Application of Molecular Topology for the Prediction of Reaction Yields and Anti-Inflammatory Activity of Heterocyclic Amidine Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sets and Studied Reactions
2.2. Molecular Descriptors
2.3. QSAR Algorithms: Multilinear Regression Analysis
2.4. Molecular Screening
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- Tanaka, K; Toda, F. Solvent-free organic synthesis. Chem. Rev 2000, 100, 1025–1074. [Google Scholar]
- Wannberg, J; Larhed, M. Increasing rates and scope of reactions: Sluggish amines in microwave-heated aminocarbonylation reactions under air. J. Org. Chem 2003, 68, 5750–5753. [Google Scholar]
- De La Hoz, A; Diaz-Ortiz, A; Moreno, A. Selectivity in organic synthesis under microwave irradiation. Curr. Org. Chem 2004, 8, 903–918. [Google Scholar]
- Ganzler, K; Salgo, A; Valko, K. Microwave extraction: A novel sample preparation method for chromatography. J. Chromatogr. A 1986, 371, 299–306. [Google Scholar]
- Barakat, M; Mahmoud, M. Recovery of platinum from spent catalyst. Hydrometallurgy 2004, 72, 179–184. [Google Scholar]
- Radoiu, MT; Martin, DI; Calinescu, I. Emission control of SO2 and NOx by irradiation methods. J. Hazard. Mater 2003, 97, 145–158. [Google Scholar]
- Zhu, YJ; Wang, WW; Qi, RJ; Hu, XL. Microwave-assisted synthesis of single-crystalline tellurium nanorods and nanowires in ionic liquids. Angew. Chem 2004, 116, 1434–1438. [Google Scholar]
- LidstroÈm, P; Tierney, J; Wathey, B; Westman, J. Microwave assisted organic synthesis—A review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar]
- De Julian-Ortiz, JV; Galvez, J; Munoz-Collado, C; Garcia-Domenech, R; Gimeno-Cardona, C. Virtual combinatorial syntheses and computational screening of new potential anti-herpes compounds1. J. Med. Chem 1999, 42, 3308–3314. [Google Scholar]
- Garcia-Domenech, R; Galvez, J; De Julian-Ortiz, JV; Pogliani, L. Some new trends in chemical graph theory. Chem. Rev 2008, 108, 1127–1169. [Google Scholar]
- Kier, LB; Hall, LH. The nature of structure-activity relationships and their relation to molecular connectivity. Eur. J. Med. Chem 1977, 12, 307–312. [Google Scholar]
- De Julián-Ortiz, J; De Gregorio Alapont, C; Ríos-Santamarina, I; García-Doménech, R; Gálvez, J. Prediction of properties of chiral compounds by molecular topology. J. Mol. Graph. Model 1998, 16, 14–18. [Google Scholar]
- Golbraikh, A; Bonchev, D; Tropsha, A. Novel ZE-isomerism descriptors derived from molecular topology and their application to QSAR analysis. J. Chem. Inf. Comput. Sci 2002, 42, 769–787. [Google Scholar]
- Gálvez, J; Gálvez-Llompart, M; García-Domenech, R. Application of molecular topology for the prediction of the reaction times and yields under solvent-free conditions. Green Chem 2010, 12, 1056–1061. [Google Scholar]
- Galvez-Llompart, M; Giner, M; Recio, C; Candeletti, S; Garcia-Domenech, R. Application of molecular topology to the search of novel NSAIDs: Experimental validation of activity. Lett. Drug Des. Discov 2010, 7, 438–445. [Google Scholar]
- Sondhi, SM; Rani, R; Gupta, P; Agrawal, S; Saxena, A. Synthesis, anticancer, and anti-inflammatory activity evaluation of methanesulfonamide and amidine derivatives of 3, 4-Diaryl-2-Imino-4-Thiazolines. Mol. Divers 2009, 13, 357–366. [Google Scholar]
- Han, FS; Osajima, H; Cheung, M; Tokuyama, H; Fukuyama, T. Novel structural motifs consisting of chiral thiazolines: Synthesis, molecular recognition, and anticancer activity. Chem. Eur. J 2007, 13, 3026–3038. [Google Scholar]
- Li, W; Wang, Z; Gududuru, V; Zbytek, B; Slominski, AT; Dalton, JT; Miller, DD. Structure-activity relationship studies of arylthiazolidine amides as selective cytotoxic agents for melanoma. Anticancer Res 2007, 27, 883–888. [Google Scholar]
- Turan-Zitouni, G; Sivaci, D; Kaplancikli, Z; Özdemir, A. Synthesis and antimicrobial activity of some pyridinyliminothiazoline derivatives. Il Farmaco 2002, 57, 569–572. [Google Scholar]
- Ashok, M; Holla, BS; Kumari, NS. Convenient one pot synthesis of some novel derivatives of Thiazolo [2, 3-b] dihydropyrimidinone possessing 4-Methylthiophenyl moiety and evaluation of their antibacterial and antifungal activities. Eur. J. Med. Chem 2007, 42, 380–385. [Google Scholar]
- Bedi, P; Mahajan, MP; Kapoor, VK. Amidine derived 1, 3-Diazabuta-1, 3-Dienes as potential antibacterial and antifungal agents. Bioorg. Med. Chem. Lett 2004, 14, 3821–3824. [Google Scholar]
- Sondhi, SM; Dinodia, M; Kumar, A. Synthesis, anti-inflammatory and analgesic activity evaluation of some amidine and hydrazone derivatives. Bioorg. Med. Chem 2006, 14, 4657–4663. [Google Scholar]
- Khanna, IK; Yu, Y; Huff, RM; Weier, RM; Xu, X; Koszyk, FJ; Collins, PW; Cogburn, JN; Isakson, PC; Koboldt, CM. Selective Cyclooxygenase-2 inhibitors: Heteroaryl modified 1, 2-Diarylimidazoles are potent, orally active anti-inflammatory agents. J. Med. Chem 2000, 43, 3168–3185. [Google Scholar]
- Sondhi, SM; Rani, R; Roy, P; Agrawal, S; Saxena, A. Conventional and microwave assisted synthesis of small molecule based biologically active heterocyclic amidine derivatives. Eur. J. Med. Chem 2010, 45, 902–908. [Google Scholar]
- Winter, CA; Risley, EA; Nuss, GW. Carrageenin-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med 1962, 111, 544–547. [Google Scholar]
- Wiener, H. Structural determination of paraffin boiling points. J. Am. Chem. Soc 1947, 69, 17–20. [Google Scholar]
- Wiener, H. Correlation of heats of isomerization, and differences in heats of vaporization of isomers, among the paraffin hydrocarbons. J. Am. Chem. Soc 1947, 69, 2636–2638. [Google Scholar]
- Moreau, G; Broto, P. Autocorrelation of molecular structures. Application to sar studies. Nouv. J. Chim 1980, 4, 757–764. [Google Scholar]
- Bonchev, D; Mekenyan, O. A topological approach to the calculation of the Pi-electron energy and energy gap of infinite conjugated polymers. Z. Naturforsch. A 1980, 35A, 739–747. [Google Scholar]
- DRAGON for Windows (Software for Molecular Descriptor Calculations); Version 5.4; TALETE SRL: Milano, Italy, 2006.
- Hocking, R. Criteria for selection of a subset regression: Which one should be used? Technometrics 1972, 14, 967–970. [Google Scholar]
- Besalu, E. Fast computation of cross-validated properties in full linear leave-many-out procedures. J. Math. Chem 2001, 29, 191–204. [Google Scholar]
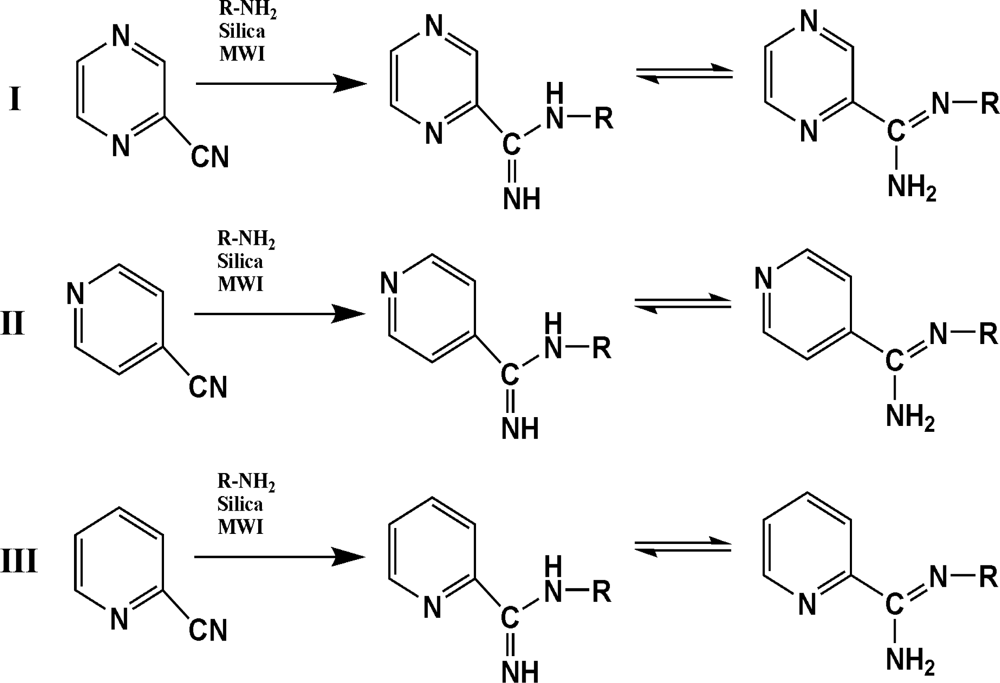




| Synthesis | Comp. | R | (%) Yield | (%)AA* |
|---|---|---|---|---|
| I | 3a |  | 93 | 36.6 |
| I | 3b |  | 91 | 25.4 |
| I | 3c |  | 80 | 21.8 |
| I | 3d |  | 90 | 32.0 |
| I | 3f |  | 85 | 23.9 |
| II | 4a | 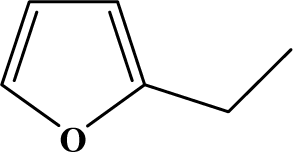 | 90 | 15.2 |
| II | 4b |  | 82 | 22.1 |
| II | 4c | 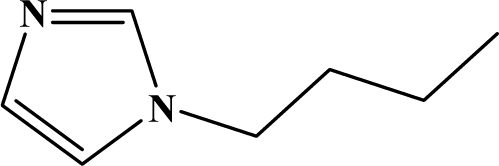 | 80 | 10 |
| II | 4d |  | 85 | 31.0 |
| II | 4e |  | 93 | 33.8 |
| II | 4f | 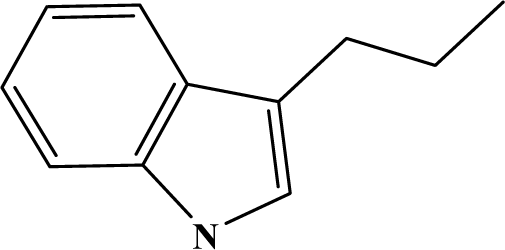 | 80 | 28.2 |
| III | 5a |  | 85 | 28.7 |
| III | 5b |  | 86 | 26.8 |
| III | 5c |  | 86 | 18.8 |
| III | 5d |  | 90 | 28.2 |
| III | 5f |  | 85 | 23.6 |
| Symbol | Name | Definition | Refs. |
|---|---|---|---|
| Pol | Polarity number | Number of pairs of vertexes at topological distance equal to 3 | [26,27] |
| ATSkv k = 1–8 | Moreau-Broto autocorrelation index of order k weighed by Van der Waals volumes | v, Van der Waals volume □, Kronecker delta dij, topological distance between i-atom and k-atom k, index order | [28] |
| EEigkd k = 1–15 | K-st eigenvalue from weighted edge adjacency matrix | K-st eigenvalue from edge adjacency matrix weighted by dipole moments of atoms | [29] |
| EEigkr k = 1–15 | K-st eigenvalue from weighted edge adjacency matrix | K-st eigenvalue from edge adjacency matrix weighted by the resonance integral | [29] |
| Comp. | Yieldexpa (%) | Yieldcalcb (%) | Yieldcalc (cv)c (%) | AAexpa (%) | AAcalcd (%) | AAcalc (cv)e (%) |
|---|---|---|---|---|---|---|
| 3a | 93 | 92 | 92 | 36.6 | 37.1 | 37.2 |
| 3b | 91 | 89 | 88 | 25.4 | 32.6 | 36.4 |
| 3c | 80 | 83 | 83 | 21.8 | 20.7 | 19.9 |
| 3d | 90 | 91 | 91 | 32.0 | 25.9 | 25.2 |
| 3f | 85 | 86 | 86 | 2.9 | 25.6 | 26.4 |
| 4a | 90 | 90 | 90 | 15.2 | 18.7 | 21.6 |
| 4b | 82 | 82 | 82 | 22.1 | 21.1 | 21.0 |
| 4c | 80 | 78 | 78 | 10.0 | 12.2 | 14.1 |
| 4d | 85 | 89 | 89 | 31.0 | 32.0 | 32.1 |
| 4e | 93 | 90 | 89 | 33.8 | 32.0 | 31.7 |
| 4f | 80 | 81 | 82 | 28.2 | 27.0 | 26.4 |
| 5a | 85 | 87 | 87 | 28.7 | 24.1 | 23.3 |
| 5b | 86 | 88 | 89 | 26.8 | 25.8 | 25.8 |
| 5c | 86 | 86 | 86 | 18.8 | 15.2 | 13.9 |
| 5d | 90 | 86 | 86 | 28.2 | 30.6 | 30.9 |
| 5f | 85 | 83 | 83 | 23.6 | 23.3 | 23.2 |

| Sinthesis | Comp. | R | Yieldcalca (%) | AAcalcb (%) |
|---|---|---|---|---|
| II | 6a |  | 89.3 | 31.1 |
| II | 6b |  | 95.9 | 31.4 |
| II | 6c |  | 97.8 | 31.4 |
| II | 6d | 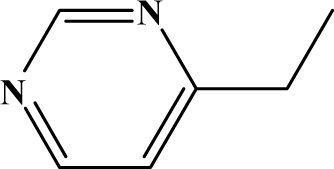 | 98.2 | 31.6 |
| II | 7a | 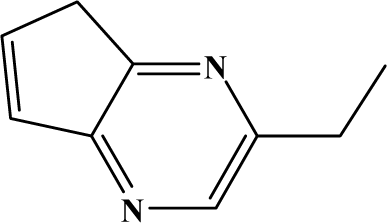 | 80.3 | 29.8 |
| II | 7b |  | 89.5 | 27.2 |
| II | 7c | 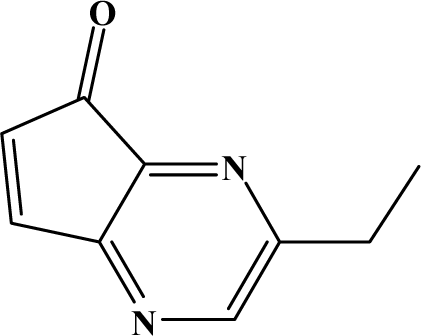 | 72.7 | 42.2 |
| II | 7d |  | 93.8 | 65.3 |
| II | 7e |  | 94.9 | 69.9 |
| II | 7f | 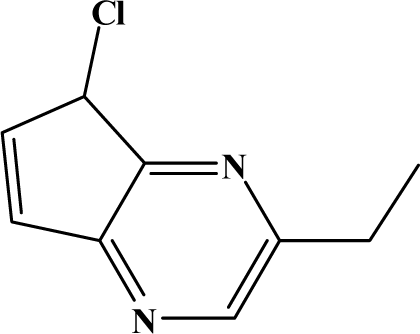 | 86.0 | 57.9 |
| II | 7g |  | 85.3 | 56.1 |
| II | 8a |  | 96.3 | 67.1 |
| II | 8b | 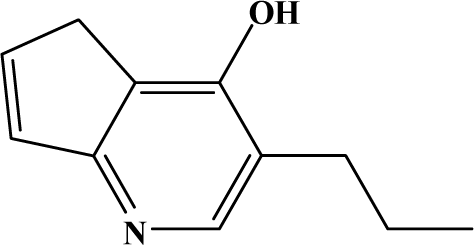 | 96.2 | 59.3 |
| II | 8c |  | 83.9 | 47.5 |
| II | 8d |  | 85.7 | 48.9 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pla-Franco, J.; Gálvez-Llompart, M.; Gálvez, J.; García-Domenech, R. Application of Molecular Topology for the Prediction of Reaction Yields and Anti-Inflammatory Activity of Heterocyclic Amidine Derivatives. Int. J. Mol. Sci. 2011, 12, 1281-1292. https://doi.org/10.3390/ijms12021281
Pla-Franco J, Gálvez-Llompart M, Gálvez J, García-Domenech R. Application of Molecular Topology for the Prediction of Reaction Yields and Anti-Inflammatory Activity of Heterocyclic Amidine Derivatives. International Journal of Molecular Sciences. 2011; 12(2):1281-1292. https://doi.org/10.3390/ijms12021281
Chicago/Turabian StylePla-Franco, Jordi, María Gálvez-Llompart, Jorge Gálvez, and Ramón García-Domenech. 2011. "Application of Molecular Topology for the Prediction of Reaction Yields and Anti-Inflammatory Activity of Heterocyclic Amidine Derivatives" International Journal of Molecular Sciences 12, no. 2: 1281-1292. https://doi.org/10.3390/ijms12021281
APA StylePla-Franco, J., Gálvez-Llompart, M., Gálvez, J., & García-Domenech, R. (2011). Application of Molecular Topology for the Prediction of Reaction Yields and Anti-Inflammatory Activity of Heterocyclic Amidine Derivatives. International Journal of Molecular Sciences, 12(2), 1281-1292. https://doi.org/10.3390/ijms12021281





