for AIDS-related diarrhoea in zambia-clinical, parasitological and mucosal responses. Alim. Pharmacol. Ther 2002, 16, 595–601. [Google Scholar]
- Pandey, VK; Upadhay, M; Dev Gupta, V; Tandon, M. Benzimidazolyl quinolinyl mercaptotriazoles as potential antimicrobial and antiviral agents. Acta Pharm 2005, 55, 47–56. [Google Scholar]
- Beaulieu, PL; Bousquet, Y; Gauthier, J; Gillard, J; Marquis, M; McKercher, G; Pellerin, C; Valois, S; Kukolj, G. Non-nucleoside benzimidazole-based allosteric inhibitors of the hepatitis C virus NS5B polymerase: Inhibition of subgenomic hepatitis C virus RNA replicons in Huh-7 cells. J. Med. Chem 2004, 47, 6884–6892. [Google Scholar]
- Patel, PD; Patel, MR; Kaushik-Basu, N; Talele, TT. 3D QSAR and molecular docking studies of benzimidazole derivatives as hepatitis C virus NS5B polymerase inhibitors. J. Chem. Inf. Model 2008, 48, 42–55. [Google Scholar]
- Hansch, C; Leo, A; Hoekman, DH. Exploring QSAR, Fundamentals and Application in Chemistry and Biology; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Hansch, C; Leo, A; Hoekman, DH. Exploring QSAR, Hydrophobic, Electronic and Steric Constants; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Podunavac-Kuzmanović, SO; Markov, SL; Barna, DJ. Relationship between the lipophilicity and antifungal activity of some benzimidazole derivatives. J. Theor. Comp. Chem 2007, 6, 687–698. [Google Scholar]
- Podunavac-Kuzmanović, SO; Cvetković, DD. Anion Effect on antimicrobial activity of metal complexes with benzimidazole derivative. CICEQ 2007, 13, 68–71. [Google Scholar]
- Perišić-Janjić, NU; Podunavac-Kuzmanović, SO. RPTLC study of QSRR and QSAR for some benzimidazole derivatives. J. Planar. Chromatogr 2008, 21, 135–141. [Google Scholar]
- Podunavac-Kuzmanović, SO; Cvetković, DD; Barna, DJ. The effect of lipophilicity on antibacterial activity of some 1-benzylbenzimidazole derivatives. J. Serb. Chem. Soc 2008, 73, 967–978. [Google Scholar]
- Podunavac-Kuzmanović, SO; Cvetković, DD; Barna, DJ. The effect of lipophilicity on antifungal activity of some 2-amino and 2-methylbenzimidazole derivatives. Chem. Listy 2008, 102, 753–756. [Google Scholar]
- Podunavac-Kuzmanović, SO; Cvetković, DD; Barna, DJ. QSAR Models for predicting the antibacterial activity of some 1-benzylbenzimidazole derivatives. Chem. Listy 2008, 102, 757–761. [Google Scholar]
- Podunavac-Kuzmanović, SO; Cvetković, DD. Antimicrobial investigations of nickel(II) complexes with some 1-benzylbenzimidazole derivatives. Chem. Listy 2008, 102, 762–764. [Google Scholar]
- Podunavac-Kuzmanović, SO; Leovac, VM; Cvetković, DD. Antibacterial activity of cobalt(ii) complexes with some benzimidazole derivatives. J. Serb. Chem. Soc 2008, 73, 1153–1160. [Google Scholar]
- Topliss, JG; Edwards, RP. Chance factors in studies of quantitative structure-activity relationships. J. Med. Chem 1979, 22, 1238–1244. [Google Scholar]
- Snedecor, GW; Cochran, WG. Statistical Methods; Oxford and IBH: New Delhi, India, 1967; p. 381. [Google Scholar]
- Chaltterjee, S; Hadi, AS; Price, B. Regression Analysis by Examples; Wiley VCH: New York, USA, 2000. [Google Scholar]
- Diudea, MV. QSPR/QSAR Studies for Molecular Descriptors; Nova Science: Huntingdon, New York, USA, 2000. [Google Scholar]
- Golbraikh, A; Tropsha, J. Beware of q2! J. Mol. Graph. Mod 2002, 20, 269–276. [Google Scholar]
- Maggiora, G. On outliers and activity cliffs-why QSAR often disappoints. J. Chem. Inf. Model 2006, 46, 1535–1535. [Google Scholar]
- Vlaović, Đ; Čanadanović-Brunet, J; Balaž, J; Juranić, I; Đoković, D; Mackenzie, K. Synthesys, antibacterial and antifungal activities of some new benzimidazoles. Biosci. Biotech. Biochem 1992, 56, 199–206. [Google Scholar]
- NCCLS Approval Standard Document M2-A7; National Commitee for Clinical Laboratory Standards: Vilanova, PA, USA, 2000.
- NCCLS Approval Standard Document M7-A5; National Commitee for Clinical Laboratory Standards: Vilanova, PA, USA, 2000.
- HyperChem 7.5; Hypercube, Inc: Waterloo, ON, Canada.
- Todescini, R; Consonni, V; Mauri, A; Pavan, M.
- .

| Cmpd | R1 | R2 | Cmpd | R1 | R2 |
|---|---|---|---|---|---|
| 1 | NH2 | H | 8 | CH3 | H |
| 2 | NH2 |  | 9 | CH3 |  |
| 3 | NH2 | 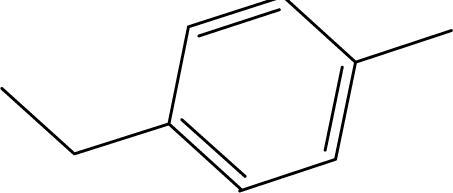 | 10 | CH3 |  |
| 4 | NH2 |  | 11 | CH3 |  |
| 5 | NH2 |  | 12 | CH3 |  |
| 6 | NH2 |  | 13 | CH3 |  |
| 7 | NH2 | 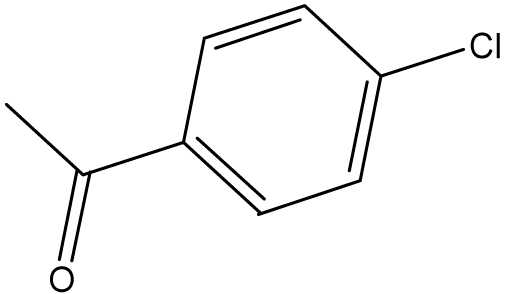 | 14 | CH3 |  |
| Compound | MIC (μg/mL) | log1/cMIC |
|---|---|---|
| 1 | 50 | 3.425 |
| 2 | 25 | 3.951 |
| 3 | 12.5 | 4.278 |
| 4 | 6.25 | 4.615 |
| 5 | 50 | 3.676 |
| 6 | 12.5 | 4.303 |
| 7 | 6.25 | 4.638 |
| 8 | 100 | 3.121 |
| 9 | 50 | 3.648 |
| 10 | 25 | 3.975 |
| 11 | 12.5 | 4.312 |
| 12 | 100 | 3.373 |
| 13 | 25 | 4.000 |
| 14 | 12.5 | 4.335 |
| Ampicillin | 12.5 | 4.446 |
| Sultamicillin | 0.78 | 5.787 |
| Cmpd | MR | P | MV | HE | MW | TE | SAG | DM | ClogP |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 43.63 | 15.13 | 430.33 | −11.28 | 133.15 | −1.21 | 292.48 | 1.54 | −0.61 |
| 2 | 77.28 | 26.63 | 675.88 | −7.12 | 223.28 | −9.75 | 416.78 | 1.45 | 0.65 |
| 3 | 81.56 | 28.46 | 728.44 | −5.95 | 237.30 | −9.81 | 442.99 | 1.53 | 0.80 |
| 4 | 81.99 | 28.55 | 712.38 | −6.72 | 257.72 | −9.81 | 437.70 | 1.48 | 0.43 |
| 5 | 77.21 | 26.71 | 666.31 | −7.67 | 237.29 | 29.80 | 409.30 | 2.16 | 0.07 |
| 6 | 81.49 | 28.55 | 720.15 | −6.52 | 251.29 | 29.61 | 437.71 | 2.13 | 0.22 |
| 7 | 80.61 | 28.64 | 710.59 | −7.35 | 271.71 | 30.38 | 434.96 | 2.88 | 0.44 |
| 8 | 44.83 | 15.62 | 450.85 | −4.75 | 132.16 | 10.35 | 304.78 | 1.36 | −0.51 |
| 9 | 78.48 | 27.11 | 693.35 | −2.73 | 222.29 | 1.34 | 423.77 | 1.32 | 0.75 |
| 10 | 82.76 | 28.94 | 745.41 | −1.61 | 236.32 | 1.23 | 453.96 | 1.45 | 0.90 |
| 11 | 83.19 | 29.04 | 737.17 | −2.44 | 256.73 | 1.63 | 448.98 | 1.69 | 0.53 |
| 12 | 78.41 | 27.20 | 686.80 | −3.68 | 236.27 | 53.89 | 422.33 | 2.40 | 0.17 |
| 13 | 82.69 | 29.03 | 741.13 | −2.53 | 250.30 | 53.68 | 452.01 | 2.42 | 0.32 |
| 14 | 81.81 | 29.12 | 731.39 | −3.36 | 270.71 | 54.42 | 447.07 | 2.86 | 0.54 |
| Model | Coefficient | Error | n | r | s | F | |
|---|---|---|---|---|---|---|---|
| 1 | Intercept | 2.0228 | 0.4432 | 14 | 0.7910 | 0.2999 | 20.053 |
| MW | 0.0085 | 0.0019 | |||||
| 2 | Intercept | 1.88448 | 0.3965 | 14 | 0.8587 | 0.2624 | 15.437 |
| MW | 0.0098 | 0.0018 | |||||
| TE | −0.0068 | 0.0031 | |||||
| Model | PRESS | SSY | PRESS/SSY | SPRESS | r2CV | r2adj |
|---|---|---|---|---|---|---|
| 1 | 1.4444 | 2.8835 | 0.5009 | 0.3212 | 0.4991 | 0.5944 |
| 2 | 1.2609 | 2.8835 | 0.4373 | 0.3001 | 0.5627 | 0.6895 |
| Cmpd | log1/cMICexp. | Model 1 | Model 2 | ||
|---|---|---|---|---|---|
| log1/cMIC pred. | Residuals | log1/cMIC pred. | Residuals | ||
| 15 | 4.328 | 4.287 | 0.041 | 4.298 | 0.030 |
| 16 | 4.278 | 4.040 | 0.238 | 4.030 | 0.248 |
| 17 | 4.314 | 4.213 | 0.101 | 4.233 | 0.081 |
| 18 | 4.333 | 4.312 | 0.021 | 4.337 | −0.004 |
| 19 | 4.352 | 4.414 | −0.062 | 4.442 | −0.090 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Podunavac-Kuzmanović, S.O.; Cvetković, D.D.; Barna, D.J. QSAR Analysis of 2-Amino or 2-Methyl-1-Substituted Benzimidazoles Against Pseudomonas aeruginosa. Int. J. Mol. Sci. 2009, 10, 1670-1682. https://doi.org/10.3390/ijms10041670
Podunavac-Kuzmanović SO, Cvetković DD, Barna DJ. QSAR Analysis of 2-Amino or 2-Methyl-1-Substituted Benzimidazoles Against Pseudomonas aeruginosa. International Journal of Molecular Sciences. 2009; 10(4):1670-1682. https://doi.org/10.3390/ijms10041670
Chicago/Turabian StylePodunavac-Kuzmanović, Sanja O., Dragoljub D. Cvetković, and Dijana J. Barna. 2009. "QSAR Analysis of 2-Amino or 2-Methyl-1-Substituted Benzimidazoles Against Pseudomonas aeruginosa" International Journal of Molecular Sciences 10, no. 4: 1670-1682. https://doi.org/10.3390/ijms10041670
APA StylePodunavac-Kuzmanović, S. O., Cvetković, D. D., & Barna, D. J. (2009). QSAR Analysis of 2-Amino or 2-Methyl-1-Substituted Benzimidazoles Against Pseudomonas aeruginosa. International Journal of Molecular Sciences, 10(4), 1670-1682. https://doi.org/10.3390/ijms10041670





