In Vitro Antioxidant Activities of Sulfated Derivatives of Polysaccharides Extracted from Auricularia auricular
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Reagents
2.3. Extraction of A. auricula-judae Polysaccharide
2.4. Fraction of A. auricula-judae Polysaccharide
2.5. Sulfation of AAAP and NAAP
2.6. Determination of Degree of Sulfating (DS)
2.7. Amino Acid Analysis
2.8. Analytical High-Performance Liquid Chromatography (HPLC)
2.9. Infrared Spectroscopy Analysis
2.10. Superoxide Anion Scavenging Activity
2.11. Hydroxyl Radical Scavenging Activity
2.12. ABTS Radical Scavenging Assay
2.13. Assay of Lipid Peroxidation
3. Results and Discussion
3.1. Preparation
3.2. Determination of DS
3.3. Amino Acid Analysis by Amino Acid Analyzer
3.4. Molecular Mass Distribution
3.5. Fourier Transform Infrared (FT-IR) Spectroscopy Analysis
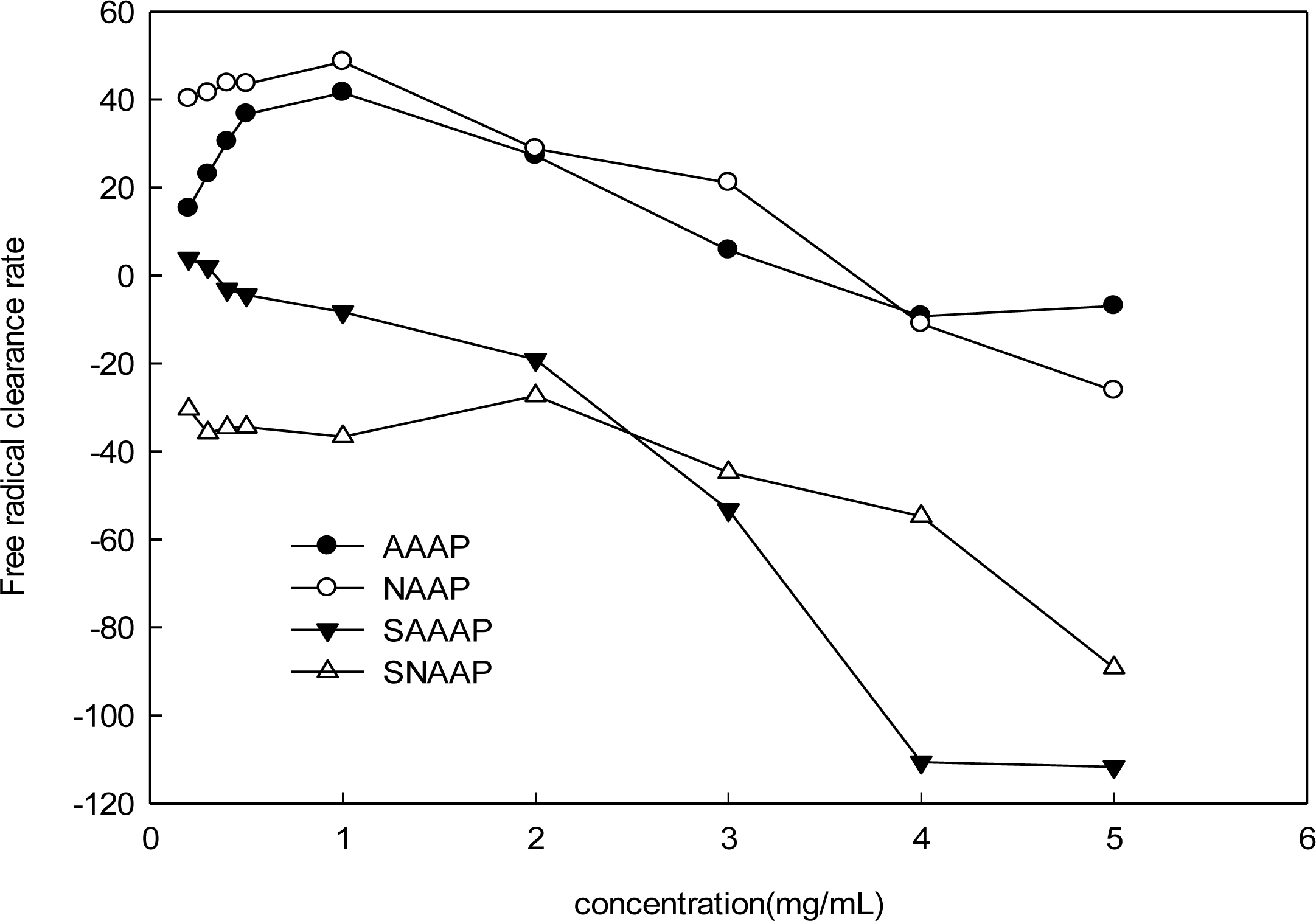
3.6. Scavenging Activity of Superoxide Radical Analysis
3.7. Scavenging Activity of Hydroxyl Radical Analysis
3.8. ABTS Radical Scavenging Assay
3.9. Assay of Lipid Peroxidation
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare that they have no conflict of interest.
References
- Hadi, SM; Bhat, SH; Azmi, AS; Hanif, S; Shamim, U; Ullah, MF. Oxidative breakage of cellular DNA by plant polyphenols: A putative mechanism for anticancer properties. Semin. Cancer Biol 2007, 17, 370–376. [Google Scholar]
- Mantle, D; Eddeb, F; Pickering, A. Comparison of relative antioxidant activities of British medicinal plant species in vitro. J. Ethnopharmacol 2000, 72, 47–51. [Google Scholar]
- Heo, SJ; Kim, JP; Jung, WK; Lee, NH; Kang, HS; Jun, EM; Park, SH; Kang, SM; Lee, YJ; Park, PJ; et al. Identification of chemical structure and free radical scavenging activity of diphlorethohydroxycarmalol isolated from a brown alga, Ishige okamurae. J. Microbiol. Biotechnol 2008, 18, 676–681. [Google Scholar]
- Kang, HS; Kim, KR; Jun, EM; Park, SH; Lee, TS; Suh, JW; Kim, JP. Cyathuscavins A, B, and C, new free radical scavengers with DNA protection activity from the Basidiomycete Cyathus stercoreus. Bioorg. Med. Chem. Lett 2008, 18, 4047–4050. [Google Scholar]
- Lü, JM; Peter, HL; Yao, QZ; Chen, CY. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med 2010, 14, 840–860. [Google Scholar]
- Tsiapali, E; Whaley, S; Kalbfleisch, J; Ensley, HE; Browder, IW; Williams, DL. Glucans exhibit weak antioxidant activity, but stimulate macrophage free radical activity. Free Radic. Biol. Med 2001, 30, 393–402. [Google Scholar]
- Pang, ZJ; Chen, Y; Zhou, M. Polysaccharide krestin enhances manganese superoxide dismutase activity and mRNA expression in mouse peritoneal macrophages. Am. J. Chin. Med 2000, 28, 331–341. [Google Scholar]
- Halliwell, B; Aeschbach, R; Loliger, J; Aruoma, I. The characterization of antioxidants. Food Chem. Toxicol 1995, 33, 601–617. [Google Scholar]
- Halliwell, B; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol 2004, 142, 231–255. [Google Scholar]
- Liu, YH; Chun, HL; Tan, HN; Zhao, T; Cao, JC; Wang, FS. Sulfation of a polysaccharide obtained from Phellinus ribis and potential biological activities of the sulfated derivatives. Carbohydr. Polym 2009, 77, 370–375. [Google Scholar]
- Nie, XH; Shi, BJ; Ding, YT; Tao, WY. Preparation of a chemically sulfated polysaccharide derived from Grifola frondosa and its potential biological activities. Int. J. Biol. Macromol 2006, 39, 228–233. [Google Scholar]
- Mao, WJ; Li, HY; Li, Y; Zhang, HJ; Qi, XH; Sun, HH; Chen, Y; Guo, SD. Chemical characteristic and anticoagulant activity of the sulfated polysaccharide isolated from Monostroma latissimum (Chlorophyta). Int. J. Biol. Macromol 2009, 44, 70–74. [Google Scholar]
- Zhang, HZ; Mao, WJ; Fang, F; Li, HY; Sun, HH; Chen, Y; Qi, XH. Chemical characteristics and anticoagulant activities of a sulfated polysaccharide and its fragments from Monostroma latissimum. Carbohydr. Polym 2008, 71, 428–434. [Google Scholar]
- Chattopadhyay, K; Ghosh, T; Pujol, CA; Carlucci, MJ; Damonte, EB; Ray, B. Polysaccharides from Gracilaria corticata: Sulfation, chemical characterization and anti-HSV activities. Int. J. Biol. Macromol 2008, 43, 346–351. [Google Scholar]
- Zou, C; Du, YM; Li, Y; Yang, JH; Feng, T; Zhang, L; Kennedy, JF. Preparation of lacquer polysaccharide sulfates and their antioxidant activity in vitro. Carbohyd. Polym 2008, 73, 322–331. [Google Scholar]
- Yan, PS; Luo, XC; Zhou, Q. RAPD molecular differentiation of the cultivated strains of the jelly mushrooms, Auricularia auricula and A. polytricha. World J. Microbiol. Biotechnol 2004, 20, 795–799. [Google Scholar]
- Chang, JS; Kim, HJ; Bae, JT; Park, SH; Kim, SE; Kim, OM; Lee, KR. Inhibition effects of A. auricula-judae-judae methanol extract on lipid peroxidation and liver damage in benzo(α)pyrene-treated mice. J. Korean Soc. Food Sci. Nutr 1998, 27, 712–717. [Google Scholar]
- Mau, JL; Chao, GR; Wu, KT. Antioxidant properties of methanolic extracts from several ear mushrooms. J. Agric. Food Chem 2001, 49, 5461–5467. [Google Scholar]
- Luo, YC; Chen, G; Li, B; Ji, BP; Guo, Y; Tian, F. Evaluation of anti-oxidative and hypolipidemic properties of a novel functional diet formulation of Auricularia auricula and Hawthorn. Innovat. Food Sci. Emerg. Tech 2009, 10, 215–221. [Google Scholar]
- Wu, Q; Tan, ZP; Liu, HD; Gao, L; Wu, SJ; Luo, JW; Zhang, WZ; Zhao, TL; Yu, JF; Xu, XH. Chemical characterization of Auricularia auricula polysaccharides and its pharmacological effect on heart anti-oxidant enzyme activities and left ventricle ejection fraction in aged mice. Int. J. Biol. Macromol 2010, 46, 284–288. [Google Scholar]
- Zhang, H; Wang, ZY; Zhang, Z; Wang, X. Purified Auricularia auricular-judae polysaccharide (AAP I-a) prevents oxidative stress in an ageing mouse model. Carbohydr. Polym 2011, 84, 638–648. [Google Scholar]
- Wang, J; Liu, L; Zhang, QB; Zhang, ZS; Qi, HM; Li, PC. Synthesized oversulphated, acetylated and benzoylated derivatives of fucoidan extracted from Laminaria japonica and their potential antioxidant activity in vitro. Food Chem 2009, 114, 1285–1290. [Google Scholar]
- Nishikimi, M; Appaji, RN; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Comm 1972, 46, 849–854. [Google Scholar]
- Smironoff, N; Cumbes, Q. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar]
- Halliwell, B; Gutteridge, JMC; Aruoma, OI. The deoxyribose method: A sample test tube assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem 1987, 165, 215–219. [Google Scholar]
- Ajandouz, EH; Tchiakpe, LS; Ore, FD; Benajiba, A; Puigserver, A. Effects of pH on Caramelization and Maillard Reaction Kinetics in Fructose-Lysine Model Systems. J. Food Sci 2001, 66, 926–931. [Google Scholar]
- Duh, PD; Du, PC; Yen, G. Action of methanolic extract of mung bean hulls as inhibitors of lipid peroxidation and non-lipid oxidative damage. Food Chem. Toxicol 1999, 37, 1055–1061. [Google Scholar]
- Mizuno, T. Development of antitumor polysaccharides from mushroom fungi. Foods Food Ingredients J. Jpn 1996, 167, 69–85. [Google Scholar]
- Yang, LQ; Zhang, LM. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym 2009, 76, 349–361. [Google Scholar]
- Staob, AM. Removal of proteins from polysaccharides methods. Carbohydr Chem 1965. [Google Scholar]
- Mähner, C; Lechner, MD; Nordmeier, E. Synthesis and characterisation of dextran and pullulan sulphate. Carbohydr. Res 2001, 331, 203–208. [Google Scholar]
- Kardošová, A; Machová, E. Antioxidant activity of medicinal plant polysaccharides. Fitoterapia 2006, 77, 367–373. [Google Scholar]
- Li, XM; Li, XL; Zhou, AG. Evaluation of antioxidant activity of the polysaccharides extracted from Lycium barbarum fruits in vitro. Eur. Polymer J 2007, 43, 488–497. [Google Scholar]
- Xu, WT; Zhang, FF; Luo, YB; Ma, LY; Kou, XH; Huang, KL. Antioxidant activity of a water-soluble polysaccharide purified from Pteridium aquilinum. Carbohydr. Res 2009, 344, 217–222. [Google Scholar]
- Fan, LS; Zhang, SH; Yu, L; Ma, L. Evaluation of antioxidant property and quality of breads containing Auricularia auricula polysaccharide flour. Food Chem 2006, 101, 1158–1163. [Google Scholar]
- Yoon, SJ; Yu, MA; Pyun, YR; Hwang, JK; Chuc, DC; Junejac, LR; Mourão, PAS. The nontoxic mushroom Auricularia auricula contains a polysaccharide with anticoagulant activity mediated by anti-thrombin. Thromb. Res 2003, 112, 151–158. [Google Scholar]
- Zhang, LN; Yang, LQ. Properties of A. auricula-judae β-d-glucan in dilute solution. Biopolymers 1995, 36, 695–700. [Google Scholar]
- Xing, RE; Yu, HH; Liu, S; Zhang, WW; Zhang, QB; Li, ZE; Peng, CL. Antioxidant activity of differently regioselective chitosan sulfates in vitro. Bioorg. Med. Chem 2005, 13, 1387–1392. [Google Scholar]
- Zhu, XW; Raina, AK; Lee, HG; Casadesus, G; Smith, MA; Perry, G. Oxidative stress signalling in Alzheimer’s disease. Brain Res 2004, 1000, 32–39. [Google Scholar]
- Ak, T; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact 2008, 174, 27–37. [Google Scholar]
- Shon, MY; Kim, TH; Sung, NJ. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chem 2003, 82, 593–597. [Google Scholar]
- Yagi, K. Lipid peroxides and human disease. Chem. Phys. Lipids 1987, 45, 337–341. [Google Scholar]
- Westerlund, C; Ostlund-Lindqvist, AM; Sainsbury, M; Shertzer, HG; Sjöquist, PO. Characterization of novel indenoindoles. Part I. Structure-activity relationships in different model systems of lipid peroxidation. Biochem. Pharmacol 1996, 51, 1397–1402. [Google Scholar]
- Tepe, B; Sokmen, M; Akpulat, HA; Yumrutas, O; Sokmen, A. Screening of antioxidative properties of the methanolic extracts of Pelargoniumendlicherianum Fenzl., Verbascum wiedemannianum Fisch. and Mey., Sideritislibanotica Labill. subsp. Linearis (Bentham) Borm., Centaurea mucronifera DC. and Hieracium cappadocicum Feyn from Turkish flora. Food Chem 2006, 98, 9–13. [Google Scholar]






| Amino Acids | SAAAP | SNAAP | Amino Acids | SAAAP | SNAAP | |
|---|---|---|---|---|---|---|
| Cys | 0.78 | 1.42 | Ile | not detected | not detected | |
| Met | not detected | not detected | Leu | 0.28 | 0.39 | |
| Asp | 0.25 | 0.32 | Tyr | 0.25 | 0.35 | |
| Thr | 0.19 | 0.25 | Phe | not detected | not detected | |
| Ser | 0.19 | 0.26 | Lys | not detected | not detected | |
| Glu | 0.41 | 0.57 | His | 0.08 | 0.10 | |
| Gly | 0.09 | 0.12 | Arg | not detected | not detected | |
| Ala | 0.19 | 0.23 | Pro | not detected | not detected | |
| Val | 0.16 | not detected | total amino acids | 2.87 | 4.01 | |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, H.; Wang, Z.-Y.; Yang, L.; Yang, X.; Wang, X.; Zhang, Z. In Vitro Antioxidant Activities of Sulfated Derivatives of Polysaccharides Extracted from Auricularia auricular. Int. J. Mol. Sci. 2011, 12, 3288-3302. https://doi.org/10.3390/ijms12053288
Zhang H, Wang Z-Y, Yang L, Yang X, Wang X, Zhang Z. In Vitro Antioxidant Activities of Sulfated Derivatives of Polysaccharides Extracted from Auricularia auricular. International Journal of Molecular Sciences. 2011; 12(5):3288-3302. https://doi.org/10.3390/ijms12053288
Chicago/Turabian StyleZhang, Hua, Zhen-Yu Wang, Lin Yang, Xin Yang, Xue Wang, and Zhi Zhang. 2011. "In Vitro Antioxidant Activities of Sulfated Derivatives of Polysaccharides Extracted from Auricularia auricular" International Journal of Molecular Sciences 12, no. 5: 3288-3302. https://doi.org/10.3390/ijms12053288
APA StyleZhang, H., Wang, Z.-Y., Yang, L., Yang, X., Wang, X., & Zhang, Z. (2011). In Vitro Antioxidant Activities of Sulfated Derivatives of Polysaccharides Extracted from Auricularia auricular. International Journal of Molecular Sciences, 12(5), 3288-3302. https://doi.org/10.3390/ijms12053288





