Chemistry of Secondary Polyphenols Produced during Processing of Tea and Selected Foods
Abstract
:1. Introduction
2. Secondary Polyphenols Produced by Oxidation
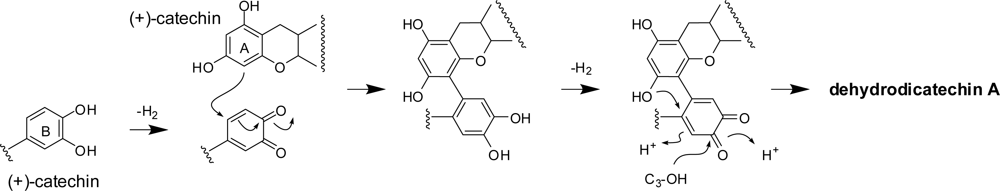
2.1. Oxidation of (+)-catechin and (−)-epicatechin
2.2. Black Tea Polyphenols
2.3. Whisky
3. Secondary Polyphenols Produced by Reaction with Coexisting Compounds
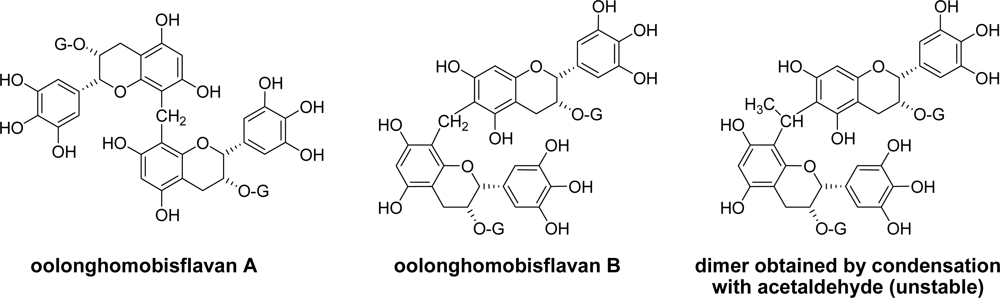
3.1. Oolong Tea and Black Tea
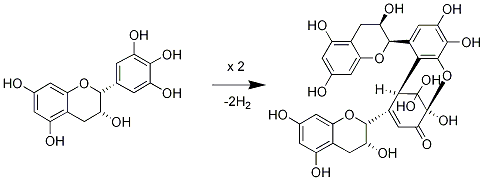
3.2. Insolubilization of Proanthocyanidins in Persimmon Fruits
3.3. Cinnamon Bark
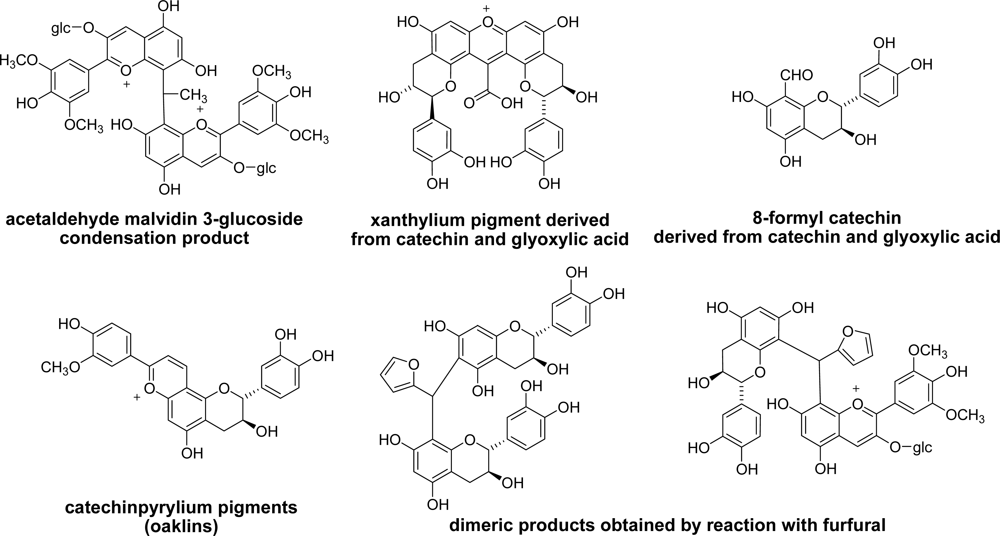
3.4. Application of Catechin-aldehyde Conjugation
3.5. Reaction of Polyphenols with Aldehyde in Other Foods
3.6. Cacao and Coffee
4. Conclusions
References and Notes
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. .Sci. Food Agric 2006, 86, 2010–2037. [Google Scholar]
- Ghosh, D; Scheepens, A. Vascular action of polyphenols. Mol. Nutr. Food Res 2009, 53, 322–331. [Google Scholar]
- Rasmussen, SE; Frederiksen, H; Krogholm, KS; Poulsen, L. Dietary proanthocyanidins: Occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol. Nutr. Food Res 2005, 49, 159–174. [Google Scholar]
- Beecher, GR. Proanthocyanidins: Biological activities associated with human health. Pharm. Biol 2004, 42, 2–20. [Google Scholar]
- Prior, RL; Gu, L. Occurrence and biological significance of proanthocyanidins in the American diet. Phytochemistry 2005, 66, 2264–2280. [Google Scholar]
- Törrönen, R. Sources and health effects of dietary ellagitannins. In Chemistry and Biology of Ellagitannins, An Underestimated Class of Bioactive Plant Polyphenols; Quideau, S, Ed.; World Scientific: Singapore, 2009; pp. 298–315. [Google Scholar]
- Yokozawa, T; Chen, CP; Rhyu, DY; Tanaka, T; Park, JC; Kitani, K. Potential of sanguiin H-6 against oxidative damage in renal mitochondria and apoptosis mediated by peroxynitrite in vivo. Nephron 2002, 92, 133–141. [Google Scholar]
- Weinges, K; Ebert, W; Huthwelker, D; Mattauch, H; Perner, J. Oxidative coupling of phenols. II. Structure and mechanism of the formation of dehydrodicatechin A. Justus Liebigs Ann. Chem 1969, 726, 114–124. [Google Scholar]
- Guyot, S; Vercauteren, J; Cheynier, V. Structural determination of colourless and yellow dimers resulting from (+)-catechin coupling catalysed by grape polyphenoloxidase. Phytochemistry 1996, 42, 1279–1288. [Google Scholar]
- Tanaka, T; Mine, C; Inoue, K; Matsuda, M; Kouno, I. Synthesis of theaflavin from epicatechin and epigallocatechin by plant homogenates and role of epicatechin quinone in the synthesis and degradation of theaflavin. J. Agric. Food Chem 2002, 50, 2142–2148. [Google Scholar]
- Callemien, D; Collin, S. Involvement of flavanoids in beer color instability during storage. J. Agric. Food Chem 2007, 55, 9066–9073. [Google Scholar]
- Lopez-Serrano, M; Ros Barcelo, A. Reversed-phase and size-exclusion chromatography as useful tools in the resolution of peroxidase-mediated (+)-catechin oxidation products. J. Chromatogr. A 2001, 919, 267–273. [Google Scholar]
- Karioti, A; Bilia, AR; Gabbiani, C; Messori, L; Skaltsa, H. Proanthocyanidin glycosides from the leaves of Quercus ilex L. (Fagaceae). Tetrahedron Lett 2009, 50, 1771–1776. [Google Scholar]
- Tanaka, T; Kataoka, M; Tsuboi, N; Kouno, I. New monoterpene glycoside esters and phenolic constituents of Paeoniae Radix, and increase of water solubility of proanthocyanidins in the presence of paeoniflorin. Chem. Pharm. Bull 2000, 48, 201–207. [Google Scholar]
- Yoshikado, N; Taniguchi, S; Kasajima, N; Ohashi, F; Doi, K; Shibata, T; Yoshida, T; Hatano, T. Uncariagambiriine and gambircatechol: Novel constituents of Uncaria gambir leaves. Heterocycles 2009, 77, 793–800. [Google Scholar]
- Shahidi, F; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2004; pp. 241–250. [Google Scholar]
- Takino, Y; Imagawa, H; Horikawa, H; Tanaka, A. Studies on the mechanism of the oxidation of tea leaf catechins. Part III. Formation of a reddish orange pigment and its spectral relationship to some benzotropolone derivatives. Agric. Biol. Chem 1964, 28, 64–71. [Google Scholar]
- Takino, Y; Ferretti, A; Flanagan, V; Gianturco, M; Vogel, M. The structure of theaflavin, a polyphenol of black tea. Tetrahedron Lett 1965, 4019–4025. [Google Scholar]
- Collier, PD; Bryce, T; Mallows, R; Thomas, PE; Frost, DJ; Korver, O; Wilkins, CK. The theaflavins of black tea. Tetrahedron 1973, 29, 125–142. [Google Scholar]
- Haslam, E. Quinone tannin and oxidative polymerization. In Practical Polyphenolics from Structure to Molecular Recognition and Physiological Action; Cambridge University Press: Cambridge, UK, 1998; pp. 335–373. [Google Scholar]
- Tanaka, T; Betsumiya, Y; Mine, C; Kouno, I. Theanaphthoquinone, a novel pigment oxidatively derived from theaflavin during tea-fermentation. Chem Commun 2000, 1365–1366. [Google Scholar]
- Jhoo, J-W; Lo, C-Y; Li, S; Sang, S; Ang, CYW; Heinze, TM; Ho, C-T. Stability of black tea polyphenol, theaflavin, and identification of theanaphthoquinone as its major radical reaction product. J. Agric. Food Chem 2005, 53, 6146–6150. [Google Scholar]
- Tanaka, T; Mine, C; Kouno, I. Structures of two new oxidation products of green tea polyphenols generated by model tea fermentation. Tetrahedron 2002, 58, 8851–8856. [Google Scholar]
- Tanaka, T; Inoue, K; Betsumiya, Y; Mine, C; Kouno, I. Two types of oxidative dimerization of the black tea polyphenol theaflavin. J. Agric. Food Chem 2001, 49, 5785–5789. [Google Scholar]
- Menet, M-C; Sang, S; Yang, CS; Ho, C-T; Rosen, RT. Analysis of theaflavins and thearubigins from black tea extract by MALDI-TOF mass spectrometry. J. Agric. Food Chem 2004, 52, 2455–2461. [Google Scholar]
- Sang, S; Tian, S; Meng, X; Stark, RE; Rosen, RT; Yang, CS; Ho, C-H. Theadibenzotropolone, a new type pigment from enzymatic oxidation of (−)-epicatechin and (−)-epigallocatechin gallate and characterized from black tea using LC/MS/MS. Tetrahedron Lett 2002, 43, 7129–7133. [Google Scholar]
- Sang, S; Lambert, JD; Tian, S; Hong, J; Hou, Z; Ryu, J-H; Stark, RE; Rosen, RT; Huang, M-T; Yang, CS; Ho, C-T. Enzymatic synthesis of tea theaflavin derivatives and their anti-inflammatory and cytotoxic activities. Bioorg. Med. Chem 2004, 12, 459–467. [Google Scholar]
- Lewis, JR; Davis, AL; Cai, Y; Davies, AP; Wilkins, JPG; Pennington, M. Theaflavate B, isotheaflavin-3′-O-gallate and neotheaflavin-3-O-gallate: Three polyphenolic pigments from black tea. Phytochemistry 1998, 49, 2511–2519. [Google Scholar]
- Wan, X; Nursten, HE; Cai, Y; Davis, AL; Wilkins, JPG; Davies, AP. A new type of tea pigment—From the chemical oxidation of epicatechin gallate and isolated from tea. J. Sci. Food Agric 1997, 74, 401–408. [Google Scholar]
- Kusano, R; Tanaka, T; Matsuo, Y; Kouno, I. Structures of epicatechin gallate trimer and tetramer produced by enzymatic oxidation. Chem. Pharm. Bull 2007, 55, 1768–1772. [Google Scholar]
- Roberts, EAH. Oxidation-reduction potentials in tea fermentation. Chem Ind 1957, 1354–1355. [Google Scholar]
- Imagawa, H; Takino, Y. Studies on the oxidation of myricetin glycosides by tea oxidase. Agric. Biol. Chem 1962, 26, 541–542. [Google Scholar]
- Tanaka, T; Watarumi, S; Matsuo, Y; Kamei, M; Kouno, I. Production of theasinensins A and D, epigallocatechin gallate dimers of black tea, by oxidation-reduction dismutation of dehydrotheasinensin A. Tetrahedron 2003, 59, 7939–7947. [Google Scholar]
- Tanaka, T; Mine, C; Watarumi, S; Fujioka, T; Mihashi, K; Zhang, Y-J; Kouno, I. Accumulation of epigallocatechin quinone dimers during tea fermentation and formation of theasinensins. J. Nat. Prod 2002, 65, 1582–1587. [Google Scholar]
- Nonaka, G; Kawahara, O; Nishioka, I. Tannins and related compounds. XV. A new class of dimeric flavan-3-ol gallates, theasinensins A and B, and proanthocyanidin gallates from green tea leaf (1). Chem. Pharm. Bull 1983, 31, 3906–3914. [Google Scholar]
- Hashimoto, F; Nonaka, G; Nishioka, I. Tannins and related compounds. LXIX. Isolation and structure elucidation of B,B′-linked bisflavanoids, theasinensins D-G and oolongtheanin from oolong tea (2). Chem. Pharm. Bull 1988, 36, 1676–1684. [Google Scholar]
- Li, Y; Tanaka, T; Kouno, I. Oxidative coupling of the pyrogallol B-ring with a galloyl group during enzymatic oxidation of epigallocatechin 3-O-gallate. Phytochemistry 2007, 68, 1081–1088. [Google Scholar]
- Matsuo, Y; Tanaka, T; Kouno, I. A new mechanism for oxidation of epigallocatechin and production of benzotropolone pigments. Tetrahedron 2006, 62, 4774–4783. [Google Scholar]
- Nonaka, G; Hashimoto, F; Nishioka, I. Tannins and related compounds. XXXVI. Isolation and structures of theaflagallins, new red pigments from black tea. Chem. Pharm. Bull 1986, 34, 61–65. [Google Scholar]
- Matsuo, Y; Tanaka, T; Kouno, I. Production mechanism of proepitheaflagallin, a precursor of benzotropolone-type black tea pigment, derived from epigallocatechin via a bicyclo[3.2.1]octane-type intermediate. Tetrahedron Lett 2009, 50, 1348–1351. [Google Scholar]
- Matsuo, Y; Yamada, Y; Tanaka, T; Kouno, I. Enzymatic oxidation of gallocatechin and epigallocatechin: Effects of C-ring configuration on the reaction products. Phytochemistry 2008, 69, 3054–3061. [Google Scholar]
- Davis, AL; Lewis, JR; Cai, Y; Powell, C; Davis, AP; Wilkins, JPG; Pudney, P; Clifford, MN. A polyphenolic pigment from black tea. Phytochemistry 1997, 46, 1397–1402. [Google Scholar]
- Matsuo, Y; Li, Y; Watarumi, A; Tanaka, T; Kouno, I. Production mechanism of black tea pigments via bicyclo[3.2.1]octane-type intermediates derived from epigallocatechins. Symposium on the Chemistry of Natural Products, Sappro, Japan, September 19–21, 2007; pp. 299–304.
- Tanaka, T; Matsuo, Y; Kouno, I. A novel black tea pigment and two new oxidation products of epigallocatechin-3-O-gallate. J. Agric. Food Chem 2005, 53, 7571–7578. [Google Scholar]
- Luczaj, W; Skrzydlewska, E. Antioxidative properties of black tea. Prevent. Med 2005, 40, 910–918. [Google Scholar]
- Way, T-Z; Lee, H-H; Kao, M-C; Lin, J-K. Black tea polyphenol theaflavins inhibit aromatase activity and attenuate tamoxifen resistance in HER2/-transfected human breast cancer cells through tyrosine kinase suppression. Eur. J. Cancer 2004, 40, 2165–2174. [Google Scholar]
- Krishnan, R; Maru, GB. Inhibitory effect(s) of polymeric black tea polyphenol fractions on the formation of [3H]-B(a)P-derived DNA adducts. J. Agric. Food Chem 2004, 52, 4261–4269. [Google Scholar]
- Maity, S; Ukil, A; Karmakar, A; Datta, N; Chaudhuri, T; Vedasiromoni, JR; Ganguly, DK; Das, PK. Thearubigin, the major polyphenol of black tea, ameliorates mucosal injury in trinitrobenzene sulfonic acid-induced colitis. Eur. J. Pharmacol 2003, 470, 103–112. [Google Scholar]
- Aneja, R; Odoms, K; Denenberg, AG; Wong, HR. Theaflavin, a black tea extract, is a novel anti-inflammatory compound. Crit. Care Med 2004, 32, 2097–2103. [Google Scholar]
- Mulder, TPJ; van Platerink, CJ; Wijnand Schuyl, PJ; van Amelsvoort, JMM. Analysis of theaflavins in biological fluids using liquid chromatography–electrospray mass spectrometry. J. Chromatogr. B 2001, 760, 271–279. [Google Scholar]
- Haslam, E. Molecular recognition-phenols and polyphenols. In Practical Polyphenolics from Structure to Molecular Recognition and Physiological Action; Cambridge University Press: Cambridge, UK, 1988; pp. 138–177. [Google Scholar]
- Zhang, Y-J; Tanaka, T; Betsumiya, Y; Kusano, R; Matsuo, A; Ueda, T; Kouno, I. Association of tannins and related polyphenols with the cyclic peptide gramicidin S. Chem. Pharm. Bull 2002, 50, 258–262. [Google Scholar]
- Inokuchi, J; Okabe, H; Yamaichi, T; Nagamatsu, A; Nonaka, G; Nishioka, I. Inhibitors of angiotensin-converting enzyme in crude drugs. II. Chem. Pharm. Bull 1985, 33, 264–269. [Google Scholar]
- Hara, Y; Honda, M. The inhibition of α-amylase by tea polyphenols. Agric. Biol. Chem 1990, 54, 1939–1945. [Google Scholar]
- Matsui, T; Tanaka, T; Tamura, S; Toshima, A; Tamaya, K; Miyata, Y; Tanaka, K; Matsumoto, K. α-Glucosidase inhibitory profile of catechins and theaflavins. J. Agric. Food Chem 2007, 55, 99–105. [Google Scholar]
- Nakai, M; Fukui, Y; Asami, A; Toyoda-Ono, Y; Iwashita, T; Shibata, H; Mitsunaga, T; Hashimoto, F; Kiso, Y. Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro. J. Agric. Food Chem 2005, 53, 4593–4598. [Google Scholar]
- Haslam, E. Thoughts on thearubigins. Phytochemistry 2003, 64, 61–73. [Google Scholar]
- Kusano, R; Andou, H; Fujieda, M; Tanaka, T; Matsuo, Y; Kouno, I. Polymer-like polyphenols of black tea and their lipase and amylase inhibitory activities. Chem. Pharm. Bull 2008, 56, 266–272. [Google Scholar]
- Roberts, EAH. Economic importance of flavonoid substances: Tea fermentation. In The Chemistry of Flavonoid Compounds; Pergamon Press: Oxford, UK, 1962; pp. 468–512. [Google Scholar]
- Krishnan, R; Maru, GB. Isolation and analyses of polymeric polyphenol fractions from black tea. Food Chem 2006, 94, 331–340. [Google Scholar]
- Ishii, T; Mori, T; Tanaka, T; Mizuno, D; Yamaji, R; Kumazawa, S; Nakayama, T; Akagawa, M. Covalent modification of proteins by green tea polyphenol epigallocatechin-3-gallate through autoxidation. Free Radical Biol. Med 2008, 45, 1384–1394. [Google Scholar]
- Long, LH; Clement, MV; Halliwell, B. Artifacts in cell culture: Rapid generation of hydrogen peroxide on addition of (−)-epigallocatechin, (−)-epigallocatechin gallate, (+)-catechin, and quercetin to commonly used cell culture media. Biochem. Biophys. Res. Commun 2000, 273, 50–53. [Google Scholar]
- Akagawa, M; Shigemitsu, T; Suyama, K. Production of hydrogen peroxide by polyphenols and polyphenol-rich beverages under quasi-physiological conditions. Biosci. Biotec. Biochem 2003, 67, 2632–2640. [Google Scholar]
- Kondo, K; Kurihara, M; Miyata, N; Suzuki, T; Toyoda, M. Mechanistic studies of catechins as antioxidants against radical oxidation. Arch. Biochem. Biophys 1999, 362, 79–86. [Google Scholar]
- Kondo, K; Kurihara, M; Miyata, N; Suzuki, T; Toyoda, M. Scavenging mechanisms of (−)-epigallocatechin gallate and (−)-epicatechin gallate on peroxyl radicals and formation of superoxide during the inhibitory action. Free Radical Biol. Med 1999, 27, 855–863. [Google Scholar]
- Subramanian, N; Venkatesh, P; Ganguli, S; Sinkar, VP. Role of polyphenol oxidase and peroxidase in the generation of black tea theaflavins. J. Agric. Food Chem 1999, 47, 2571–2578. [Google Scholar]
- Nakayama, T; Ichiba, M; Kuwabara, M; Kajiya, K; Kumazawa, S. Mechanisms and structural specificity of hydrogen peroxide formation during oxidation of catechins. Food Sci. Technol. Res 2002, 8, 261–267. [Google Scholar]
- Herve du Penhoat, CLM; Michon, VMF; Peng, S; Viriot, C; Scalbert, A; Gage, D. Structural elucidation of new dimeric ellagitannins from Quercus robur L, Roburins A-E. J. Chem. Soc.: Perkin Trans 1991, 7, 1653–1660. [Google Scholar]
- Cadahia, E; Varea, S; Munoz, L; Fernandez de Simon, B; Garcia-Vallejo, MC. Evolution of ellagitannins in Spanish, French, and American oak woods during natural seasoning and toasting. J. Agric. Food Chem 2001, 49, 3677–3684. [Google Scholar]
- Fujieda, M; Tanaka, T; Suwa, Y; Koshimizu, S; Kouno, I. Isolation and structure of whiskey polyphenols produced by oxidation of oak wood ellagitannins. J. Agric. Food Chem 2008, 56, 7305–7310. [Google Scholar]
- Saucier, C; Jourdes, M; Glories, Y; Quideau, S. Extraction, detection, and quantification of flavano-ellagitannins and ethylvescalagin in a bordeaux red wine aged in oak barrels. J. Agric. Food Chem 2006, 54, 7349–7354. [Google Scholar]
- Glabasnia, A; Hofmann, T. Identification and sensory evaluation of dehydro- and deoxy-ellagitannins formed upon toasting of oak wood (Quercus alba L.). J. Agric. Food Chem 2007, 55, 4109–4118. [Google Scholar]
- Hashimoto, F; Nonaka, G; Nishioka, I. Tannins and related compounds. XC. 8-C-ascorbyl (−)-epigallocatechin 3-O-gallate and novel dimeric flavan-3-ols, oolonghomobisflavans A and B, from oolong tea. (3). Chem. Pharm. Bull 1989, 37, 3255–3263. [Google Scholar]
- Tanaka, T; Takahashi, R; Kouno, I; Nonaka, G. Chemical evidence for the de-astringency (insolubilization of tannins) of persimmon fruit. J Chem Soc Perkin Trans 1994, 3013–3022. [Google Scholar]
- Matsuo, T; Itoo, S. A model experiment for de-astringency of persimmon fruit with high carbon dioxide treatment: In vitro gelation of kaki-tannin by reacting with acetaldehyde. Agric. Biol. Chem 1982, 46, 683–689. [Google Scholar]
- Co, H; Sanderson, GW. Biochemistry of tea fermentation: Conversion of amino acids to black tea aroma constituents. J. Food Sci 1970, 35, 160–164. [Google Scholar]
- Roberts, GR; Sanderson, GW. Changes undergone by free amino acids during the manufacture of black tea. J. Sci. Food Agric 1966, 17, 182–188. [Google Scholar]
- Saijo, R; Takeo, T. Formation of aldehydes from amino acids by tea leaves extracts. Agric. Biol. Chem 1970, 34, 227–233. [Google Scholar]
- Rizzi, GP. Formation of strecker aldehydes from polyphenol-derived quinones and α-amino acids in a nonenzymic model system. J. Agric. Food Chem 2006, 54, 1893–1897. [Google Scholar]
- Tanaka, T; Watarumi, S; Fujieda, M; Kouno, I. New black tea polyphenol having N-ethyl-2-pyrrolidinone moiety derived from tea amino acid theanine: Isolation, characterization and partial synthesis. Food Chem 2005, 93, 81–87. [Google Scholar]
- Sugiura, A; Tomana, T. Relationship of ethanol production by seeds of different types of Japanese persimmons and their tannin content. Hortscience 1983, 18, 319–321. [Google Scholar]
- Pesis, E; Ben-Arie, R. Involvement of acetaldehyde and ethanol accumulation during induced deastringency of persimmon fruits. J. Food Sci 1984, 49, 896–899. [Google Scholar]
- Tanaka, T; Matsuo, Y; Yamada, Y; Kouno, I. Structure of polymeric polyphenols of cinnamon bark deduced from condensation products of cinnamaldehyde with catechin and procyanidin. J. Agric. Food Chem 2008, 56, 5864–5870. [Google Scholar]
- Yanagida, A; Shoji, T; Shibusawa, Y. Separation of proanthocyanidins by degree of polymerization by means of size-exclusion chromatography and related techniques. J. Biochei. Biophys. Meth 2003, 56, 311–322. [Google Scholar]
- Yokozawa, T; Dong, E; Nakagawa, T; Kashiwagi, H; Nakagawa, H; Takeuchi, S; Chung, HY. In vitro and in vivo studies on the radical-scavenging activity of tea. J. Agric. Food Chem 1998, 46, 2143–2150. [Google Scholar]
- Nanjo, F; Mori, M; Goto, K; Hara, Y. Radical scavenging activity of tea catechins and their related compounds. Biosci., Biotech. Biochem 1999, 63, 1621–1623. [Google Scholar]
- Nakagawa, T; Yokozawa, T; Sano, M; Takeuchi, S; Kim, M; Minamoto, S. Activity of (−)-epigallocatechin 3-O-gallate against oxidative stress in rats with adenine-induced renal failure. J. Agric. Food Chem 2004, 52, 2103–2107. [Google Scholar]
- Yokozawa, T; Cho, EJ; Hara, Y; Kitani, K. Antioxidative activity of green tea treated with radical initiator 2,2′-azobis(2-amidinopropane) dihydrochloride. J. Agric. Food Chem 2000, 48, 5068–5073. [Google Scholar]
- Tanaka, T; Kusano, R; Kouno, I. Synthesis and antioxidant activity of novel amphipathic derivatives of tea polyphenol. Bioorg. Med. Chem. Lett 1998, 8, 1801–1806. [Google Scholar]
- Kajiya, K; Hojo, HI; Suzuki, M; Nanjo, F; Kumazawa, S; Nakayama, T. Relationship between antibacterial activity of (+)-catechin derivatives and their interaction with a model membrane. J. Agric. Food Chem 2004, 52, 1514–1519. [Google Scholar]
- Fudouji, R; Tanaka, T; Taguri, T; Matsuo, Y; Kouno, I. Coupling reactions of catechins with natural aldehydes and allyl alcohols and radical scavenging activities of the triglyceride-soluble products. J. Agric. Food Chem 2009, 57, 6417–6424. [Google Scholar]
- Es-Safi, N-E; Fulcrand, H; Cheynier, V; Moutounet, M. Studies on the acetaldehyde-induced condensation of (−)-epicatechin and malvidin 3-O-glucoside in a model solution system. J. Agric. Food Chem 1999, 47, 2096–2102. [Google Scholar]
- Atanasova, V; Fulcrand, H; Gueraevé, CL; Cheynier, V; Moutounet, M. Structure of a new dimeric acetaldehyde malvidin 3-glucoside condensation product. Tetrahedron Lett 2002, 43, 6151–6153. [Google Scholar]
- Es-Safi, N-E; Gueraevé, CL; Labarbe, B; Fulcrand, H; Cheynier, V; Moutounet, M. Structure of a new xanthylium salt derivative. Tetrahedron Lett 1999, 40, 5869–5872. [Google Scholar]
- Es-Safi, N-E; Gueraevé, CL; Cheynier, V; Moutounet, M. New phenolic compounds obtained by evolution of (+)-catechin and glyoxylic acid in hydroalcoholic medium. Tetrahedron Lett 2000, 41, 1917–1921. [Google Scholar]
- Sousa, C; Mateus, N; Perez-Alonso, J; Cantos-Buelga, C; de Freitas, V. Preliminary study of oaklins, a new class of brick-red catechinpyrylium pigments resulting from the reaction between catechin and wood aldehydes. J. Agric. Food Chem 2005, 53, 9249–9256. [Google Scholar]
- Nonier, M-F; Pianet, I; Laguerre, M; Vivas, N; de Gaulejac, NV. Condensation products derived from flavan-3-ol oak wood aldehydes reaction 1. Structural investigation. Anal. Chim. Acta 2006, 563, 76–83. [Google Scholar]
- Es-Safi, N-E; Cheynier, V; Moutounet, M. Interactions between cyanidin 3-O-glucoside and furfural derivatives and their impact on food color changes. J. Agric. Food Chem 2002, 50, 5586–5595. [Google Scholar]
- Es-Safi, N-E; Cheynier, V; Moutounet, M. Study of the reactions between (+)-catechin and furfural derivatives in the presence or absence of anthocyanins and their implication in food color change. J. Agric. Food Chem 2000, 48, 5946–5954. [Google Scholar]
- Wollgast, J; Anklam, E. Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int 2000, 33, 423–447. [Google Scholar]
- Porter, LJ; Ma, Z; Chan, BG. Cacao procyanidins: Major flavanoids and identification of some minor metabolites. Phytochemistry 1991, 30, 1657–1663. [Google Scholar]
- Hatano, T; Miyatake, H; Natsume, M; Osakabe, N; Takizawa, T; Ito, H; Yoshida, T. Proanthocyanidin glycosides and related polyphenols from cacao liquor and their antioxidant effects. Phytochemistry 2002, 59, 749–758. [Google Scholar]
- Frank, O; Blumberg, S; Kunert, C; Zehentbauer, G; Hofmann, T. Structure determination and sensory analysis of bitter-tasting 4-vinylcatechol oligomers and their identification in roasted coffee by means of LC-MS/MS. J. Agric. Food Chem 2007, 55, 1945–1954. [Google Scholar]
- Zucker, WV. Tannins: Does structure determine function? An ecological perspective. Am. Nat 1983, 121, 335–365. [Google Scholar]
- Robbins, CT; Hanley, TA; Hagerman, AE; Hjeljord, O; Baker, DL; Schwartz, CC; Mautz, WW. Role of tannins in defending plants against ruminants: Reduction in protein availability. Ecology 1987, 68, 98–107. [Google Scholar]
- Barbehenn, RV; Jones, CP; Hagerman, AE; Karonen, M; Salminen, J-P. Ellagitannins have greater oxidative activities than condensed tannins and galloyl glucoses at high pH: Potential impact on caterpillars. J. Chem. Ecol 2006, 32, 2253–2267. [Google Scholar]

















© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tanaka, T.; Matsuo, Y.; Kouno, I. Chemistry of Secondary Polyphenols Produced during Processing of Tea and Selected Foods. Int. J. Mol. Sci. 2010, 11, 14-40. https://doi.org/10.3390/ijms11010014
Tanaka T, Matsuo Y, Kouno I. Chemistry of Secondary Polyphenols Produced during Processing of Tea and Selected Foods. International Journal of Molecular Sciences. 2010; 11(1):14-40. https://doi.org/10.3390/ijms11010014
Chicago/Turabian StyleTanaka, Takashi, Yosuke Matsuo, and Isao Kouno. 2010. "Chemistry of Secondary Polyphenols Produced during Processing of Tea and Selected Foods" International Journal of Molecular Sciences 11, no. 1: 14-40. https://doi.org/10.3390/ijms11010014
APA StyleTanaka, T., Matsuo, Y., & Kouno, I. (2010). Chemistry of Secondary Polyphenols Produced during Processing of Tea and Selected Foods. International Journal of Molecular Sciences, 11(1), 14-40. https://doi.org/10.3390/ijms11010014