High Yield of Wax Ester Synthesized from Cetyl Alcohol and Octanoic Acid by Lipozyme RMIM and Novozym 435
Abstract
:1. Introduction
2. Results and Discussion
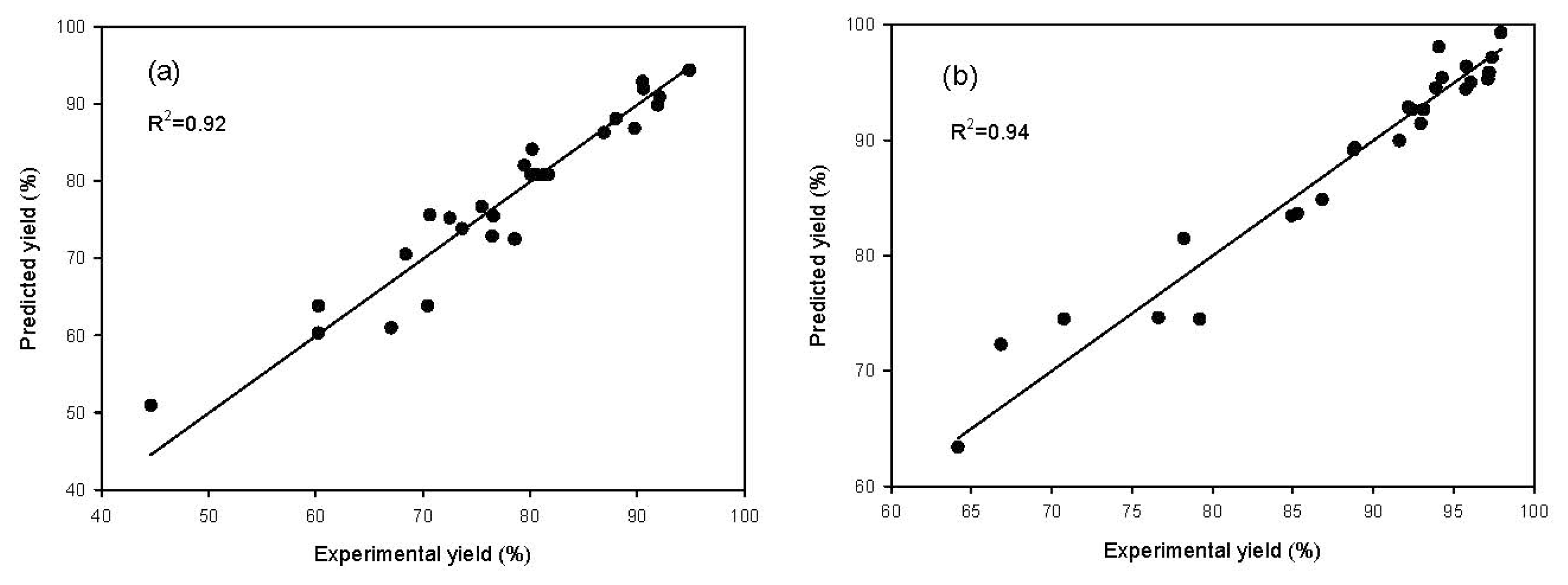
2.1. Model Fitting
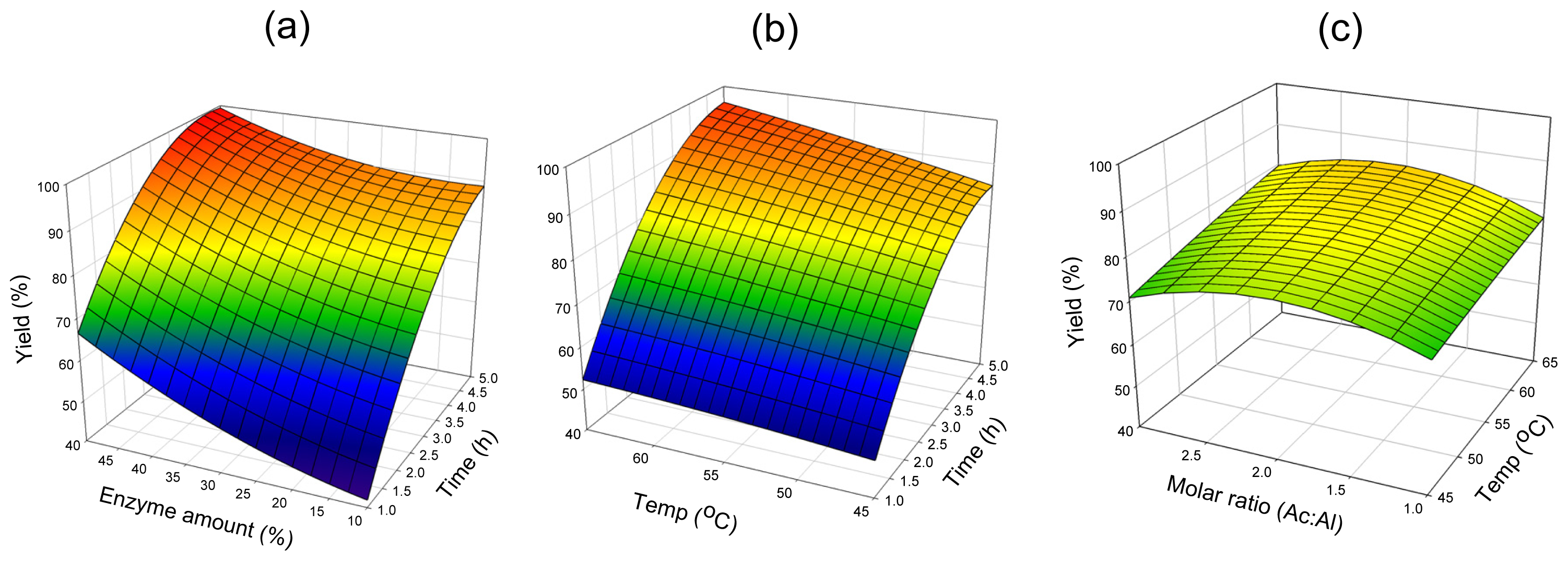
2.2. Mutual Effect of Parameters
2.2.1. Lipozyme® RMIM
2.2.2. Novozym® 435
2.3. Attaining Optimum Conditions
3. Experimental Section
3.1. Materials
3.2. Experimental Design
3.3. Enzymatic Esterification
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Sellami, M.; Aissa, I.; Frikha, F.; Gargouri, Y.; Miled, N. Immobilized Rhizopus oryzae lipase catalyzed synthesis of palm stearin and cetyl alcohol wax esters: Optimization by response surface methodology. BMC Biotechnol 2011, 11, 68. [Google Scholar]
- Huynh, L.H.; Do, Q.D.; Kasim, N.S.; Ju, Y.H. Isolation and analysis of wax esters from activated sludge. Bioresour. Technol 2011, 102, 9518–9523. [Google Scholar]
- Vali, S.R.; Ju, Y.H.; Kaimal, T.N.B.; Chern, Y.T. A process for the preparation of food-grade rice bran wax and the determination of its composition. J. Am. Oil Chem. Soc 2005, 82, 57–64. [Google Scholar]
- Ieda, N.; Mantri, K.; Miyata, Y.; Ozaki, A.; Komura, K.; Sugi, Y. Esterification of long-chain acids and alcohols catalyzed by ferric chloride hexahydrate. Ind. Eng. Chem. Res 2008, 47, 8631–8638. [Google Scholar]
- Hadzir, N.M.; Basri, M.; Rahman, M.B.A.; Razak, C.N.A.; Rahman, R.N.Z.A.; Salleh, A.B. Enzymatic alcoholysis of triolein to produce wax ester. J. Chem. Technol. Biotechnol 2001, 76, 511–515. [Google Scholar]
- Salis, A.; Solinas, V.; Monduzzi, M. Wax esters synthesis from heavy fraction of sheep milk fat and cetyl alcohol by immobilised lipases. J. Mol. Catal. B-Enzym 2003, 21, 167–174. [Google Scholar]
- Fernández-Lorente, G.; Betancor, L.; Carrascosa, A.V.; Guisán, J.M. Release of omega-3 fatty acids by the hydrolysis of fish oil catalyzed by lipases immobilized on hydrophobic supports. J. Am. Oil Chem. Soc 2011, 88, 1173–1178. [Google Scholar]
- Xin, J.Y.; Wang, Y.; Liu, T.; Lin, K.; Chang, L.; Xia, C.G. Biosysthesis of corn starch palmitate by lipase Novozym 435. Int. J. Mol. Sci 2012, 13, 7226–7236. [Google Scholar]
- Chen, H.C.; Kuo, C.H.; Twu, Y.K.; Chen, J.H.; Chang, C.M.J.; Liu, Y.C.; Shieh, C.J. A continuous ultrasound-assisted packed-bed bioreactor for the lipase-catalyzed synthesis of caffeic acid phenethyl ester. J. Chem. Technol. Biotechnol 2011, 86, 1289–1294. [Google Scholar]
- Rahman, N.F.A.; Basri, M.; Rahman, M.B.A.; Rahman, R.N.Z.R.A.; Salleh, A.B. High yield lipase-catalyzed synthesis of Engkabang fat esters for the cosmetic industry. Bioresour. Technol. 2011, 102, 2168–2176. [Google Scholar] [Green Version]
- Ramírez Fajardo, A.; Akoh, C.C.; Lai, O.M. Lipase-catalyzed incorporation of n-3 PUFA into palm oil. J. Am. Oil Chem. Soc 2003, 80, 1197–1200. [Google Scholar]
- Rodrigues, R.C.; Fernandez-Lafuente, R. Lipase from Rhizomucor miehei as an industrial biocatalyst in chemical process. J. Mol. Catal. B Enzym 2010, 64, 1–22. [Google Scholar]
- Domínguez De María, P.; Carboni-Oerlemans, C.; Tuin, B.; Bargeman, G.; van Der Meer, A.; van Gemert, R. Biotechnological applications of Candida antarctica lipase A: State-of-the-art. J. Mol. Catal. B Enzym 2005, 37, 36–46. [Google Scholar]
- Knez, Ž.; Laudani, C.G.; Habulin, M.; Reverchon, E. Exploiting the pressure effect on lipase-catalyzed wax ester synthesis in dense carbon dioxide. Biotechnol. Bioeng. 2007, 97, 1366–1375. [Google Scholar]
- Kuo, C.H.; Ju, H.Y.; Chu, S.W.; Chen, J.H.; Chang, C.M.J.; Liu, Y.C.; Shieh, C.J. Optimization of lipase-catalyzed synthesis of cetyl octanoate in supercritical carbon dioxide. J. Am. Oil Chem. Soc 2012, 89, 103–110. [Google Scholar]
- Zarevúcka, M.; Wimmer, Z. Plant products for pharmacology: Application of enzymes in their transformations. Int. J. Mol. Sci 2008, 9, 2447–2473. [Google Scholar]
- Gandhi, N.N.; Patil, N.S.; Sawant, S.B.; Joshi, J.B.; Wangikar, P.P.; Mukesh, D. Lipase-catalyzed esterification. Catal. Rev 2000, 42, 439–480. [Google Scholar]
- Kuo, C.H.; Chen, G.J.; Twu, Y.K.; Liu, Y.C.; Shieh, C.J. Optimum lipase immobilized on diamine-grafted PVDF membrane and its characterization. Ind. Eng. Chem. Res 2012, 51, 5141–5147. [Google Scholar]
- Kuo, C.H.; Liu, Y.C.; Chang, C.M.J.; Chen, J.H.; Chang, C.; Shieh, C.J. Optimum conditions for lipase immobilization on chitosan-coated Fe3O4 nanoparticles. Carbohydr. Polym 2012, 87, 2538–2545. [Google Scholar]
- Zhao, L.; He, Y.; Deng, X.; Xia, X.; Liang, J.; Yang, G.; Li, W.; Wang, H. Ultrasound-assisted extraction of syringin from the bark of Ilex rotunda thumb using response surface methodology. Int. J. Mol. Sci 2012, 13, 7607–7616. [Google Scholar]
- Wang, Y.; Sun, D.; Chen, H.; Qian, L.; Xu, P. Fatty acid composition and antioxidant activity of tea (Camellia sinensis L.) seed oil extracted by optimized supercritical carbon dioxide. Int. J. Mol. Sci 2011, 12, 7708–7719. [Google Scholar]
- Guo, Z.; Shen, L.; Ji, Z.; Wu, W. Enhanced production of a novel cyclic hexapeptide antibiotic (NW-G01) by Streptomyces alboflavus 313 using response surface methodology. Int. J. Mol. Sci 2012, 13, 5230–5241. [Google Scholar]
- Shieh, C.J.; Liao, H.F.; Lee, C.C. Optimization of lipase-catalyzed biodiesel by response surface methodology. Bioresour. Technol 2003, 88, 103–106. [Google Scholar]
- Yadav, G.D.; Devi, K.M. Enzymatic synthesis of perlauric acid using Novozym 435. Biochem. Eng. J 2002, 10, 93–101. [Google Scholar]
- Sun, S.; Shan, L.; Jin, Q.; Liu, Y.; Wang, X. Solvent-free synthesis of glyceryl ferulate using a commercial microbial lipase. Biotechnol. Lett 2007, 29, 945–949. [Google Scholar]
- Basri, M.; Rahman, R.N.; Ebrahimpour, A.; Salleh, A.B.; Gunawan, E.R.; Rahman, M.B.A. Comparison of estimation capabilities of response surface methodology (RSM) with artificial neural network (ANN) in lipase-catalyzed synthesis of palm-based wax ester. BMC Biotechnol 2007, 7, 53. [Google Scholar]
- Chowdary, G.; Prapulla, S. Enzymatic synthesis of ethyl hexanoate by transesterification. Int. J. Food Sci. Technol 2003, 38, 127–133. [Google Scholar]
- Hari Krishna, S.; Karanth, N. Lipase-catalyzed synthesis of isoamyl butyrate: A kinetic study. Biochim. Biophys. Aata 2001, 1547, 262–267. [Google Scholar]
- Liaw, E.T.; Liu, K.J. Synthesis of terpinyl acetate by lipase-catalyzed esterification in supercritical carbon dioxide. Bioresour. Technol 2010, 101, 3320–3324. [Google Scholar]
- Yadav, G.D.; Borkar, I.V. Kinetic modeling of immobilized lipase catalysis in synthesis of n-butyl levulinate. Ind. Eng. Chem. Res 2008, 47, 3358–3363. [Google Scholar]

| Treatment No. a | Factor | Lipozyme® RMIM yield Y1 (%) | Novozym® 435 yield Y2 (%) | |||
|---|---|---|---|---|---|---|
| Time X1 (h) | Temp X2 (°C) | Molar ratio X3 (Ac:Al) b | Enzyme Amount X4 (%) c | |||
| 1 | 1(4) b | −1(50) | 1(2.5) | −1(20) | 81.32 ± 0.76 | 92.96 ± 0.21 |
| 2 | 1(4) | −1(50) | −1(1.5) | −1(20) | 79.45 ± 4.25 | 84.93 ± 2.02 |
| 3 | 0(3) | 0(55) | 0(2.0) | 2(50) | 90.49 ± 1.06 | 96.07 ± 0.10 |
| 4 | 1(4) | −1(50) | −1(1.5) | 1(40) | 88.01 ± 1.89 | 93.90 ± 1.26 |
| 5 | 0(3) | 2(65) | 0(2.0) | 0(30) | 80.22 ± 3.72 | 94.09 ± 2.40 |
| 6 | −1(2) | −1(50) | −1(1.5) | 1(40) | 68.40 ± 0.69 | 86.83 ± 2.80 |
| 7 | 1(4) | 1(60) | 1(2.5) | 1(40) | 94.86 ± 1.90 | 97.39 ± 0.57 |
| 8 | −1(2) | −1(50) | 1(2.5) | 1(40) | 76.47 ± 2.02 | 92.18 ± 0.83 |
| 9 | 0(3) | 0(55) | 0(2.0) | 0(30) | 81.71 ± 2.60 | 92.41 ± 0.66 |
| 10 | −1(2) | −1(50) | −1(1.5) | −1(20) | 67.05 ± 2.26 | 64.16 ± 0.39 |
| 11 | 2(5) | 0(55) | 0(2.0) | 0(30) | 92.11 ± 0.29 | 95.78 ± 0.09 |
| 12 | −1(2) | 1(60) | −1(1.5) | −1(20) | 60.25 ± 1.86 | 79.20 ± 0.89 |
| 13 | −2(1) | 0(55) | 0(2.0) | 0(30) | 44.62 ± 1.93 | 70.75 ± 0.47 |
| 14 | −1(2) | 1(60) | 1(2.5) | −1(20) | 70.44 ± 0.98 | 85.29 ± 3.01 |
| 15 | 0(3) | 0(55) | 0(2.0) | −2(10) | 72.50 ± 0.46 | 66.83 ± 1.36 |
| 16 | 1(4) | 1(60) | 1(2.5) | −1(20) | 86.90 ± 1.34 | 97.22 ± 1.74 |
| 17 | 0(3) | 0(55) | −2(1.0) | 0(30) | 73.67 ± 3.62 | 78.23 ± 2.24 |
| 18 | −1(2) | −1(50) | 1(2.5) | −1(20) | 60.22 ± 0.85 | 76.64 ± 1.93 |
| 19 | 0(3) | 0(55) | 2(3.0) | 0(30) | 70.65 ± 2.68 | 94.28 ± 0.86 |
| 20 | 0(3) | −2(45) | 0(2.0) | 0(30) | 75.49 ± 0.89 | 88.77 ± 0.41 |
| 21 | 1(4) | 1(60) | −1(1.5) | −1(20) | 89.75 ± 0.93 | 91.62 ± 0.29 |
| 22 | 1(4) | 1(60) | −1(1.5) | 1(40) | 90.58 ± 1.54 | 95.76 ± 0.63 |
| 23 | −1(2) | 1(60) | −1(1.5) | 1(40) | 78.56 ± 0.64 | 88.85 ± 2.20 |
| 24 | −1(2) | 1(60) | 1(2.5) | 1(40) | 76.58 ± 2.86 | 97.15 ± 0.97 |
| 25 | 0(3) | 0(55) | 0(2.0) | 0(30) | 80.65 ± 1.05 | 92.40 ± 2.06 |
| 26 | 1(4) | −1(50) | 1(2.5) | 1(40) | 91.91 ± 1.28 | 97.95 ± 0.11 |
| 27 | 0(3) | 0(55) | 0(2.0) | 0(30) | 80.08 ± 1.63 | 93.14 ± 1.47 |
| Source | Lipozyme® RMIM | Novozym® 435 | ||
|---|---|---|---|---|
| Sum of squares | p-Value | Sum of squares | p-Value | |
| Linear | 2951.08 | <0.0001 * | 1907.39 | <0.0001 * |
| Quadratic | 233.75 | 0.0887 b | 177.31 | 0.0222 a |
| Crossproduct | 26.51 | 0.9713 b | 197.06 | 0.0422 a |
| Total Model | 3211.35 | 0.0001 a | 2281.77 | <0.0001 * |
| Lack of Fit | 267.29 | 0.0252 a | 124.00 | 0.0144 a |
| Pure Error | 1.36 | 0.36 | ||
| Total Error | 268.65 | 124.36 | ||
| Factor | Lipozyme® RMIM | Novozym® 435 | ||
|---|---|---|---|---|
| Sum of squares | p-Value | Sum of squares | p-Value | |
| Time (X1) | 2542.85 | <0.0001 a | 928.55 | <0.0001 a |
| Temperature (X2) | 87.83 | 0.5800 | 189.73 | 0.0304 a |
| Molar ratio (X3) | 63.90 | 0.7214 | 340.11 | 0.0037 a |
| Enzyme amount (X4) | 503.89 | 0.0153 a | 1045.50 | <0.0001 a |
| Item | X1 (h) | X2 (°C) | X3 (Ac:Al) a | X4 (%) | Predicted yield (%) | Experimental yield (%) |
|---|---|---|---|---|---|---|
| Lipozyme® RMIM | 4.00 | 57.51 | 2.12 | 46.41 | 97.56 ± 3.61 | 94.21 ± 1.56 |
| Novozym® 435 | 3.65 | 57.84 | 2.35 | 34.38 | 98.39 ± 1.61 | 98.24 ± 0.11 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kuo, C.-H.; Chen, H.-H.; Chen, J.-H.; Liu, Y.-C.; Shieh, C.-J. High Yield of Wax Ester Synthesized from Cetyl Alcohol and Octanoic Acid by Lipozyme RMIM and Novozym 435. Int. J. Mol. Sci. 2012, 13, 11694-11704. https://doi.org/10.3390/ijms130911694
Kuo C-H, Chen H-H, Chen J-H, Liu Y-C, Shieh C-J. High Yield of Wax Ester Synthesized from Cetyl Alcohol and Octanoic Acid by Lipozyme RMIM and Novozym 435. International Journal of Molecular Sciences. 2012; 13(9):11694-11704. https://doi.org/10.3390/ijms130911694
Chicago/Turabian StyleKuo, Chia-Hung, Hsin-Hung Chen, Jiann-Hwa Chen, Yung-Chuan Liu, and Chwen-Jen Shieh. 2012. "High Yield of Wax Ester Synthesized from Cetyl Alcohol and Octanoic Acid by Lipozyme RMIM and Novozym 435" International Journal of Molecular Sciences 13, no. 9: 11694-11704. https://doi.org/10.3390/ijms130911694
APA StyleKuo, C.-H., Chen, H.-H., Chen, J.-H., Liu, Y.-C., & Shieh, C.-J. (2012). High Yield of Wax Ester Synthesized from Cetyl Alcohol and Octanoic Acid by Lipozyme RMIM and Novozym 435. International Journal of Molecular Sciences, 13(9), 11694-11704. https://doi.org/10.3390/ijms130911694







