Enzymatic Properties and Mutational Studies of Chalcone Synthase from Physcomitrella patens
Abstract
:1. Introduction
2. Results and Discussions
2.1. Purification and Characterization of Recombinant Chalcone Synthase
2.2. Mutational Studies to Study Factors That Effect the Substrate Specificity of PpCHS
3. Materials and Method
3.1. Expression and Purification of Recombinant Chalcone Synthase
3.2. Standard Assay Conditions of CHS
3.3. Characterization of Purified Chalcone Synthase
3.3.1. The Effect of Substrate Specificity
3.3.2. The Effect of pH on Chalcone Synthase Activity
3.3.3. Thermal Stability of Chalcone Synthase Activity
3.4. Preparation of CHS Mutants
3.5. Protein Expression in E.coli and Enzyme Extracts
3.6. HPLC Analysis of Mutant’s Reaction Products
3.7. Sequence Analysis and Homology Modeling
3.7.1. Validation and Analysis of Model
3.7.2. Building Mutants
4. Conclusions
Acknowledgment
References
- Jiang, C.; Schrommer, C.K.; Kim, S.Y.; Suh, D.Y. Cloning and characterization of chalcone synthase fron the moss, Physcomitrella patens. Phytochemistry 2006, 67, 2531–2540. [Google Scholar]
- Harashima, S.; Takano, H.; Ono, K.; Takio, S. Chalcone synthase-like gene in liverwort, Marchantia palacea var diptera. Plant Cell Reprod 2004, 23, 167–173. [Google Scholar]
- Pang, Y.; Shen, G.; Wu, W.; Liu, X.; Lin, J.; Tan, F.; Sun, X.; Tang, K. Characterization and expression of chalcone syntahse gene from Gikgo biloba. Plant Sci 2005, 168, 1525–1531. [Google Scholar]
- Schaefer, D.G.; Zryd, J.-P. The moss Physcomitrella patens, Now and Then. Plant Physiol 2001, 127, 1430–1438. [Google Scholar]
- Wolf, L.; Rizzini, L.; Stracke, R.; Ulm, R.; Rensing, S.A. The molecular and physiological responses of Physcomitrella patens to Ultraviolet-B-radiation. Plant Physiol 2010, 153, 1123–1134. [Google Scholar]
- Koduri, P.K.; Gordon, G.S.; Barker, E.I.; Colpitts, C.C.; Ashton, N.W.; Suh, D.Y. Genome-wide analysis of the chalcone synthase superfamily genes of Physcomitrella patens. Plant Mol. Biol 2009, 72, 247–263. [Google Scholar]
- Jez, J.M.; Noel, J.P. Mechanism of Chalcone Synthase. pKa of the catalytic cysteine and the role of the conserved histidine in a plant polyketide synthase. J. Biol. Chem 2000, 275, 39640–39646. [Google Scholar]
- Morita, H.; Noguchi, H.; Schroder, J.; Abe, I. Novel polyketide synthesized with a higher plant stilbene synthase. European Journal of Biochemistry 2001, 268, 3759–3766. [Google Scholar]
- Ferrer, J.L.; Bowman, M.E.; Dixon, R.A.; Noel, J.P. Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. Nat. Struct. Biol 1999, 6, 775–784. [Google Scholar]
- Zakaria, I.I.; Rahman, R.N.Z.; Salleh, A.B.; Mahiran, B. Bacteriocin-release protein mediated secretory expression of recombinant chalcone synthase in Escherichia coli. Appl. Biochem. Biotechnol 2011, 165, 737–747. [Google Scholar]
- Zuurbier, K.W.M.; Leser, J.; Berger, T.; Hofte, A.J.P.; Schroder, G.; Verpoorte, R.; Schroder, J. 4-hyroxy-2-pyrone formation by chalcone synthase and stilbene synthase with nonphysiological substrates. Phytochemisty 1998, 49, 1945–1951. [Google Scholar]
- Schuz, R.; Heller, W.; Hahlbrock, K. Substrate specificity of chalcone synthase from Petroselinum hortense. J. Biol. Chem 1983, 258, 6730–6734. [Google Scholar]
- Funa, N.; Ohnishi, Y.; Ebizuka, Y.; Horinouchi, S. Alteration of reaction and substrate specificity of a bacterial type III polyketide synthase by site-directed mutagenesis. Biochem. J 2002, 367, 781–789. [Google Scholar]
- Raharjo, T.J.; Chang, W.T.; Choi, Y.H.; Peltenburg-Looman, A.M.G.; Verpoorte, R. Olivetol as product of a polyketide synthase in Cannabis Sativa L. Plant Sci 2004, 166, 381–385. [Google Scholar]
- Suh, D.-Y.; Kagami, J.; Fukuma, K.; Sankawa, U. Evidence for catalytic Cysteine-Histidine dyad in chalcone synthase. Biochem. Biophys. Res. Commun 2000, 275, 725–730. [Google Scholar]
- Suh, D.-Y.; Fukuma, K.; Kagami, J.; Yamazaki, Y.; Shibuya, M.; Ebizuka, Y.; Sankawa, U. Identification of amino acid residues important in the cyclization reactions of chalcone and stilbene synthases. Biochem. J 2000, 350, 229–235. [Google Scholar]
- Mallika, V.; Sivakumar, K.C.; Soniya, E.V. Implifications and physicochemical analyses of selected proteins of type III polyketide synthase family. Evol. Bioinforma 2011, 7, 41–53. [Google Scholar]
- Lanz, T.; Tropf, S.; Marner, F.J.; Schroder, J.; Schroder, G. The role of cysteines in polyketide synthases. Site-directed mutagenesis of resveratrol and chalcone synthases, two key enzymes in different plant-specific pathways. J. Biol. Chem 1991, 266, 9971–9976. [Google Scholar]
- Fukuma, K.; Neuls, E.D.; Ryberg, J.M.; Suh, D.-Y.; Sankawa, U. Mutational analysis of conserved outer sphere arginine residue of chalcone synthase. J. Biochem 2007, 142, 731–739. [Google Scholar]
- MODELER Accelrys, San Diego. Available online: http://www.accelrys.com accessed on 1 May 2012.
- Sali, A.; Blundell, TL. Comparative protein modeling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar]
- Procheck, version 3.5.4; European Bioinformatics Institute: Heidelberg, Germany, 1994. Available online: http://www.biochem.ucl.ac.uk/~roman/procheck.html accesed on 21 September 2011.
- Errat, version 2.0; UCLA-DOE: Los Angeles, CA, USA, 2012. Available online: http://www.doe-mbi.ucla.edu/errat_server.html accessed on 21 September 2011.
- Zheng, Z.; Zuo, Z.; Liu, Z.; Tsai, K.; Liu, A.; Zou, G. Construction of a 3D model nattokinase, a novel fibrinolytic enzyme from Bacillus natto. A novel nucleophilic catalytic mechanism for nattokinase. J. Mol. Graphics Modell 2005. 2005, 23, 373–380. [Google Scholar]
- Guerois, R.; Nielsen, J.E.; Serrano, L. Predicting changes in the stability of proteins and protein complexes: A study of more than 1000 mutations. J. Mol. Biol 2002, 320, 369–87. [Google Scholar]
- Colovos, C.; Yeates, T.O. Verification of protein structures: Patterns of non-bonded atomic interaction. Protein Sci 1992, 2, 1511–1519. [Google Scholar]
- YASARA, Biosciences GmbH. Available online: http://www.yasara.org accessed on 1 May 2012.











| Type/Mutants | Dyad Interactions (Distance) | Cavity Volume |
|---|---|---|
| Recombinant PpCHS |  Cys-His : 4.0 Å | 216.31 Å3 |
| Cys 170 Arg Mutant |  No interaction detected | 195.53 Å3 |
| Cys 170 Ser Mutant | 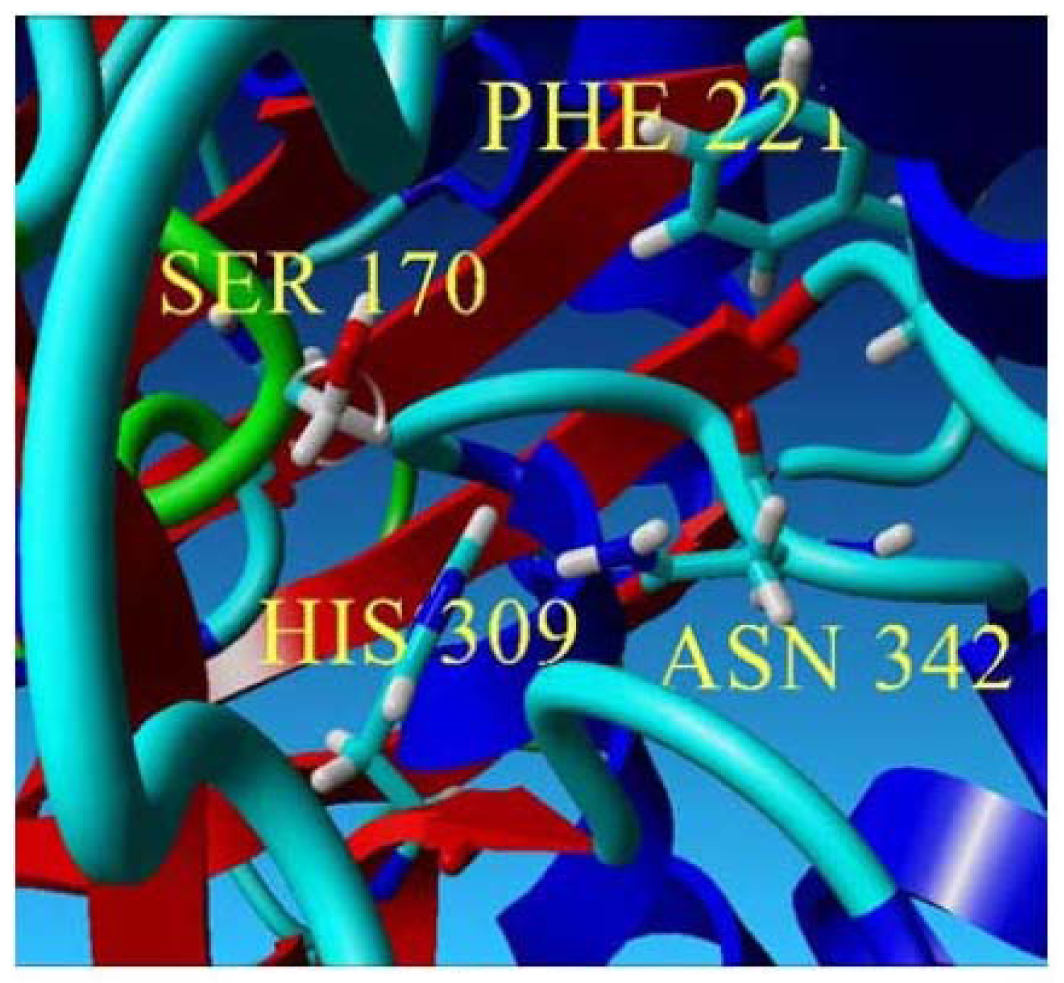 Ser-His : 3.931 Å | 231.23 Å3 |
| Substitutions (Residues) | Total Energy (kcal/mol) |
|---|---|
| Wild type reference (Cys 170) | −66.62 |
| Cys 170 Gly mutant | −66.72 |
| Cys 170 Ala mutant | −66.87 |
| Cys 170 Leu mutant | −66.78 |
| Cys 170 Val mutant | −65.54 |
| Cys 170 Ile mutant | −65.83 |
| Cys 170 Pro mutant | −69.28 |
| Cys 170 Arg mutant | −65.03 |
| Cys 170 Thr mutant | −65.59 |
| Cys 170 Ser mutant | −66.97 |
| Cys 170 Met mutant | −68.13 |
| Cys 170 Lys mutant | −65.53 |
| Cys 170 Glu mutant | −67.44 |
| Cys 170 Gln mutant | −67.05 |
| Cys 170 Asp mutant | −66.72 |
| Cys 170 Asn mutant | −65.68 |
| Cys 170 Trp mutant | −63.97 |
| Cys 170 Tyr mutant | −66.45 |
| Cys 170 Phe mutant | −66.07 |
| Cys 170 His mutant | −64.15 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Raja Abdul Rahman, R.N.Z.; Zakaria, I.I.; Salleh, A.B.; Basri, M. Enzymatic Properties and Mutational Studies of Chalcone Synthase from Physcomitrella patens. Int. J. Mol. Sci. 2012, 13, 9673-9691. https://doi.org/10.3390/ijms13089673
Raja Abdul Rahman RNZ, Zakaria II, Salleh AB, Basri M. Enzymatic Properties and Mutational Studies of Chalcone Synthase from Physcomitrella patens. International Journal of Molecular Sciences. 2012; 13(8):9673-9691. https://doi.org/10.3390/ijms13089673
Chicago/Turabian StyleRaja Abdul Rahman, Raja Noor Zaliha, Iffah Izzati Zakaria, Abu Bakar Salleh, and Mahiran Basri. 2012. "Enzymatic Properties and Mutational Studies of Chalcone Synthase from Physcomitrella patens" International Journal of Molecular Sciences 13, no. 8: 9673-9691. https://doi.org/10.3390/ijms13089673
APA StyleRaja Abdul Rahman, R. N. Z., Zakaria, I. I., Salleh, A. B., & Basri, M. (2012). Enzymatic Properties and Mutational Studies of Chalcone Synthase from Physcomitrella patens. International Journal of Molecular Sciences, 13(8), 9673-9691. https://doi.org/10.3390/ijms13089673




