Gelam Honey Scavenges Peroxynitrite During the Immune Response
Abstract
:1. Introduction
2. Results
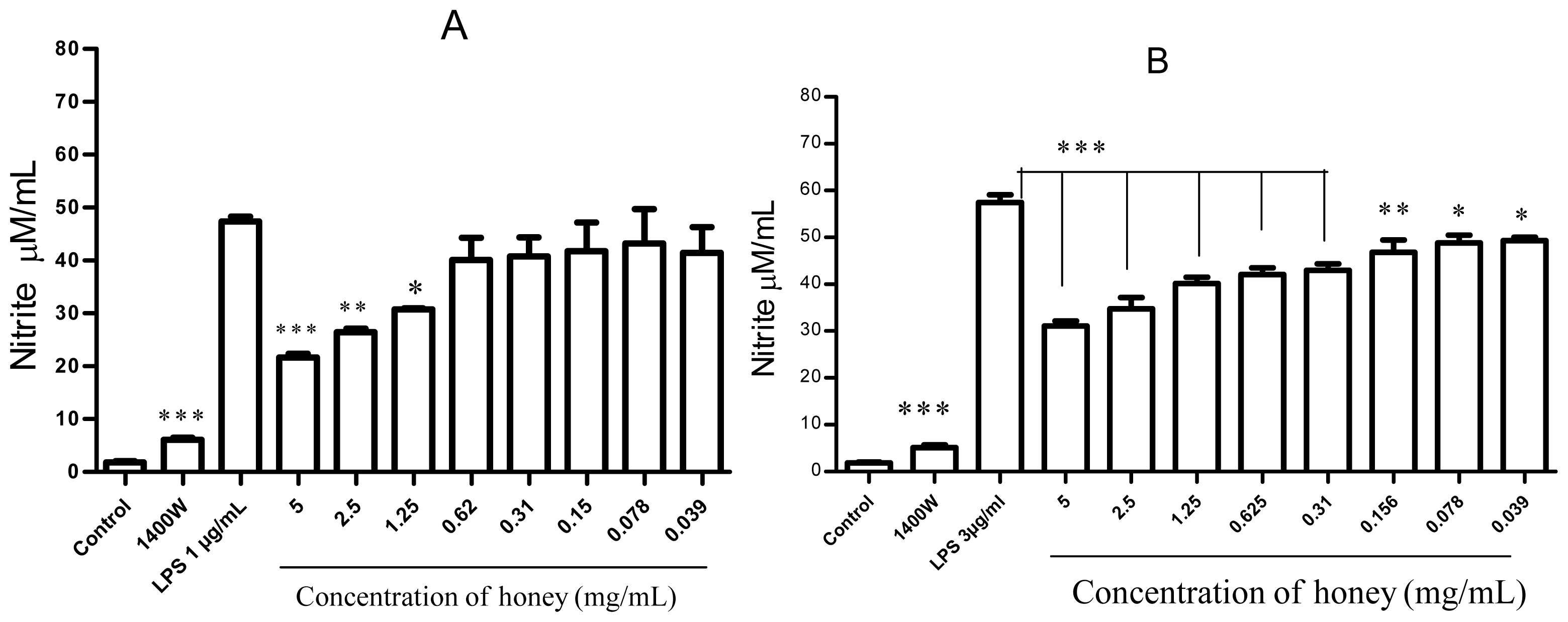
2.1. Effect of Gelam Honey on Untreated and LPS/IFN-γ-Stimulated Cells
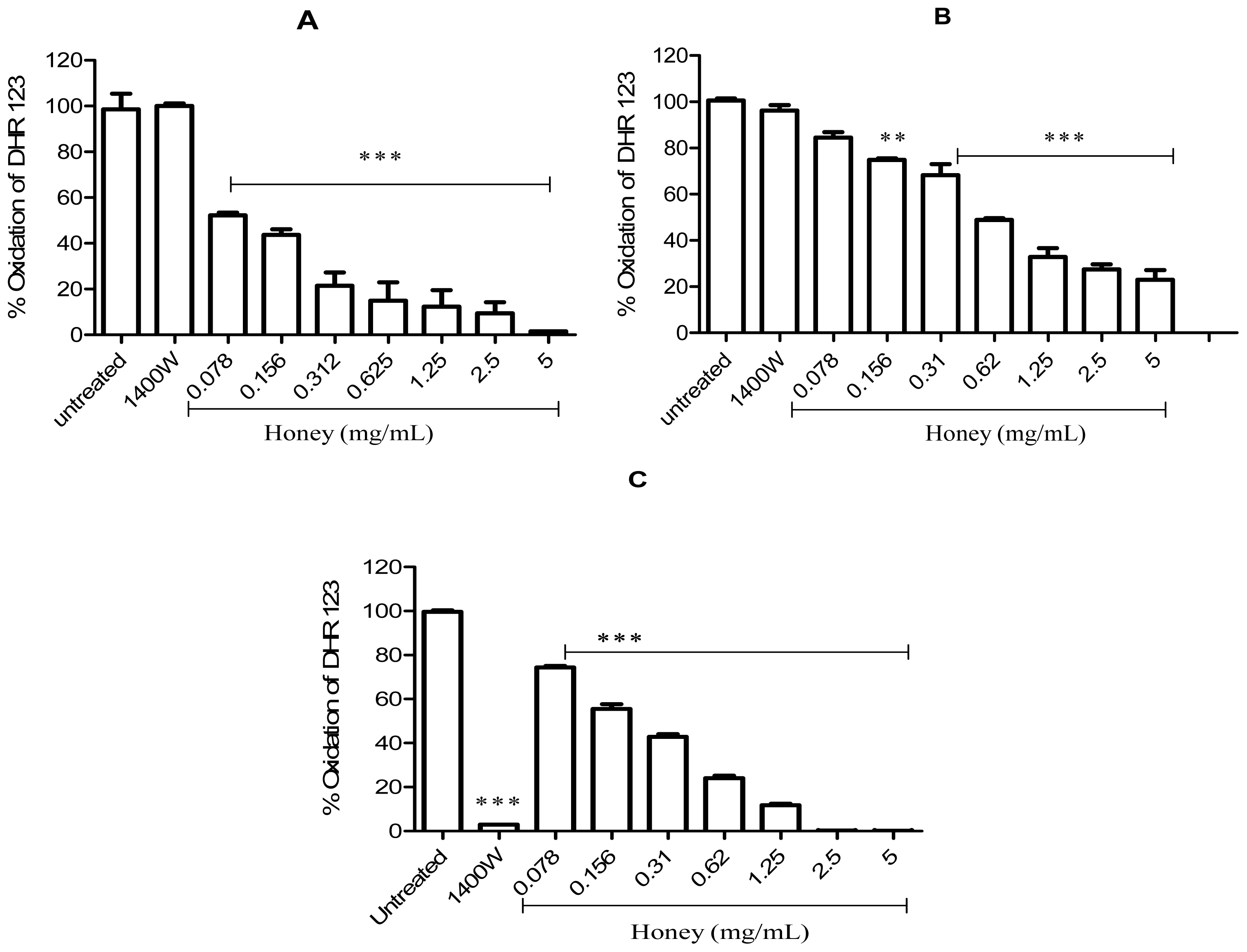
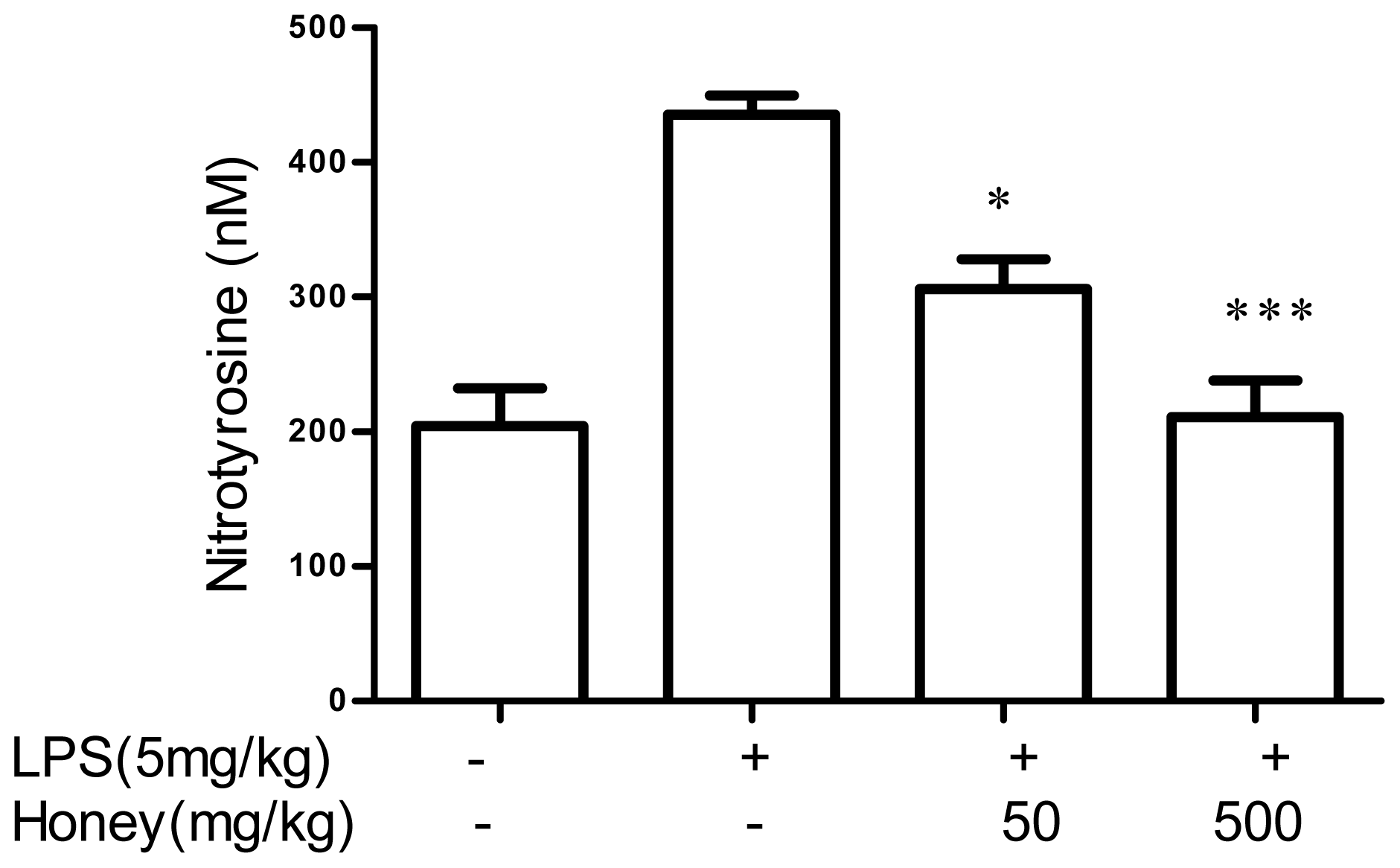
2.2. Effect of Gelam Honey on Peroxynitrite in vitro and in vivo
3. Discussion
4. Experimental Section
4.1. Preparation of Gelam Honey
4.2. Animals
4.3. Cell Culture and Reagents
4.4. LPS/IFN-γ Stimulation of RAW 264.7 Cells
4.5. Measurement of Mitochondrial Respiration
4.6. Nitric Oxide Assay
4.7. DHR-123 Oxidation Assay
4.7.1. DHR-123 Oxidation Using SIN-1
4.7.2. DHR-123 Oxidation Using Peroxynitrite
4.7.3. DHR-123 Oxidation by LPS/IFN-γ-Treated RAW 264.7 Cells
4.8. Induction of an Immune Response in LPS-Stimulated Rats and the Effects of Honey
4.9. Statistical Analysis
5. Conclusion
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Sampath, V.; Radish, A.C.; Eis, A.L.; Broniowska, K.; Hogg, N.; Konduri, G.G. Attenuation of lipopolysaccharide-induced oxidative stress and apoptosis in fetal pulmonary artery endothelial cells by hypoxia. Free Radical Biol. Med 2009, 46, 663–671. [Google Scholar]
- Kedzierska, K.; Crowe, S.M. Cytokines and hiv-1: Interactions and clinical implications. Antiviral Chem. Chemother 2001, 12, 133–150. [Google Scholar]
- Hanna, M.G., Jr. Immunologic aspects of bcg-mediated regression of established tumors and metastases in guinea pigs. Semin. Oncol. 1974, 1, 319–335. [Google Scholar]
- Sawa, T.; Ohshima, H. Nitrative DNA damage in inflammation and its possible role in carcinogenesis. Nitric Oxide 2006, 14, 91–100. [Google Scholar]
- Kimura, H.; Hokari, R.; Miura, S.; Shigematsu, T.; Hirokawa, M.; Akiba, Y.; Kurose, I.; Higuchi, H.; Fujimori, H.; Tsuzuki, Y.; et al. Increased expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in colonic mucosa of patients with active ulcerative colitis. Gut 1998, 42, 180–187. [Google Scholar]
- Han, K.Y.; Kwon, T.H.; Lee, T.H.; Lee, S.J.; Kim, S.H.; Kim, J. Suppressive effects of lithospermum erythrorhizon extracts on lipopolysaccharide-induced activation of ap-1 and nf-kappab via mitogen-activated protein kinase pathways in mouse macrophage cells. BMB Rep 2008, 41, 328–333. [Google Scholar]
- Dannenberg, A.J.; Altorki, N.K.; Boyle, J.O.; Dang, C.; Howe, L.R.; Weksler, B.B.; Subbaramaiah, K. Cyclo-oxygenase 2: A pharmacological target for the prevention of cancer. Lancet Oncol 2001, 2, 544–551. [Google Scholar]
- Petros, A.; Bennett, D.; Vallance, P. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet 1991, 338, 1557–1558. [Google Scholar]
- Szabo, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov 2007, 6, 662–680. [Google Scholar]
- Zouki, C.; Jozsef, L.; Ouellet, S.; Paquette, Y.; Filep, J.G. Peroxynitrite mediates cytokine-induced il-8 gene expression and production by human leukocytes. J. Leukoc. Biol 2001, 69, 815–824. [Google Scholar]
- Szabo, C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol. Lett 2003, 140–141, 105–112. [Google Scholar]
- Lanone, S.; Manivet, P.; Callebert, J.; Launay, J.M.; Payen, D.; Aubier, M.; Boczkowski, J.; Mebazaa, A. Inducible nitric oxide synthase (nos2) expressed in septic patients is nitrated on selected tyrosine residues: Implications for enzymic activity. Biochem. J 2002, 366, 399–404. [Google Scholar]
- Whiteman, M.; Ketsawatsakul, U.; Halliwell, B. A reassessment of the peroxynitrite scavenging activity of uric acid. Ann. N. Y. Acad. Sci 2002, 962, 242–259. [Google Scholar]
- Scott, G.S.; Spitsin, S.V.; Kean, R.B.; Mikheeva, T.; Koprowski, H.; Hooper, D.C. Therapeutic intervention in experimental allergic encephalomyelitis by administration of uric acid precursors. Proc. Natl. Acad. Sci. USA 2002, 99, 16303–16308. [Google Scholar]
- Robinson, K.M.; Morre, J.T.; Beckman, J.S. Triuret: A novel product of peroxynitrite-mediated oxidation of urate. Arch. Biochem. Biophys 2004, 423, 213–217. [Google Scholar]
- Scott, G.S.; Cuzzocrea, S.; Genovese, T.; Koprowski, H.; Hooper, D.C. Uric acid protects against secondary damage after spinal cord injury. Proc. Natl. Acad. Sci. USA 2005, 102, 3483–3488. [Google Scholar]
- Sanz, M.L.; Sanz, J.; Martinez-Castro, I. Gas chromatographic-mass spectrometric method for the qualitative and quantitative determination of disaccharides and trisaccharides in honey. J. Chromatogr. A 2004, 1059, 143–148. [Google Scholar]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S. Honey—A novel antidiabetic agent. Int. J. Biol. Sci 2012, 8, 913–934. [Google Scholar]
- Munstedt, K.; Bohme, M.; Hauenschild, A.; Hrgovic, I. Consumption of rapeseed honey leads to higher serum fructose levels compared with analogue glucose/fructose solutions. Eur. J. Clin. Nutr 2011, 65, 77–80. [Google Scholar]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S. Fructose might contribute to the hypoglycemic effect of honey. Molecules 2012, 17, 1900–1915. [Google Scholar]
- Munstedt, K.; Sheybani, B.; Hauenschild, A.; Bruggmann, D.; Bretzel, R.G.; Winter, D. Effects of basswood honey, honey-comparable glucose-fructose solution, and oral glucose tolerance test solution on serum insulin, glucose, and c-peptide concentrations in healthy subjects. J. Med. Food 2008, 11, 424–428. [Google Scholar]
- Gomes, T.; Feas, X.; Iglesias, A.; Estevinho, L.M. Study of organic honey from the northeast portugal. Molecules 2011, 16, 5374–5386. [Google Scholar]
- Martos, I.; Ferreres, F.; Yao, L.; D’Arcy, B.; Caffin, N.; Tomas-Barberan, F.A. Flavonoids in monospecific eucalyptus honeys from australia. J. Agric. Food Chem 2000, 48, 4744–4748. [Google Scholar]
- Bertoncelj, J.; Dobersek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of slovenian honey. Food Chem 2007, 105, 822–828. [Google Scholar]
- Mohamed, M.; Sulaiman, S.A.; Jaafar, H.; Sirajudeen, K.N. Antioxidant protective effect of honey in cigarette smoke-induced testicular damage in rats. Int. J. Mol. Sci 2011, 12, 5508–5521. [Google Scholar]
- Moskaug, J.O.; Carlsen, H.; Myhrstad, M.C.; Blomhoff, R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr 2005, 81, 277S–283S. [Google Scholar]
- Morais, M.; Moreira, L.; Feas, X.; Estevinho, L.M. Honeybee-collected pollen from five portuguese natural parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem. Toxicol 2011, 49, 1096–1101. [Google Scholar]
- Hung, H.C.; Joshipura, K.J.; Jiang, R.; Hu, F.B.; Hunter, D.; Smith-Warner, S.A.; Colditz, G.A.; Rosner, B.; Spiegelman, D.; Willett, W.C. Fruit and vegetable intake and risk of major chronic disease. J. Natl. Cancer Inst 2004, 96, 1577–1584. [Google Scholar]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Cardetti, M.; Keen, C.L. Honey with high levels of antioxidants can provide protection to healthy human subjects. J. Agric. Food Chem 2003, 51, 1732–1735. [Google Scholar]
- Tartibian, B.; Maleki, B.H. The effects of honey supplementation on seminal plasma cytokines, oxidative stress biomarkers, and antioxidants during 8 weeks of intensive cycling training. J. Androl 2012, 33, 449–461. [Google Scholar]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of northeast portugal honey. Food Chem. Toxicol 2008, 46, 3774–3779. [Google Scholar]
- Chaudhuri, S.; Banerjee, A.; Basu, K.; Sengupta, B.; Sengupta, P.K. Interaction of flavonoids with red blood cell membrane lipids and proteins: Antioxidant and antihemolytic effects. Int. J. Biol. Macromol 2007, 41, 42–48. [Google Scholar]
- Pawlikowska-Pawlega, B.; Gruszecki, W.I.; Misiak, L.E.; Gawron, A. The study of the quercetin action on human erythrocyte membranes. Biochem. Pharmacol 2003, 66, 605–612. [Google Scholar]
- Mendes, L.; de Freitas, V.; Baptista, P.; Carvalho, M. Comparative antihemolytic and radical scavenging activities of strawberry tree (arbutus unedo l.) leaf and fruit. Food Chem. Toxicol 2011, 49, 2285–2291. [Google Scholar]
- Valente, M.J.; Baltazar, A.F.; Henrique, R.; Estevinho, L.; Carvalho, M. Biological activities of portuguese propolis: Protection against free radical-induced erythrocyte damage and inhibition of human renal cancer cell growth in vitro. Food Chem. Toxicol 2011, 49, 86–92. [Google Scholar]
- Beretta, G.; Orioli, M.; Facino, R.M. Antioxidant and radical scavenging activity of honey in endothelial cell cultures (ea.Hy926). Planta Med 2007, 73, 1182–1189. [Google Scholar]
- Blasa, M.; Candiracci, M.; Accorsi, A.; Piacentini, M.P.; Albertini, M.C.; Piatti, E. Raw millefiori honey is packed full of antioxidants. Food Chem 2006, 97, 217–222. [Google Scholar]
- Aljadi, A.M.; Kamaruddin, M.Y. Evaluation of the phenolic contents and antioxidant capacities of two malaysian floral honeys. Food Chem 2004, 85, 513–518. [Google Scholar]
- Kassim, M.; Achoui, M.; Mansor, M.; Yusoff, K.M. The inhibitory effects of gelam honey and its extracts on nitric oxide and prostaglandin e(2) in inflammatory tissues. Fitoterapia 2010, 81, 1196–1201. [Google Scholar]
- Kassim, M.; Achoui, M.; Mustafa, M.R.; Mohd, M.A.; Yusoff, K.M. Ellagic acid, phenolic acids, and flavonoids in malaysian honey extracts demonstrate in vitro anti-inflammatory activity. Nutr. Res 2010, 30, 650–659. [Google Scholar]
- Kassim, M.; Yusoff, K.M.; Ong, G.; Sekaran, S.; Yusof, M.Y.; Mansor, M. Gelam honey inhibits lipopolysaccharide-induced endotoxemia in rats through the induction of heme oxygenase-1 and the inhibition of cytokines, nitric oxide, and high-mobility group protein b1. Fitoterapia 2012, 83, 1054–1059. [Google Scholar]
- Kassim, M.; Mansor, M.; Al-Abd, N.; Yusoff, K.M. Gelam honey has a protective effect against lipopolysaccharide (lps)-induced organ failure. Int. J. Mol. Sci 2012, 13, 6370–6381. [Google Scholar]
- Yao, L.K.; Abdul Razak, S.L.; Nazhirah, I.; Ng, C.F.; Asyraf, M.H.; Asgar, M.; Nursyahirah, M.S.; Aan, G.J.; Jubri, Z. Malaysian gelam honey reduces oxidative damage and modulates antioxidant enzyme activities in young and middle aged rats. J. Med. Plant Res 2011, 5, 5618–5625. [Google Scholar]
- Fang, F.C. Perspectives series: Host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig 1997, 99, 2818–2825. [Google Scholar]
- Jagtap, P.; Szabo, C. Poly(adp-ribose) polymerase and the therapeutic effects of its inhibitors. Nat. Rev. Drug Discov 2005, 4, 421–440. [Google Scholar]
- Virag, L.; Szabo, E.; Gergely, P.; Szabo, C. Peroxynitrite-induced cytotoxicity: Mechanism and opportunities for intervention. Toxicol. Lett 2003, 140–141, 113–124. [Google Scholar]
- Shacka, J.J.; Sahawneh, M.A.; Gonzalez, J.D.; Ye, Y.Z.; D’Alessandro, T.L.; Estevez, A.G. Two distinct signaling pathways regulate peroxynitrite-induced apoptosis in pc12 cells. Cell Death Differ 2006, 13, 1506–1514. [Google Scholar]
- Virag, L.; Marmer, D.J.; Szabo, C. Crucial role of apopain in the peroxynitrite-induced apoptotic DNA fragmentation. Free Radic Biol. Med 1998, 25, 1075–1082. [Google Scholar]
- Zhuang, S.; Simon, G. Peroxynitrite-induced apoptosis involves activation of multiple caspases in hl-60 cells. Am. J. Physiol. Cell Physiol 2000, 279, C341–C351. [Google Scholar]
- Vicente, S.; Perez-Rodriguez, R.; Olivan, A.M.; Martinez Palacian, A.; Gonzalez, M.P.; Oset-Gasque, M.J. Nitric oxide and peroxynitrite induce cellular death in bovine chromaffin cells: Evidence for a mixed necrotic and apoptotic mechanism with caspases activation. J. Neurosci. Res 2006, 84, 78–96. [Google Scholar]
- Kim, Y.M.; Chung, H.T.; Simmons, R.L.; Billiar, T.R. Cellular non-heme iron content is a determinant of nitric oxide-mediated apoptosis, necrosis, and caspase inhibition. J. Biol. Chem 2000, 275, 10954–10961. [Google Scholar]
- Stadler, J.; Bergonia, H.A.; Di Silvio, M.; Sweetland, M.A.; Billiar, T.R.; Simmons, R.L.; Lancaster, J.R., Jr. Nonheme iron-nitrosyl complex formation in rat hepatocytes: Detection by electron paramagnetic resonance spectroscopy. Arch. Biochem. Biophys. 1993, 302, 4–11. [Google Scholar]
- Kazmierski, W.M.; Wolberg, G.; Wilson, J.G.; Smith, S.R.; Williams, D.S.; Thorp, H.H.; Molina, L. Iron chelates bind nitric oxide and decrease mortality in an experimental model of septic shock. Proc. Natl. Acad. Sci. USA 1996, 93, 9138–9141. [Google Scholar]
- Kim, Y.M.; Chung, H.T.; Kim, S.S.; Han, J.A.; Yoo, Y.M.; Kim, K.M.; Lee, G.H.; Yun, H.Y.; Green, A.; Li, J.; et al. Nitric oxide protects pc12 cells from serum deprivation-induced apoptosis by cgmp-dependent inhibition of caspase signaling. J. Neurosci 1999, 19, 6740–6747. [Google Scholar]
- Boese, M.; Mordvintcev, P.I.; Vanin, A.F.; Busse, R.; Mulsch, A. S-nitrosation of serum albumin by dinitrosyl-iron complex. J. Biol. Chem 1995, 270, 29244–29249. [Google Scholar]
- Zhang, X.; Chen, J.; Graham, S.H.; Du, L.; Kochanek, P.M.; Draviam, R.; Guo, F.; Nathaniel, P.D.; Szabo, C.; Watkins, S.C.; et al. Intranuclear localization of apoptosis-inducing factor (aif) and large scale DNA fragmentation after traumatic brain injury in rats and in neuronal cultures exposed to peroxynitrite. J. Neurochem 2002, 82, 181–191. [Google Scholar]
- Nin, N.; Cassina, A.; Boggia, J.; Alfonso, E.; Botti, H.; Peluffo, G.; Trostchansky, A.; Batthyany, C.; Radi, R.; Rubbo, H.; et al. Septic diaphragmatic dysfunction is prevented by mn(iii)porphyrin therapy and inducible nitric oxide synthase inhibition. Intensive Care Med 2004, 30, 2271–2278. [Google Scholar]
- Trujillo, M.; Budde, H.; Pineyro, M.D.; Stehr, M.; Robello, C.; Flohe, L.; Radi, R. Trypanosoma brucei and trypanosoma cruzi tryparedoxin peroxidases catalytically detoxify peroxynitrite via oxidation of fast reacting thiols. J. Biol. Chem 2004, 279, 34175–34182. [Google Scholar]
- Dubuisson, M.; Vander Stricht, D.; Clippe, A.; Etienne, F.; Nauser, T.; Kissner, R.; Koppenol, W.H.; Rees, J.F.; Knoops, B. Human peroxiredoxin 5 is a peroxynitrite reductase. FEBS Lett 2004, 571, 161–165. [Google Scholar]
- Rhee, S.G.; Chae, H.Z.; Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol. Med 2005, 38, 1543–1552. [Google Scholar]
- Spitsin, S.V.; Scott, G.S.; Kean, R.B.; Mikheeva, T.; Hooper, D.C. Protection of myelin basic protein immunized mice from free-radical mediated inflammatory cell invasion of the central nervous system by the natural peroxynitrite scavenger uric acid. Neurosci. Lett 2000, 292, 137–141. [Google Scholar]
- Hooper, D.C.; Spitsin, S.; Kean, R.B.; Champion, J.M.; Dickson, G.M.; Chaudhry, I.; Koprowski, H. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. USA 1998, 95, 675–680. [Google Scholar]
- Szabo, C.; Ferrer-Sueta, G.; Zingarelli, B.; Southan, G.J.; Salzman, A.L.; Radi, R. Mercaptoethylguanidine and guanidine inhibitors of nitric-oxide synthase react with peroxynitrite and protect against peroxynitrite-induced oxidative damage. J. Biol. Chem 1997, 272, 9030–9036. [Google Scholar]
- Ploner, F.; Radermacher, P.; Theisen, M.; Tugtekin, I.F.; Matejovic, M.; Stehr, A.; Szabo, C.; Southan, G.J.; Georgieff, M.; Bruckner, U.B.; et al. Effects of combined selective inos inhibition and peroxynitrite blockade during endotoxemia in pigs. Shock 2001, 16, 130–136. [Google Scholar]
- Lancel, S.; Tissier, S.; Mordon, S.; Marechal, X.; Depontieu, F.; Scherpereel, A.; Chopin, C.; Neviere, R. Peroxynitrite decomposition catalysts prevent myocardial dysfunction and inflammation in endotoxemic rats. J. Am. Coll. Cardiol 2004, 43, 2348–2358. [Google Scholar]
- Klotz, L.O.; Sies, H. Defenses against peroxynitrite: Selenocompounds and flavonoids. Toxicol. Lett 2003, 140–141, 125–132. [Google Scholar]
- Daiber, A.; Zou, M.H.; Bachschmid, M.; Ullrich, V. Ebselen as a peroxynitrite scavenger in vitro and ex vivo. Biochem. Pharmacol 2000, 59, 153–160. [Google Scholar]
- Noiri, E.; Nakao, A.; Uchida, K.; Tsukahara, H.; Ohno, M.; Fujita, T.; Brodsky, S.; Goligorsky, M.S. Oxidative and nitrosative stress in acute renal ischemia. Am. J. Physiol. Renal Physiol 2001, 281, F948–F957. [Google Scholar]
- Crow, J.P. Peroxynitrite scavenging by metalloporphyrins and thiolates. Free Radic Biol. Med 2000, 28, 1487–1494. [Google Scholar]
- Briviba, K.; Roussyn, I.; Sharov, V.S.; Sies, H. Attenuation of oxidation and nitration reactions of peroxynitrite by selenomethionine, selenocystine and ebselen. Biochem. J 1996, 319, 13–15. [Google Scholar]
- Tabuchi, Y.; Sugiyama, N.; Horiuchi, T.; Furusawa, M.; Furuhama, K. Ebselen, a seleno-organic compound, protects against ethanol-induced murine gastric mucosal injury in both in vivo and in vitro systems. Eur. J. Pharmacol 1995, 272, 195–201. [Google Scholar]
- Batinic-Haberle, I.; Spasojevic, I.; Stevens, R.D.; Hambright, P.; Neta, P.; Okado-Matsumoto, A.; Fridovich, I. New class of potent catalysts of O2.-dismutation. Mn(iii) ortho-methoxyethylpyridyland di-ortho-methoxyethylimidazolylporphyrins. Dalton Trans 2004, 1696–1702. [Google Scholar]
- Wang, W.; Jittikanont, S.; Falk, S.A.; Li, P.; Feng, L.; Gengaro, P.E.; Poole, B.D.; Bowler, R.P.; Day, B.J.; Crapo, J.D.; et al. Interaction among nitric oxide, reactive oxygen species, and antioxidants during endotoxemia-related acute renal failure. Am. J. Physiol. Renal Physiol 2003, 284, F532–F537. [Google Scholar]
- Brodsky, S.V.; Gealekman, O.; Chen, J.; Zhang, F.; Togashi, N.; Crabtree, M.; Gross, S.S.; Nasjletti, A.; Goligorsky, M.S. Prevention and reversal of premature endothelial cell senescence and vasculopathy in obesity-induced diabetes by ebselen. Circ. Res 2004, 94, 377–384. [Google Scholar]
- Gealekman, O.; Brodsky, S.V.; Zhang, F.; Chander, P.N.; Friedli, C.; Nasjletti, A.; Goligorsky, M.S. Endothelial dysfunction as a modifier of angiogenic response in zucker diabetic fat rat: Amelioration with ebselen. Kidney Int 2004, 66, 2337–2347. [Google Scholar]
- Crow, J.P. Catalytic antioxidants to treat amyotropic lateral sclerosis. Expert Opin. Investig. Drugs 2006, 15, 1383–1393. [Google Scholar]
- Beckman, J.S. Oxidative damage and tyrosine nitration from peroxynitrite. Chem. Res. Toxicol 1996, 9, 836–844. [Google Scholar]
- Pannala, A.S.; Razaq, R.; Halliwell, B.; Singh, S.; Rice-Evans, C.A. Inhibition of peroxynitrite dependent tyrosine nitration by hydroxycinnamates: Nitration or electron donation? Free Radic Biol. Med 1998, 24, 594–606. [Google Scholar]
- Kassim, M.; Mansor, M.; Achoui, M.; Ong, G.S.Y.; Sekaran, S.D.; Yusoff, K.M. Honey as an immunomodulator during sepsis in animal models. Crit. Care J 2009, 13, P40. [Google Scholar]
- Hussein, S.Z.; Mohd Yusoff, K.; Makpol, S.; Mohd Yusof, Y.A. Gelam honey inhibits the production of proinflammatory, mediators no, pge2, tnf-α, and il-6 in carrageenan-induced acute paw edema in rats. Evid.-Based Complement. Altern. Med 2012, 2012, 13. [Google Scholar]
- Laskin, D.L.; Pendino, K.J. Macrophages and inflammatory mediators in tissue injury. Annu. Rev. Pharmacol. Toxicol 1995, 35, 655–677. [Google Scholar]
- Cheng, Y.W.; Chang, C.Y.; Lin, K.L.; Hu, C.M.; Lin, C.H.; Kang, J.J. Shikonin derivatives inhibited lps-induced nos in raw 264.7 cells via downregulation of mapk/nf-kappab signaling. J. Ethnopharmacol 2008, 120, 264–271. [Google Scholar]
- Sandur, S.K.; Ichikawa, H.; Sethi, G.; Ahn, K.S.; Aggarwal, B.B. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) suppresses nf-kappab activation and nf-kappab-regulated gene products through modulation of p65 and ikappabalpha kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J. Biol. Chem 2006, 281, 17023–17033. [Google Scholar]
- Muijsers, R.B.; van Den Worm, E.; Folkerts, G.; Beukelman, C.J.; Koster, A.S.; Postma, D.S.; Nijkamp, F.P. Apocynin inhibits peroxynitrite formation by murine macrophages. Br. J. Pharmacol 2000, 130, 932–936. [Google Scholar]
- Achoui, M.; Appleton, D.; Abdulla, M.A.; Awang, K.; Mohd, M.A.; Mustafa, M.R. In vitro and in vivo anti-inflammatory activity of 17-O-acetylacuminolide through the inhibition of cytokines, nf-kappab translocation and ikkbeta activity. PLoS One 2010, 5, e15105. [Google Scholar]


© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kassim, M.; Mansor, M.; Suhaimi, A.; Ong, G.; Yusoff, K.M. Gelam Honey Scavenges Peroxynitrite During the Immune Response. Int. J. Mol. Sci. 2012, 13, 12113-12129. https://doi.org/10.3390/ijms130912113
Kassim M, Mansor M, Suhaimi A, Ong G, Yusoff KM. Gelam Honey Scavenges Peroxynitrite During the Immune Response. International Journal of Molecular Sciences. 2012; 13(9):12113-12129. https://doi.org/10.3390/ijms130912113
Chicago/Turabian StyleKassim, Mustafa, Marzida Mansor, Anwar Suhaimi, Gracie Ong, and Kamaruddin Mohd Yusoff. 2012. "Gelam Honey Scavenges Peroxynitrite During the Immune Response" International Journal of Molecular Sciences 13, no. 9: 12113-12129. https://doi.org/10.3390/ijms130912113
APA StyleKassim, M., Mansor, M., Suhaimi, A., Ong, G., & Yusoff, K. M. (2012). Gelam Honey Scavenges Peroxynitrite During the Immune Response. International Journal of Molecular Sciences, 13(9), 12113-12129. https://doi.org/10.3390/ijms130912113



