Synthesis, Structure Optimization and Antifungal Screening of Novel Tetrazole Ring Bearing Acyl-Hydrazones
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biology
2.2.1. MIC and MFC
2.2.2. Disc Diffusion Halo Assays
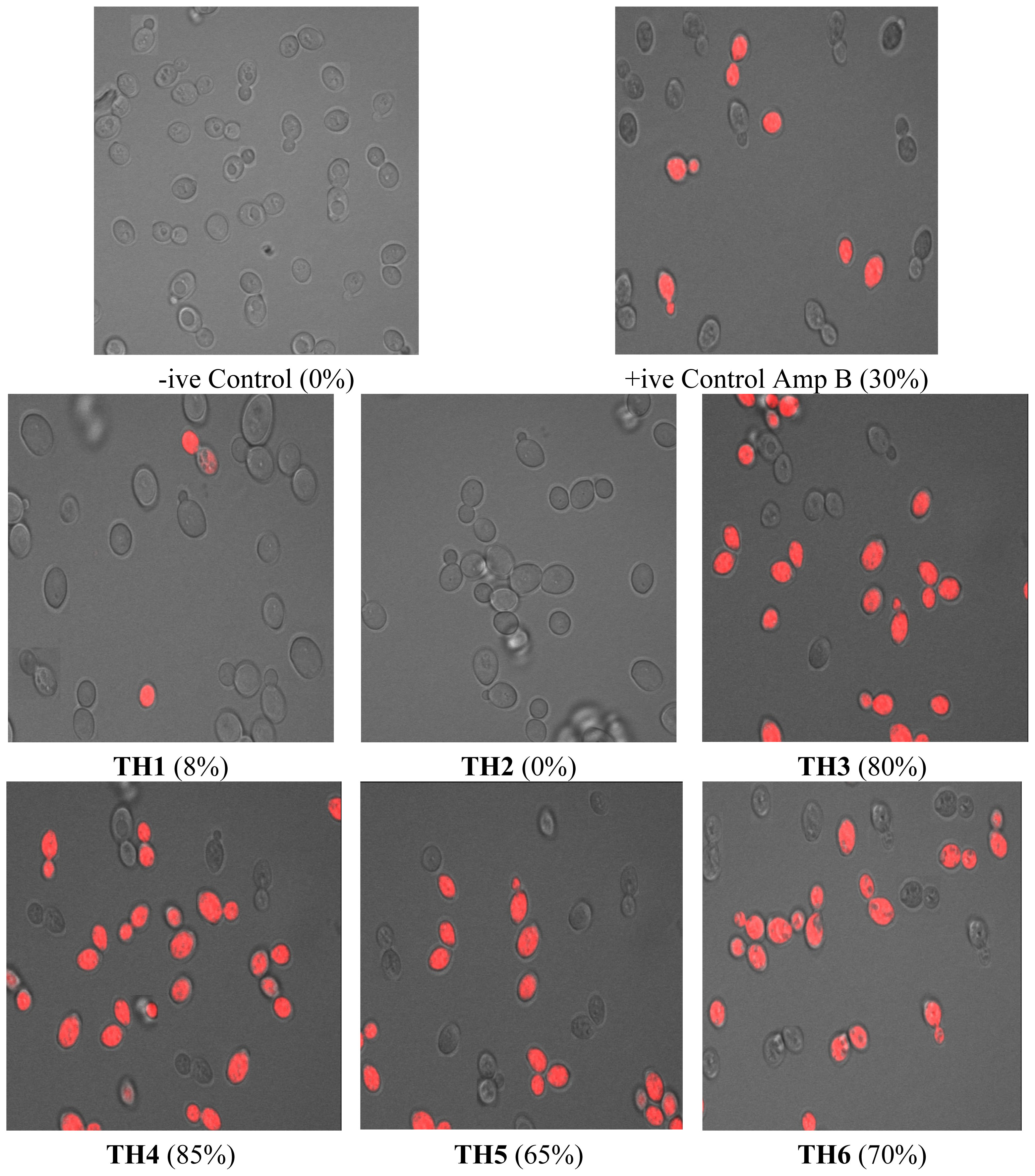
2.2.3. Confocal Microscopy Analysis
2.2.4. Sterol Quantitation
3. Materials and Methods
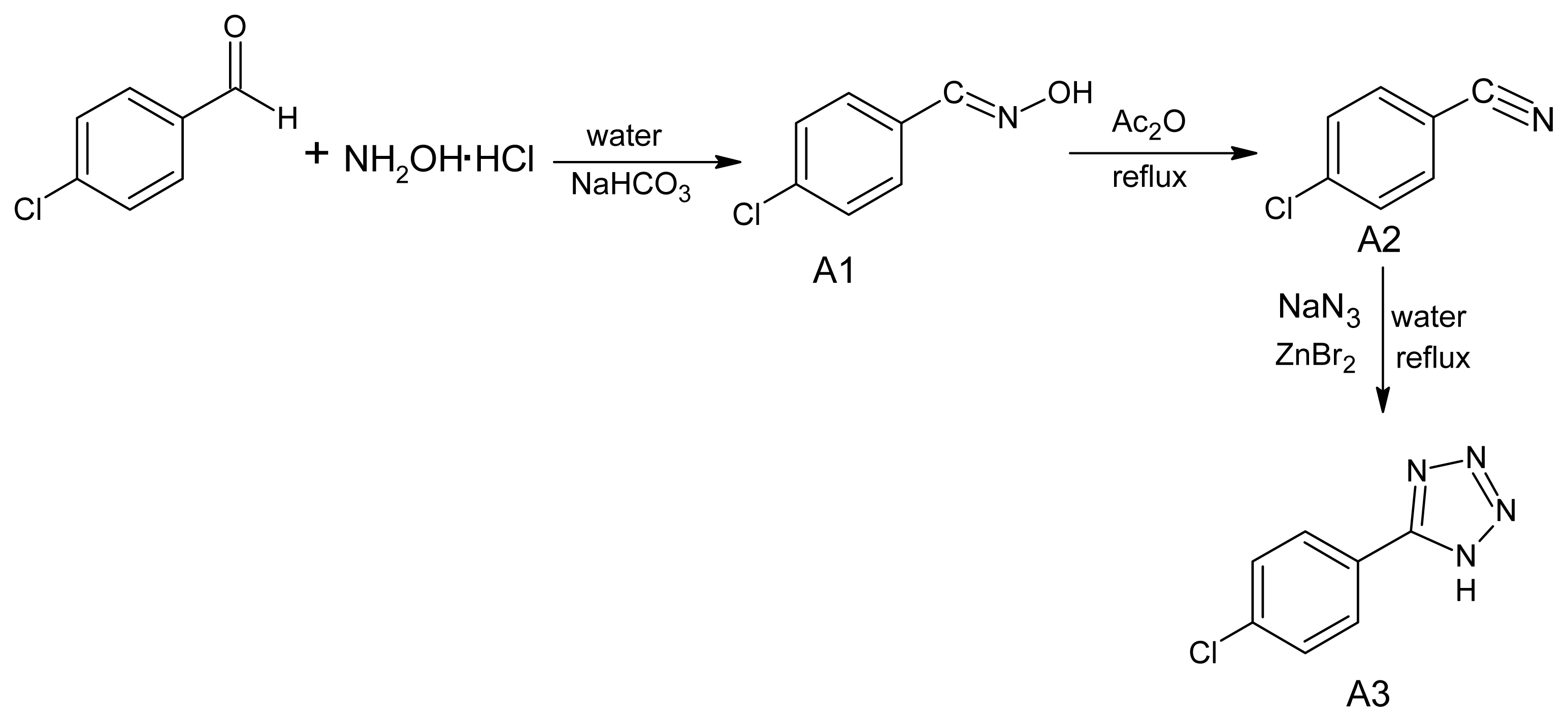
3.1. Synthesis of 5-(4-Chlorophenyl)-1H-tetrazole (A3)
3.2. Synthesis of ethyl [5-(4-Chlorophenyl)-tetrazol-1-yl]acetate (A4)
3.3. Synthesis of 2-[5-(4-Chlorophenyl)-tetrazol-1-yl]acetohydrazide (A5)
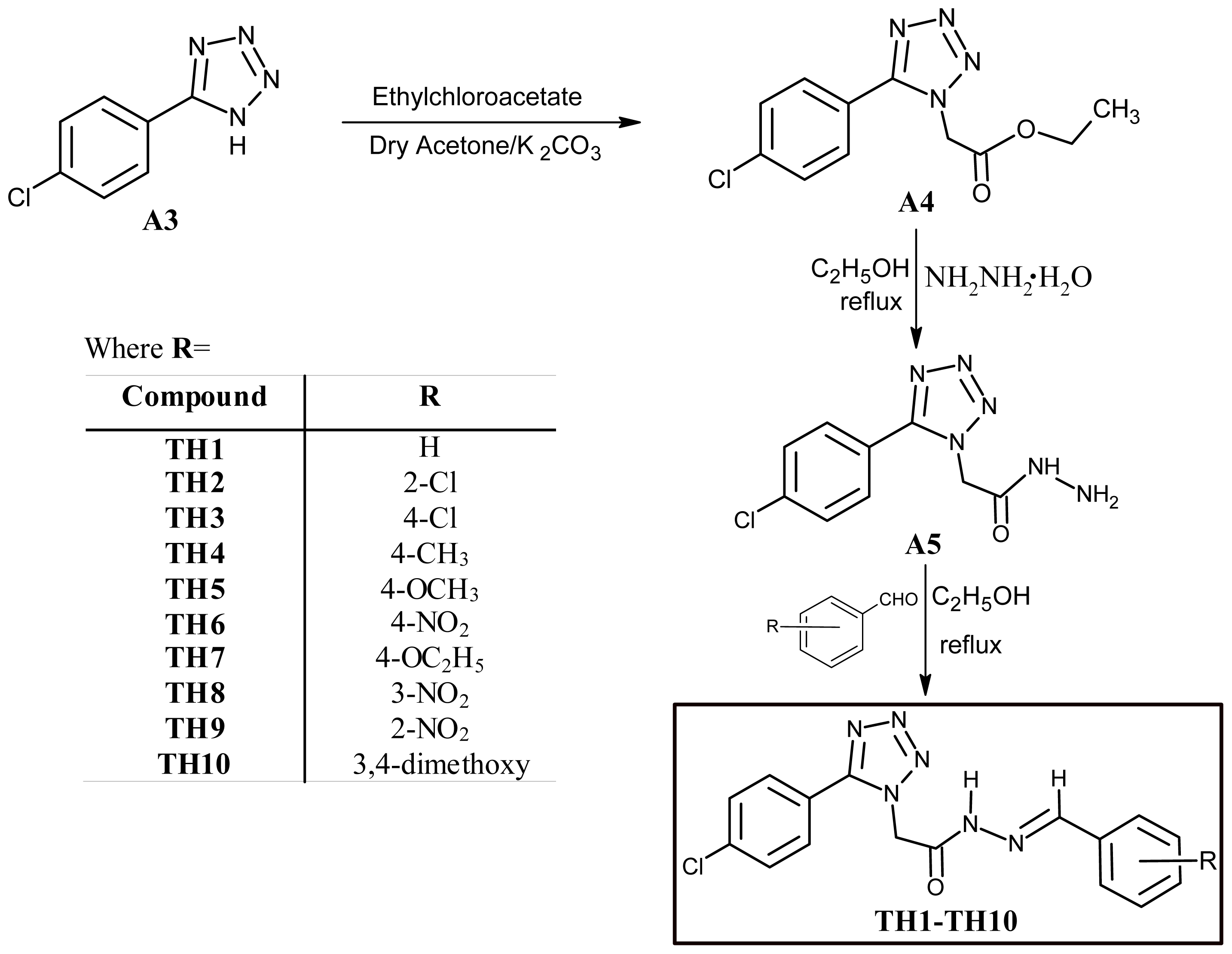
3.4. Experimental Procedure for the Synthesis of Acyl-hydrazones (TH1–TH10)
3.5. 2-[5-(4-Chlorophenyl)-tetrazol-1-yl]-N′-[(E)-phenylmethylidene]-acetohydrazide (TH1)
3.6. N′-[(E)-(2-Chlorophenyl)methylidene]-2-[5-(2-chlorophenyl)-tetrazol-1-yl]-acetohydrazide (TH2)
3.7. N′-[(E)-(2-Chlorophenyl)methylidene]-2-[5-(4-chlorophenyl)-tetrazol-1-yl]-acetohydrazide (TH3)
3.8. 2-[5-(4-Chlorophenyl)-tetrazol-1-yl]-N′-[(E)-(4-ethylphenyl)methylidene]-acetohydrazide (TH4)
3.9. 2-[5-(4-Chlorophenyl)-tetrazol-1-yl]-N′-[(E)-(4-methoxyphenyl)methylidene]-acetohydrazide (TH5)
3.10. 2-[5-(4-Chlorophenyl)-tetrazol-1-yl]-N′-[(E)-(4-nitrophenyl)methylidene]-acetohydrazide (TH6)
3.11. 2-[5-(4-Chlorophenyl)-tetrazol-1-yl]-N′-[(E)-(4-ethoxyphenyl)methylidene]-acetohydrazide (TH7)
3.12. 2-[5-(4-Chlorophenyl)-tetrazol-1-yl]-N′-[(E)-(3-nitrophenyl)methylidene]-acetohydrazide (TH8)
3.13. 2-[5-(4-Chlorophenyl)-tetrazol-1-yl]-N′-[(E)-(2-nitrophenyl)methylidene]-acetohydrazide (TH9)
3.14. 2-[5-(4-Chlorophenyl)-tetrazol-1-yl]-N′-[(E)-(3,4-dimethoxyphenyl)methylidene]-acetohydrazide (TH10)
4. Antifungal Studies
4.1. Strains and Media
4.2. Determination of MIC and MFC
4.3. Disc Diffusion Halo Assays
4.4. Confocal Scanning Laser Microscopy
4.5. Sterol Quantitation Assay
5. Conclusions
Acknowledgments
References
- Chi, H.; Yang, Y.; Shang, S.; Chen, K.; Yeh, K.; Chang, F.; Lin, J. Candida albicans versus non-albicans bloodstream infections: The comparison of risk factors and outcome. J. Microbiol. Immunol. Infect 2011, 44, 369–375. [Google Scholar]
- Fidel, P.L., Jr. Candida-host interactions in HIV disease: Relationships in oropharyngeal candidiasis. Adv. Dent. Res. 2006, 19, 80–84. [Google Scholar]
- Pfaller, M.A.; Diekema, D.J. Rare and emerging opportunistic fungal pathogens: Concern for resistance beyond Candida albicans and Aspergillus fumigatus. J. Clinic. Microbiol 2004, 42, 4419–4431. [Google Scholar]
- Cohen, B. Amphotericin B toxicity and lethality: A tale of two channels. Int. J. Pharm 1998, 162, 95–106. [Google Scholar]
- Sanglard, D. Resistance of human fungal pathogens to antifungal drugs. Curr. Opinion Microbiol 2002, 5, 379–385. [Google Scholar]
- Marr, K.A.; White, T.C.; van Burik, J.A.; Bowden, R.A. Development of fluconazole resistance in Candida albicans causing disseminated infection in a patient undergoing marrow transplantation. Clin. Infect. Dis 1997, 25, 908–910. [Google Scholar]
- Menozzi, G.; Mosti, L.; Fossa, P.; Musiu, C.; Murgioni, C.; La Colla, P. Synthesis and biological evaluation of azole derivatives, analogues of bifonazole, with a phenylisoxazolyl or phenylpyrimidinyl moiety. II Farmaco 2001, 56, 633–640. [Google Scholar]
- Liu, F.; Luo, X.Q.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y. Synthesis and antifungal activity of novel sulfoxide derivatives containing trimethoxyphenyl substituted 1,3,4-thiadiazole and 1,3,4-oxadiazole moiety. Bioorg. Med. Chem 2008, 16, 3632–3639. [Google Scholar]
- Giraud, F.; Loge, C.; Pagniez, F.; Crepin, D.; Le Pape, P.; Le Borgne, M. Design, synthesis, and evaluation of 1-(N-benzylamino)-2-phenyl-3-(1H-1,2,4-triazol-1-yl)propan-2-ols as antifungal agents. Bioorg. Med. Chem. Lett 2008, 18, 1820–1824. [Google Scholar]
- Mesenzani, O.; Massarotti, A.; Giustiniano, M.; Pirali, T.; Bevilacqua, V.; Caldarelli, A.; Canonico, P.; Sorba, G.; Novellino, E.; Genazzani, A.A.; et al. Replacement of the double bond of antitubulin chalcones with triazoles and tetrazoles: Synthesis and biological evaluation. Bioorg. Med. Chem. Lett 2011, 21, 764–768. [Google Scholar]
- Webster, S.P.; Binnie, M.; McConnell, K.M.M.; Sooy, K.; Ward, P.; Greaney, M.F.; Vinter, A.; Pallin, T.D.; Dyke, H.J.; Gill, M.I.A.; et al. Modulation of 11β-hydroxysteroid dehydrogenase type 1 activity by 1,5-substituted 1H-tetrazoles. Bioorg. Med. Chem. Lett 2010, 20, 3265–3271. [Google Scholar]
- Rostom, S.A.F.; Ashour, H.M.A.; Abd El Razik, H.A.; Abd El Fattah, A.H.; El-Din, N.N. Azole antimicrobial pharmacophore-based tetrazoles: Synthesis and biological evaluation as potential antimicrobial and anticonvulsant agents. Bioorg. Med. Chem 2009, 17, 2410–2422. [Google Scholar]
- Matysiak, J.; Niewiadomy, A.; Krajewska-Kulak, E.; Macik-Niewiadomy, G. Synthesis of some 1-(2,4-dihydroxythiobenzoyl)imidazoles, -imidazolines and -tetrazoles and their potent activity against Candida species. II Farmaco 2003, 58, 455–461. [Google Scholar]
- Upadhayaya, R.S.; Jain, S.; Sinha, N.; Kishore, N.; Chandra, R.; Arora, S.K. Synthesis of novel substituted tetrazoles having antifungal activity. Eur. J. Med. Chem 2004, 39, 579–592. [Google Scholar]
- De Souza, A.O.; Pedrosa, M.T.; Alderete, J.B.; Cruz, A.F.; Prado, M.A.; Alves, R.B.; Silva, C.L. Cytotoxicity, antitumoral and antimycobacterial activity of tetrazole and oxadiazole derivatives. Pharmazie 2005, 60, 396–397. [Google Scholar]
- Rajasekaran, A.; Murugesan, S.; AnandaRajagopal, K. Antibacterial, antifungal and anticonvulsant evaluation of novel newly synthesized 1-[2-(1H-tetrazol-5-yl)ethyl]-1H-benzo[d][1,2,3]triazoles. Arch. Pharm. Res 2006, 29, 535–540. [Google Scholar]
- Kuçukguzel, S.G.; Oruc, E.E.; Rollas, S.; Sahin, F.; Ozbek, A. Synthesis, characterisation and biological activity of novel 4-thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Eur. J. Med. Chem 2002, 37, 197–206. [Google Scholar]
- Cristina, M.M.; Ovidiu, O.; Alina, P.; Brînduşa, T.; Philippe, V.; Adrian, P.; Ovidiu, C.; Marius, B.; Raluca, P. Synthesis and anti-inflammatory evaluation of some new acyl-hydrazones bearing 2-aryl-thiazole. Eur. J. Med. Chem 2011, 46, 526–534. [Google Scholar]
- Avaji, P.G.; Kumar, C.H.V.; Patil, S.A.; Shivananda, K.N.; Nagaraju, C. Synthesis, spectral characterization, in vitro microbiological evaluation and cytotoxic activities of novel macrocyclic bis hydrazone. Eur. J. Med.Chem 2009, 44, 3552–3559. [Google Scholar]
- Zang, Y.; Zang, L.; Liu, L.; Guo, J.; Wu, D.; Xu, G.; Wang, X.; Jia, D. Anticancer activity, structure, and theoretical calculation of N-(1-phenyl-3-methyl-4-propyl-pyrazolone-5)-salicylidene hydrazone and its copper(II) complex. Inorg. Chim. Acta 2010, 363, 289–293. [Google Scholar]
- Lima, L.M.; Frattani, F.S.; Dos Santos, J.L.; Castro, H.C.; Fraga, C.A.M.; Zingali, R.B.; Barreiro, E.J. Synthesis and anti-platelet activity of novel arylsulfonate-acylhydrazone derivatives, designed as antithrombotic candidates. Eur. J. Med. Chem 2008, 43, 348–356. [Google Scholar]
- Chornous, V.A.; Bratenko, M.K.; Vovk, M.V.; Sidorchuk, I.I. Synthesis and antimicrobial activity of Pyrazole-4-carboxylic acid hydrazides and N-(4-Pyrazoyl)hydrazones of and heteroaromatic aldehydes. Pharm. Chem. J 2001, 35, 203–205. [Google Scholar]
- Demko, Z.P.; Sharpless, K.B. Preparation of 5-Substituted 1H-Tetrazoles from nitriles in water. J. Org. Chem 2001, 66, 7945–7950. [Google Scholar]
- Chauviere, G.; Bouteille, B.; Enanga, B.; Albuquerque, C.; Croft, S.L.; Dumas, M.; Perie, J. Synthesis and biological activity of nitro heterocycles analogous to megazol, a lead. J. Med. Chem 2003, 46, 427–440. [Google Scholar]
- Ansari, K.F.; Lal, C. Synthesis, physicochemical properties and antimicrobial activity of some benzimidazole derivatives. Eur. J. Med. Chem 2009, 44, 4028–4033. [Google Scholar]
- Lupetti, A.; Danesi, R.; Campa, M.; Del-Tacca, M.; Kelly, S. Molecular basis of resistance to antifungals. Trends Mol. Med 2002, 8, 76–81. [Google Scholar]



| Mean MIC/MFC (mg L−1) | ||||||
|---|---|---|---|---|---|---|
| Compound | Standard lab strains (n = 3) | Clinical strains (n = 11) | Resistant strains (n = 3) | |||
| MIC | MFC | MIC | MFC | MIC | MFC | |
| A1 | 64 | na | 64 | na | - | - |
| A2 | na | na | na | na | - | - |
| A3 | na | na | na | na | - | - |
| A4 | na | na | na | na | - | - |
| A5 | na | na | na | na | - | - |
| TH1 | 6 | na | 6 | na | 12 | 64 |
| TH2 | na | na | na | na | na | na |
| TH3 | 62 | 128 | 62 | 128 | 128 | 256 |
| TH4 | 4 | 16 | 8 | 16 | 4 | 16 |
| TH5 | 8 | 64 | 16 | 64 | 16 | 128 |
| TH6 | 4 | 64 | 8 | 128 | 4 | 128 |
| TH7 | 16 | 32 | 16 | 32 | 64 | 64 |
| TH8 | 2 | na | 8 | na | 64 | na |
| TH9 | 4 | na | 4 | na | 32 | na |
| TH10 | 32 | na | 32 | na | 64 | na |
| Classification of isolates standard, n = 3 | Type of isolate |
|---|---|
| ATCC 10261 | C. albicans |
| ATCC 90028 | C. albicans |
| ATCC 750 | C. tropicalis |
| Clinical susceptible, n = 11 | |
| Candidemia, VVC *, UTI (n = 6) | C. albicans (3), C. tropicalis (1), C. glabrata (1), C. krusei (1) |
| Cutaneous (n = 3) | C. albicans (2), C. tropicalis (1) |
| Oropharyngeal (n = 2) | C. albicans (1), C. tropicalis (1) |
| Clinical Resistant, n = 3 | |
| Candidemia, VVC * | C. albicans (1), C. glabrata (1), C. krusei (1) |
| Test compounds −ive Control | Mean ergosterol content * of susceptible Candida cells 0.0181 ± 0.0045 | Mean ergosterol content * of resistant Candida cells 0.0201 ± 0.0034 | |
|---|---|---|---|
| TH1 | ½ MIC | 0.0135 ± 0.0013(25) ** | 0.0115 ± 0.0012 (43) ** |
| MIC | 0.00012 ± 0.0001(99) ** | 0.0009 ± 0.0004 (96) ** | |
| TH2 | ½ MIC | 0.0167 ± 0.0034(8) ** | 0.0171 ± 0.0021 (15) ** |
| MIC | 0.0107 ± 0.0079 (41) ** | 0.0055 ± 0.001 (73) ** | |
| TH3 | ½ MIC | 0.0087 ± 0.002(52) ** | 0.0093 ± 0.0024 (54) ** |
| MIC | 0.00011 ± 0.0001(99) ** | 0.00149 ± 0.00012 (93) ** | |
| TH4 | ½ MIC | 0.0027 ± 0.0028 (85) ** | 0.0061 ± 0.0034 (70) ** |
| MIC | 0 ± 0 (100) ** | 0 ± 0 (100) ** | |
| TH5 | ½ MIC | 0.0059 ± 0.0034(67) ** | 0.0049 ± 0.0011 (76) ** |
| MIC | 0.00044 ± 0.0002(97) ** | 0.00039 ± 0.0001(98) ** | |
| TH6 | ½ MIC | 0.0046 ± 0.0017(75) ** | 0.0122 ± 0.001 (40) ** |
| MIC | 0.00041 ± 0.0002(98) ** | 0 ± 0 (100) ** | |
| TH7 | ½ MIC | 0.0066 ± 0.003(63) ** | 0.0109 ± 0.001 (46) ** |
| MIC | 0.0006 ± 0.0003(96) ** | 0.0022 ± 0.0011 (89) ** | |
| TH8 | ½ MIC | 0.0062 ± 0.0019(66) ** | 0.014 ± 0.004 (30) ** |
| MIC | 0.00018 ± 0.0001(99) ** | 0 ± 0 (100) ** | |
| TH9 | ½ MIC | 0.0127 ± 0.0024(29) ** | 0.0102 ± 0.01 (50) ** |
| MIC | 0.00041 ± 0.0003(99) ** | 0.0028 ± 0.0004 (86) ** | |
| TH10 | ½ MIC | 0.0144 ± 0.0028(21) ** | 0.0089 ± 0.0008 (56) ** |
| MIC | 0.00052 ± 0.0003(97) ** | 0.001 ± 0.0003(95) ** | |
| A1 | 124 mg L−1 | 0.0045 ± 0.0011 (75) ** | - |
| A2 | 124 mg L−1 | 0.00016 ± 0.0001(49) ** | - |
| A3 | 124 mg L−1 | 0.00010 ± 0.0001(99) ** | - |
| A4 | 124 mg L−1 | 0.00039 ± 0.0001(98) ** | - |
| A5 | 124 mg L−1 | 0.00044 ± 0.0002(97) ** | - |
| +ive Control | 0 ± 0 (100) ** | 0.0147 (27) ** | |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Malik, M.A.; Al-Thabaiti, S.A.; Malik, M.A. Synthesis, Structure Optimization and Antifungal Screening of Novel Tetrazole Ring Bearing Acyl-Hydrazones. Int. J. Mol. Sci. 2012, 13, 10880-10898. https://doi.org/10.3390/ijms130910880
Malik MA, Al-Thabaiti SA, Malik MA. Synthesis, Structure Optimization and Antifungal Screening of Novel Tetrazole Ring Bearing Acyl-Hydrazones. International Journal of Molecular Sciences. 2012; 13(9):10880-10898. https://doi.org/10.3390/ijms130910880
Chicago/Turabian StyleMalik, Maqsood Ahmad, Shaeel Ahmed Al-Thabaiti, and Manzoor A. Malik. 2012. "Synthesis, Structure Optimization and Antifungal Screening of Novel Tetrazole Ring Bearing Acyl-Hydrazones" International Journal of Molecular Sciences 13, no. 9: 10880-10898. https://doi.org/10.3390/ijms130910880
APA StyleMalik, M. A., Al-Thabaiti, S. A., & Malik, M. A. (2012). Synthesis, Structure Optimization and Antifungal Screening of Novel Tetrazole Ring Bearing Acyl-Hydrazones. International Journal of Molecular Sciences, 13(9), 10880-10898. https://doi.org/10.3390/ijms130910880





