Miniaturization in Biocatalysis
Abstract
:1. Introduction
2. Miniature Systems
2.1. Multi-well Plates as Bioreactors
2.2. Microfluidic Systems
2.2.1. Materials
2.2.2. Flow in Microfluidic Devices
2.2.3. Applications of Microreactors in Biocatalysis
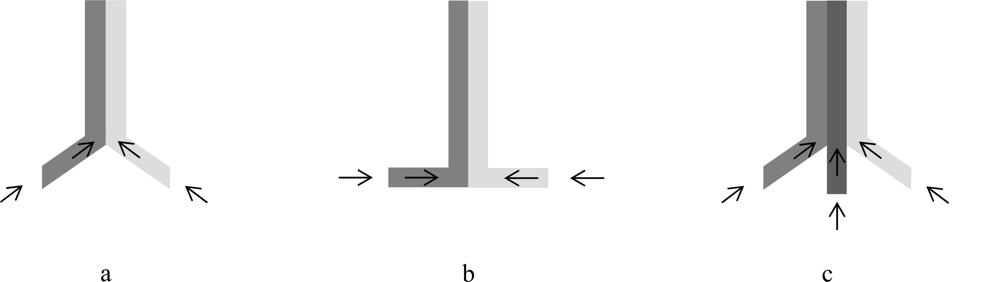
2.2.4. Visualization and Quantification of Fluid Flow
3. Conclusions
Acknowledgments
References and Notes
- Straathof, A. Quantitative analysis of industrial biotransformations. In Industrial Biotransformations, 2nd ed; Liese, A, Seebach, K, Wandrey, C, Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 515–520. [Google Scholar]
- Bommarius, AS; Riebel, BR. Biocatalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004. [Google Scholar]
- Illanes, A. Enzyme Biocatalysis—Principles and Applications; Springer: New York, NY, USA, 2008. [Google Scholar]
- Alcalde, M; Ferrer, M; Plou, F; Ballesteros, A. Environmental biocatalysis: from remediation with enzymes to novel green processes. Trends Biotechnol 2006, 24, 281–287. [Google Scholar] [Green Version]
- Kim, J; Jia, H; Wang, P. Challenges in biocatalysis for enzyme-based biofuel cells. Biotechnol. Adv 2006, 24, 296–308. [Google Scholar]
- Lorenz, P; Eck, J. Screening for Novel Industrial Biocatalysts. Eng. Life Sci 2004, 4, 501–504. [Google Scholar]
- Bornscheuer, UT; Buchholz, K. Highlights in biocatalysis–Historical landmarks and current trends. Eng Life Sci 2005, 5, 309–323. [Google Scholar]
- McNamara, CJ; Mitchell, R. Microbial deterioration of historic stone. Front. Ecol. Env 2005, 8, 445–451. [Google Scholar]
- Jaeger, KE; Eggert, T; Eipper, A; Reetz, MT. Directed evolution and the creation of enantioselective biocatalysts. Appl. Microbiol. Biotechnol 2001, 55, 519–530. [Google Scholar]
- Eijsink, VGH; Gaseidnes, S; Borchert, TV; van den Burg, B. Directed evolution of enzyme stability. Biomolecul. Eng 2005, 22, 21–30. [Google Scholar]
- Johannes, TW; Zhao, H. Directed evolution of enzymes and biosynthetic pathways. Curr. Op. Microbiol 2006, 9, 261–267. [Google Scholar]
- Dalby, P. Engineering enzymes for biocatalysis. Recent Pat Biotechnol 2007, 1, 1–9. [Google Scholar]
- Bershtein, S; Tawfik, DS. Advances in laboratory evolution of enzymes. Curr. Op. Chem. Biol 2008, 12, 151–158. [Google Scholar]
- Rozzell, JD. Commercial scale biocatalysis: Myths and realities. Bioorg. Med. Chem 1999, 7, 2253–2261. [Google Scholar]
- Rasor, JP; Voss, E. Enzyme-catalyzed processes in pharmaceutical industry. Appl. Catal. A: General 2001, 221, 145–158. [Google Scholar]
- Schoemaker, HE; Mink, D; Wubbolts, MG. Dispelling the myths–biocatalysis in industrial synthesis. Science 2003, 299, 1694–1697. [Google Scholar]
- Lye, GJ; Ayazi-Shamlou, P; Baganz, F; Dalby, PA; Woodley, JM. Accelerated design of bioconversion processes using automated microscale processing techniques. Trends Biotechnol 2003, 21, 29–37. [Google Scholar]
- Micheletti, M; Lye, GJ. Microscale bioprocess optimisation. Curr. Op. Biotechnol 2006, 17, 611–618. [Google Scholar]
- Urban, PL; Goodall, DM; Bruce, NC. Enzymatic microreactors in chemical analysis and kinetic studies. Biotechnol. Adv 2006, 24, 42–57. [Google Scholar]
- Miyazaki, M; Maeda, H. Microchannel enzyme reactors and their applications for processing. Trends Biotechnol 2006, 24, 463–470. [Google Scholar]
- Hessel, V; Knobloch, C; Löwe, H. Review on patents in microreactor and micro process engineering. Recent Pat. Chem. Eng 2008, 1, 1–16. [Google Scholar]
- Watts, P; Wiles, C. Recent advances in synthetic micro reaction technology. Chem. Commun 2007, 5, 443–467. [Google Scholar]
- Marques, MPC; Cabral, JMS; Fernandes, P. High throughput in biotechnology: From shakeflasks to fully instrumented microfermentors. Recent Pat. Biotechnol 2009, 3, 124–140. [Google Scholar]
- Doig, SD; Pickering, SCR; Lye, GJ; Woodley, J. The use of microscale processing technologies for quantification of biocatalytic Baeyer-Villiger oxidation kinetics. Biotechnol. Bioeng 2002, 80, 42–49. [Google Scholar]
- Robertson, DE; Steer, BA. Recent progress in biocatalyst discovery and optimization. Curr. Op. Chem. Biol 2004, 8, 141–149. [Google Scholar]
- Ferreira-Torres, C; Micheletti, M; Lye, GJ. Microscale process evaluation of recombinant biocatalyst libraries: application to Baeyer–Villiger monooxygenase catalysed lactone synthesis. Bioprocess Biosyst. Eng 2005, 28, 83–93. [Google Scholar]
- van Beilen, JB; Holtackers, R; Lüscher, D; Bauer, U; Witholt, B; Duetz, WA. Biocatalytic production of perillyl alcohol from limonene by using a novel Mycobacterium sp. cytochrome P450 alkane hydroxylase expressed in Pseudomonas putida. Appl. Environ. Microbiol 2005, 71, 1737–1744. [Google Scholar]
- Brandt, B; Hidalgo, A; Bornscheuer, UT. Immobilization of enzymes in microtiter plate scale. Biotechnol. J 2006, 1, 582–587. [Google Scholar]
- Konarzycka-Besslera, M; Jaeger, K-E. Select the best: novel biocatalysts for industrial applications. Trends Biotechnol 2006, 24, 248–250. [Google Scholar]
- Marques, MPC; de Carvalho, CCCR; Claudino, MJC; Cabral, JMS; Fernandes, P. On the feasibility of the microscale approach for a multi-step biotransformation: sitosterol side-chain cleavage. J. Chem. Technol. Biotechnol 2007, 82, 856–863. [Google Scholar]
- Miller, OJ; Hibbert, EG; Ingram, CU; Lye, GJ; Dalby, PA. Optimisation and evaluation of a generic microplate-based HPLC screen for transketolase activity. Biotechnol. Lett 2007, 29, 1759–1770. [Google Scholar]
- Chen, BH; Micheletti, M; Baganz, F; Woodley, JM; Lye, GJ. An efficient approach to bioconversion kinetic model generation based on automated microscale experimentation integrated with model driven experimental design. Chem. Eng. Sci 2009, 64, 403–409. [Google Scholar]
- Kliche, S; Räuchle, K; Bertau, M; Reschetilowski, W. Ganzzell-Biokatalyse mittels Saccharomyces cerevisiae im Mikroreaktor. Chem. Ing. Tech 2009, 81, 343–347. [Google Scholar]
- Laurent, N; Haddoub, R; Flitsch, S. Enzyme catalysis on solid surfaces. Trends Biotechnol 2008, 26, 328–337. [Google Scholar]
- Fernandes, P; Cabral, JMS. Microlitre/millilitre shaken bioreactors in fermentative and biotransformation processes – A review. Biocat. Biotrans 2006, 24, 237–252. [Google Scholar]
- Zhang, Z; Perozziello, G; Boccazzi, P; Sinskey, AJ; Geschke, O; Jensen, KF. Micro-bioreactors for Bioprocess Development. JALA 2007, 12, 143–151. [Google Scholar]
- Woodley, JM. New opportunities for biocatalysis: making pharmaceutical processes greener. Trends Biotechnol 2008, 26, 321–327. [Google Scholar]
- Titchener-Hooker, NJ; Dunnill, P; Hoare, M. Micro biochemical engineering to accelerate the design of industrial-scale downstream processes for biopharmaceutical proteins. Biotechnol. Bioeng 2008, 100, 473–487. [Google Scholar]
- Tustian, AD; Salte, H; Willoughby, NA; Hassan, I; Rose, MH; Baganz, F; Hoare, M; Titchener-Hooker, NJ. Adapted ultra scale-down approach for predicting the centrifugal separation behavior of high cell density cultures. Biotechnol. Prog 2007, 23, 1404–1410. [Google Scholar]
- Chhatre, S; Titchener-Hooker, NJ. Microscale methods for high-throughput chromatography development in the pharmaceutical industry. J. Chem. Technol. Biotechnol 2009, 84, 927–940. [Google Scholar]
- Bensch, M; Selbach, B; Hubbuch, J. High throughput screening techniques in downstream processing: Preparation, characterization and optimization of aqueous two-phase systems. Chem. Eng. Sci 2007, 62, 2011–2021. [Google Scholar]
- Znidarsic-Plazl, P; Plazl, I. Steroid extraction in a microchannel system—mathematical modelling and Experiments. Lab Chip 2007, 7, 883–889. [Google Scholar]
- Jackson, NB; Liddell, JM; Lye, GJ. An automated microscale technique for the quantitative and parallel analysis of microfiltration operations. J. Memb. Sci 2006, 276, 31–41. [Google Scholar]
- Tao, J; Zhao, L; Ran, N. Recent advances in developing chemoenzymatic processes for active pharmaceutical ingredients. Org. Proc. Res. Dev 2007, 11, 259–267. [Google Scholar]
- Brunati, M; Marinelli, F; Bertolini, C; Gandolfi, R; Daffonchio, D; Molinari, F. Biotransformations of cinnamic and ferulic acid with actinomycetes. Enzyme Microb. Technol 2004, 34, 3–9. [Google Scholar]
- Reymond, J-L; Fluxa, VS; Maillard, N. Enzyme assays. Chem. Commun 2009, 1, 34–46. [Google Scholar]
- Matosevic, S; Micheletti, M; Woodley, JM; Lye, GJ; Baganz, F. Quantification of kinetics for enzyme-catalysed reactions: implications for diffusional limitations at the 10 mL scale. Biotechnol. Lett 2008, 30, 995–1000. [Google Scholar]
- John, GT; Heinzle, E. Quantitative screening method for hydrolases in microplates using pH indicators: determination of kinetic parameters by dynamic pH monitoring. Biotechnol. Bioeng 2001, 72, 620–627. [Google Scholar]
- Bertram, M; Manschot-Lawrence, C; Flöter, E; Bornscheuer, UT. A microtiter plate-based assay method to determine fat quality. Eur. J. Lipid Sci. Technol 2007, 109, 180–185. [Google Scholar]
- Rachinskiy, K; Schultze, H; Boy, M; Bornscheuer, U; Büchs, J. “Enzyme Test Bench,” a high-throughput enzyme characterization technique including the long-term stability. Biotechnol. Bioeng 2009, 103, 305–322. [Google Scholar]
- Marques, MPC; Carvalho, F; Magalhães, S; Cabral, JMS; Fernandes, P. Screening for suitable solvents as substrate carriers for the microbial side-chain cleavage of sitosterol using microtitre plates. Proc. Biochem 2009, 44, 174–180. [Google Scholar]
- Brandt, B; Hidalgo, A; Bornscheuer, UT. Immobilization of enzymes in microtiter plate scale. Biotechnol. J 2006, 1, 582–587. [Google Scholar]
- Stahl, S; Greasham, R; Chartrain, M. Implementation of a rapid microbial screening procedure for biotransformation activities. J. Biosci. Bioeng 2000, 89, 367–371. [Google Scholar]
- Doig, SD; Diep, A; Baganz, F. Characterisation of a novel miniature bubble column bioreactor for high throughput cell cultivation. Biochem. Eng. J 2005, 23, 97–105. [Google Scholar]
- Hermann, R; Lehmann, M; Büchs, J. Characterization of gas-liquid mass transfer phenomena in microtiter plates. Biotechnol. Bioeng 2003, 81, 178–186. [Google Scholar]
- Duetz, WA; Witholt, B. Oxygen transfer by orbital shaking of square vessels and deepwell microtiter plates of various dimensions. Biochem. Eng. J 2004, 17, 181–185. [Google Scholar]
- Kensy, F; Zimmermann, HF; Knabben, I; Anderlei, T; Trauthwein, H; Dingerdissen, U; Büchs, J. Oxygen transfer phenomena in 48-well microtiter plates: Determination by optical monitoring of sulfite oxidation and verification by real-time measurement during microbial growth. Biotechnol. Bioeng 2005, 89, 698–708. [Google Scholar]
- Linek, V; Kordac, M; Moucha, T. Evaluation of the optical sulfite oxidation method for the determination of the interfacial mass transfer area in small-scale bioreactors. Biochem. Eng. J 2006, 26, 264–268. [Google Scholar]
- Funke, M; Diederichs, S; Kensy, F; Muller, C; Buchs, J. The baffled microtiter plate: increased oxygen transfer and improved online monitoring in small scale fermentations. Biotechnol. Bioeng 2009, 103, 1118–1128. [Google Scholar]
- Lamping, SR; Zhang, H; Allen, B; Shamlou, PA. Design of a prototype miniature bioreactor for high throughput automated bioprocessing. Chem. Eng. Sci 2003, 58, 747–758. [Google Scholar]
- Doig, SD; Ortiz-Ochoa, K; Ward, JM; Baganz, F. Characterization of Oxygen Transfer in Miniature and Lab-Scale bubble column bioreactors and comparison of microbial growth performance based on constant kLa. Biotechnol. Prog 2005, 21, 1175–1182. [Google Scholar]
- Puskeiler, R; Kusterer, A; John, GT; Weuster-Botz, D. Miniature bioreactors for automated high-throughput bioprocess design (HTBD): Reproducibility of parallel fed-batch cultivations with Escherichia coli. Biotechnol. Appl. Biochem 2005, 42, 227–235. [Google Scholar]
- Islam, RS; Tisi, D; Levy, MS; Lye, GJ. Scale-up of Escherichia coli growth and recombinant protein expression conditions from microwell to laboratory and pilot scale based on matched kLa, Biotechnol. Bioeng 2008, 99, 1128–1139. [Google Scholar]
- Fernandes, P; Cabral, JMS. Microlitre/millilitre shaken bioreactors in fermentative and biotransformation processes – A review. Biocatal. Biotrans 2006, 24, 237–252. [Google Scholar]
- Duetz, WA. Microtiter plates as mini-bioreactors: miniaturization of fermentation methods. Trends Microbiol 2007, 15, 469–475. [Google Scholar]
- Cottingham, MG; Bain, CD; Vaux, DJT. Rapid method for measurement of surface tension in multi-well plates. Lab. Invest 2004, 84, 523–529. [Google Scholar]
- Bałdyga, J; Bourne, JR; Hearn, SJ. Interaction between chemical reactions and mixing on various scales. Chem. Eng. Sci 1997, 52, 457–466. [Google Scholar]
- Rao, AR; Bimlesh, K. Scale up parameter for surface aeration systems. Int. J. Chem. Reactor Eng 2008, 66, 1–14. [Google Scholar]
- Sharma, MM. New directions in chemical engineering. J. Indian Inst. Sci 1991, 71, 457–473. [Google Scholar]
- Skurtys, O; Aguilera, JM. Applications of microfluidic devices in food engineering. Food Biophys 2008, 3, 1–15. [Google Scholar]
- Geyer, K; Codée, JDC; Seeberger, PH. Microreactors as tools for synthetic chemists – The chemists round-bottomed flask of the 21st century? Chem. Eur. J 2006, 12, 8434–8442. [Google Scholar]
- Barrow, D. Properties and use of microreactors. In Microreactors in Organic Synthesis and Catalysis; Wirth, T, Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 43–57. [Google Scholar]
- Miyazaki, M; Honda, T; Yamaguchi, H; Briones, MPP; Maeda, H. Enzymatic processing in microfluidic reactors. Biotechnol Gen Eng Rev 2008, 25, 405–428. [Google Scholar]
- Jensen, JF. Silicon-based microchemical systems: Characteristics and applications. MRS Bull 2006, 31, 101–107. [Google Scholar]
- Whitesides, GM. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar]
- Jensen, KF. Microreaction engineering - is small better? Chem. Eng. Sci 2001, 56, 293–303. [Google Scholar]
- Park, S; Jeong, Y; Kim, J; Choi, K; Kim, HC; Chung, DS; Chun, K. Fabricaton of poly(dimethylsiloxane) microlens for laser-induced fluorescence detection. Jpn. J. Appl. Phys 2006, 45, 5614–5617. [Google Scholar]
- Lee, JN; Park, C; Whitesides, GM. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem 2003, 75, 6544–6554. [Google Scholar]
- Xia, Y; Whitesides, GM. Soft Lithography. 1998. Annu. Rev. Mater. Sci 1998, 28, 153–194. [Google Scholar]
- Strike, DJ; Fiaccabrino, G-C; Koudelka-Hep, M; de Rooij, NF. Enzymatic microreactor using Si, glass and EPON SU-8. Biomed. Microdev 2000, 2, 175–178. [Google Scholar]
- Chen, EJ; Lee, YM; Selikson, PL. A simulation study of logistics activities in a chemical plant. Simul. Model. Pract. Th 2002, 10, 235–245. [Google Scholar]
- Hesse, V; Löwe, H. Organic synthesis with microstructured reactors. Chem. Eng. Technol 2005, 28, 267–284. [Google Scholar]
- Kirschneck, D; Tekautz, G. Integration of a microreactor in an existing production plant. Chem. Eng. Technol 2007, 30, 305–308. [Google Scholar]
- Zhang, X; Stefanick, S; Villani, FJ. Application of microreactor technology in process development. Org. Process Res. Dev 2004, 8, 455–460. [Google Scholar]
- Hartman, RL; Jensen, KF. Microchemical systems for continuous-flow synthesis. Lab Chip 2009, 9, 2495–2507. [Google Scholar]
- Watts, P; Wiles, C. Recent advances in synthetic micro reaction technology. Chem. Commun 2007, 5, 443–467. [Google Scholar]
- Gokhale, SV; Tayal, RK; Jayaraman, VK; Kulkarni, BD. Microchannel reactors: Applications and use in process development. Int. J. Chem. React. Eng 2005, 3, 1–53. [Google Scholar]
- Mary, P; Studer, V; Tabeling, P. Microfluidic droplet-based liquid-liquid extraction. Anal. Chem 2008, 80, 2680–2687. [Google Scholar]
- Kralj, JG; Sahoo, HR; Jensen, KF. Integrated continuous microfluidic liquid–liquid extraction. Lab Chip 2007, 7, 256–263. [Google Scholar]
- Xu, JH; Li, SW; Tan, J; Luo, GS. Correlations of droplet formation in T-junction microfluidic devices: from squeezing to dripping. Microfluid. Nanofluid 2008, 5, 711–717. [Google Scholar]
- Jakeway, SC; de Mello, AJ; Russel, EL. Miniaturized total analysis systems for biological analysis Fresenius. J. Anal. Chem 2000, 366, 525–539. [Google Scholar]
- Aubin, J; Fletcher, DF; Bertrand, J; Xuereb, C. Characterization of the mixing quality in micromixers. Chem. Eng. Technol 2003, 26, 1262–1270. [Google Scholar]
- Nguyen, N-T; Wu, Z. Micromixers – A review. J. Micromech. Microeng 2005, 15, R1–R16. [Google Scholar]
- Mason, BP; Price, KE; Steinbacher, JL; Bogdan, AR; McQuade, DT. Greener approaches to organic synthesis using microreactor technology. Chem. Rev 2007, 107, 2300–2318. [Google Scholar]
- Tisma, M; Zelic, B; Vasic-Racki, D; Znidarsic-Plazl, P; Plazl, I. Modelling of laccase-catalyzed L-DOPA oxidation in a microreactor. Chem. Eng. J 2009, 149, 383–388. [Google Scholar]
- Znidarsic-Plazl, P; Plazl, I. Modelling and experimental studies on lipase-catalyzed isoamyl acetate synthesis in a microreactor. Proc. Biochem 2009, 44, 1115–1121. [Google Scholar]
- Kanno, K; Maeda, H; Izumo, S; Ikuno, M; Takeshita, K; Tashiro, A; Fujii, M. Rapid enzymatic transglycosylation and oligosaccharide synthesis in a microchip reactor. Lab Chip 2000, 2, 15–18. [Google Scholar]
- Ristenpart, WD; Wan, J; Stone, HA. Enzymatic reactions in microfluidic devices: Michaelis-menten kinetics. Anal. Chem 2008, 80, 3270–3276. [Google Scholar]
- Pohar, A; Plazl, I; Znidarsic–Plazl, P. Lipase-catalyzed synthesis of isoamyl acetate in an ionic liquid/n–heptane two-phase system at the microreactor scale. Lab Chip 2009, 9, 3385–3390. [Google Scholar]
- Koch, K; van den Ber, RJF; Nieuwland, PJ; Wijtmans, R; Wubbolts, MG; Schoemaker, HE; Rutjes, FPJT; van Hest, JCM. Enzymatic synthesis of optically pure cyanohydrins in microchannels using a crude cell lysate. Chem. Eng. J 2008, 135S, S89–S92. [Google Scholar]
- Koch, K; van den Ber, RJF; Nieuwland, PJ; Wijtmans, R; Schoemaker, HE; van Hest, JCM; Rutjes, FPJT. Enzymatic enantioselective C–C-bond formation in microreactors. Biotechnol. Bioeng 2008, 99, 1028–1033. [Google Scholar]
- Swarts, JW; Vossenberg, P; Meerman, MH; Janssen, AEM; Boom, RM. Comparison of two-phase lipase-catalyzed esterification on micro and bench scale. Biotechnol. Bioeng 2008, 99, 855–861. [Google Scholar]
- Maruyama, T; Uchida, J-I; T Ohkawa, T; Futami, T; Katayama, K; Nishizawa, K-i; Sotowa, K-i; Kubota, F; Kamiyaa, N; Goto, M. Enzymatic degradation of p-chlorophenol in a two-phase flow microchannel system. Lab Chip 2003, 3, 308–312. [Google Scholar]
- Buchholz, K; Kasche, V; Bornscheuer, UT. Biocatalysts and Enzyme Technology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Illanes, A; Altamirano, C. Enzyme Reactors. In Enzyme Biocatalysis—Principles and Applications; Illanes, A, Ed.; Springer: New York, NY, USA, 2008; pp. 205–251. [Google Scholar]
- Gódia, F; Sola, C. Fluidized-bed bioreactors. Biotechnol. Prog 1995, 11, 479–497. [Google Scholar]
- Krenková, J; Foret, F. Immobilized microfluidic enzymatic reactors. Electrophoresis 2004, 25, 3550–3563. [Google Scholar]
- Hickey, AM; Ngamsom, B; Wiles, C; Greenway, GM; Watts, P; Littlechild, JA. A microreactor for the study of biotransformations by a cross-linked γ-lactamase enzyme. Biotechnol. J 2009, 4, 510–516. [Google Scholar]
- Hickey, AM; Marle, L; McCreedy, T; Watts, P; Greenway, GM; Littlechild, JA. Immobilization of thermophilic enzymes in miniaturized flow reactors. Biochem. Soc. Trans 2007, 35, 1621–1623. [Google Scholar]
- Wiles, C; Hammond, MJ; Watts, P. The development and evaluation of a continuous flow process for the lipase-mediated oxidation of alkenes. Beilstein J. Org. Chem 2009, 5, 27–39. [Google Scholar]
- Honda, T; Miyazaki, M; Nakamura, H; Maeda, H. Facile preparation of an enzyme-immobilized microreactor using a cross-linking enzyme membrane on a microchannel surface. Adv. Synth. Catal 2006, 348, 2163–2171. [Google Scholar]
- He, P; Greenway, G; Haswell, SJ. Development of enzyme immobilized monolith micro-reactors integrated with microfluidic electrochemical cell for the evaluation of enzyme kinetics. Microfluid Nanofluid 2009. [Google Scholar]
- Thomsen, MS; Pölt, P; Nidetzky, B. Development of a microfluidic immobilised enzyme reactor. Chem. Commun 2007, 24, 2527–2529. [Google Scholar]
- Jones, F; Lu, Z; Elmore, BB. Development of novel microscale system as immobilized enzyme bioreactor. Appl. Biochem. Biotechnol 2002, 98, 627–640. [Google Scholar]
- Schwarz, A; Thomsen, M; Nidetzky, B. Enzymatic synthesis of β-glucosylglycerol using a continuous-flow microreactor containing thermostable β-glycoside hydrolase CelB immobilized on coated microchannel walls. Biotechnol. Bioeng 2009, 103, 865–872. [Google Scholar]
- Thomsen, MS; Nidetzky, B. Coated-wall microreactor for continuous biocatalytic transformations using immobilized enzymes. Biotechnol. J 2009, 4, 98–107. [Google Scholar]
- Honda, T; Miyazaki, M; Nakamura, H; Maeda, H. Immobilization of enzymes on a microchannel surface through cross-linking polymerization. Chem. Comm 2005, 40, 5062–5064. [Google Scholar]
- Koh, W-G; Pishko, M. Immobilization of multi-enzyme microreactors inside microfluidic devices. Sensors Actuat. B 2005, 106, 335–342. [Google Scholar]
- Kawakami, K; Sera, Y; Sakai, S; Ono, T; Ijima, H. Development and Characterization of a Silica Monolith Immobilized Enzyme Micro-bioreactor. Ind. Eng. Chem. Res 2005, 44, 236–240. [Google Scholar]
- Togo, M; Takamura, A; Asai, T; Kaji, H; Nishizawa, M. Structural studies of enzyme-based microfluidic biofuel cells. J. Power Sources 2008, 178, 53–58. [Google Scholar]
- Brody, JP; Yager, P; Goldstein, RE; Austin, RH. Biotechnology at low reynolds numbers. Biophys. J 1996, 71, 3430–3441. [Google Scholar]
- Hosokawa, K; Fuji, T; Nojima, T; Shoji, S; Yotsumoto, A; Endo, I. Microbiochemical reactors for enzymatic reactions including cell-free mRNA translation. Micromechatronics 2000, 1, 85–98. [Google Scholar]
- Ulber, R; Frerichs, J-G; Beutel, S. Optical sensor systems for bioprocess monitoring. Anal. Bioanal. Chem 2003, 376, 342–348. [Google Scholar]
- King, C; Walsh, E; Grimes, R. PIV measurements of flow within plugs in a microchannel. Microfluid. Nanofluid 2007, 3, 463–472. [Google Scholar]
- Pohar, A; Plazl, I. Laminar to turbulent transition and heat transfer in a microreactor: mathematical modeling and experiments. Ind. Eng. Chem. Res 2008, 47, 7447–7455. [Google Scholar]
- Li, X; van der Steen, G; van Dedem, GWK; van der Wielen, LAM; van Leeuwen, M; van Gulik, WM; Heijnen, WM; Ottens, M; Krommenhoek, EE; Gardeniers, JGE; van den Berg, A. Application of direct fluid flow oscillations to improve mixing in microbioreactors. AIChE J 2009, 55, 2725–2736. [Google Scholar]
- Lindken, R; Rossi, M; Große, S; Westerweel, J. Micro-Particle Image Velocimetry (μPIV): Recent developments, applications, and guidelines. Lab Chip 2009, 9, 2551–2567. [Google Scholar]
- Weuster-Botz, D; Puskeiler, R; Kusterer, A; Kaufmann, K; John, GT; Arnold, M. Methods and milliliter scale devices for high-throughput bioprocess design. Bioprocess Biosyst. Eng 2005, 28, 109–119. [Google Scholar]
- Zhang, H; Williams-Dalson, W; Keshavarz-Moore, E; Shamlou, PA. Computational fluid dynamics (CFD) analysis of mixing and gas-liquid mass transfer in shake flasks. Biotechnol. Appl. Biochem 2005, 41, 1–8. [Google Scholar]
- Kashid, MN; Agar, DW; Turek, S. CFD modelling of mass transfer with and without chemical reaction in the liquid-liquid slug flow capillary microreactor. Chem. Eng. Sci 2007, 62, 5102–5109. [Google Scholar]


| Advantages | Criticisms | Comments |
|---|---|---|
| High selectivity of enzymes (substrate, stereo-, region- and functional group selectivity | Narrow range of substrates for a given enzyme | The remarkable chemical selectivity of enzymes favors production of single stereoisomers, minimizes side reactions, eases downstream and reduces pollution. Although some enzymes seem limited to a single substrate (viz catalase) many others, namely hydrolases, act on a wide range of substrates |
| Operation under mild conditions | Enzymes are limited to aqueous environments | The ability to act as catalyst at atmospheric pressure and relatively low temperatures (as compared to chemical catalysts) decreases production costs Several enzymes are active in non-aqueous environments, and occasionally present novel activities under such media. |
| Environmentally friendly | Enzymes only accept low substrate loadings. | As proteins, enzyme catalysts are fully biodegradable, and present no relevant hazard for humans (but for occasional allergic reactions), unlike most chemical catalysts. Biocatalysis has low energy demands, hence minimizing emissions of greenhouse gases Although in nature enzymes are typically faced with low titers, they have been shown to perform efficiently under high substrate concentrations |
| High catalytic efficiency | Enzymes are too expensive | High turn-over numbers, If the cost issue is addressed on a price per kg basis of an enzyme and a transition metal this remark is not obvious, far from it. Yet, and although enzymes are major players in some area, such as detergents, where low cost is a major asset, their use in the production of plenty of commodities as well as in the energy sector (viz. biodiesel) is far from the choice in industry |
| Enzymes can be modified to enhance activity, selectivity, stability | High sensitivity of enzymes and operation in a limited range of pH and temperature | Although low stability of enzymes is often claimed, many enzymes display high operational stability. Enzyme immobilization has partly contributed to this, as well as to widen the mode of operation, albeit at an increase in production costs. Adequate processing of the exhausted immobilized biocatalyst may bring along further costs |
| Microreactor | Bioconversion system | Comments | Ref. |
|---|---|---|---|
| 96-well plate | Hydrolysis of 4-nitrophenyl acetate to 4-nitrophenol and acetic acid catalyzed by free penicillin acylase | Evaluation of kinetic parameters. Design of experiments based on the color change of pH indicator along the time course of the reaction | [48] |
| 24-round and 96-round and 96-square deep well plates | Baeyer-Villiger oxidation of bicyclo[3.2.0]hept-2en-6-one to (-)-(1S,5R)-2-oxabicyclo[3.3.0]oct-6-ene-3-one and (-)-(1S,5R)-3-oxabicyclo[3.3.0]oct-6-ene-2-one catalyzed by free whole cells of Escherichia coli with cyclohexanone monooxygenase activity | Evaluation of operational parameters (viz. well shape, shaking frequency, biocatalyst concentration, filling volume) in the outcome of the bioconversion. Glycerol was used as source of reducing power for regeneration of the NADP+/NADPH system. Validation of the “sacrificial well” approach. Comparison of kinetics in multi-well plate and stirred reactor. Quantification of substrate/products by GC | [24] |
| 24-square well plates | Sitosterol side-chain cleavage to 4-androstene-3,17-dione (AD) using whole resting cells of Mycobacterium sp. NRRL B-3805 | Establishes the feasibility of microtiter plates as platforms for the characterization of multi-enzyme bioconversion systems and as tools for solvent selection in complex bioconversion systems. Highlights some key operational parameters that have to be considered (viz. evaporation, chemical interaction of solvent and plate material) | [30,51] |
| 24-square and 96-round well plates | Production of L-erythrulose from lithium hydroxypyruvate and glycolaldehyde using E. coli lysates with transketolase activity | Evaluation of the statistical significance of initial reaction rate data at multi-well scale. Effect of mixing in the bioconversion pattern. Further validation of the “sacrificial well” approach. Quantification of substrate/products by HPLC | [47] |
| 96-round well plates | Ester hydrolysis catalyzed by esterase | Establishes a multi-well platform for the fast characterization of biocatalysts. Relies on fluorescence techniques for on-line monitoring of the product formed. A mathematical model was developed, which allows for relating the pH-shift that takes place during the reaction, and the concentration of the resulting product. | [50] |
| 96-well plates | Alcoholysis of p-nitrophenyl acetate with 1-propanol promoted by a esterase in anhydrous environment | Screening for suitable methodologies for enzyme immobilization in multi-well plates | [52] |
| Microreactor | Bioconversion system | Comments | Ref. |
|---|---|---|---|
| Chip type microreactor, made of glass, with Y-junctions at the inlet and at the outlet, continuous mode of operation. Oxygen (half) saturated L-DOPA and laccase solutions fed from each inflow | Oxidation of L-DOPA with laccase in full aqueous media | High (roughly 90%) conversion yields were obtained for residence times under 2 minutes. Model predictions, based in the reaction-diffusion equation, provided a good approach to experimental data | [95] |
| Chip type microreactor, made of glass, with Y-junctions at the inlet and at the outlet, continuous mode of operation. n-Hexane and substrates; and buffered enzyme solution fed from each inflow. In the Y-shaped outlet buffer and n-heptane phases were recovered | Synthesis of isoamyl acetate in n-heptane/buffer catalyzed by lipase, using acetic acid as acyl donor | Faster reaction rates were observed in the microfluidic system, when compared to batch runs. Model simulations obtained by numerical solution of non-linear systems provided a good fit to experimental data | [96] |
| Chip type microreactor, made of Poly(methyl methacrylate), PMMA, with Y-junction at the inlet, continuous mode of operation | Hydrolysis of p-nitrophenyl-β-D-galactopyranoside and transgalactosylation on p-nitrophenyl-2-acetamide-2-deoxy-β-D-glucopyranoside, both promoted by galactosidase | Hydrolysis was performed in fully buffered media, whereas transgalactosyation was performed in buffer-acetonitrile solvent system, to minimize reverse reactions. Both reactions were enhanced as compared to the batch system | [97] |
| Chip type microreactors, made of PDMS, with Y-junction at the inlet, continuous mode of operation | Bioluminiscent reaction promoted by luciferase | The reaction was performed in full aqueous media, with luciferin/luciferase and ATP solutions fed to each side of the junction. The microfluidic technique allowed for the determination of Michaelis-Menten rate constants with a single experiment | [98] |
| Chip type microreactor, made of glass, with Y- or Ψ-junction at the inlet, and Y- and single junction at the outlet, respectively, continuous mode of operation. The Ψ-junction was used for the separate inflow of ionic liquid (IL), enzyme and isoamyl alcohol; IL acetic anhydride and enzyme; and n-heptane | Synthesis of isoamyl acetate in n-heptane/1-butyl-3-methylpyridinium dicyanamide, catalyzed by lipase, and with acetic anhydride as acyl donor | Lipase was retained in the interface given its amphiphilic nature. The system allowed for simultaneous esterification and product recovery, showed a 3-fold increase in reaction rate when compared to conventional batch runs, and higher productivity. Parallel or slug flow could be observed depending on the relative flow rate of the ionic liquid and of the organic solvent | [99] |
| Chip type microreactor, made of glass, with Y-junctions at the inlet and at the outlet, continuous mode of operation. Aqueous phase with enzyme and KCN, and organic phase containing aldehyde were fed from each inflow | Synthesis of optically pure cyanohydrins using a cell lysate containing S-selective hydroxynitrile lyase | The crude cell lysate allowed for enantioselective synthesis of cyanohydrins in microchannels with a reaction rate and selectivity only achieved in larger batch mode under intense shaking, where a stable emulsion was formed. No clogging of the microchannels was observed | [100, 101] |
| Chip type microreactor, made of glass, with Y-junctions at the inlet and a single outlet, continuous mode of operation. Aqueous phase containing the enzyme and n-decane containing substrates were fed from each inflow | Esterification of propionic acid and n-butanol catalyzed by lipase | Kinetic parameters obtained in microfluidic system matched those obtained in conventional batch mode of operation. Activation and inactivation patterns were also similar in both scales | [102] |
| Chip type microreactor, made of glass, with Y-junctions at the inlet and a single outlet, continuous mode of operation. Aqueous phase containing the enzyme and iso-octane containing substrates were fed from each inflow | Dehalogenation of p-chlorophenol catalyzed by laccase | The surface of the microchannel was partially modified with octadecylsilane groups to provide a hydrophobic nature, and thus phase separation at the outlet of the microchannel | [103] |
| Microreactor | Bioconversion system | Comments | Ref. |
|---|---|---|---|
| Capillary tubes with a frit at one end, packed with dried cross-linked (+)-γ-lactamase, mixed with controlled pore glass (120–200 mesh, 500-Å nominal pore diameter) in a 1:1 ratio | Conversion of benzamide to benzoic acid using γ-lactamase. Other substrates were screened, namely amides, with γ-lactam emerging as the preferred substrate, although the immobilized enzyme easily hydrolyzed several aromatic amides | The immobilized enzyme was stable for 6 h at 80 °C and kinetic constants were determined in the microreactor. Packing the capillary tubes with the cross-linked enzyme without controlled pore glass led to prohibitive back pressure levels | [108] |
| Same as above, or monolith microreactor. Immobilization in monoliths was achieved by binding to the surface epoxide groups | Conversion of N-benzoyl-l-phenylalanine into l-phenylalanine catalyzed by l-aminoacylase | CLEA capillary column reactor and monolith reactos allowed for 100% conversion at 20ºC and 40 °C respctively, well below the optimum temperatue of 85 °C | [109] |
| Capillary glass tube Novozym® 435 | Conversion of 1-methyl-cyclohexene to 1-methyl-cyclohexene oxide and epoxidation of alkenes in the presence of hydrogen peroxide | Effective transfer of a batch process to a packed-bed flow reactor, allowing for a significant reduction in reaction time. Furthermore, the flow reactor allowed for hydrogen peroxide to be used over prolonged periods of time | [110] |
| Cross-linked enzyme aggregates, CLEA, immobilized onto the inner wall of poly(tetrafluoroethylene), PTFE, microtubes (0.25 mm inner diameter) | Hydrolysis of acetyl-d,l-phenylalanine catalyzed byimmobilized acylase | Successful implementation in a microreactor configuration of a methodology for preparing CLEA applicable to electronegative enzymes | [111] |
| Capillary glass microreactor containing a silica monolith. Glucose oxidase or choline oxidase were separately immobilized on the surface of the polyethylenimine (PEI) coated monolith | Conversion of d-glucose to d-gluconic acid catalyzed by glucose oxidase or conversion of choline to choline acetate catalyzed by choline oxidase | Simple method for preparation of monolith with controlled porosity, allowing for low pressure drop and avoiding mass transfer limitations. Enzyme immobilization was effective on the PEI-activated surface of the monolith, through interaction due to the electronegative and the electropositive nature of the former and the later. Kinetic constants were easily established since on-chip electrochemical detection allowed fast monitoring of enzyme kinetics | [112] |
| Chip type microreactor, made of PDMS, supplemented with pyrogenic silicic acid as a filler and simultaneously providing hydroxy groups for surface chemistry. Enzyme was covalently immobilized on silanised walls of the microchannels by coupling with glutardialdehyde | Hydrolysis of lactose catalyzed by β-glycosidase. Operated in continuous mode and in aqueous phase | The microstructured enzyme reactor was effectively tested in continuous production. A residence time above 33 minutes was required to achive a conversion yield of 100 mM substrate in excess of 60%. The system endured 5 days of continuous operation | [113] |
| Chip type microreactor, made of PDMS, with the enzyme entrapped in the PDMS crosslinked matrix | Hydrolysis of urea catalyzed by urease. Operated in continuous mode and in aqueous phase | Urea conversion significantly decreased for flow rate above 0.064 cm3 min−1 for and initial substrate concentration of 100 mM. Promising results were also referred for operation with glucoamylase in starch hydrolysis | [114] |
| Stainless steel plate with 34 linear channels. Full volume of the reactor of 25 microL. The walls of each channel were coated with a layer of □-aluminum oxide for covalent immobilization of the enzyme. The layer was derivatized with derivatized with (3-aminopropyl)triethoxysilane and the amino groups activated with glutardialdehyde | Transglucosylation reactions catalyzed by β-glucosidase. 2-Nitrophenyl-β-d-glucoside, oNPGlc, or cellobiose were used as donor substrates and glycerol as acceptor, to obtain β-glucosylglycerol in two stereochemical forms, 1-O-β-d-glucosyl-rac-glycerol and 2-O-β-d-glucosyl-sn-glycerol. Hydrolysis of lactose to galactose and glucose with β–glucosidase. In both cases, reactions were performed in aqueous environment and in continuous mode | Residence times were within 0.2 to 90 s. High yields of βGG, roughly of 60% and 80%, based on cellobiose and oNPGlc converted, respectively. Near exhaustion of substrate (80%), yields about 120 mM of βGG from the reaction of 250 mM cellobiose and 1 M glycerol. Determination of kinetic parameters for lactose hydrolysis. Sustained hydrolysis of lactose (100 mM) at 80 °C was observed for 4 days, corresponding to a space-time yield of 500 mg glucose mL−1h−1 at a stable conversion in excess of 70%. | [115, 116] |
| Microreactor composed of PTFE tubing (0.5 mm inner diameter) with enzyme covalently linked. Glutaraldehyde and paraformaldehyde were used as crosslinkers | Hydrolysis of N-glutaryl-l-phenylalanine p-nitroanilide catalyzed by α-chymotrypsin. Operated in continuous mode and in aqueous phase | Hydrolysis yield was kept at 90% and above for a substrate concentration of 1 mM, in a continuous flow (4 μL min−1) for some days | [117] |
| Chip type microreactor with microchannels in PDMS with enzyme-containing poly(ethylene glycol) (PEG) hydrogel microstructures fabricated in microfluidic channels | Hydrolysis of p-nitrophenylphosphate with alkaline phosphatase. Operated in continuous mode and in aqueous phase. | A pH sensitive fluorophore was incorporated in the hydrogel microstructures to allow for reaction through the variation of the emission intensity ratio with pH. The immobilization approach system was reported to be also effective when applied to the applied to urea hydrolysis by urease. | [118] |
| Capillary poly(ether ether ketone) (PEEK) tubes, with inner diameters within 0.1–2.0 mm, filled with silica monolith-entrapped enzyme, produced by sol-gel methodology, from tetramethoxysilane and methyltrimethoxysilane | Transesterification between (S)-(-)-glycidol and vinyl n-butyrate catalyzed by a protease. Continuous operation in organic environment | The microreactor outperformed the batch reactor used for control regarding conversion, when operating at higher flow rates (from the total range of 4.0 × 10−4 to 5.0 mLmin−1). No changes in conversion were observed at a given superficial liquid velocity with variations in tube diameter. Moreover, the conversion increased with a decrease in the enzyme content. The whole suggested mass transfer limitations | [119] |
| Chip type microreactor, made of PDMS as a microfuidic fuel cell. Three enzymes were immobilized alongside the bottom wall of the single stream channel. Bilirubin oxidase (BOD)-adsorbed O2 cathode and a glucose anode prepared by co-immobilization of glucose dehydrogenase (GDH), diaphorase (Dp) and vitamin K3-modified poly-L-lysine, VK3-PLL. | Oxygen reduction catalyzed by BOD; Reduction of VK3/oxidation of NAD+ catalyzed by Dp and NADH regeneration catalyzed by GDH | The cell performance, based on output current, increased with channel height. However, the volume density of current and power were enhanced when cell height decreased | [120] |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fernandes, P. Miniaturization in Biocatalysis. Int. J. Mol. Sci. 2010, 11, 858-879. https://doi.org/10.3390/ijms11030858
Fernandes P. Miniaturization in Biocatalysis. International Journal of Molecular Sciences. 2010; 11(3):858-879. https://doi.org/10.3390/ijms11030858
Chicago/Turabian StyleFernandes, Pedro. 2010. "Miniaturization in Biocatalysis" International Journal of Molecular Sciences 11, no. 3: 858-879. https://doi.org/10.3390/ijms11030858
APA StyleFernandes, P. (2010). Miniaturization in Biocatalysis. International Journal of Molecular Sciences, 11(3), 858-879. https://doi.org/10.3390/ijms11030858




