Enabling Anticancer Therapeutics by Nanoparticle Carriers: The Delivery of Paclitaxel
Abstract
:1. Introduction
2. Polymer Nanocarriers
3. Carbon Nanocarriers
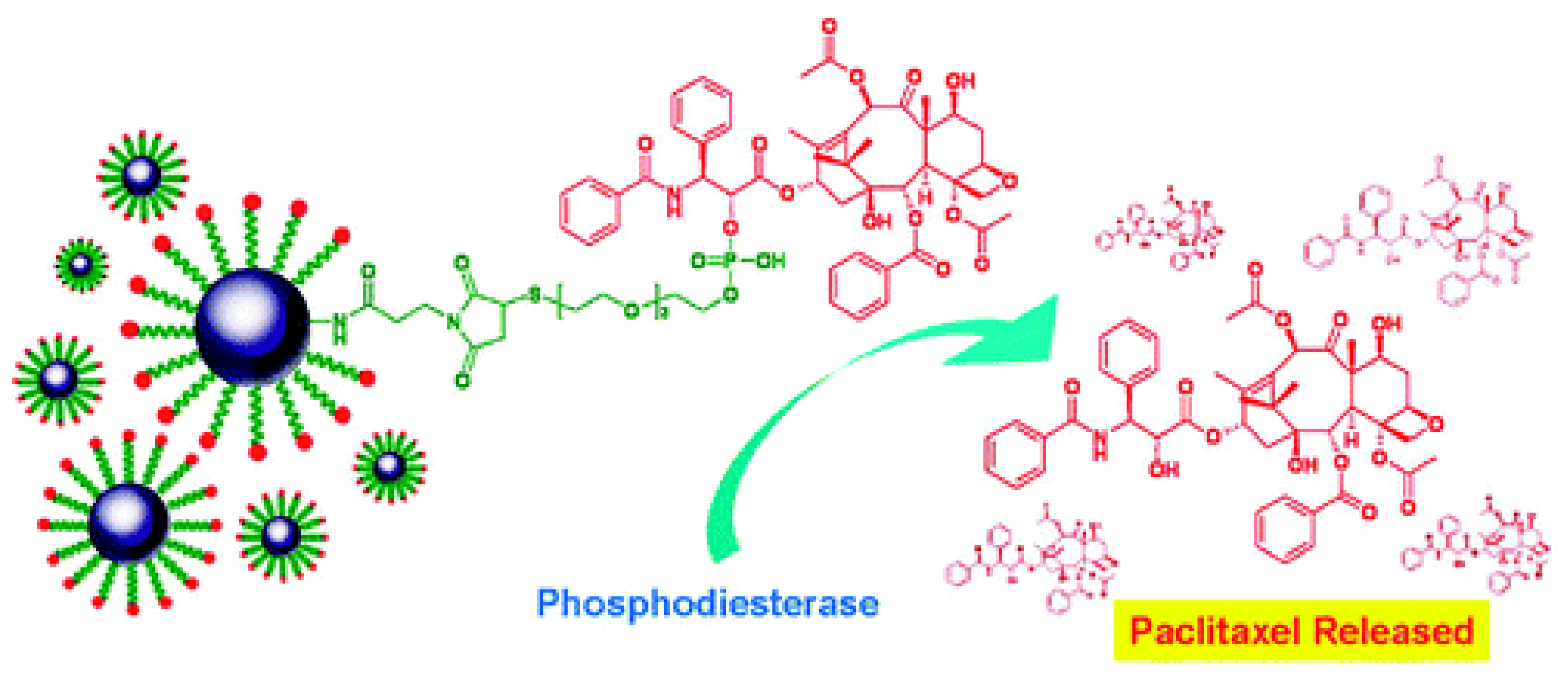
4. Magnetic Nanocarriers
5. Gold Nanocarriers
6. Other Nanocarriers
7. Concluding Remarks
Acknowledgements
References
- Lopes, NM; Adams, EG; Pitts, TW; Bhuyan, BK. Cell kill kinetics and cell cycle effects of taxol on human and hamster ovarian cell lines. Cancer Chemother. Pharmacol 1993, 32, 235–242. [Google Scholar]
- Rowinsky, EK; Cazenave, LA; Donehower, RC. Taxol: A novel investigational antimicrotubule agent. J. Natl. Cancer 1990, 82, 1247–1259. [Google Scholar]
- Jordan, MA; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar]
- Kingston, DGI. Tubulin-interactive natural products as anticancer agents (1). J. Nat. Prod 2009, 72, 507–515. [Google Scholar]
- Mugabe, C; Hadaschik, BA; Kainthan, RK; Brooks, DE; So, AI; Gleave, ME; Burt, HM. Paclitaxel incorporated in hydrophobically derivatized hyperbranched polyglycerols for intravesical bladder cancer therapy. BJU Int 2009, 103, 978–986. [Google Scholar]
- Gelderblom, H; Verweij, J; Nooter, K; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar]
- Knemeyer, I; Wientjes, MG; Au, JLS. Cremophor reduces paclitaxel penetration into bladder wall during intravesical treatment. Cancer Chemother. Pharmacol 1999, 44, 241–248. [Google Scholar]
- Singla, AK; Garg, A; Aggarwal, D. Paclitaxel and its formulations. Int. J. Pharm 2002, 235, 179–192. [Google Scholar]
- Wong, J; Brugger, A; Khare, A; Chaubal, M; Papadopoulos, P; Rabinow, B; Kipp, J; Ning, J. Suspensions for intravenous (IV) injection: A review of development, preclinical and clinical aspects. Adv. Drug Deliv. Rev 2008, 60, 939–954. [Google Scholar]
- Skwarczynski, M; Hayashi, Y; Kiso, Y. Paclitaxel prodrugs: Toward smarter delivery of anticancer agents. J. Med. Chem 2006, 49, 7253–7269. [Google Scholar]
- Torchilin, V. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res 2007, 24, 1–16. [Google Scholar]
- Tekade, RK; Kumar, PV; Jain, NK. Dendrimers in oncology: An expanding horizon. Chem. Rev 2008, 109, 49–87. [Google Scholar]
- Musacchio, TV; Laquintana, A; Latrofa, G; Trapani, VP. Torchilin, PEG-PE micelles loaded with paclitaxel and surface-modified by a PBR-ligand: Synergistic anticancer effect. Mol. Pharm 2008, 6, 468–479. [Google Scholar]
- Kedar, U; Phutane, P; Shidhaye, S; Kadam, V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine 2010, 6, 714–729. [Google Scholar]
- Hawkins, MJ; Soon-Shiong, P; Desai, N. Protein nanoparticles as drug carriers in clinical medicine. Adv. Drug Deliv. Rev 2008, 60, 876–885. [Google Scholar]
- Jain, RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar]
- Acharya, S; Sahoo, SK. PLGA nanoparticles containing various anticancer agents and tumor delivery by EPR effect. Adv. Drug Deliv. Rev 2011, 63, 170–183. [Google Scholar]
- Yang, R; Yang, SG; Shim, WS; Cui, F; Cheng, G; Kim, IW; Kim, DD; Chung, SJ; Shim, CK. Lung-specific delivery of paclitaxel by chitosan-modified PLGA nanoparticles via transient formation of microaggregates. J. Pharm. Sci 2009, 98, 970–984. [Google Scholar]
- Danhier, F; Lecouturier, N; Vroman, B; Jérōme, C; Marchand-Brynaert, J; Feron, O; Préat, V. Paclitaxel-loaded PEGylated PLGA-based nanoparticles: In vitro and in vivo evaluation. J. Control. Release 2009, 133, 11–17. [Google Scholar]
- Bhardwaj, V; Ankola, DD; Gupta, SC; Schneider, M; Lehr, CM; Kumar, MNVR. PLGA nanoparticles stabilized with cationic surfactant: Safety studies and application in oral delivery of paclitaxel to treat chemical-induced breast cancer in rat. Pharm. Res 2009, 26, 2495–2503. [Google Scholar]
- Lee, E; Lee, J; Lee, IH; Yu, M; Kim, H; Chae, SY; Jon, S. Conjugated chitosan as a novel platform for oral delivery of paclitaxel. J. Med. Chem 2008, 51, 6442–6449. [Google Scholar]
- Patil, Y; Sadhukha, T; Ma, L; Panyam, J. Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tariquidar overcomes tumor drug resistance. J. Control. Release 2009, 136, 21–29. [Google Scholar]
- Milane, LJ; Duan, Z; Amiji, MM. Development of EGFR-targeted polymer blend nanocarriers for paclitaxel/lonidamine delivery to treat multi-drug resistance in human breast and ovarian tumor cells. Mol. Pharm 2011, 8, 185–203. [Google Scholar]
- Danhier, F; Vroman, B; Lecouturier, N; Crokart, N; Pourcelle, V; Freichels, H; Jérôme, C; Marchand-Brynaert, J; Feron, O; Préat, V. Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with Paclitaxel. J. Control. Release 2009, 140, 166–173. [Google Scholar]
- Zhang, Y; Bai, YH; Yan, B. Functionalized carbon nanotubes for potential medicinal applicatons. Drug Discov. Today 2010, 15, 428–435. [Google Scholar]
- Wu, HC; Chang, X; Liu, L; Zhao, F; Zhao, Y. Chemistry of carbon nanotubes in biomedical applications. J. Mater. Chem 2010, 20, 1036–1052. [Google Scholar]
- Zhou, HY; Mu, QX; Gao, NN; Liu, AF; Xing, YH; Gao, SL; Zhang, Q; Qu, GB; Chen, YY; Liu, G; Zhang, B; Yan, B. A nano-combinatorial library strategy for the discovery of nanotubes with reduced protein-binding, cytotoxicity, and immune response. Nano Lett 2008, 8, 859–865. [Google Scholar]
- Liu, Z; Tabakman, S; Welsher, K; Dai, H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res 2009, 2, 85–120. [Google Scholar]
- Partha, R; Mitchell, LR; Lyon, JL; Joshi, PP; Conyers, JL. Buckysomes: Fullerene-based nanocarriers for hydrophobic molecule delivery. ACS Nano 2008, 2, 1950–1958. [Google Scholar]
- Green, MR; Manikhas, GM; Orlov, S; Afanasyev, B; Makhson, AM; Bhar, P; Hawkins, MJ. Abraxane®, a novel Cremophor®-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann. Oncol 2006, 17, 1263–1268. [Google Scholar]
- Liu, Z; Chen, K; Davis, C; Sherlock, S; Cao, Q; Chen, X; Dai, H. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res 2008, 68, 6652–6660. [Google Scholar]
- Berlin, JM; Leonard, AD; Pham, TT; Sano, D; Marcano, DC; Yan, S; Fiorentino, S; Milas, ZL; Kosynkin, DV; Price, BK. Effective drug delivery, in vitro and in vivo, by carbon-based nanovectors noncovalently loaded with unmodified paclitaxel. ACS Nano 2010, 4, 4621–4636. [Google Scholar]
- Lay, CL; Liu, HQ; Tan, HR; Liu, Y. Delivery of paclitaxel by physically loading onto poly(ethylene glycol)(PEG)-graftcarbon nanotubes for potent cancer therapeutics. Nanotechnology 2010, 21, 065101. [Google Scholar]
- Griset, AP; Walpole, J; Liu, R; Gaffey, A; Colson, YL; Grinstaff, MW. Expansile nanoparticles: Synthesis, characterization, and in vivo efficacy of an acid-responsive polymeric drug delivery system. J. Am. Chem. Soc 2009, 131, 2469–2471. [Google Scholar]
- Guo, Y; Shi, D; Cho, H; Dong, Z; Kulkarni, A; Pauletti, GM; Wang, W; Lian, J; Liu, W; Ren, L. In vivo imaging and drug storage by quantum-dot-conjugated carbon nanotubes. Adv. Funct. Mater 2008, 18, 2489–2497. [Google Scholar]
- Hilder, TA; Hill, JM. Probability of encapsulation of paclitaxel and doxorubicin into carbon nanotubes. Micro Nano Lett 2008, 3, 41–49. [Google Scholar]
- Chen, J; Wong, SS; Ojima, I. Carbon nanotube-based drug delivery systems and methods of making same. US Patent 20100021471, 2008. [Google Scholar]
- Okon, E; Pouliquen, D; Okon, P; Kovaleva, ZV; Stepanova, TP; Lavit, SG; Kudryavtsev, BN; Jallet, P. Biodegradation of magnetite dextran nanoparticles in the rat: A histologic and biophysical study. Lab. Invest 1994, 71, 895–903. [Google Scholar]
- Yin, H; Yu, S; Casey, PS; Chow, GM. Synthesis and properties of poly(D,L-lactide) drug carrier with maghemite nanoparticles. Mater. Sci. Eng. C 2010, 30, 618–623. [Google Scholar]
- Johannsen, M; Gneveckow, U; Eckelt, L; Feussner, A; Waldfner, N; Scholz, R; Deger, S; Wust, P; Loening, SA; Jordan, A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial technique. Int. J. Hyperth 2005, 21, 637–647. [Google Scholar]
- Alexiou, C; Arnold, W; Klein, RJ; Parak, FG; Hulin, P; Bergemann, C; Erhardt, W; Wagenpfeil, S; Lübbe, AS. Locoregional cancer treatment with magnetic drug targeting. Cancer Res 2000, 60, 6641–6648. [Google Scholar]
- Lübbe, AS; Bergemann, C; Riess, H; Schriever, F; Reichardt, P; Possinger, K; Matthias, M; Dörken, B; Herrmann, F; Gürtler, R. Clinical experiences with magnetic drug targeting: a phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res 1996, 56, 4686–4693. [Google Scholar]
- Sonvico, F; Mornet, S; Vasseur, S; Dubernet, C; Jaillard, D; Degrouard, J; Hoebeke, J; Duguet, E; Colombo, P; Couvreur, P. Folate-conjugated iron oxide nanoparticles for solid tumor targeting as potential specific magnetic hyperthermia mediators: Synthesis, physicochemical characterization, and in vitro experiments. Bioconjugate Chem 2005, 16, 1181–1188. [Google Scholar]
- Kohler, N; Sun, C; Wang, J; Zhang, M. Methotrexate-modified superparamagnetic nanoparticles and their intracellular uptake into human cancer cells. Langmuir 2005, 21, 8858–8864. [Google Scholar]
- Zhang, JL; Srivastava, RS; Misra, RDK. Core-shell magnetite nanoparticles surface encapsulated with smart stimuli-responsive polymer: Synthesis, characterization, and LCST of viable drug-targeting delivery system. Langmuir 2007, 23, 6342–6351. [Google Scholar]
- Santra, S; Kaittanis, C; Grimm, J; Perez, JM. Drug/dye-loaded, multifunctional iron oxide nanoparticles for combined targeted cancer therapy and dual optical/magnetic resonance imaging. Small 2009, 5, 1862–1868. [Google Scholar]
- Hua, MY; Yang, HW; Chuang, CK; Tsai, RY; Chen, WJ; Chuang, KL; Chang, YH; Chuang, HC; Pang, ST. Magnetic-nanoparticle-modified paclitaxel for targeted therapy for prostate cancer. Biomaterials 2010, 31, 7355–7363. [Google Scholar]
- Chorny, M; Fishbein, I; Yellen, BB; Alferiev, IS; Bakay, M; Ganta, S; Adamo, R; Amiji, M; Friedman, G; Levy, RJ. Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields. Proc. Natl. Acad. Sci 2010, 107, 8346–8351. [Google Scholar]
- Hwu, JR; Lin, YS; Josephrajan, T; Hsu, MH; Cheng, FY; Yeh, CS; Su, WC; Shieh, DB. Targeted Paclitaxel by conjugation to iron oxide and gold nanoparticles. J. Am. Chem. Soc 2008, 131, 66–68. [Google Scholar]
- Tapan, KJ; Marco, AM; Sanjeeb, KS; Diandra, LLP; Vinod, L. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol. Pharm 2005, 2, 194–205. [Google Scholar]
- Jain, TK; Richey, J; Strand, M; Leslie-Pelecky, DL; Flask, CA; Labhasetwar, V. Magnetic nanoparticles with dual functional properties: Drug delivery and magnetic resonance imaging. Biomaterials 2008, 29, 4012–4021. [Google Scholar]
- Cho, HS; Dong, Z; Pauletti, GM; Zhang, J; Xu, H; Gu, H; Wang, L; Ewing, RC; Huth, C; Wang, F; Shi, DL. Fluorescent, superparamagnetic nanospheres for drug storage, targeting, and imaging: a multifunctional nanocarrier system for cancer diagnosis and treatment. ACS Nano 2010, 4, 5398–5404. [Google Scholar]
- Johnson, B; Toland, B; Chokshi, R; Mochalin, V; Koutzaki, S; Polyak, B. Magnetically responsive Paclitaxel-loaded biodegradable nanoparticles for treatment of vascular disease: Preparation, characterization and in vitro evaluation of anti-proliferative potential. Curr. Drug Deliv 2010, 7, 263–273. [Google Scholar]
- Henglein, A. Radiolytic preparation of ultrafine colloidal gold particles in aqueous solution: Optical spectrum, controlled growth, and some chemical reactions. Langmuir 1999, 15, 6738–6744. [Google Scholar]
- Oh, KS; Kim, RS; Lee, J; Kim, D; Cho, SH; Yuk, SH. Gold/chitosan/pluronic composite nanoparticles for drug delivery. J. Appl. Polym. Sci 2008, 108, 3239–3244. [Google Scholar]
- Kuo, TR; Hovhannisyan, VA; Chao, YC; Chao, SL; Chiang, SJ; Lin, SJ; Dong, CY; Chen, CC. Multiple release kinetics of targeted drug from gold nanorod embedded polyelectrolyte conjugates induced by near-infrared laser irradiation. J. Am. Chem. Soc 2010, 132, 14163–14171. [Google Scholar]
- You, J; Shao, R; Wei, X; Gupta, S; Li, C. Near-Infrared light triggers release of paclitaxel from biodegradable microspheres: Photothermal effect and enhanced antitumor activity. Small 2010, 6, 1022–1031. [Google Scholar]
- Vivero-Escoto, JL; Slowing, II; Wu, CW; Lin, VSY. Photoinduced intracellular controlled release drug delivery in human cells by gold-capped mesoporous silica nanosphere. J. Am. Chem. Soc 2009, 131, 3462–3463. [Google Scholar]
- Yezhelyev, MV; Gao, X; Xing, Y; Al-Hajj, A; Nie, S; O’Regan, RM. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol 2006, 7, 657–667. [Google Scholar]
- Nyman, DW; Campbell, KJ; Hersh, E; Long, K; Richardson, K; Trieu, V; Desai, N; Hawkins, MJ; Von Hoff, DD. Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J. Clin. Oncol 2005, 23, 7785–7793. [Google Scholar]
- Tekade, RK; Kumar, PV; Jain, NK. Dendrimers in oncology: An expanding horizon. Chem. Rev 2008, 109, 49–87. [Google Scholar]
- Orringer, DA; Koo, YE; Chen, T; Kopelman, R; Sagher, O; Philbert, MA. Small solutions for big problems: the application of nanoparticles to brain tumor diagnosis and therapy. Clin. Pharmacol. Ther 2009, 85, 531–534. [Google Scholar]
- Dong, X; Mattingly, CA; Tseng, MT; Cho, MJ; Liu, Y; Adams, VR; Mumper, RJ. Doxorubicin and paclitaxel-loaded lipid-based nanoparticles overcome multidrug resistance by inhibiting P-glycoprotein and depleting ATP. Cancer Res 2009, 69, 3918–3926. [Google Scholar]
- Luo, J; Xiao, K; Li, Y; Lee, JS; Shi, L; Tan, YH; Xing, L; Holland, CR; Liu, GY; Lam, KS. Well-defined, size-tunable, multifunctional micelles for efficient paclitaxel delivery for cancer treatment. Bioconjugate Chem 2010, 21, 1216–1224. [Google Scholar]
- Zhao, Z; He, M; Yin, L; Bao, J; Shi, L; Wang, B; Tang, C; Yin, C. Biodegradable nanoparticles based on linoleic acid and poly(β-malic acid) double grafted chitosan derivatives as carriers of anticancer drugs. Biomacromolecules 2009, 10, 565–572. [Google Scholar]
- Ganta, S; Amiji, M. Coadministration of paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol. Pharm 2009, 6, 928–939. [Google Scholar]
- Liu, Y; Huang, L; Liu, F. Paclitaxel nanocrystals for overcoming multidrug resistance in cancer. Mol. Pharm 2010, 7, 863–869. [Google Scholar]
- Reddy, LH. Drug delivery to tumours: Recent strategies. J. Pharm. Pharmacol 2005, 57, 1231–1242. [Google Scholar]
- Cho, K; Wang, X; Nie, S; Chen, ZG; Shin, DM. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res 2008, 14, 1310–1316. [Google Scholar]
- Wong, HL; Bendayan, R; Rauth, AM; Xue, HY; Babakhanian, K; Wu, XY. A mechanistic study of enhanced doxorubicin uptake and retention in multidrug resistant breast cancer cells using a polymer-lipid hybrid nanoparticle system. J. Pharmacol. Exp. Ther 2006, 317, 1372–1381. [Google Scholar]
- Zhang, X; Chen, J; Zheng, Y; Gao, X; Kang, Y; Liu, J; Cheng, M; Sun, H; Xu, C. Follicle-stimulating hormone peptide can facilitate paclitaxel nanoparticles to target ovarian carcinoma in vivo. Cancer Res 2009, 69, 6506–6514. [Google Scholar]
- Bilensoy, E; Gürkaynak, O; Dogan, AL; Hincal, AA. Safety and efficacy of amphiphilic-cyclodextrin nanoparticles for paclitaxel delivery. Int. J. Pharm 2008, 347, 163–170. [Google Scholar]
- Wang, Y; Xin, D; Liu, K; Zhu, M; Xiang, J. Heparin-paclitaxel conjugates as drug delivery system: synthesis, self-assembly property, drug release, and antitumor activity. Bioconjugate Chem 2009, 20, 2214–2221. [Google Scholar]
- Pathak, P; Prasad, GL; Meziani, MJ; Joudeh, AA; Sun, YP. Nanosized paclitaxel particles from supercritical carbon dioxide processing and their biological evaluation. Langmuir 2007, 23, 2674–2679. [Google Scholar]
- Peer, D; Karp, JM; Hong, S; Farokhzad, OC; Margalit, R; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol 2007, 2, 751–760. [Google Scholar]








© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, Y.; Zhang, B.; Yan, B. Enabling Anticancer Therapeutics by Nanoparticle Carriers: The Delivery of Paclitaxel. Int. J. Mol. Sci. 2011, 12, 4395-4413. https://doi.org/10.3390/ijms12074395
Liu Y, Zhang B, Yan B. Enabling Anticancer Therapeutics by Nanoparticle Carriers: The Delivery of Paclitaxel. International Journal of Molecular Sciences. 2011; 12(7):4395-4413. https://doi.org/10.3390/ijms12074395
Chicago/Turabian StyleLiu, Yongjin, Bin Zhang, and Bing Yan. 2011. "Enabling Anticancer Therapeutics by Nanoparticle Carriers: The Delivery of Paclitaxel" International Journal of Molecular Sciences 12, no. 7: 4395-4413. https://doi.org/10.3390/ijms12074395
APA StyleLiu, Y., Zhang, B., & Yan, B. (2011). Enabling Anticancer Therapeutics by Nanoparticle Carriers: The Delivery of Paclitaxel. International Journal of Molecular Sciences, 12(7), 4395-4413. https://doi.org/10.3390/ijms12074395



