Scutellaria barbata D. Don Inhibits Tumor Angiogenesis via Suppression of Hedgehog Pathway in a Mouse Model of Colorectal Cancer
Abstract
:1. Introduction
2. Results and Discussion
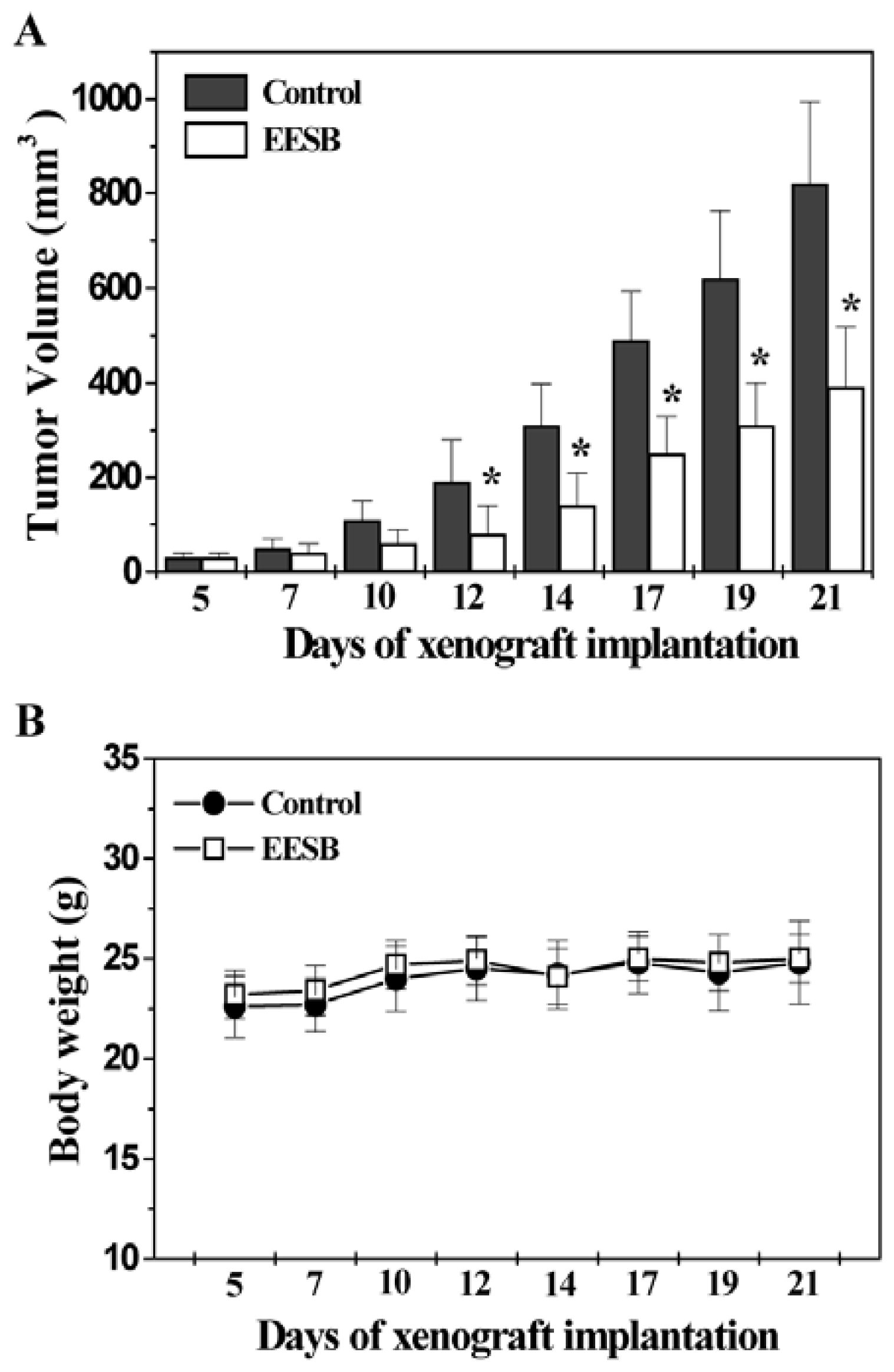
2.1. EESB Inhibits Tumor Growth in CRC Xenograft Mice
2.2. EESB Inhibits Tumor Angiogenesis in CRC Xenograft Mice
2.3. EESB Suppresses SHH Signaling Pathway in CRC Xenograft Mice
2.4. EESB Inhibits the Expression of VEGF-A and VEGFR2 in CRC Xenograft Mice
3. Experimental Section
3.1. Materials and Reagents
3.2. Preparation of Ethanol Extract from Scutellaria barbata D. Don
3.3. HPLC Analysis
3.4. Cell Culture
3.5. Animals
3.6. In vivo Nude Mouse Xenograft Formation
3.7. Immunohistochemistry Analysis
3.8. RNA Extraction and RT-PCR Analysis
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no financial or commercial conflict of interest.
References
- Folkman, J. Angiogenesis. Annu. Rev. Med 2006, 57, 1–18. [Google Scholar]
- Cook, K.M.; Figg, W.D. Angiogenesis inhibitors: Current strategies and future prospects. CA Cancer J. Clin 2010, 60, 222–243. [Google Scholar]
- Ingham, P.W.; Nakano, Y.; Seger, C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat. Rev. Genet 2011, 12, 393–406. [Google Scholar]
- Ruiz i Altaba, A. Gli proteins encode context-dependent positive and negative functions: Implications for development and disease. Development 1999, 126, 3205–3216. [Google Scholar]
- Theunissen, J.W.; de Sauvage, F.J. Paracrine Hedgehog signaling in cancer. Cancer Res 2009, 69, 6007–6010. [Google Scholar]
- Mazumdar, T.; DeVecchio, J.; Shi, T.; Jones, J.; Agyeman, A.; Houghton, J.A. Hedgehog signaling drives cellular survival in human colon carcinoma cells. Cancer Res 2011, 71, 1092–1102. [Google Scholar]
- Lum, L.; Beachy, P.A. The Hedgehog response network: Sensors, switches, and routers. Science 2004, 304, 1755–1759. [Google Scholar]
- Varjosalo, M.; Taipale, J. Hedgehog: Functions and mechanisms. Genes Dev 2008, 22, 2454–2472. [Google Scholar]
- Pola, R.; Ling, L.E.; Silver, M.; Corbley, M.J.; Kearney, M.; Pepinsky, R.B.; Shapiro, R.; Taylor, F.R.; Baker, D.P.; Asahara, T. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat. Med 2001, 7, 706–711. [Google Scholar]
- Claret, S.; Sanial, M.; Plessis, A. Evidence for a novel feedback loop in the Hedgehog pathway involving Smoothened and Fused. Curr. Biol 2007, 17, 1326–1333. [Google Scholar]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar]
- Jain, R.K. Tumor angiogenesis and accessibility: Role of vascular endothelial growth factor. Semin. Oncol 2002, 29, 3–9. [Google Scholar]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med 2003, 9, 669–676. [Google Scholar]
- Shigami, S.I.; Arii, S.; Furutani, M.; Niwano, M.; Harada, T.; Mizumoto, M.; Mori, A.; Onodera, H.; Imamura, M. Predictive value of vascular endothelial growth factor (VEGF) in metastasis and prognosis of human colorectal cancer. Br. J. Cancer 1998, 78, 1379–1384. [Google Scholar]
- Gille, H.; Kowalski, J.; Li, B.; LeCouter, J.; Moffat, B.; Zioncheck, T.F.; Pelletier, N.; Ferrara, N. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J. Biol. Chem 2001, 276, 3222–3230. [Google Scholar]
- Gorlick, R.; Bertino, J.R. Drug resistance in colon cancer. Semin. Oncol 1999, 26, 606–611. [Google Scholar]
- Longley, D.B.; Allen, W.L.; Johnston, P.G. Drug resistance, predictive markers and pharmacogenomics in colorectal cancer. Biochim. Biophys. Acta 2006, 1766, 184–196. [Google Scholar]
- Boose, G.; Stopper, H. Genotoxicity of several clinically used topoisomerase II inhibitors. Toxicol. Lett 2000, 116, 7–16. [Google Scholar]
- Gordaliza, M. Natural products as leads to anticancer drugs. Clin. Transl. Oncol 2007, 9, 767–776. [Google Scholar]
- Ji, H.F.; Li, X.J.; Zhang, H.Y. Natural products and drug discovery. EMBO Rep 2009, 10, 194–200. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the Peoples Republic of China. Chin. Med. Sci. Technol. Press 2010, 1, 109–110.
- Tan, P.; Lu, B.Z.; Bao, W.L. Analysis on the clinical application of Scutellaria barbata D. Don in the anti-cancer therapy. J. Jiangxi Tradit. Chin. Med 2006, 37, 57–58. [Google Scholar]
- Qian, B. Clinical effect of anticancer Chinese medicine. Shanghai Transl. Publ. House 1987, 111–112. [Google Scholar]
- Cha, Y.Y.; Lee, E.O.; Lee, H.J.; Park, Y.D.; Ko, S.G.; Kim, D.H.; Kim, H.M.; Kang, I.C.; Kim, S.H. Methylene chloride fraction of Scutellaria barbata induces apoptosis in human U937 leukemia cells via the mitochondrial signaling pathway. Clin. Chim. Acta 2004, 348, 41–48. [Google Scholar]
- Suh, S.J.; Yoon, J.W.; Lee, T.K.; Jin, U.H.; Kim, S.L.; Kim, M.S.; Kwon, D.Y.; Lee, Y.C.; Kim, C.H. Chemoprevention of Scutellaria bardata on human cancer cells and tumorigenesis in skin cancer. Phytother. Res 2007, 21, 135–141. [Google Scholar]
- Zhao, Z.; Holle, L.; Song, W.; Wei, Y.; Wagner, T.E.; Yu, X. Antitumor and anti-angiogenic activities of Scutellaria barbata extracts in vitro are partially mediated by inhibition of Akt/protein kinase B. Mol. Med. Rep 2012, 5, 788–792. [Google Scholar]
- Marconett, C.N.; Morgenstern, T.J.; San Roman, A.K.; Sundar, S.N.; Singhal, A.K.; Firestone, G.L. BZL101, a phytochemical extract from the Scutellaria barbata plant, disrupts proliferation of human breast and prostate cancer cells through distinct mechanisms dependent on the cancer cell phenotype. Cancer Biol. Ther 2010, 10, 397–405. [Google Scholar]
- Dai, Z.J.; Liu, X.X.; Tang, W.; Xue, Q.; Wang, X.J.; Ji, Z.Z.; Kang, H.F.; Diao, Y. Antitumor and immune-modulating effects of Scutellaria barbata extract in mice bearing hepatocarcinoma H22 cells-derived tumor. Nan Fang Yi Ke Da Xue Xue Bao 2008, 28, 1835–1837. [Google Scholar]
- Wong, B.Y.; Nguyen, D.L.; Lin, T.; Wong, H.H.; Cavalcante, A.; Greenberg, N.M.; Hausted, R.P.; Zheng, J. Chinese Medicinal herb Scutellaria barbata modulates apoptosis and cell survival in murine and human prostate cancer cells and tumor development in TRAMP mice. Eur. J. Cancer Prev 2009, 18, 331–341. [Google Scholar]
- Goh, D.; Lee, Y.H.; Ong, E.S. Inhibitory effects of a chemically standardized extract from Scutellaria barbata in human colon cancer cell lines, LoVo. J. Agric. Food Chem 2005, 53, 8197–8204. [Google Scholar]
- Wei, L.H.; Chen, Y.Q.; Lin, J.M.; Zhao, J.Y.; Chen, X.Z.; Xu, W.; Liu, X.X.; Sferra, T.J.; Peng, J. Scutellaria barbata D. Don induces apoptosis of human colon carcinoma cell via activation of the mitochondrion-dependent pathway. J. Med. Plants Res 2011, 5, 1962–1970. [Google Scholar]
- Wei, L.H.; Lin, J.M.; Xu, W.; Hong, Z.F.; Liu, X.X.; Peng, J. Scutellaria barbata D. Don inhibits tumor angiogenesis via suppressing proliferation, migration and tube formation of endothelial cells and downregulating the expression of VEGF-A in cancer cells. J. Med. Plants Res 2011, 5, 3260–3268. [Google Scholar]





© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wei, L.; Lin, J.; Xu, W.; Cai, Q.; Shen, A.; Hong, Z.; Peng, J. Scutellaria barbata D. Don Inhibits Tumor Angiogenesis via Suppression of Hedgehog Pathway in a Mouse Model of Colorectal Cancer. Int. J. Mol. Sci. 2012, 13, 9419-9430. https://doi.org/10.3390/ijms13089419
Wei L, Lin J, Xu W, Cai Q, Shen A, Hong Z, Peng J. Scutellaria barbata D. Don Inhibits Tumor Angiogenesis via Suppression of Hedgehog Pathway in a Mouse Model of Colorectal Cancer. International Journal of Molecular Sciences. 2012; 13(8):9419-9430. https://doi.org/10.3390/ijms13089419
Chicago/Turabian StyleWei, Lihui, Jiumao Lin, Wei Xu, Qiaoyan Cai, Aling Shen, Zhenfeng Hong, and Jun Peng. 2012. "Scutellaria barbata D. Don Inhibits Tumor Angiogenesis via Suppression of Hedgehog Pathway in a Mouse Model of Colorectal Cancer" International Journal of Molecular Sciences 13, no. 8: 9419-9430. https://doi.org/10.3390/ijms13089419
APA StyleWei, L., Lin, J., Xu, W., Cai, Q., Shen, A., Hong, Z., & Peng, J. (2012). Scutellaria barbata D. Don Inhibits Tumor Angiogenesis via Suppression of Hedgehog Pathway in a Mouse Model of Colorectal Cancer. International Journal of Molecular Sciences, 13(8), 9419-9430. https://doi.org/10.3390/ijms13089419



