Titration Calorimetry Standards and the Precision of Isothermal Titration Calorimetry Data
Abstract
:1. Introduction
2. Results and Discussion
2.1. Enthalpy standards
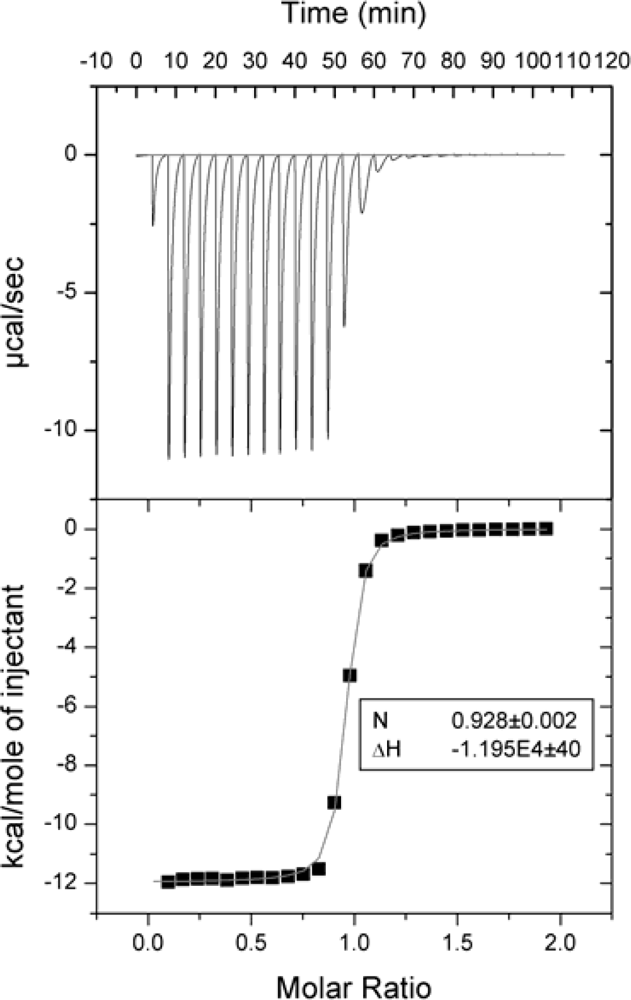
2.2. Comparison of data obtained using several microcalorimeters
2.3. Uncertainties of protein-ligand binding enthalpies determined by several microcalorimeters
3. Experimental Section
4. Conclusions
Acknowledgments
References and Notes
- Wiseman, T; Williston, S; Brandts, JF; Lin, LN. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal. Biochem 1989, 179, 131–137. [Google Scholar]
- Lundqvist, T. The devil is still in the details — driving early drug discovery forward with biophysical experimental methods. Curr. Opin. Drug Discov. Devel 2005, 8, 513–519. [Google Scholar]
- Perozzo, R; Folkers, G; Scapozza, L. Thermodynamics of protein-ligand interactions: History, presence, and future aspects. J. Recept. Signal. Transduct. Res 2004, 24, 1–52. [Google Scholar]
- Ward, WH; Holdgate, GA. Isothermal titration calorimetry in drug discovery. Prog. Med. Chem 2001, 38, 309–376. [Google Scholar]
- Liu, CC; Richard, AJ; Datta, K; LiCata, VJ. Prevalence of temperature-dependent heat capacity changes in protein-DNA interactions. Biophys. J 2008, 94, 3258–3265. [Google Scholar]
- Dragan, AI; Li, Z; Makeyeva, EN; Milgotina, EI; Liu, Y; Crane-Robinson, C; Privalov, PL. Forces driving the binding of homeodomains to DNA. Biochemistry 2006, 45, 141–151. [Google Scholar]
- Matulis, D; Rouzina, I; Bloomfield, VA. Thermodynamics of cationic lipid binding to DNA and DNA condensation: Roles of electrostatics and hydrophobicity. J. Am. Chem. Soc 2002, 124, 7331–7342. [Google Scholar]
- Tsamaloukas, A; Szadkowska, H; Heerklotz, H. Thermodynamic comparison of the interactions of cholesterol with unsaturated phospholipid and sphingomyelins. Biophys. J 2006, 90, 4479–4487. [Google Scholar]
- Hansen, LD; Russell, DJ; Choma, CT. From biochemistry to physiology: The calorimetry connection. Cell. Biochem. Biophys 2007, 49, 125–140. [Google Scholar]
- Velazquez Campoy, A; Freire, E. ITC in the post-genomic era…? Priceless. Biophys. Chem 2005, 115, 115–124. [Google Scholar]
- Ladbury, JE. Application of isothermal titration calorimetry in the biological sciences: Things are heating up! Biotechniques 2004, 37, 885–887. [Google Scholar]
- Chen, X; Lin, Y; Liu, M; Gilson, MK. The Binding Database: data management and interface design. Bioinformatics 2002, 18, 130–139. [Google Scholar]
- Chen, X; Liu, M; Gilson, MK. BindingDB: A web-accessible molecular recognition database. Comb. Chem. High. Throughput Screen 2001, 4, 719–725. [Google Scholar]
- Liu, T; Lin, Y; Wen, X; Jorissen, RN; Gilson, MK. BindingDB: A web-accessible database of experimentally determined protein-ligand binding affinities. Nucleic Acids Res 2007, 35, D198–D201. [Google Scholar]
- Olsson, TS; Williams, MA; Pitt, WR; Ladbury, JE. The thermodynamics of protein-ligand interaction and solvation: Insights for ligand design. J. Mol. Biol 2008, 384, 1002–1017. [Google Scholar]
- Myszka, DG; Abdiche, YN; Arisaka, F; Byron, O; Eisenstein, E; Hensley, P; Thomson, JA; Lombardo, CR; Schwarz, F; Stafford, W; Doyle, ML. The ABRF-MIRG’02 study: Assembly state, thermodynamic, and kinetic analysis of an enzyme/inhibitor interaction. J. Biomol. Technol 2003, 14, 247–269. [Google Scholar]
- Navratilova, I; Papalia, GA; Rich, RL; Bedinger, D; Brophy, S; Condon, B; Deng, T; Emerick, AW; Guan, HW; Hayden, T; Heutmekers, T; Hoorelbeke, B; McCroskey, MC; Murphy, MM; Nakagawa, T; Parmeggiani, F; Qin, X; Rebe, S; Tomasevic, N; Tsang, T; Waddell, MB; Zhang, FF; Leavitt, S; Myszka, DG. Thermodynamic benchmark study using Biacore technology. Anal. Biochem 2007, 364, 67–77. [Google Scholar]
- Wadso, I. Needs for standards in isothermal microcalorimetry. Thermochim. Acta 2000, 347, 73–77. [Google Scholar]
- Tellinghuisen, J. Stupid statistics! Methods Cell Biol 2008, 84, 737–780. [Google Scholar]
- Tellinghuisen, J. Statistical error in isothermal titration calorimetry. Methods Enzymol 2004, 383, 245–282. [Google Scholar]
- Mizoue, LS; Tellinghuisen, J. Calorimetric vs. van’t Hoff binding enthalpies from isothermal titration calorimetry: Ba2+-crown ether complexation. Biophys. Chem 2004, 110, 15–24. [Google Scholar]
- Horn, JR; Russell, D; Lewis, EA; Murphy, KP. Van’t Hoff and calorimetric enthalpies from isothermal titration calorimetry: are there significant discrepancies? Biochemistry 2001, 40, 1774–1778. [Google Scholar]
- Mizoue, LS; Tellinghuisen, J. The role of backlash in the “first injection anomaly” in isothermal titration calorimetry. Anal. Biochem 2004, 326, 125–127. [Google Scholar]
- Tellinghuisen, J. Calibration in isothermal titration calorimetry: heat and cell volume from heat of dilution of NaCl(aq). Anal. Biochem 2007, 360, 47–55. [Google Scholar]
- Dean, JA. Lange’s Handbook of Chemistry; McGraw-Hill, Inc: New York, USA, 1999. [Google Scholar]
- Christensen, JJ; Hansen, LD; Izatt, RM. Handbook of Proton Ionizations Heats; Wiley-Interscience: Hoboken, NJ, USA, 1976. [Google Scholar]
- Dudutiene, V; Baranauskiene, L; Matulis, D. Benzimidazo[1,2-c][1,2,3]thiadiazole-7-sulfonamides as inhibitors of carbonic anhydrase. Bioorg. Med. Chem. Lett 2007, 17, 3335–3338. [Google Scholar]
- Krishnamurthy, VM; Kaufman, GK; Urbach, AR; Gitlin, I; Gudiksen, KL; Weibel, DB; Whitesides, GM. Carbonic anhydrase as a model for biophysical and physical-organic studies of proteins and protein-ligand binding. Chem. Rev 2008, 108, 946–1051. [Google Scholar]
- Matulis, D; Todd, MJ. Thermodynamics - Structure Correlations of Sulfonamide Inhibitor Binding to Carbonic Anhydrase. In Biocalorimetry 2; Ladbury, JE, Doyle, ML, Eds.; Wiley: New York, USA, 2004; pp. 107–132. [Google Scholar]
- Matulis, D; Kranz, JK; Salemme, FR; Todd, MJ. Thermodynamic stability of carbonic anhydrase: measurements of binding affinity and stoichiometry using ThermoFluor. Biochemistry 2005, 44, 5258–5266. [Google Scholar]
- Baker, BM; Murphy, KP. Evaluation of linked protonation effects in protein binding reactions using isothermal titration calorimetry. Biophys. J 1996, 71, 2049–2055. [Google Scholar]
- Baker, BM; Murphy, KP. Dissecting the energetics of a protein-protein interaction: The binding of ovomucoid third domain to elastase. J. Mol. Biol 1997, 268, 557–569. [Google Scholar]
- Cimmperman, P; Baranauskiene, L; Jachimoviciute, S; Jachno, J; Torresan, J; Michailoviene, V; Matuliene, J; Sereikaite, J; Bumelis, V; Matulis, D. A quantitative model of thermal stabilization and destabilization of proteins by ligands. Biophys. J 2008, 95, 3222–3231. [Google Scholar]



| Cell contents | Syringe contents | Temperature, °C, other conditions | ΔH (kJ·mol−1) from the literature [25,26] | ΔCp (J·mol−1·K−1) from the literature [25,26] |
|---|---|---|---|---|
| 0.5 mM HNO3 | 5 mM Tris base | 25 °C, 100 mM NaCl | −47.45 | +73.01 |
| 0.5 mM HNO3 | 5 mM NaOH | 25 °C, 100 mM NaCl | −55.81 | +223.85 |
| 0.2 mM NaCl | 2 mM AgNO3 | 25 °C | −65.72 | +165.39 |
| 0.2 mM NaBr | 2 mM AgNO3 | 25 °C | −84.75 | +172.38 |
| 0.2 mM NaI | 2 mM A1gNO3 | 25 °C | −110.9 | +177.32 |
| Reaction | Literature values (Table 1), kJ·mol−1 | ΔH, kJ·mol−1, obtained in our laboratory withthese calorimeters | ||
|---|---|---|---|---|
| VP-ITC | ITC200 | Nano ITC-III | ||
| Tris-base + HNO3 → Tris-acidic + …, 25 °C | −47.45 | −47.8 ± 1.0 | −48.4 ± 2.1 | −41.9 ± 0.6 |
| Tris-base + HNO3 → Tris-acidic + …, 13 °C | −48.33 | −47.5 ± 0.8 | −47.2 ± 3.4 | −44.4 ± 0.1 |
| Tris-base + HNO3 → Tris-acidic + …, 37 °C | −46.57 | −46.9 ± 0.9 | −50.0 ± 2.8 | −40.8 ± 0.1 |
| NaOH + HNO3 → H2O + …, 25 °C | −55.81 | −52.6 ± 1.8 | ND | −47.2 ± 2.3 |
| AgNO3 + NaCl → AgCl↓ + …, 25 °C | −65.72 | −57.9 ± 2.0 | ND | −52.3 ± 2.6 |
| AgNO3 + NaBr → AgBr↓ + …, 25 °C | −84.75 | −84.4 ± 3.1 | ND | ND |
| AgNO3 + NaI → AgI↓ + …, 25 °C | −110.9 | −110.0 ± 1.7 | −103.4 ± 10.8 | ND |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Baranauskienė, L.; Petrikaitė, V.; Matulienė, J.; Matulis, D. Titration Calorimetry Standards and the Precision of Isothermal Titration Calorimetry Data. Int. J. Mol. Sci. 2009, 10, 2752-2762. https://doi.org/10.3390/ijms10062752
Baranauskienė L, Petrikaitė V, Matulienė J, Matulis D. Titration Calorimetry Standards and the Precision of Isothermal Titration Calorimetry Data. International Journal of Molecular Sciences. 2009; 10(6):2752-2762. https://doi.org/10.3390/ijms10062752
Chicago/Turabian StyleBaranauskienė, Lina, Vilma Petrikaitė, Jurgita Matulienė, and Daumantas Matulis. 2009. "Titration Calorimetry Standards and the Precision of Isothermal Titration Calorimetry Data" International Journal of Molecular Sciences 10, no. 6: 2752-2762. https://doi.org/10.3390/ijms10062752
APA StyleBaranauskienė, L., Petrikaitė, V., Matulienė, J., & Matulis, D. (2009). Titration Calorimetry Standards and the Precision of Isothermal Titration Calorimetry Data. International Journal of Molecular Sciences, 10(6), 2752-2762. https://doi.org/10.3390/ijms10062752




