Enhancement of Mechanical and Thermal Properties of Polycaprolactone/Chitosan Blend by Calcium Carbonate Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results of Nanoparticle Characterization
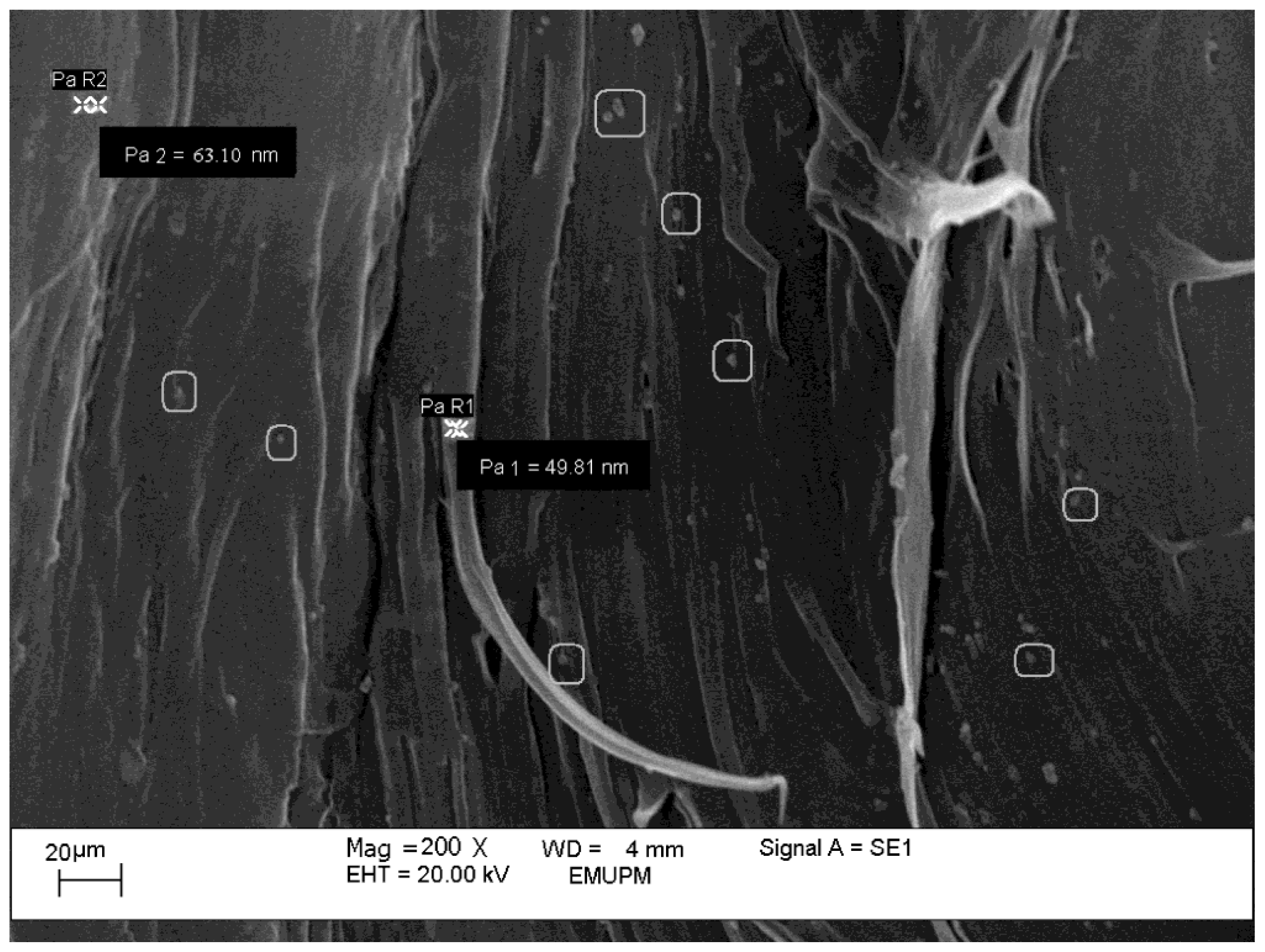
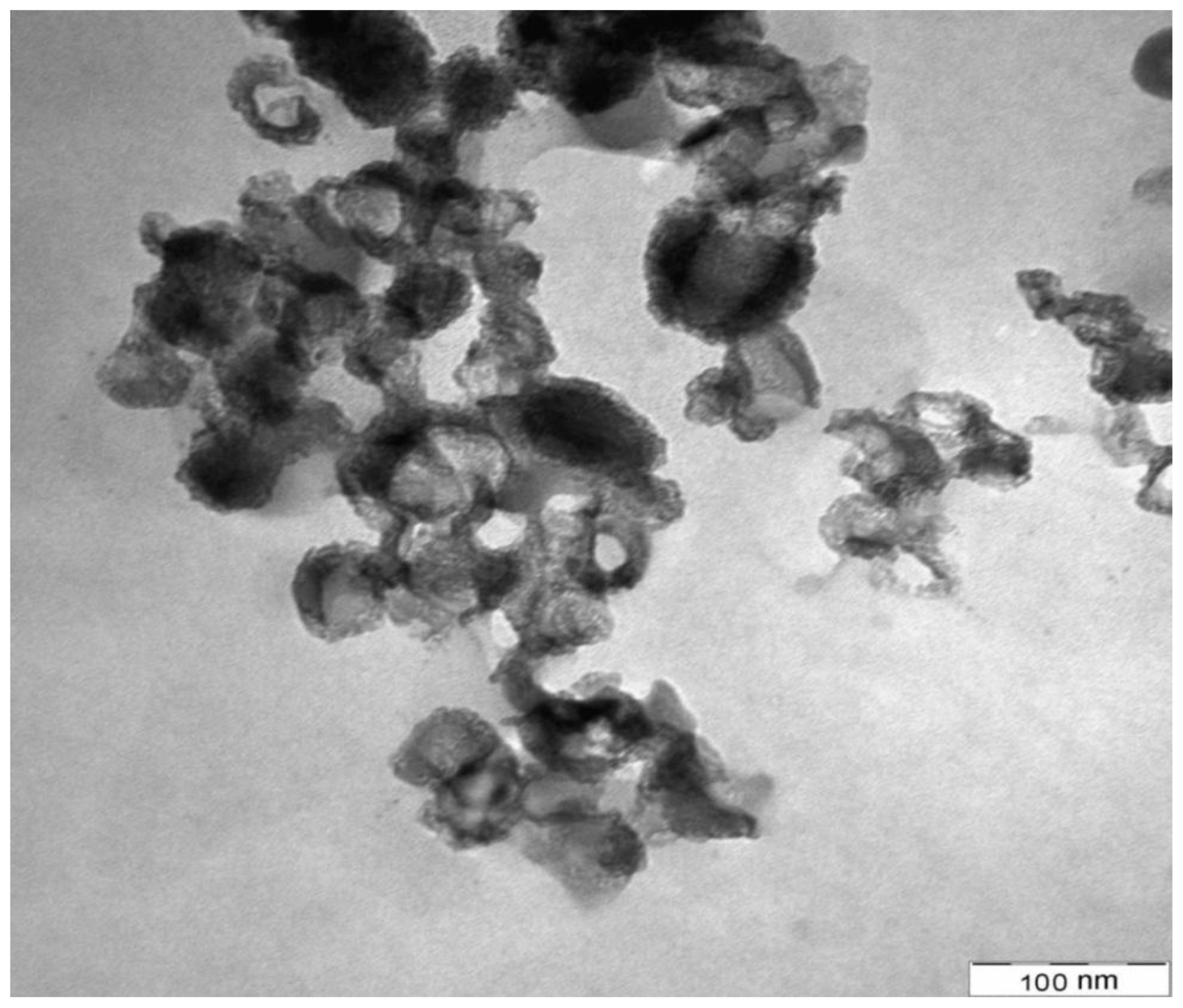
2.1.1. Electron Microscopic Analysis
2.1.2. Fourier Transform Infrared Characterization (FTIR)
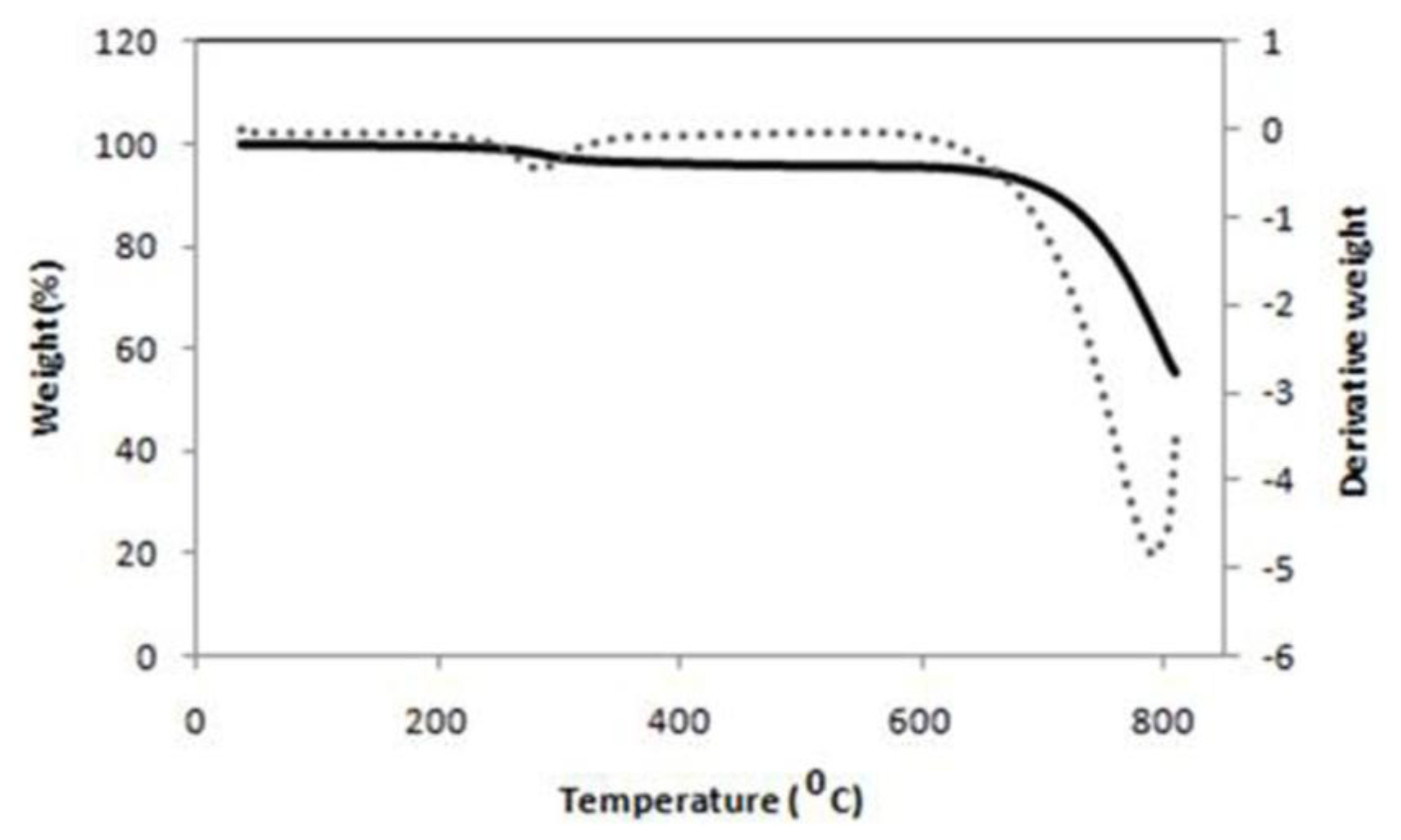
2.1.3. Thermogravimetric Analysis
2.2. Results of Nanocomposites Characterization
2.2.1. Mechanical Properties of Nanocomposites
2.2.2. Thermal Properties of Nanocomposites
2.2.3. Morphology Observation
3. Experimental Section
3.1. Materials
3.2. Preparation of Nanocomposites
3.3. Characterizations
4. Conclusions
Acknowledgments
References
- Fu, X.; Qutubuddin, S. Polymer-clay nanocomposites: Exfoliation of organophilic montmorillonite nanolayers in polystyrene. Polymer 2001, 42, 807–813. [Google Scholar]
- Zhang, W.; Chen, D.; Zhao, Q.; Fang, Y. Different vinyl acetate content on the morphology and properties of eva/clay nanocomposites. Polymer 2003, 44, 7953–7961. [Google Scholar]
- Ma, C.G.; Mai, Y.L.; Rong, M.Z.; Ruan, W.H.; Zhang, M.Q. Phase structure and mechanical properties of ternary polypropylene/elastomer/nano-CaCO3 composites. Compos. Sci. Technol 2007, 67, 2997–3005. [Google Scholar]
- Gorna, K.; Hund, M.; Vucak, M.; Gröhn, F.; Wegner, G. Amorphous calcium carbonate in form of spherical nanosized particles and its application as fillers for polymers. Mater. Sci. Eng. A 2008, 477, 217–225. [Google Scholar]
- Liang, J.Z. Evaluation of dispersion of nano-CaCO3 particles in polypropylene matrix based on fractal method. Compos. Part A 2007, 38, 1502–1506. [Google Scholar]
- Xie, X.L.; Liu, Q.X.; Li, R.K.Y.; Zhou, X.P.; Zhang, Q.X.; Yu, Z.Z.; Mai, Y.W. Rheological and mechanical properties of PVC/CaCO3 nanocomposites prepared by in situ polymerization. Polymer 2004, 45, 6665–6673. [Google Scholar]
- Di Lorenzo, M.L.; Errico, M.E.; Avella, M. Thermal and morphological characterization of poly (ethylene terephthalate)/calcium carbonate nanocomposites. J. Mater. Sci 2002, 37, 2351–2358. [Google Scholar]
- Gilbert, M. Surface Treatments for Particulate Fillers in Plastics. In Plastics Additives: An A–Z Reference; Pritchard, G., Ed.; Chapman and Hall: London, UK, 1998; pp. 590–603. [Google Scholar]
- Bartczak, Z.; Argon, A.S.; Cohen, R.E.; Weinberg, M. Toughness mechanism in semicrystalline polymer blends: II. High-density polyethylene toughened with calcium carbonate filler particles. Polymer 1999, 40, 2347–2365. [Google Scholar]
- Zuiderduin, W.C.J.; Westzaan, C.; Huétink, J.; Gaymans, R.J. Toughening of polypropylene with calcium carbonate particles. Polymer 2003, 44, 261–275. [Google Scholar]
- Jiang, L.; Zhang, J.; Wolcott, M.P. Comparison of polylactide/nano-sized calcium carbonateand polylactide/montmorillonite composites: Reinforcing effects and toughening mechanisms. Polymer 2007, 48, 7632–7644. [Google Scholar]
- Jiang, L.; Lam, Y.C.; Tam, K.C.; Chua, T.H.; Sim, G.W.; Ang, L.S. Strengthening acrylonitrile-butadiene-styrene (ABS) with nano-sized and micron-sized calcium carbonate. Polymer 2005, 46, 243–252. [Google Scholar]
- Okada, M. Chemical syntheses of biodegradable polymers. Prog. Polym. Sci 2002, 27, 87–133. [Google Scholar]
- Cao, A.; Okamura, T.; Ishiguro, C.; Nakayama, K.; Inoue, Y.; Masuda, T. Studies on synthesis and physical characterization of biodegradable aliphatic poly (butylene succinate-co-ɛ-caprolactone)s. Polymer 2002, 43, 671–679. [Google Scholar]
- Wu, C.S. Physical properties and biodegradability of maleated-polycaprolactone/starch composite. Polym. Degrad. Stab 2003, 80, 127–134. [Google Scholar]
- Wu, C.S. A comparison of the structure, thermal properties, and biodegradability of polycaprolactone/chitosan and acrylic acid grafted polycaprolactone/chitosan. Polymer 2005, 46, 147–155. [Google Scholar]
- Averous, L.; Moro, L.; Dole, P.; Fringant, C. Properties of thermoplastic blends: starch-polycaprolactone. Polymer 2000, 41, 4157–4167. [Google Scholar]
- Ikejima, T.; Inoue, Y. Crystallization behavior and environmental biodegradability of the blend films of poly (3-hydroxybutyric acid) with chitin and chitosan. Carbohydr. Polym 2000, 41, 351–356. [Google Scholar]
- Then, Y.Y.; Ibrahim, N.A.; Wan Yunus, W.M.Z. Enhancement of tensile strength and flexibility of polycaprolactone/tapioca starch blends by octadecylamine modified clay. J. Polym. Environ 2011, 19, 535–539. [Google Scholar]
- Wan, Y.; Lu, X.; Dalai, S.; Zhang, J. Thermophysical properties of polycaprolactone/chitosan blend membranes. Thermochim. Acta 2009, 487, 33–38. [Google Scholar]
- Calil, M.R.; Gaboardi, F.; Guedes, C.G.F.; Rosa, D.S. Comparison of the biodegradation of poly (ɛ-caprolactone), cellulose acetate and their blends by the Sturm test and selected cultured fungi. Polym. Test 2006, 25, 597–604. [Google Scholar]
- Rao, S.B.; Sharma, C.P. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J. Biomed. Mater. Res 1997, 34, 21–28. [Google Scholar]
- Ray, S.S.; Bousmina, M. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog. Polym. Sci 2005, 50, 962–1079. [Google Scholar]
- Wang, S.F.; Shen, L.; Tong, Y.J.; Chen, L.; Phang, I.Y.; Lim, P.Q.; Liu, T.X. Biopolymer chitosan/montmorillonite nanocomposites: Preparation and characterization. Polym. Degrad. Stab 2005, 90, 123–131. [Google Scholar]
- Casariego, A.; Souza, B.W.S.; Cerqueira, M.A.; Teixeira, J.A.; Cruz, L.; Díaz, R.; Vicente, A.A. Chitosan/clay films’ properties as affected by biopolymer and clay micro/nanoparticles’ concentrations. Food Hydrocolloids 2009, 23, 1895–1902. [Google Scholar]
- Sarasam, A.; Madihally, S.V. Characterization of chitosan-polycaprolactone blends for tissue engineering applications. Biomaterials 2005, 26, 5500–5508. [Google Scholar]
- Ahmad, M.B.; Tay, M.Y.; Shameli, K.; Hussein, M.Z.; Lim, J.J. Green synthesis and characterization of silver/chitosan/polyethylene glycol nanocomposites without any reducing agent. Int. J. Mol. Sci 2011, 12, 4872–4884. [Google Scholar]
- Abdolmohammadi, S.; Wan Yunus, W.M.Z.; Rahman, M.Z.; Ibrahim, N.A. Effect of organoclay on mechanical and thermal properties of polycaprolactone/chitosan/montmorillonite nanocomposites. J. Reinf. Plast. Compos 2011, 30, 1045–1054. [Google Scholar]
- Liu, L.; Li, Y.; Liu, H.; Fang, Y. Synthesis and characterization of chitosan-graftpolycaprolactone copolymers. Eur. Polym. J 2004, 40, 2739–2744. [Google Scholar]
- Shan, D.; Wang, S.; Xue, H.; Cosnier, S. Direct electrochemistry and electrocatalysis of hemoglobin entrapped in composite matrix based on chitosan and CaCO3 nanoparticles. Electrochem. Commun 2007, 9, 529–534. [Google Scholar]
- Gao, Y.; Liu, L.; Zhang, Z. Mechanical performance of nano-CaCO3 filled polystyrene composites. Acta Mech. Solida Sin 2009, 22, 555–562. [Google Scholar]
- Chan, C.M.; Wu, J.; Li, J.X.; Cheung, Y.K. Polypropylene/calcium carbonate nanocomposites. Polymer 2002, 43, 2981–2992. [Google Scholar]
- Chen, N.; Wan, C.; Zhang, Y.; Zhang, Y. Effect of nano-CaCO3 on mechanical properties of PVC and PVC Blendex blend. Polym. Test 2004, 23, 169–174. [Google Scholar]
- Reynaud, E.; Jouen, T.; Gauthier, C.; Vigier, G.; Varlett, J. Nanofillers in polymeric matrix: A study on silica reinforced PA. Polymer 2001, 42, 8759–8768. [Google Scholar]
- Zhang, Q.X.; Yu, Z.Z.; Xie, X.L.; Mai, Y.W. Crystallization and impact energy of polypropylene/CaCO3 nanocomposites with nonionic modifier. Polymer 2004, 45, 5985–5994. [Google Scholar]
- Baskaran, R.; Sarojadevi, M.; Vijayakumar, C.T. Mechanical and thermal properties of unsaturated polyester/calcium carbonate nanocomposites. J. Reinf. Plast. Compos 2011, 30, 1549–1556. [Google Scholar]
- Shimpi, N.G.; Verma, J.; Mishra, S. Dispersion of nano CaCO3 on PVC and its influence on mechanical and thermal properties. J. Compos. Mater 2010, 44, 211–219. [Google Scholar]
- Fuad, M.Y.A.; Hanim, H.; Zarina, R.; Ishak, Z.A.M.; Hassan, A. Polypropylene/calcium carbonate nanocomposites—Effects of processing techniques and maleated polypropylene compatibiliser. eXPRESS Polym. Lett 2010, 4, 611–620. [Google Scholar]
- Yang, K.; Yang, Q.; Li, G.; Sun, Y.; Feng, D. Morphology and mechanical properties of polypropylene/calcium carbonate nanocomposites. Mater. Lett 2006, 60, 805–809. [Google Scholar]







| Composite | Tensile Strength (MPa) | Elongation at Break (%) | Modulus (MPa) |
|---|---|---|---|
| PCL/chitosan | 15.05 ± 0.84 | 848.00 ± 6.87 | 160.26 ± 4.15 |
| PCL/chitosan/0.5 wt% nano CaCO3 | 17.31 ± 0.92 | 915.00 ± 7.84 | 190.58 ± 3.20 |
| PCL/chitosan/1 wt% nano CaCO3 | 20.18 ± 0.96 | 1215.00 ± 8.35 | 214.91 ± 3.53 |
| PCL/chitosan/3 wt% nano CaCO3 | 14.65 ± 0.73 | 751.00 ± 6.54 | 231.80 ± 3.89 |
| PCL/chitosan/5 wt% nano CaCO3 | 12.16 ± 0.67 | 460.00 ± 5.31 | 247.21 ± 5.36 |
| PCL/chitosan/7 wt% nano CaCO3 | 9.18 ± 0.54 | 124.00 ± 4.57 | 278.60 ± 4.53 |
| PCL/Chitosan | 100/0 | 90/10 | 80/20 | 70/30 | 60/40 | 50/50 |
|---|---|---|---|---|---|---|
| Tensile Strength | 20.59 | 15.05 | 13.23 | 10.80 | 8.50 | 4.89 |
| Elongation at break (%) | 1300.00 | 848.60 | 505.47 | 320.10 | 208.12 | 87.34 |
| Tensile Modulus | 130.26 | 160.26 | 168.50 | 178.32 | 186.64 | 195.10 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abdolmohammadi, S.; Siyamak, S.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Rahman, M.Z.A.; Azizi, S.; Fatehi, A. Enhancement of Mechanical and Thermal Properties of Polycaprolactone/Chitosan Blend by Calcium Carbonate Nanoparticles. Int. J. Mol. Sci. 2012, 13, 4508-4522. https://doi.org/10.3390/ijms13044508
Abdolmohammadi S, Siyamak S, Ibrahim NA, Yunus WMZW, Rahman MZA, Azizi S, Fatehi A. Enhancement of Mechanical and Thermal Properties of Polycaprolactone/Chitosan Blend by Calcium Carbonate Nanoparticles. International Journal of Molecular Sciences. 2012; 13(4):4508-4522. https://doi.org/10.3390/ijms13044508
Chicago/Turabian StyleAbdolmohammadi, Sanaz, Samira Siyamak, Nor Azowa Ibrahim, Wan Md Zin Wan Yunus, Mohamad Zaki Ab Rahman, Susan Azizi, and Asma Fatehi. 2012. "Enhancement of Mechanical and Thermal Properties of Polycaprolactone/Chitosan Blend by Calcium Carbonate Nanoparticles" International Journal of Molecular Sciences 13, no. 4: 4508-4522. https://doi.org/10.3390/ijms13044508
APA StyleAbdolmohammadi, S., Siyamak, S., Ibrahim, N. A., Yunus, W. M. Z. W., Rahman, M. Z. A., Azizi, S., & Fatehi, A. (2012). Enhancement of Mechanical and Thermal Properties of Polycaprolactone/Chitosan Blend by Calcium Carbonate Nanoparticles. International Journal of Molecular Sciences, 13(4), 4508-4522. https://doi.org/10.3390/ijms13044508




