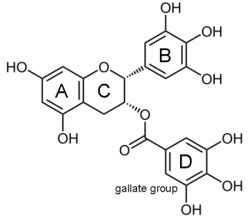
Epigallocatechin-3-gallate (EGCG) for Clinical Trials: More Pitfalls than Promises?
Abstract
:1. Introduction
2. Responders vs. Non-Responders
2.1. Monitoring Left Ventricular Wall Thickness and Function
2.2. Assessment of Plasma Levels of EGCG
3. Bioavailability of EGCG
3.1. Factors Influencing EGCG Bioavailability
3.1.1. EGCG Oxidation Starts Already during Direct Contact with Air
3.1.2. EGCG May Undergo Gastrointestinal Inactivation
3.1.3. EGCG Undergoes Liver Metabolism
3.1.4. EGCG Blood Transport and Stabilization Is Affected by the Level of Serum Albumin
3.2. Optimizing EGCG Bioavailability
3.2.1. Humidity and Temperature
3.2.2. Intake with Empty Stomach
3.2.3. Hard Waters
3.2.4. Vitamin C
3.2.5. Fish Oil
3.2.6. Piperine
4. Side Effects of EGCG
4.1. Anxiolytic Activity
4.2. Hypoglycemic Activity
4.3. Hypochromic Anemia
4.4. Liver and Kidney Failure
5. Conclusions
Acknowledgments
References
- Hunstein, W. Epigallocatechin-3-gallate in AL amyloidosis: A new therapeutic option? Blood 2007, 110, 2216. [Google Scholar]
- Mereles, D; Buss, SJ; Hardt, SE; Hunstein, W; Katus, HA. Effects of the main green tea polyphenol epigallocatechin-3-gallate on cardiac involvement in patients with AL amyloidosis. Clin Res Cardiol 2010, 99, 483–490. [Google Scholar]
- Ehrnhoefer, DE; Bieschke, J; Boeddrich, A; Herbst, M; Masino, L; Lurz, R; Engemann, S; Pastore, A; Wanker, EE. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat Struct Mol Biol 2008, 5, 558–566. [Google Scholar]
- Bieschke, J; Russ, J; Friedrich, RP; Ehrnhoefer, DE; Wobst, H; Neugebauer, K; Wanker, EE. EGCG remodels mature α-synuclein and amyloid-β fibrils and reduces cellular toxicity. Proc Natl Acad Sci USA 2010, 107, 7710–7715. [Google Scholar]
- Hauber, I; Hohenberg, H; Holstermann, B; Hunstein, W; Hauber, J. The main green tea polyphenol epigallocatechin-3-gallate counteracts semen-mediated enhancement of HIV infection. Proc Natl Acad Sci USA 2009, 106, 9033–9038. [Google Scholar]
- Hunstein, W. AL-amyloidosis and epigallocatechin-3-gallate: 4 years and 5 months later. 2011. Available online: http://www.hunstein-egcg.de accessed on 13 July 2011.
- Merlini, G. Personal communication, University of Pavia: Pavia, Italy, 2011.
- PubMed Home Page. Available online: http://www.pubmed.org accessed on 13 July 2011.
- Chen, D; Wan, SB; Yang, H; Yuan, J; Chan, TH; Dou, QP. EGCG, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment. Adv Clin Chem 2011, 53, 55–177. [Google Scholar]
- Saeki, K; Hayakawa, S; Isemura, M; Miyase, T. Importance of a pyrogallol-type structure in catechin compounds for apoptosis-inducing activity. Phytochemistry 2000, 53, 391–394. [Google Scholar]
- Ishii, T; Ichikawa, T; Minoda, K; Kusaka, K; Ito, S; Suzuki, Y; Akagawa, M; Mochizuki, K; Goda, T; Nakayama, T. Human serum albumin as an antioxidant in the oxidation of (−)-epigallocatechin gallate: Participation of reversible covalent binding for interaction and stabilization. Biosci Biotechnol Biochem 2011, 75, 100–106. [Google Scholar]
- Wang, X; Song, KS; Guo, QX; Tian, WX. The galloyl moiety of green tea catechins is the critical structural feature to inhibit fatty-acid synthase. Biochem Pharmacol 2003, 66, 2039–2047. [Google Scholar]
- Byun, EH; Fujimura, Y; Yamada, K; Tachibana, H. TLR4 signaling inhibitory pathway induced by green tea polyphenol epigallocatechin-3-gallate through 67-kDa laminin receptor. J Immunol 2010, 185, 33–45. [Google Scholar]
- Byun, EH; Omura, T; Yamada, K; Tachibana, H. Green tea polyphenol epigallocatechin-3-gallate inhibits TLR2 signaling induced by peptidoglycan through the polyphenol sensing molecule 67-kDa laminin receptor. FEBS Lett 2011, 585, 814–820. [Google Scholar]
- Shammas, MA; Neri, P; Koley, H; Batchu, RB; Bertheau, RC; Munshi, V; Prabhala, R; Fulciniti, M; Tai, YT; Treon, SP; et al. Specific killing of multiple myeloma cells by (−)-epigallocatechin-3-gallate extracted from green tea: Biologic activity and therapeutic implications. Blood 2006, 108, 2804–2810. [Google Scholar]
- Gertz, MA; Comenzo, R; Falk, RH; Fermand, JP; Hazenberg, BP; Hawkins, PN; Merlini, G; Moreau, P; Ronco, P; Sanchorawala, V; et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis. Am J Hematol 2004, 79, 319–328. [Google Scholar]
- Lee, MJ; Maliakal, P; Chen, L; Meng, X; Bondoc, FY; Prabhu, S; Lambert, G; Mohr, S; Yang, CS. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomark Prev 2002, 11, 1025–1032. [Google Scholar]
- Altland, K; Schreiner, R; Hunstein, W. Of Green Tea, Black Pepper, and Amyloidoses. 2010. Available online: http://www.ukgm.de/ugm_2/deu/ugi_hum/EGCG_Piperin_engl.pdf accessed on 13 July 2011.
- Auger, C; Mullen, W; Hara, Y; Crozier, A. Bioavailability of polyphenon E flavan-3-ols in humans with an ileostomy. J Nutr 2008, 138, 1535S–1542S. [Google Scholar]
- Stalmach, A; Troufflard, S; Serafini, M; Crozier, A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol Nutr Food Res 2009, 53, S44–S53. [Google Scholar]
- Roowi, S; Stalmach, A; Mullen, W; Lean, ME; Edwards, CA; Crozier, A. Green tea flavan-3-ols: Colonic degradation and urinary excretion of catabolites by humans. J Agric Food Chem 2010, 58, 1296–1304. [Google Scholar]
- Li, N; Taylor, LS; Mauer, LJ. Degradation kinetics of catechins in green tea powder: Effects of temperature and relative humidity. J Agric Food Chem 2011, 59, 6082–6060. [Google Scholar]
- Lorenz, M; Jochmann, N; von Krosigk, A; Martus, P; Baumann, G; Stangl, K; Stangl, V. Addition of milk prevents vascular protective effects of tea. Eur Heart J 2007, 28, 219–223. [Google Scholar]
- Lu, H; Meng, X; Yang, CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug Metab Dispos 2003, 31, 572–579. [Google Scholar]
- Chow, HH; Cai, Y; Hakim, IA; Crowell, JA; Shahi, F; Brooks, CA; Dorr, RT; Hara, Y; Alberts, DS. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res 2003, 9, 3312–3319. [Google Scholar]
- Peters, CM; Green, RJ; Janle, EM; Ferruzzi, MG. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res Int 2010, 43, 95–102. [Google Scholar]
- Giunta, B; Hou, H; Zhu, Y; Salemi, J; Ruscin, A; Shytle, RD; Tan, J. Fish oil enhances anti-amyloidogenic properties of green tea EGCG in Tg2576 mice. Neurosci Lett 2010, 471, 134–138. [Google Scholar]
- Bose, M; Hao, X; Ju, J; Husain, A; Park, S; Lambert, JD; Yang, CS. Inhibition of tumorigenesis in ApcMin/+ mice by a combination of (−)-epigallocatechin-3-gallate and fish oil. J Agric Food Chem 2007, 55, 7695–7700. [Google Scholar]
- Shirai, N; Suzuki, H. Effects of simultaneous intakes of fish oil and green tea extracts on plasma, glucose, insulin, C-peptide, and adiponectin and on liver lipid concentrations in mice fed low- and high-fat diets. Ann Nutr Metab 2008, 52, 241–249. [Google Scholar]
- Lambert, JD; Hong, J; Kim, DH; Mishin, VM; Yang, CS. Piperine enhances the bioavailability of the tea polyphenol (−)-epigallocatechin-3-gallate in mice. J Nutr 2004, 134, 1948–1952. [Google Scholar]
- Dube, A; Nicolazzo, JA; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate. Eur J Pharm Sci 2010, 41, 219–225. [Google Scholar]
- Vyas, S; Sharma, M; Sharma, PD; Singh, TV. Design, semisynthesis, and evaluation of O-acyl derivatives of (−)-epigallocatechin-3-gallate as antitumor agents. J Agric Food Chem 2007, 55, 6319–6324. [Google Scholar]
- Tanaka, H; Miyoshi, H; Chuang, YC; Ando, Y; Takahashi, T. Solid-phase synthesis of epigallocatechin gallate derivatives. Angew Chem Int Ed Engl 2007, 46, 5934–5937. [Google Scholar]
- Lambert, JD; Kim, DH; Zheng, R; Yang, CS. Transdermal delivery of (−)-epigallocatechin-3-gallate, a green tea polyphenol, in mice. J Pharm Pharmacol 2006, 58, 599–604. [Google Scholar]
- Campbell, EL; Chebib, M; Johnston, GA. The dietary flavonoids apigenin and (−)-epigallocatechin gallate enhance the positive modulation by diazepam of the activation by GABA of recombinant GABA(A) receptors. Biochem Pharmacol 2004, 68, 1631–1638. [Google Scholar]
- Adachi, N; Tomonaga, S; Tachibana, T; Denbow, DM; Furuse, M. (−)-Epigallocatechin gallate attenuates acute stress responses through GABAergic system in the brain. Eur J Pharmacol 2006, 531, 171–175. [Google Scholar]
- Vignes, M; Maurice, T; Lanté, F; Nedjar, M; Thethi, K; Guiramand, J; Récasens, M. Anxiolytic properties of green tea polyphenol (−)-epigallocatechin gallate (EGCG). Brain Res 2006, 1110, 102–115. [Google Scholar]
- Collins, QF; Liu, HY; Pi, J; Liu, Z; Quon, MJ; Cao, W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J Biol Chem 2007, 282, 30143–30149. [Google Scholar]
- Anton, S; Melville, L; Rena, G. Epigallocatechin gallate (EGCG) mimics insulin action on the transcription factor FOXO1a and elicits cellular responses in the presence and absence of insulin. Cell Signal 2007, 19, 378–383. [Google Scholar]
- Li, C; Allen, A; Kwagh, J; Doliba, NM; Qin, W; Najafi, H; Collins, HW; Matschinsky, FM; Stanley, CA; Smith, TJ. Green tea polyphenols modulate insulin secretion by inhibiting glutamate dehydrogenase. J Biol Chem 2006, 281, 10214–10221. [Google Scholar]
- Zijp, IM; Korver, O; Tijburg, LB. Effect of tea and other dietary factors on iron absorption. Crit Rev Food Sci Nutr 2000, 40, 371–398. [Google Scholar]
- Hurrell, RF; Reddy, M; Cook, JD. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br J Nutr 1999, 81, 289–295. [Google Scholar]
- Ryan, P; Hynes, MJ. The kinetics and mechanisms of the complex formation and antioxidant behaviour of the polyphenols EGCg and ECG with iron (III). J Inorg Biochem 2007, 101, 585–593. [Google Scholar]
- Galati, G; Lin, A; Sultan, AM; O’Brien, PJ. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic Biol Med 2006, 40, 570–580. [Google Scholar]
- Lambert, JD; Kennett, MJ; Sang, S; Reuhl, KR; Ju, J; Yang, CS. Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food Chem Toxicol 2010, 48, 409–416. [Google Scholar]
- Bonkovsky, HL. Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis). Ann Intern Med 2006, 144, 68–71. [Google Scholar]
- Lubick, N. Endosulfan’s exit: U.S. EPA pesticide review leads to a ban. Science 2010, 328, 1466. [Google Scholar]
- Silva, MH; Beauvais, SL. Human health risk assessment of endosulfan. I: Toxicology and hazard identification. Regul Toxicol Pharmacol 2010, 56, 4–17. [Google Scholar]
- Rahimtoola, SH. Digitalis and William Withering, the clinical investigator. Circulation 1975, 52, 969–971. [Google Scholar]



© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for Clinical Trials: More Pitfalls than Promises? Int. J. Mol. Sci. 2011, 12, 5592-5603. https://doi.org/10.3390/ijms12095592
Mereles D, Hunstein W. Epigallocatechin-3-gallate (EGCG) for Clinical Trials: More Pitfalls than Promises? International Journal of Molecular Sciences. 2011; 12(9):5592-5603. https://doi.org/10.3390/ijms12095592
Chicago/Turabian StyleMereles, Derliz, and Werner Hunstein. 2011. "Epigallocatechin-3-gallate (EGCG) for Clinical Trials: More Pitfalls than Promises?" International Journal of Molecular Sciences 12, no. 9: 5592-5603. https://doi.org/10.3390/ijms12095592
APA StyleMereles, D., & Hunstein, W. (2011). Epigallocatechin-3-gallate (EGCG) for Clinical Trials: More Pitfalls than Promises? International Journal of Molecular Sciences, 12(9), 5592-5603. https://doi.org/10.3390/ijms12095592