Astragalin from Cassia alata Induces DNA Adducts in Vitro and Repairable DNA Damage in the Yeast Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Activity of CaRP
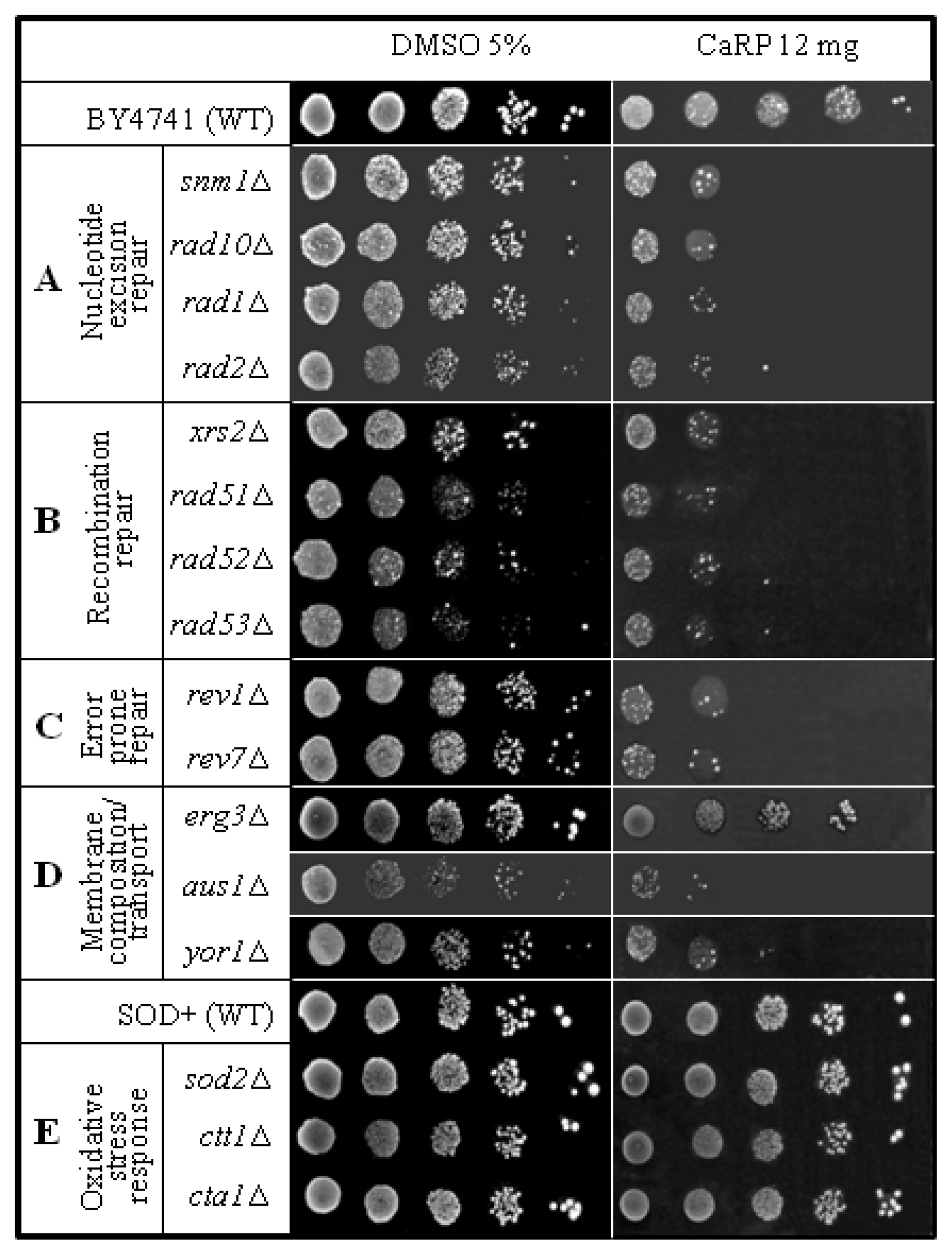
2.2. Yeast Sensitivity Assay
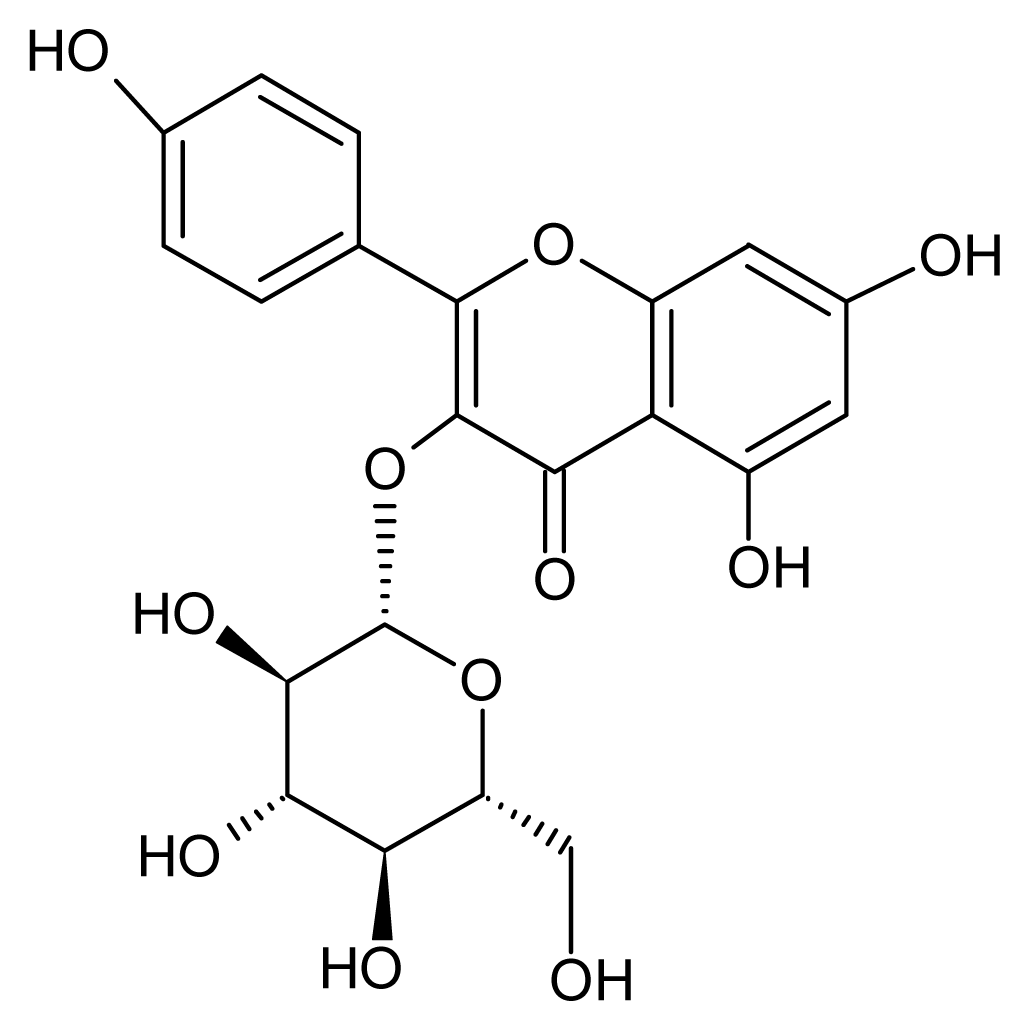
2.3. Bioactive DNA-Binding Compounds from CaRP
2.4. Structural Identification
2.5. FTIR Spectra of AST-DNA Complex
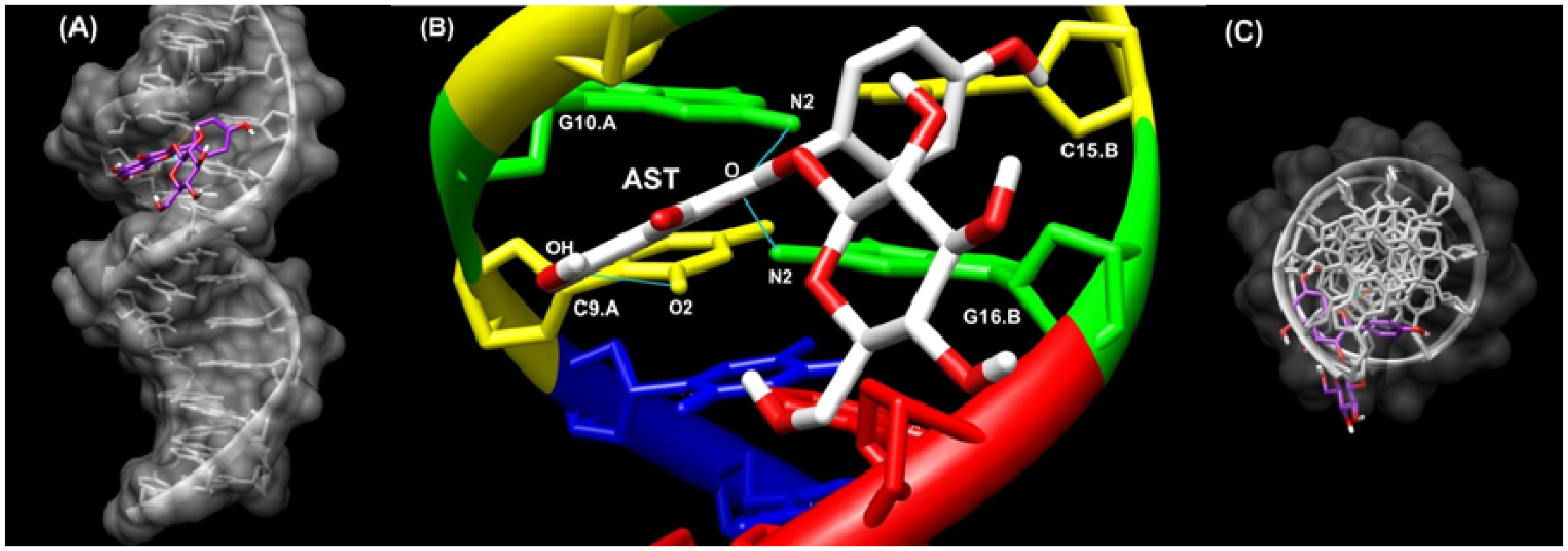
2.6. Docking Study
3. Experimental Section
3.1. Extraction Protocol
3.2. DPPH Assay
3.3. Strains and Media
3.4. Sensitivity Assay in Haploid Yeast to CaRP
3.5. HPLC Analyses
3.5.1. LC-UV DNA Binding of CaRP
3.5.2. LC-UV Microfractionation and Identification of the Fractions
3.6. FTIR Analysis
3.7. Docking Study
4. Conclusions
Acknowledgments
Abbreviations
| CaRP | Reverse phase-solid phase extract from Cassia alata leaves |
| AST | astragalin |
| MRSA | Methicillin-resistant Staphylococcus aureus |
References
- Hennebelle, T.; Weniger, B.; Joseph, H.; Sahpaz, S.; Bailleul, F. Senna alata. Fitoterapia 2009, 80, 385–393. [Google Scholar]
- Hazni, H.; Ahmad, N.; Hitotsuyanagi, Y.; Takeya, K.; Choo, C.Y. Phytochemical constituents from Cassia alata with inhibition against methicillin-resistant Staphylococcus aureus (MRSA). Planta Med 2008, 74, 1802–1805. [Google Scholar]
- Lee, H.-B.; Kim, E.-K.; Park, S.J.; Bang, S.-G.; Kim, T.G.; Chung, D.-W. Isolation and anti-inflammatory effect of astragalin synthesized by enzymatic hydrolysis of tea seed extract. J. Sci. Food Agric 2011, 91, 2315–2321. [Google Scholar]
- Wei, Y.; Xie, Q.; Fisher, D.; Sutherland, I.A. Separation of patuletin-3-O-glucoside, astrgalin, quercetin, kaempferol and isorhamnetin from Flaveria bidentis (L.) Kuntze by elution-pump-out high-performance counter-current chromatography. J. Chromatogr. A 2011, 1218, 6206–6211. [Google Scholar]
- Huang, B.; Ban, X.; Jingsheng, H.; Tong, J.; Tian, J.; Wang, Y. Hepatoprotective and antioxidant activity of ethanolic extract of edible lotus (Nelumbo nucifera Gaertn.) leaves. Food Chem 2010, 120, 873–878. [Google Scholar]
- Yang, L.; Chen, Q.; Wang, F.; Zhang, G. Antiosteoporotic compounds from seeds of Cuscuta chinensis. J. Ethnolpharmacol 2011, 135, 553–560. [Google Scholar]
- Kotani, M.; Matsumoto, M.; Fujita, A.; Higa, S.; Wang, W.; Suemura, M.; Kishimoto, T.; Tanaka, T. Persimmon leaf extract and astragalin inhibit development of dermatitis and IgE elevation in NC/Nga mice. J. Allergy Clin. Immunol 2000, 106, 159–166. [Google Scholar]
- Anuradha, R.; Krishnamoorthy, P. Effect of astragalin on the activities of membrane bound enzymes on leadacetate toxicity in albino rats. J. Chem. Pharm. Res 2011, 3, 616–620. [Google Scholar]
- Deng, S.-G.; Deng, Z.-Y.; Fan, Y.-W.; Shan, B.; Xiong, D.M. Spectroscopic investigation on the interaction between astragalin from Lotus leaf and DNA. Spectrosc. Spect. Anal 2010, 30, 476–480. [Google Scholar]
- Akinmoladun, A.C.; Obuotor, E.M.; Farombi, E.O. Evaluation of antioxidant and free radical scavenging capacities of some Nigerian indigenous medicinal plants. J. Med. Food 2010, 13, 444–451. [Google Scholar]
- Chomnawang, M.T.; Surassmo, S.; Nukoolkarn, V.S.; Gritsanapan, W. Effect of Garcinia mangostana on inflammation caused by Propionibacterium acnes. Fitoterapia 2007, 78, 401–408. [Google Scholar]
- SGD—Saccharomyces Genome Database. Available online: www.yeastgenome.org accessed on 20 February 2012.
- Pungartnik, C.; Picada, J.; Brendel, M.; Henriques, J.A.P. Further phenotypic characterization of pso mutants of Saccharomyces cerevisiae with respect to DNA repair and response to oxidative stress. Gen. Mol. Res 2002, 1, 79–89. [Google Scholar]
- Friedberg, E.C. Deoxyribonucleic acid repair in the yeast Saccharomyces cerevisiae. Microbiol Rev 1988, 52, 70–102. [Google Scholar]
- Game, J.C. DNA double-strand breaks and the RAD50-RAD57 genes in Saccharomyces. Semin. Cancer Biol 1993, 4, 73–83. [Google Scholar]
- Kunz, B.A.; Haynes, R.H. Phenomenology and genetic control of mitotic recombination in yeast. Annu. Rev. Genet 1981, 15, 57–89. [Google Scholar]
- Noll, D.M.; Mason, T.M.; Miller, P.S. Formation and repair of interstrand cross-links in DNA. Chem. Rev 2006, 106, 277–301. [Google Scholar]
- Schmidt, C.L.; Grey, M.; Schmidt, M.; Brendel, M.; Henriques, J.A. Allelism of Saccharomyces cerevisiae genes PSO6, involved in survival after 3-CPs+UVA induced damage, and ERG3, encoding the enzyme sterol C-5 desaturase. Yeast 1999, 15, 1503–1510. [Google Scholar]
- Zhou, J.L.; Qian, Z.M.; Luo, Y.D.; Tang, D.; Chen, H.; Yi, L.; Li, P. Screening and mechanism study of components targeting DNA from the Chinese herb Lonicera japonica by liquid chromatography/mass spectrometry and fluorescence spectroscopy. Biomed. Chromatogr 2008, 22, 1164–1172. [Google Scholar]
- Saito, S.T.; Trentin, D.S.; Macedo, A.J.; Pungartnik, C.; Gosmann, G.; Silveira, J.D.; Guecheva, T.N.; Henriques, J.A.P.; Brendel, M. Bio-guided fractionation shows Cassia alata extract to inhibit Staphylococcus epidermidis and Pseudomonas aeruginosa growth and biofilm formation. Evid. Based Complement. Alternat. Med. 2012, in press. [Google Scholar]
- Liu, A.; Xu, L.; Zou, Z.; Yang, S. Studies on chemical constituents from leaves of Cassia alata. Zhongguo Zhong Yao Za Zhi 2009, 34, 861–863. [Google Scholar]
- Usha, S.; Johnson, I.M.; Malathi, R. Interaction of resveratrol and genistein with nucleic acids. J. Biochem. Mol. Biol 2005, 38, 198–205. [Google Scholar]
- Alex, S.; Dupuis, P. FT-IR and Raman investigation of cadmium binding by DNA. Inorg. Chim. Acta 1989, 157, 271–281. [Google Scholar]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Infrared Spectroscopy. In Introduction to Spectroscopy: A Guide for Students of Organic Chemistry; Brooks/Cole: Belmont, CA, USA, 2001; Volume Chapter 2, pp. 14–101. [Google Scholar]
- Kumar, C.V.; Asuncion, E.H. DNA binding studies and site selective fluorescence sensitization of an anthryl probe. J. Am. Chem. Soc 1993, 115, 8547–8553. [Google Scholar]
- Singer, B.; Grunberger, D. Molecular Biology of Mutagens and Carcinogens; Plenun Press: New York, NY, USA, 1983. [Google Scholar]
- TOXNET database. Available online: http://toxnet.nlm.nih.gov/cgi-bin/sis/search accessed on 20 February 2012.
- Macindoe, G.; Mavridis, L.; Venkatraman, V.; Devignes, M.-D.; Ritchie, D.W. HexServer: Na FFT-based protein docking Server powered by graphics processors. Nucleic Acids Res 2010, 38, 445–449. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol 1995, 28, 25–30. [Google Scholar]
- Ruhland, A.; Haase, E.; Siede, W.; Brendel, M. Isolation of yeast mutants sensitive to the bifunctional alkylating agent nitrogen mustard. Mol. Gen. Genet 1981, 181, 346–351. [Google Scholar]
- Queiroz, E.F.; Wolfender, J.L.; Atindehou, K.K.; Traore, D.; Hostettmann, K. On-line identification of the antifungal constituintes of Erythrina vogelii by liquid chromatography with tandem mass spectrometry, ultraviolet absorbance detection and nuclear magnetic resonance spectrometry combined with liquid chromatographic micro-fractionation. J. Chromatogr. A 2002, 974, 123–134. [Google Scholar]
- Larsen, T.A.; Goodsell, D.S.; Cascio, D.; Grzeskowiak, K.; Dickerson, R.E. The structure of DAPI bound to DNA. J. Biomol. Struct. Dyn 1989, 7, 477–491. [Google Scholar]
- Couch, G.S.; Hendrix, D.K.; Ferrin, T.E. Nucleic acid visualization with UCSF Chimera. Nucleic. Acids Res 2006, 34, 2–5. [Google Scholar]




| Compound | IC50 (μg.mL−1) | AEAC (g) | TEAC (g) | linear range (μg.mL−1) | slope | intercept | Correlation coefficient (r) |
|---|---|---|---|---|---|---|---|
| ascorbic acid | 3.99 ± 0.09 | 1.0000 | 0.8867 | 2.0–4.7 | −12.131 | 98.414 | 0.9975 |
| Trolox | 4.50 ± 0.08 | 1.1278 | 1.0000 | 2.7–5.3 | −12.275 | 105.230 | 0.9965 |
| CaRP | 2.25 ± 0.28 | 0.5639 | 0.5000 | 2.0–4.7 | −14.733 | 83.457 | 0.9989 |
| Free AST, Free DNA and complexes | Observed changes in the vibrational frequency of the functional groups and bases (cm−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| OH | C=O | NH | PO− 2 | Gua | Thy | Ade | Cyt | |
| Free AST | 3465–3426 | 1653 | - | - | - | - | - | - |
| Free DNA | 3469–3430 | 1707, 1649 | 3469–3430 | 1237, 1098 | 1707 | 1649 | 1602 | 1484 |
| DNA-AST | 3469–3435 | 1714, 1647 | 3469–3435 | 1236, 1094 | 1714 | 1647 | 1604 | 1488 |
| strain | genotype | Protein lacking | source |
|---|---|---|---|
| BY4741 | Matα his3Δ1 leu2Δ0 lys2Δ0 ura 3Δ0 | none | EUROSCARF |
| snm1Δ | Like BY4741 except snm1Δ::KanMx4 | 5′-3′ exonuclease activity | EUROSCARF |
| rad10Δ | Like BY4741 except rad10Δ::KanMx4 | Single-stranded DNA endonuclease (with Rad1p) | EUROSCARF |
| rad1Δ | Like BY4741 except rad1Δ::KanMx4 | Single-stranded DNA endonuclease (with Rad10p) | EUROSCARF |
| rad2Δ | Like BY4741 except rad2Δ::KanMx4 | Single-stranded DNA endonuclease | EUROSCARF |
| xrs2Δ | Like BY4741 except xrs2Δ::KanMx4 | Protein required for DNA repair; Mre11 complex component | EUROSCARF |
| rad5Δ | Like BY4741 except rad5Δ::KanMx4 | DNA helicase proposed to promote replication fork regression during postreplication repair | EUROSCARF |
| rad52Δ | Like BY4741 except rad52Δ::KanMx4 | Protein that stimulates strand exchange by facilitating Rad51p binding to single-stranded DNA | EUROSCARF |
| rad53Δ | Like BY4741 except rad53Δ::KanMx4 | Protein kinase, required for cell-cycle arrest in response to DNA damage | EUROSCARF |
| rev1Δ | Like BY4741 except rev1Δ::KanMx4 | Deoxycytidyl transferase; involved in repair of abasic sites and adducted guanines in damaged DNA by translesion synthesis (TLS) | EUROSCARF |
| rev7Δ | Like BY4741 except rev7Δ::KanMx4 | Accessory subunit of DNA polymerase zeta, involved in translesion synthesis during post-replication repair | EUROSCARF |
| erg3Δ | Like BY4741 except erg3Δ::KanMx4 | C-5 sterol desaturase, catalyzes the introduction of a C-5(6) double bond into episterol, a precursor in ergosterol biosynthesis | EUROSCARF |
| aus1Δ | Like BY4741 except aus1Δ::KanMx4 | Plasma membrane sterol transporter of the ATP-binding cassette family | EUROSCARF |
| yor1Δ | Like BY4741 except yor1Δ::KanMx4 | Plasma membrane ATP-binding cassette (ABC) transporter, multidrug transporter mediates export of many different organic anions | EUROSCARF |
| SOD+ | Matα his3Δ1 leu2Δ0 trp1-289 ura3-52 | None | E. B. Gralla, Los Angeles |
| sod2Δ | Like SOD + except sod2::TRP1 | Mn superoxide dismutase | E. B. Gralla, Los Angeles |
| ctt1Δ | Like SOD + except ctt1Δ::TRP1 | cytosolic catalase T | E. B. Gralla, Los Angeles |
| cta1Δ | Like SOD + except cta1Δ::TRP1 | catalase A present in peroxissomal matrix | E. B. Gralla, Los Angeles |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saito, S.; Silva, G.; Santos, R.X.; Gosmann, G.; Pungartnik, C.; Brendel, M. Astragalin from Cassia alata Induces DNA Adducts in Vitro and Repairable DNA Damage in the Yeast Saccharomyces cerevisiae. Int. J. Mol. Sci. 2012, 13, 2846-2862. https://doi.org/10.3390/ijms13032846
Saito S, Silva G, Santos RX, Gosmann G, Pungartnik C, Brendel M. Astragalin from Cassia alata Induces DNA Adducts in Vitro and Repairable DNA Damage in the Yeast Saccharomyces cerevisiae. International Journal of Molecular Sciences. 2012; 13(3):2846-2862. https://doi.org/10.3390/ijms13032846
Chicago/Turabian StyleSaito, Samuel, Givaldo Silva, Regineide Xavier Santos, Grace Gosmann, Cristina Pungartnik, and Martin Brendel. 2012. "Astragalin from Cassia alata Induces DNA Adducts in Vitro and Repairable DNA Damage in the Yeast Saccharomyces cerevisiae" International Journal of Molecular Sciences 13, no. 3: 2846-2862. https://doi.org/10.3390/ijms13032846




