Oxidative Stress and DNA Damage in Human Gastric Carcinoma: 8-Oxo-7'8-dihydro-2'-deoxyguanosine (8-oxo-dG) as a Possible Tumor Marker
Abstract
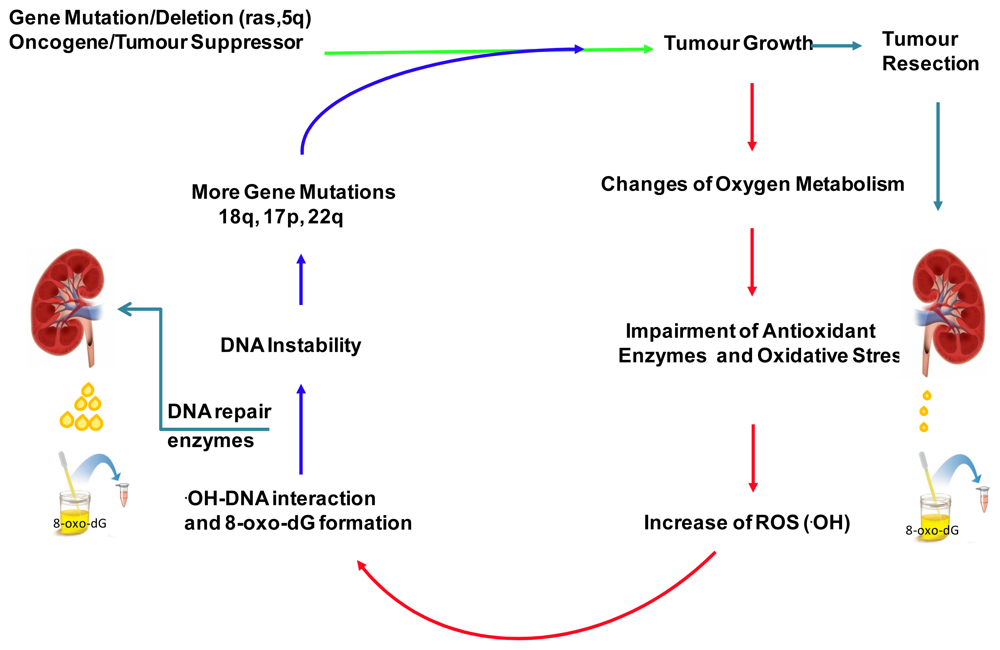
:1. Introduction
2. Results and Discussion
2.1. Oxidative Stress Status in Gastric Tumor Patients
2.2. DNA Repair Enzyme mRNA Expression
2.3. Gastrectomy Time Course Effect on Urinary and PMNC 8-oxo-dG Levels
2.4. Discussion
3. Experimental Section
3.1. Recruitment of Subjects and Biological Samples
3.2. Glutathione and Lipid Peroxidation Assay
3.3. DNA Isolation and Enzymatic Digestion
3.4. DNA 8-Oxo-7,8-dihydro-2′-deoxyguanosine Separation and Assay
3.5. Urinary 8-Oxo-7,8-dihydro-2′-deoxyguanosine Isolation and Assay
3.6. mRNA Expression of DNA Repair Enzymes
3.7. Statistical Analyses
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Sies, H. Oxidative Stress; Academic Press Inc: Orlando, FL, USA, 1985. [Google Scholar]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation and disease. FASEB J 2003, 17, 1195–1214. [Google Scholar]
- Gackowski, D.; Speina, E.; Zielinska, M.; Lowalewski, J.; Rozalski, R.; Siomek, A.; Paciorek, T.; Tudek, B.; Olinski, R. Products of oxidative DNA damage and repair as possible biomarkers of susceptibility to lung cancer. Cancer Res 2003, 63, 4899–4902. [Google Scholar]
- Andreoli, R.; Mutti, A.; Goldoni, M.; Manini, P.; Apostoli, P.; de Palma, G. Reference ranges of urinary biomarkers of oxidized guanine in (2-deoxy) ribonucleotides and nucleic acids. Free Radic. Biol. Med 2011, 50, 254–261. [Google Scholar]
- Blumberg, J. Use of biomarkers of oxidative stress in research studies. J. Nutr 2004, 134, S3188–S3189. [Google Scholar]
- Schisterman, E.F.; Faraggi, D.; Browne, R.; Freudenheim, J.; Dron, J.; Muti, P.; Armastrong, D.; Reiser, B.; Trevisan, M. Minimal and best linear combination of oxidative stress and antioxidant biomarkers to discriminate cardiovascular diseases. Nutri. Metab. Cardiovasc. Dis 2002, 12, 259–266. [Google Scholar]
- Gros, L.; Saparbaev, M.K.; Laval, J. Enzymology of the repair of free radical-induced DNA damage. Oncogene 2002, 21, 8905–8925. [Google Scholar]
- Paz-Elizur, T.; Sevlya, Z.; Leitner-Dagan, Y.; Elinger, D.; Roisman, L.; Livneh, Z. DNA repair of oxidative DNA damage in human carcinogenesis. Cancer Lett. 2008, 266, 60–72. [Google Scholar]
- Faucher, F.; Doublié, S.; Jia, Z. 8-oxoguanine DNA glycosylases: One lesion, three subfamilies. Int. J. Mol. Sci 2012, 13, 6711–6729. [Google Scholar]
- Mo, J.-Y.; Maki, H.; Sekiguchi, M. Hydrolytic elimination of a mutagenic nucleotide, 8-oxo-dGTP, by human 18-kilodalton protein: Sanitization of nucleotide pool. Proc. Natl. Acad. Sci. USA 1992, 89, 11021–11025. [Google Scholar]
- Ohtsube, T.; Nishioka, Y.; Imaiso, Y.; Iwai, S.; Shimokawa, H.; Oda, H.; Tujiwara, T.; Nakabeppu, Y. Identification of human MutY homolog (hMYH) as a repair enzyme for 2-hydroxyadenine in DNA and detection of multiple forms of hMYH located in nuclei and mitochondria. Nucleic Acids Res 2000, 28, 1355–1364. [Google Scholar]
- Thacker, J. The RAD51 family, genetic instability and cancer. Cancer Lett 2005, 219, 125–135. [Google Scholar]
- Kuchino, Y.; Mori, F.; Kasai, H.; Inoue, H.; Iwai, S.; Miura, K.; Ohtsuka, E.; Nishimura, S. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature 1987, 327, 77–79. [Google Scholar]
- Shibutani, S.; Takeshita, M.; Grollman, A.P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxo-dG. Nature 1991, 349, 431–434. [Google Scholar]
- Floyd, R.A. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis 1990, 11, 1447–1450. [Google Scholar]
- Due, M.-Q.; Carmichael, P.L.; Philips, D.H. Induction of activating mutation in the human C-Ha-ras-1 protooncogene by oxygen free radicals. Mol. Carcinog 1994, 11, 170–175. [Google Scholar]
- Hussain, F.; Aguilar, F.; Cerutti, P. Oxy-radicals induced mutagenesis of hotspot codons 248 and 249 of the human p53 gene. Oncogen 1994, 9, 2277–2281. [Google Scholar]
- Oliva, M.R.; Ripoll, F.; Muñiz, P.; Iradi, A.; Trullenque, R.; Valls, V.; Drehmer, E.; Sáez, G.T. Genetic alterations and oxidative metabolisms in sporadic colorectal tumors from a Spanish community. Molecular Carcinog 1997, 18, 232–243. [Google Scholar]
- Oltra, A.M.; Carbonell, F.; Tormos, C.; Iradi, A.; Sáez, G.T. Antioxidant enzyme activities and the production of MDA and 8-oxo-dG in chronic lymphocytic leukemia. Free Radic. Biol. Med 2001, 30, 1286–1292. [Google Scholar]
- Sánchez, M.; Torres, J.V.; Tormos, C.; Iradi, A.; Muñiz, P.; Espinosa, O.; Salvador, A.; Rodriguez-Delgado, J.; Fandos, M.; Sáez, G.T. Impairment of antioxidant enzyme, lipid peroxidation and 8-oxo-2′-deoxyguanosine in advanced epithelial ovarian carcinoma from a Spanish community. Cancer Lett 2006, 233, 28–35. [Google Scholar]
- Collado, R.; Oliver, I.; Tormos, C.; Egea, M.; Miguel, A.; Cerdá, C.; Ivars, D.; Borrego, S.; Carbonell, F.; Sáez, G.T. Early ROS-mediated DNA damage and oxidative stress biomarkers in Monoclonal B Lymphocytosis. Cancer Lett 2012, 317, 144–149. [Google Scholar]
- Kuo, H.W.; Chou, S.Y.; Hu, T.W.; Wu, F.; Chen, D.J. Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) and genetic polymorphisms in breast cancer patients. Mutation Res 2007, 631, 62–68. [Google Scholar]
- Räsänen, J.V.; Sihvo, E.I.; Ahotupa, M.O.; Farkkila, M.A.; Salo, J.A. The expression of 8-hydroxydeoxyguanosine in oesophageal tissues and tumors. Eur. J. Surg. Oncol 2007, 33, 1164–1168. [Google Scholar]
- Chen, J.H.; Yakes, F.M.; Srivastava, D.K.; Singhal, R.K.; Sobol, R.W.; Horton, J.K.; van Houten, B.; Wilson, S.H. Up-regulation of base excision repair correlates with enhanced protection against DNA damaging agent in mouse cell lines. Nucleic Acid Res. 1998, 26, 2001–2007. [Google Scholar]
- Tudek, B. Base excision repair modulation as a risk factor for human cancers. Mol. Aspects Med 2007, 28, 258–275. [Google Scholar]
- Jemal, A.; Siegal, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics 2009. CA Cancer J. Clin 2009, 59, 225–249. [Google Scholar]
- Loft, S.; Poulsen, H.E. Cancer risk and oxidative DNA damage in man. J. Mol. Med 1996, 74, 297–312. [Google Scholar]
- Ni, J.; Mei, M.; Sun, J. Oxidative DNA damage and repair in chronic gastritis and gastric cancer. Hepatogastroenterology 2012, 59, 671–675. [Google Scholar]
- Liu, R.; Yin, L.H.; Pu, Y.P. Reduced expression of human DNA genes in esophageal squamous-cell carcinoma in China. J. Toxicol. Environ. Health A 2007, 70, 956–963. [Google Scholar]
- Poplawski, T.; Arabski, M.; Kozirowska, D.; Blasinska-Morawiec, M.; Morawiec, Z.; Morawiec-Bajda, A.; Klupińska, G.; Jeziorski, A.; Chojnacki, J.; Blasiak, J. DNA damage and repair in gastric cancer: A correlation with the hOGG1 and RAD51 genes polymorphisms. Mutat. Res 2006, 601, 83–91. [Google Scholar]
- Simic, M.G. DNA markers of oxidative processes in vivo. Relevance to carcinogenesis and anticarcinogenesis. Cancer Res 1993, 54, S1918–S1923. [Google Scholar]
- Kaynar, H.M.; Meral, M.; Turhan, H.; Keles, M.; Celik, G.; Akcay, F. Glutathione peroxidase, glutathione-S-transferase, catalase, xanthine oxidase, Cu-Zn superoxide dismutase activities, total glutathione, nitric oxide and malondialdehyde levels in erythrocytes of patients with small cell and non-small cell lung cancer. Cancer Lett 2005, 227, 133–139. [Google Scholar]
- Ferraris, A.M.; Rolfo, M.; Mangerini, R.; Gaetani, G.F. Increased glutathione in chronic lymphocytic leukemia lymphocytes. Am. J. Hematol 1994, 47, 237–238. [Google Scholar]
- Blume, K.G.; Paniker, N.V.; Beutler, E. Enzymes of glutathione synthesis in patients with myeloproliferative disorders. Clin. Chim. Acta 1973, 45, 281–285. [Google Scholar]
- Ahmad, S.; Okine, L.; Le, B.; Najarian, P.; Vistica, D.T. Elevation of glutathione in phenylalanine mustard-resistant murine L1210 leukemia cells. J. Biol. Chem 1987, 262, 15048–15053. [Google Scholar]
- Toyokuni, S.; Okamoto, D.; Yodoy, J.; Hiai, H. Persistent oxidative stress in cancer. FEBS Lett 1995, 358, 1–3. [Google Scholar]
- Sun, Y. Free radicals, antioxidant enzymes and carcinogenesis. Free Radic. Biol. Med 1990, 8, 583–599. [Google Scholar]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide in human tumor cells. Cancer Res 1991, 51, 794–798. [Google Scholar]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res 2011, 711, 193–201. [Google Scholar]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA damage and disease: Induction, repair and significance. Mutat. Res 2004, 567, 1–61. [Google Scholar]
- Kondo, S.; Toyokuni, S.; Tanaka, T.; Hiai, H.; Onodera, H.; Kasai, H.; Imamura, M. Overexpression of the hOGG1 and high 8-hydroxy-2′-deoxy guanosine (8-OHdG) lyase activity in human colorectal carcinoma: Regulation mechanism of the 8-OHdG level in DNA. Clin. Cancer Res. 2000, 6, 1394–1400. [Google Scholar]
- Liguori, M.; Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages as incessant builders and destroyers of the cancer stroma. Cancers 2011, 3, 3740–3761. [Google Scholar]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation and cancer: How are they linked? Free Radic. Biol. Med 2010, 49, 1603–1616. [Google Scholar]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2004, 454, 436–444. [Google Scholar]
- Redon, C.; Dickey, J.S.; Nakamura, A.; Kareva, I.; Naf, D.; Nowsheen, S.; Kryston, T.B.; Bonner, W.M.; Georgakilas, A.G.; Sedenikova, O.A. Tumors induce complex DNA damage in distant proliferative tissues in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 17992–17997. [Google Scholar]
- Cooke, M.S.; Olinski, R.; Loft, S. Measurements and meaning of oxidatively modified DNA lesions in urine. Cancer Epidemiol. Biomarkers Prev 2008, 17, 3–14. [Google Scholar]
- Valavanidis, A.; Vlachogianni, T.; Flotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ Sci. Health. 2009, 27, 120–139. [Google Scholar]
- Hoeijmakers, J.H. Genome maintenance mechanisms for preventing cancer. Nature 2001, 411, 366–374. [Google Scholar]
- Bruner, S.D.; Norman, P.D.G.; Verdine, Y.L. Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature 2000, 403, 859–866. [Google Scholar]
- Okamoto, K.; Toyokuni, S.; Kim, W.J.; Ogava, O.; Kakehi, Y.; Arao, S.; Hiai, H.; Yoshida, O. Overexpression of human mutT homologue gene messenger RNA in renal-cell carcinoma: Evidence of persistent oxidative stress in cancer. Int. J. Cancer 1995, 65, 437–441. [Google Scholar]
- Kenny, C.H.; Pass, H.I.; Mitchell, J.B. Expression of human MutT homologue (hMTH1) protein in primary non-small-cell lung carcinomas and histologically normal surrounding tissue. Free Radic. Biol. Med 2003, 34, 1447–1457. [Google Scholar]
- Speina, E.; Arczewska, K.D.; Gackowski, D.; Zielinska, M.; Siomek, A.; Kawalewski, J.; Olinski, R.; Tudek, B.; Jusmierek, J.T. Contribution of hMTH1 to the maintenance of 8-oxoguanine levels in lung DNA of non-small-cell lung cancer patients. J. Natl. Cancer Inst 2005, 97, 384–395. [Google Scholar]
- Jewell, R.; Conway, C.; Mitra, A.; Randerson-Moor, J.; Lobo, S.; Nsengimana, J.; Harland, M.; Marples, M.; Edward, S.; Cook, M.; et al. Patterns of expression of DNA repair genes and relapse from melanoma. Clin. Cancer Res 2010, 16, 5211–5221. [Google Scholar]
- Mitra, A.; Jameson, C.; Barbachano, Y.; Sanchez, L.; Kote-Jarai, Z.; Peock, S.; Sodha, N.; Bancroft, E.; Fletcher, A.; Cooper, C.; et al. IMPACT Steering Committee and IMPACT and EMBRACE Collaborators, Eeles R, Foster CS. Overexpression of RAD51 occurs in aggressive prostatic cancer. Histopathology 2009, 55, 696–704. [Google Scholar]
- Cooke, M.S.; Evans, M.D.; Dove, R.; Rozalski, R.; Gackowski, D.; Lunec, J.; Olinski, R. DNA repair is responsible for the presence of oxidatively damaged DNA lesions in urine. Mutat. Res 2005, 574, 58–66. [Google Scholar]
- Svoboda, P.; Kasai, H. Simultaneous HPLC analysis of 8-hydroy-deoxyguanosine and 7-methylguanine in urine from humans and rodents. Anal. Biochem 2004, 334, 239–250. [Google Scholar]
- Bogdanov, M.B.; Beal, M.F.; McCabe, D.R.; Griffin, R.M.; Matson, W.R. A carbon column-based liquid chromatography electrochemical approach to routine 8-hydroxy-2′-dexyguanosine measurements in urine and other biologic matrices: A one-year evaluation of the methods. Free Radic. Biol. Med 1999, 27, 646–666. [Google Scholar]
- Collins, A.R.; Gedik, C.; Wood, S.; White, A.; Dubois, J.; Duez, P.; Rees, J.-F.; Legal, R.; Degand, L.; Loft, S.; et al. Inter-laboratory validation of procedures for measuring 8-oxo-7,8-dihydroguanine/8-oxo-7,8-dhydro-2′-deoxyguanosine in DNA. Free Radic. Res 2002, 36, 239–245. [Google Scholar]
- Evans, M.D.; Olinski, R.; Loft, S.; Cooke, M.S. Toward consensus in the analysis of urinary 8-oxo-7,8-2′-deoxyguanosine as a noninvasive biomarker of oxidative stress. FASEB J 2009, 24, 1249–1260. [Google Scholar]
- Loft, S.; Pousen, H.E.; Vistisens, K.; Knudsen, L.E. Increased urinary excretion of 8-oxo-2′-deoxyguanosine, a biomarker of oxidative DNA damage, in urban bus drivers. Mutat. Res 1999, 441, 11–19. [Google Scholar]
- Lenger, C.; Schöh, G.; Topp, H. A high-performance liquid chromatographic method for the determination of 8-oxo-7,8-2′-deoxyguanosine in urine from man and rat. Anal. Biochem 2000, 287, 65–72. [Google Scholar]
- Weiman, A.; Belling, D.; Poulsen, H. Quantification of 8-oxo-guanine and guanosine as the nucleobase, nucleoside and deoxynucleoside forms in human urine by high-performance liquid chromatography-electrospray mas spectromety. Nucleic Acid. Res 2002, 304, 4–8. [Google Scholar]
- Foksinski, M.; Gackowski, D.; Rozalski, R.; Olinski, R. Cellular level of 8-oxo-2′-deoxyguanosine in DNA does not correlate with urinary excretion of the modified base/nucleoside. Acta Biochem. Pol 2003, 50, 549–553. [Google Scholar]
- Lunec, J.; Holloway, K.A.; Cooke, M.S.; Faux, S.; Griffiths, H.R.; Evans, M.D. Urinary 8-oxo-2′-deoxyguanosine: Redox regulation of DNA repair in vivo? Free Radic. Biol. Med 2002, 33, 875–885. [Google Scholar]
- Hayakwa, H.; Taketomi, A.; Sakimi, K.; Kuwano, M.; Sekiguchi, M. Generation and elimination of 8-oxo-7,8-dihidro-2′-deoxyguanosine 5′-triphophate, a mutagenic substrate for DNA synthesis, in human cells. Biochemistry 1995, 34, 89–95. [Google Scholar]
- Cooke, M.S.; Henderson, P.T.; Evans, M.D. Sources of extracellular, oxidatively-modified DNA lesions: Implications for their measurement in urine. J. Clin. Nutr 2009, 45, 255–270. [Google Scholar]
- Carpelan-Holmström, M.; Louhimo, J.; Stenman, U.H.; Alfthan, H.; Haglund, C. CEA, CA19-9 and CA72-4 improve the diagnostic accuracy in gastrointestinal cancers. Anticancer Res 2002, 22, 2311–2316. [Google Scholar]
- Jass, J.R.; Sobin, L.H. Histological Typing of Intestinal Tumors (International Histological Classification of Tumors, WHO); Springer Verlag New York, Inc.: New York, NY, USA, 1989. [Google Scholar]
- Ahn, S.; Lee, H.J.; Hahn, S.; Kim, W.H.; Lee, K.U.; Sano, T.; Edge, S.B.; Yang, H.K. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer 2010, 116, 5592–5598. [Google Scholar]
- Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Scan. J. Clin. Lab. Invest 1968, 21, 77–89. [Google Scholar]
- Navarro, J.; Obrador, E.; Pellicer, J.A.; Asensi, M.; Viña, J.; Estrela, J.M. Blood glutathione as an index of radiation-induced oxidative stress in mice and humans. Free Radic. Biol. Med 1997, 22, 1203–1209. [Google Scholar]
- Brigelius, R.; Muckel, C.; Akerboom, T.P.; Sies, H. Identification and quantitation of glutathione in hepatic protein mixed disulfides and its relationship to glutathione disulfide. Biochem. Pharmacol 1983, 32, 2529–2534. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar]
- Gupta, R.C. Non random binding of the carcinogen N-hydroxy-2-acetylaminofluorene to repetitive sequences of rat liver DNA in vivo. Proc. Natl Acad. Sci. USA 1984, 81, 6943–6947. [Google Scholar]
- Muñíz, P.; Valls, V.; Perez-Broseta, C.; Iradi, A.; Climent, J.V.; Oliva, M.R.; Sáez, G.T. The role of 8-hydroxy-2′-deoxyguanosine in rifamycin-induced DNA damage. Free Radic. Biol. Med 1995, 18, 747–755. [Google Scholar]
- Wei, H.; Frenkel, K. Suppression of tumor promoter-induced oxidative events and DNA damage in vivo by sarcophytol A: A possible mechanism of antipromotion. Cancer Res 1992, 32, 2298–2303. [Google Scholar]
- Brown, R.K.; McBurney, A.; Kelly, F.J. Oxidative damage to DNA in patients with cystic fibrosis. Free Radic. Biol. Med 1995, 18, 801–806. [Google Scholar]
- Espinosa, O.; Jiménez-Almazán, J.; Chaves, F.J.; Tormos, C.; Clapés, S.; Iradi, A.; Salvador, A.; Fandos, M.; Redón, J.; Sáez, G.T. Urinary 8-oxo-2′-deoxyguanosine (8-oxo-dG), a reliable oxidative stress marker in hypertension. Free Rad Res 2007, 44, 546–554. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar]




| Metabolites | Controls | Gastric Cancer | p < 0.001 |
|---|---|---|---|
| 8-oxo-dG/106 dG * | 4.16 ± 0.73 | 8.43 ± 1.3 | *** |
| 8-oxo-dG (nmol/mmol creatinine) ** | 2.49 ± 1.07 | 22.29 ± 4.79 | *** |
| MDA (nmol/mg protein) * | 0.17 ± 0.06 | 0.52 ± 0.13 | *** |
| GSH (nmol/mg protein) * | 22.42 ± 3.85 | 14.00 ± 3.04 | *** |
| GSSG (nmol/mg protein) * | 4.18 ± 2.02 | 4.62 ± 1.55 | NS |
| % GSSG/GSH * | 18.65% | 33.00% | *** |
| Metabolites | Basal values | 3 Months after Surgery |
|---|---|---|
| 8-oxo-dG/106 dG * | 8.91 ± 1.02 | 9.37 ± 0.90 |
| 8-oxo-dG (nmol/mmol creatinine) ** | 18.61 ± 2.54 | 19.98 ± 3.08 |
| MDA (nmol/mg protein) * | 0.52 ± 0.09 | 0.54 ± 0.04 |
| GSH (nmol/mg protein) * | 11.54 ± 1.57 | 15.77 ± 3.97 |
| GSSG (nmol/mg protein) * | 4.43 ± 0.51 | 4.66 ± 0.45 |
| % GSSG/GSH * | 38.40% | 29.55% |
| Clinical Characteristics | Mean Values of Patients and Control Population (m–M) | ||
|---|---|---|---|
| Cancer Patients | Control Subjects | Reference Values | |
| %Female/male | 33/67 | 51/49 | - |
| Mean age | 70 (48–90) | 60 (36–90) | - |
| Hb | ♀: 10.8 (9.2–12.5) | ♀: 13.26 (11.6–16.7) | ♀: 11.5–16 g/dL |
| ♂: 11.44 (8.4–15.2) | ♂: 14 (13.6–17) | ♂: 13.5–18 g/dL | |
| Hto | ♀: 32.3 (27.1–36.9) | ♀: 40.1 (35.1–50.6) | ♀: 35%–50% |
| ♂: 34.93 (25.9–56.7) | ♂: 42.4 (41.2–51.5) | ♂: 40%–54% | |
| Leucocytes | 10.33 (3.3–24.1) | 5.7 (3.5–11) | 3.6–11.5 × 109/L |
| PCR | 5.5 (0.19–19.12) | 0.02 (0.01–0.03) | 0.00–0.05 mg/dL |
| CEA | 2.61 (0.5–14.4) | - | 0.0–3.0 ng/mL |
| CA19.9 | 9.28 (0.8–27.5) | - | 0.0–35 UI/mL |
| Tumor Grade | % |
|---|---|
| I | 2.1 |
| I B | 8.5 |
| II | 27.6 |
| II B | 2.1 |
| III A | 19.2 |
| III B | 6.5 |
| IV | 34 |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Borrego, S.; Vazquez, A.; Dasí, F.; Cerdá, C.; Iradi, A.; Tormos, C.; Sánchez, J.M.; Bagán, L.; Boix, J.; Zaragoza, C.; et al. Oxidative Stress and DNA Damage in Human Gastric Carcinoma: 8-Oxo-7'8-dihydro-2'-deoxyguanosine (8-oxo-dG) as a Possible Tumor Marker. Int. J. Mol. Sci. 2013, 14, 3467-3486. https://doi.org/10.3390/ijms14023467
Borrego S, Vazquez A, Dasí F, Cerdá C, Iradi A, Tormos C, Sánchez JM, Bagán L, Boix J, Zaragoza C, et al. Oxidative Stress and DNA Damage in Human Gastric Carcinoma: 8-Oxo-7'8-dihydro-2'-deoxyguanosine (8-oxo-dG) as a Possible Tumor Marker. International Journal of Molecular Sciences. 2013; 14(2):3467-3486. https://doi.org/10.3390/ijms14023467
Chicago/Turabian StyleBorrego, Silvia, Antonio Vazquez, Francisco Dasí, Concha Cerdá, Antonio Iradi, Carmen Tormos, Julia M. Sánchez, Leticia Bagán, Javier Boix, Cristóbal Zaragoza, and et al. 2013. "Oxidative Stress and DNA Damage in Human Gastric Carcinoma: 8-Oxo-7'8-dihydro-2'-deoxyguanosine (8-oxo-dG) as a Possible Tumor Marker" International Journal of Molecular Sciences 14, no. 2: 3467-3486. https://doi.org/10.3390/ijms14023467
APA StyleBorrego, S., Vazquez, A., Dasí, F., Cerdá, C., Iradi, A., Tormos, C., Sánchez, J. M., Bagán, L., Boix, J., Zaragoza, C., Camps, J., & Sáez, G. (2013). Oxidative Stress and DNA Damage in Human Gastric Carcinoma: 8-Oxo-7'8-dihydro-2'-deoxyguanosine (8-oxo-dG) as a Possible Tumor Marker. International Journal of Molecular Sciences, 14(2), 3467-3486. https://doi.org/10.3390/ijms14023467







