Interleukin 12 a Key Immunoregulatory Cytokine in Infection Applications
Abstract
:1. Introduction
2. IL-12 Molecular Structure and Signaling Pathway
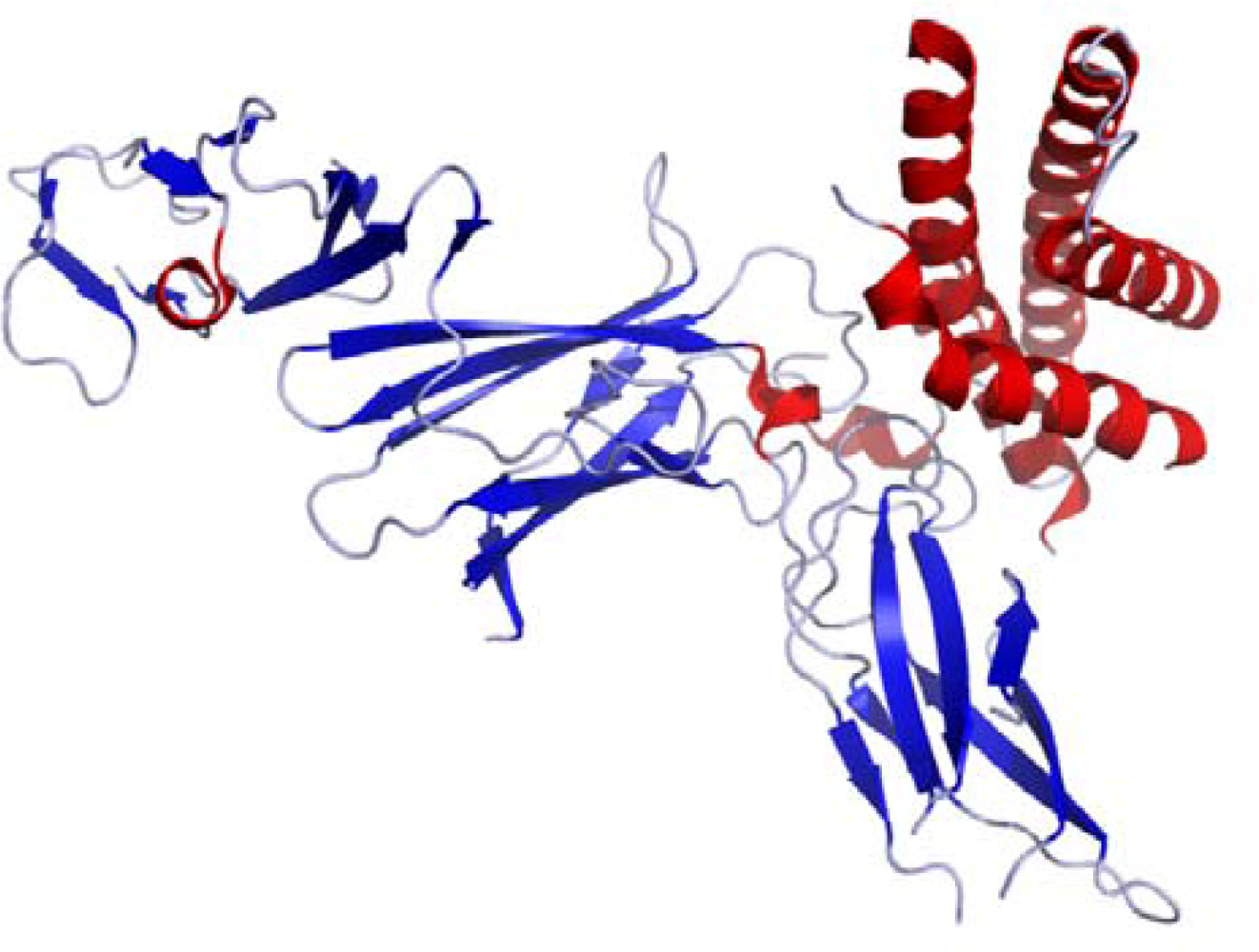
2.1. IL-12 Molecular Structure
2.2. IL-12 Family Members
2.2.1. Interleukin-23
2.2.2. Interleukin-27
2.2.3. Interleukin-35
2.3. IL-12 Signaling Pathway
3. IL-12 Biological Activities
4. IL-12 Therapeutic Applications in Infections
4.1. Viral Infections
4.1.1. Herpes simplex virus
4.1.2. Influenza virus
4.1.3. Human immunodeficiency virus (HIV)
4.2. Bacterial Infections
4.2.1. Bone infection
4.2.2. Tuberculosis
5. Conclusions and Outlook
Acknowledgments
References
- Mosmann, TR; Coffman, RL. TH1 and TH2 cells: different patterns of cytokine secretion lead to different functional properties. Annu. Rev. Immunol 1989, 7, 145–173. [Google Scholar]
- Chehimi, J; Trinchieri, G. Interleukin-12: A bridge between innate resistance and adaptive immunity with a role in infection and acquired immunodeficiency. J. Clin. Immunol 1994, 14, 149–161. [Google Scholar]
- Aggarwal, S; Ghilardi, N; Xie, MH; de Sauvage, FJ; Gurney, AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem 2003, 278, 1910–1914. [Google Scholar]
- Beadling, C; Slifka, MK. Regulation of innate and adaptive immune responses by the related cytokines IL-12, IL-23, and IL-27. Arch. Immunol. Ther. Exp 2006, 54, 15–24. [Google Scholar]
- Sinigaglia, F; D'Ambrosio, D; Panina-Bordignon, P; Rogge, L. Regulation of the IL-12/IL-12R axis: A critical step in T-helper cell differentiation and effector function. Immunol. Rev 1999, 170, 65–72. [Google Scholar]
- Merberg, DM; Wolf, SF; Clark, SC. Sequence similarity between NKSF and the IL-6/G-CSF family. Immunol. Today 1992, 13, 77–78. [Google Scholar]
- Gearing, DP; Cosman, D. Homology of the p40 subunit of natural killer cell stimulatory factor (NKSF) with the extracellular domain of the interleukin-6 receptor. Cell 1991, 66, 9–10. [Google Scholar]
- D’Andrea, A; Rengaraju, M; Valiante, NM; Chehimi, J; Kubin, M; Aste, M; Chan, SH; Kobayashi, M; Young, D; Nickbarg, E; Chizzonite, R; Wolf, SF; Trinchieri, G. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J. Exp. Med 1992, 176, 1387–1398. [Google Scholar]
- Gately, MK; Carvajal, DM; Connaughton, SE; Gillessen, S; Warrier, RR; Kolinsky, KD; Wilkinson, VL; Dwyer, CM; Higgins, GF, Jr; Podlaski, FJ; Faherty, DA; Familletti, PC; Stern, AS; Presky, DH. Interleukin-12 antagonist activity of mouse interleukin-12 p40 homodimer in vitro and in vivo. Ann. NY Acad. Sci 1996, 795, 1–12. [Google Scholar]
- Ling, P; Gately, MK; Gubler, U. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J. Immunol 1995, 154, 116–127. [Google Scholar]
- Khader, SA; Partida-Sanchez, S; Bell, G; Jelley-Gibbs, DM; Swain, S; Pearl, JE; Ghilardi, N; Desauvage, FJ; Lund, FE; Cooper, AM. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J. Exp. Med 2006, 203, 1805–1815. [Google Scholar]
- Presky, DH; Yang, H; Minetti, LJ; Chua, AO; Nabavi, N; Wu, CY; Gately, MK; Gubler, U. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc. Natl. Acad. Sci. USA 1996, 93, 14002–14007. [Google Scholar]
- Collison, LW; Vignali, DA. Interleukin-35: Odd one out or part of the family? Immunol. Rev 2008, 226, 248–262. [Google Scholar]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol 2003, 3, 133–146. [Google Scholar]
- Brombacher, F; Kastelein, RA; Alber, G. Novel IL-12 family members shed light on the orchestration of Th1 responses. Trends Immunol 2003, 24, 207–212. [Google Scholar]
- Parham, C; Chirica, M; Timans, J; Vaisberg, E; Travis, M; Cheung, J; Pflanz, S; Zhang, R; Singh, KP; Vega, F; To, W; Wagner, J; O'Farrell, AM; McClanahan, T; Zurawski, S; Hannum, C; Gorman, D; Rennick, DM; Kastelein, RA; de Waal Malefyt, R; Moore, KW. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol 2002, 168, 5699–5708. [Google Scholar]
- Oppmann, B; Lesley, R; Blom, B; Timans, JC; Xu, Y; Hunte, B; Vega, F; Yu, N; Wang, J; Singh, K; Zonin, F; Vaisberg, E; Churakova, T; Liu, M; Gorman, D; Wagner, J; Zurawski, S; Liu, Y; Abrams, JS; Moore, KW; Rennick, D; de Waal-Malefyt, R; Hannum, C; Bazan, JF; Kastelein, RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 2000, 13, 715–725. [Google Scholar]
- Kamiya, S; Owaki, T; Morishima, N; Fukai, F; Mizuguchi, J; Yoshimoto, T. An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J. Immunol 2004, 173, 3871–3877. [Google Scholar]
- Pflanz, S; Timans, JC; Cheung, J; Rosales, R; Kanzler, H; Gilbert, J; Hibbert, L; Churakova, T; Travis, M; Vaisberg, E; Blumenschein, WM; Mattson, JD; Wagner, JL; To, W; Zurawski, S; McClanahan, TK; Gorman, DM; Bazan, JF; de Waal Malefyt, R; Rennick, D; Kastelein, RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 2002, 16, 779–790. [Google Scholar]
- Collison, LW; Workman, CJ; Kuo, TT; Boyd, K; Wang, Y; Vignali, KM; Cross, R; Sehy, D; Blumberg, RS; Vignali, DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007, 450, 566–569. [Google Scholar]
- Niedbala, W; Wei, XQ; Cai, B; Hueber, AJ; Leung, BP; McInnes, IB; Liew, FY. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur. J. Immunol 2007, 37, 3021–3029. [Google Scholar]
- Bacon, CM; Petricoin, EF, III; Ortaldo, JR; Rees, RC; Larner, AC; Johnston, JA; O'Shea, JJ. Interleukin 12 induces tyrosine phosphorylation and activation of STAT4 in human lymphocytes. Proc. Natl. Acad. Sci. USA 1995, 927, 307–311. [Google Scholar]
- Mullen, AC; High, FA; Hutchins, AS; Lee, HW; Villarino, AV; Livingston, DM; Kung, AL; Cereb, N; Yao, TP; Yang, SY; Reiner, SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science 2001, 292, 1907–1910. [Google Scholar]
- Lighvani, AA; Frucht, DM; Jankovic, D; Yamane, H; Aliberti, J; Hissong, BD; Nguyen, BV; Gadina, M; Sher, A; Paul, WE; O'Shea, JJ. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc. Natl. Acad. Sci. USA 2001, 98, 15137–15142. [Google Scholar]
- Usui, T; Preiss, JC; Kanno, Y; Yao, ZJ; Bream, JH; O'Shea, JJ; Strober, W. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J. Exp. Med 2006, 203, 755–766. [Google Scholar]
- Szabo, SJ; Dighe, AS; Gubler, U; Murphy, KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med 1997, 185, 817–824. [Google Scholar]
- Boehm, U; Klamp, T; Groot, M; Howard, JC. Cellular responses to interferon-γ. Annu Rev. Immunol 1997, 15, 749–795. [Google Scholar]
- Goriely, S; Neurath, MF; Goldman, M. How microorganisms tip the balance between interleukin-12 family members. Nat. Rev. Immunol 2008, 8, 81–86. [Google Scholar]
- Gautier, G; Humbert, M; Deauvieau, F; Scuiller, M; Hiscott, J; Bates, EE; Trinchieri, G; Caux, C; Garrone, P. A type I interferon autocrine–paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J. Exp. Med 2005, 201, 1435–1446. [Google Scholar]
- Re, F; Strominger, JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem 2001, 276, 37692–37699. [Google Scholar]
- Grumont, R; Hochrein, H; O'Keeffe, M; Gugasyan, R; White, C; Caminschi, I; Cook, W; Gerondakis, S. c-Rel regulates interleukin 12 p70 expression in CD8+ dendritic cells by specifically inducing p35 gene transcription. J. Exp. Med 2001, 194, 1021–1032. [Google Scholar]
- Carmody, RJ; Ruan, Q; Liou, HC; Chen, YH. Essential roles of c-Rel in TLR-induced IL-23 p19 gene expression in dendritic cells. J. Immunol 2007, 178, 186–191. [Google Scholar]
- Wirtz, S; Becker, C; Fantini, MC; Nieuwenhuis, EE; Tubbe, I; Galle, PR; Schild, HJ; Birkenbach, M; Blumberg, RS; Neurath, MF. EBV-induced gene 3 transcription is induced by TLR signaling in primary dendritic cells via NF-κB activation. J. Immunol 2005, 174, 2814–2824. [Google Scholar]
- Watford, WT; Moriguchi, M; Morinobu, A; O'Shea, JJ. The biology of IL-12: Coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev 2003, 14, 361–368. [Google Scholar]
- Airoldi, I; Guglielmino, R; Carra, G; Corcione, A; Gerosa, F; Taborelli, G; Trinchieri, G; Pistoia, V. The interleukin-12 and interleukin-12 receptor system in normal and transformed human B lymphocytes. Haematologica 2002, 87, 434–442. [Google Scholar]
- Aragane, Y; Riemann, H; Bhardwaj, RS; Schwarz, A; Sawada, Y; Yamada, H; Luger, TA; Kubin, M; Trinchieri, G; Schwarz, T. IL-12 is expressed and released by human keratinocytes and epidermoid carcinoma cell lines. J. Immunol 1994, 153, 5366–5372. [Google Scholar]
- Bost, KL; Ramp, WK; Nicholson, NC; Bento, JL; Marriott, I; Hudson, MC. Staphylococcus aureus infection of mouse or human osteoblasts induces high levels of interleukin-6 and interleukin-12 production. J. Infect. Dis 1999, 180, 1912–1920. [Google Scholar]
- Aste-Amezaga, M; Ma, X; Sartori, A; Trinchieri, G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J. Immunol 1998, 160, 5936–5944. [Google Scholar]
- Segal, BM; Dwyer, BK; Shevach, EM. An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease. J. Exp. Med 1998, 187, 537–546. [Google Scholar]
- Bonecchi, R; Bianchi, G; Bordignon, PP; D'Ambrosio, D; Lang, R; Borsatti, A; Sozzani, S; Allavena, P; Gray, PA; Mantovani, A; Sinigaglia, F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med 1998, 187, 129–134. [Google Scholar]
- Estaquier, J; Idziorek, T; Zou, W; Emilie, D; Farber, CM; Bourez, JM; Ameisen, JC. T helper type 1/T helper type 2 cytokines and T cell death: Preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO-1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus-infected persons. J. Exp. Med 1995, 182, 1759–1767. [Google Scholar]
- Austrup, F; Vestweber, D; Borges, E; Löhning, M; Bräuer, R; Herz, U; Renz, H; Hallmann, R; Scheffold, A; Radbruch, A; Hamann, A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature 1997, 385, 81–83. [Google Scholar]
- Loetscher, M; Loetscher, P; Brass, N; Meese, E; Moser, B. Lymphocyte-specific chemokine receptor CXCR3: Regulation, chemokine binding and gene localization. Eur. J. Immunol 1998, 28, 3696–3705. [Google Scholar]
- Gazzinelli, RT; Wysocka, M; Hayashi, S; Denkers, EY; Hieny, S; Caspar, P; Trinchieri, G; Sher, A. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol 1994, 1536, 2533–2543. [Google Scholar]
- Gately, MK; Chizzonite, R; Presky, DH. Measurement of human and mouse interleukin-12. Curr Protoc Immunol 2001, 6.16.1–6.16.15. [Google Scholar]
- Wilkinson, VL; Warrier, RR; Truitt, TP; Nunes, P; Gately, MK; Presky, DH. Characterization of anti-mouse IL-12 monoclonal antibodies and measurement of mouse IL-12 by ELISA. J. Immunol. Methods 1996, 189, 15–24. [Google Scholar]
- Shiratori, I; Matsumoto, M; Tsuji, S; Nomura, M; Toyoshima, K; Seya, T. Molecular cloning and functional characterization of guinea pig IL-12. Int. Immunol 2001, 13, 1129–1139. [Google Scholar]
- Shiratori, I; Suzuki, Y; Oshiumi, H; Begum, NA; Ebihara, T; Matsumoto, M; Hazekim, K; Kodama, K; Kashiwazaki, Y; Seya, T. Recombinant interleukin-12 and interleukin-18 antitumor therapy in a guinea-pig hepatoma cell implant model. Cancer Sci 2007, 98, 1936–1942. [Google Scholar]
- Novelli, F; Casanova, JL. The role of IL-12, IL-23 and IFN-gamma in immunity to viruses. Cytokine Growth Factor Rev 2004, 15, 367–377. [Google Scholar]
- Hendricks, RL; Janowicz, M; Tumpey, TM. Critical role of corneal Langerhans cells in the CD4- but not CD8-mediated immunopathology in herpes simplex virus-1-infected mouse corneas. J. Immunol 1992, 148, 2522–2529. [Google Scholar]
- Matsuo, R; Kobayashi, M; Herndon, DN; Pollard, RB; Suzuki, F. Interleukin-12 protects thermally injured mice from herpes simplex virus type 1 infection. J. Leukoc. Biol 1996, 59, 623–630. [Google Scholar]
- Harandi, AM; Svennerholm, B; Holmgren, J; Eriksson, K. Interleukin-12 (IL-12) and IL-18 are important in innate defense against genital herpes simplex virus type 2 infection in mice but are not required for the development of acquired gamma interferon-mediated protective immunity. J. Virol 2001, 75, 6705–6709. [Google Scholar]
- Bhardwaj, N; Seder, RA; Reddy, A; Feldman, MV. IL-12 in conjunction with dendritic cells enhances antiviral CD8+ CTL responses in vitro. J. Clin. Invest 1996, 98, 715–722. [Google Scholar]
- Gross, PA; Hermogenes, AW; Sacks, HS; Lau, J; Levandowski, RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann. Int. Med 1995, 123, 518–527. [Google Scholar]
- de Boer, T; van Dissel, JT; Kuijpers, TW; Rimmelzwaan, GF; Kroon, FP; Ottenhoff, TH. Influenza virus vaccination induces interleukin-12/23 receptor beta 1 (IL-12/23R beta 1)-independent production of gamma interferon (IFN-gamma) and humoral immunity in patients with genetic deficiencies in IL-12/23R beta 1 or IFN-gamma receptor I. Clin. Vaccine Immunol 2008, 15, 1171–1175. [Google Scholar]
- Maggi, E; Mazzetti, M; Ravina, A; Annunziato, F; de Carli, M; Piccinni, MP; Manetti, R; Carbonari, M; Pesce, AM; del Prete, G. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells. Science 1994, 265, 244–248. [Google Scholar]
- Balter, M. Elusive HIV-suppressor factors found. Science 1995, 270, 1560–1561. [Google Scholar]
- Hudson, MC; Ramp, WK; Nicholson, NC; Williams, AS; Nousiainen, MT. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb. Pathog 1995, 6, 409–419. [Google Scholar]
- Almeida, RA; Matthews, KR; Cifrian, E; Guidry, AJ; Oliver, SP. Staphylococcus aureus invasion of bovine mammary epithelial cells. J. Dairy Sci 1996, 79, 1021–1026. [Google Scholar]
- Marriott, I. Osteoblast responses to bacterial pathogens: A previously unappreciated role for bone-forming cells in host defense and disease progression. Immunol. Res 2004, 30, 291–308. [Google Scholar]
- Torti, DC; Feldman, SR. Interleukin-12, interleukin-23, and psoriasis: Current prospects. J. Am. Acad. Dermato 2007, 57, 1059–1068. [Google Scholar]
- Hultgren, OH; Stenson, M; Tarkowski, A. Role of IL-12 in Staphylococcus aureus-triggered arthritis and sepsis. Arthritis Res 2001, 3, 41–47. [Google Scholar]
- Sutterwala, FS; Noel, GJ; Clynes, R; Mosser, DM. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J. Exp. Med 1997, 185, 1977–1985. [Google Scholar]
- Marth, T; Neurath, M; Cuccherini, BA; Strober, W. Defects of monocyte interleukin 12 production and humoral immunity in Whipple's disease. Gastroenterology 1997, 113, 442–448. [Google Scholar]
- O'Sullivan, ST; Lederer, JA; Horgan, AF; Chin, DH; Mannick, JA; Rodrick, M. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann. Surg 1995, 222, 482–490. [Google Scholar]
- Napolitano, LM; Napolitano, LM; Koruda, MJ; Meyer, AA; Baker, CC. The impact of femur fracture with associated soft tissue injury on immune function and intestinal permeability. Shock 1996, 5, 202–207. [Google Scholar]
- Huynh, T; Lemasters, JJ; Bracey, LW; Baker, CC. Proinflammatory Kupffer cell alterations after femur fracture trauma and sepsis in rats. Shock 2000, 14, 555–560. [Google Scholar]
- Spolarics, Z; Siddiqi, M; Siegel, JH; Garcia, ZC; Stein, DS; Denny, T; Deitch, EA. Depressed interleukin-12-producing activity by monocytes correlates with adverse clinical course and a shift toward Th2-type lymphocyte pattern in severely injured male trauma patients. Crit. Care Med 2003, 31, 1722–1729. [Google Scholar]
- Li, B; Jiang, B; Boyce, BM; Lindsey, BA. Multilayer polypeptide nanoscale coatings incorporating IL-12 for the prevention of biomedical device-associated infections. Biomaterials 2009, 30, 2552–2558. [Google Scholar]
- Li, B; Jiang, B; Dietz, MJ; Smith, ES; Clovis, NB; Rao, KMK. Evaluation of local MCP-1 and IL-12 nanocoatings for infection prevention in open fractures. J. Orthop. Res 2010, 28, 48–54. [Google Scholar]
- Murray, HW. Gamma interferon, cytokine-induced macrophage activation, and antimicrobial host defense. In vitro, in animal models, and in humans. Diagn. Microbiol. Infect. Dis 1990, 13, 411–421. [Google Scholar]
- Worth, LL; Jia, SF; Zhou, Z; Chen, L; Kleinerman, ES. Intranasal therapy with an adenoviral vector containing the murine interleukin-12 gene eradicates osteosarcoma lung metastases. Clin. Cancer Res 2000, 6, 3713–3718. [Google Scholar]
- Lenzi, R; Edwards, R; June, C; Seiden, MV; Garcia, ME; Rosenblum, M; Freedman, RS. Phase II study of intraperitoneal recombinant interleukin-12 (rhIL-12) in patients with peritoneal carcinomatosis (residual disease < 1 cm) associated with ovarian cancer or primary peritoneal carcinoma. J. Transl. Med 2007, 5, 66. [Google Scholar]
- Egilmez, NK; Jong, YS; Sabel, MS; Jacob, JS; Mathiowitz, E; Bankert, RB. In situ tumor vaccination with interleukin-12-encapsulated biodegradable microspheres: Iinduction of tumor regression and potent antitumor immunity. Cancer Res 2000, 60, 3832–3837. [Google Scholar]
- Bajetta, E; Del Vecchio, M; Mortarini, R; Nadeau, R; Rakhit, A; Rimassa, L; Fowst, C; Borri, A; Anichini, A; Parmiani, G. Pilot study of subcutaneous recombinant human interleukin 12 in metastatic melanoma. Clin. Cancer Res 1998, 4, 75–85. [Google Scholar]
- Daud, AI; DeConti, RC; Andrews, S; Urbas, P; Riker, AI; Sondak, VK; Munster, PN; Sullivan, DM; Ugen, KE; Messina, JL; Heller, R. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J. Clin. Oncol 2008, 26, 5896–5903. [Google Scholar]
- O'Suilleabhain, C; O'Sullivan, ST; Kelly, JL; Lederer, J; Mannick, JA; Rodrick, ML. Interleukin-12 treatment restores normal resistance to bacterial challenge after burn injury. Surgery 1996, 120, 290–296. [Google Scholar]
- Orme, IM; Miller, ES; Roberts, AD; Furney, SK; Griffin, JP; Dobos, KM; Chi, D; Rivoire, B; Brennan, PJ. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J. Immunol 1992, 148, 189–196. [Google Scholar]
- Flynn, JL; Goldstein, MM; Triebold, KJ; Sypek, J; Wolf, S; Bloom, BR. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J. Immunol 1995, 155, 2515–2524. [Google Scholar]
- Cooper, AM; Khader, SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol. Rev 2008, 226, 191–204. [Google Scholar]
- Jiang, B; Li, B. Tunable drug incorporation and release from polypeptide multilayer nanofilms. Int. J. Nanomed 2009, 4, 37–53. [Google Scholar]
- Zhao, Q; Li, B. pH-controlled drug loading and release from biodegradable microcapsules. Nanomed.: Nanotechnol. Biol. Med 2008, 4, 302–310. [Google Scholar]
- Haynie, DT; Palath, N; Liu, Y; Li, B; Pargaonkar, N. Biomimetic nanotechnology: Inherent reversible stabilization of polypeptide microcapsules. Langmuir 2005, 21, 1136–1138. [Google Scholar]




| • Promote naive CD4+ T cells to differentiate into Th1 |
| • Enhance the generation and activity of cytotoxic T lymphocytes |
| • Induce IFN-γ production by (i) NK cell, T cell, DC, and MΦ, (ii) cooperating with B7/CD28 interaction, and (iii) synergizing with IL-18 |
| • Increase MΦ antimicrobial activity |
| • Prime DC activation to induce more IL-12 production |
| • Induce functional adhesion molecule expression on Th1 cells and influence T cell trafficking |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hamza, T.; Barnett, J.B.; Li, B. Interleukin 12 a Key Immunoregulatory Cytokine in Infection Applications. Int. J. Mol. Sci. 2010, 11, 789-806. https://doi.org/10.3390/ijms11030789
Hamza T, Barnett JB, Li B. Interleukin 12 a Key Immunoregulatory Cytokine in Infection Applications. International Journal of Molecular Sciences. 2010; 11(3):789-806. https://doi.org/10.3390/ijms11030789
Chicago/Turabian StyleHamza, Therwa, John B. Barnett, and Bingyun Li. 2010. "Interleukin 12 a Key Immunoregulatory Cytokine in Infection Applications" International Journal of Molecular Sciences 11, no. 3: 789-806. https://doi.org/10.3390/ijms11030789
APA StyleHamza, T., Barnett, J. B., & Li, B. (2010). Interleukin 12 a Key Immunoregulatory Cytokine in Infection Applications. International Journal of Molecular Sciences, 11(3), 789-806. https://doi.org/10.3390/ijms11030789




